Abstract
Background
Gene expression analysis is useful for assessing cellular behavior and may improve our understanding of the initial cellular response to mechanical load leading to tendon degeneration. This study assessed gene expression of MMP-1 and MMP-3, genes associated with matrix degradation, in tendons exposed to cyclic loads within physiologic range.
Methods
Six flexor tendons from each of ten New Zealand White rabbits were harvested and randomly assigned to one of the following six groups: load deprived for 18 hours; cyclically loaded for 18 hours to a peak stress of 2 MPa; 3 MPa; 4 MPa; 5 MPa; or snap frozen in liquid nitrogen. MMP-1, MMP-3 and 18s mRNA expression was measured by qRT-PCR.
Results
No significant differences in MMP-1 mRNA expression levels were found between loading groups. MMP-3 expression was significantly inhibited (48%) in tendons cyclically loaded to a peak stress of 4 MPa in comparison to load deprived tendons, however, when peak stress was increased to 5 MPa, expression was no longer significantly lower compared to stress shielded tendons.
Interpretation
The results suggest a ‘U’ shape relationship between load and MMP-3 expression. The lack of change in MMP-1 expression with loading was unexpected as inhibition of MMP-1 in response to mechanical load has been demonstrated in previous studies. In conclusion, we demonstrate that MMP-3 expression is modulated by cyclic load and is sensitive to load magnitude. MMP-1 mRNA expression is not significantly modulated by cyclic load in this model.
Introduction
Tendon overuse injuries are characterized by soreness, pain, and limited range of motion (Jozsa and Kannus, 1997). These injuries are slow to heal, difficult to treat and often recurrent presenting a burden to athletes (Kannus, 1997) and a significant source of disability to workers (NRC, 2001).
Matrix metalloproteinase (MMP), a group of tightly regulated zinc dependent enzymes, play a crucial role in the normal development, repair and remodeling of connective tissues. MMP’s, which include interstitial collagenase 1 (MMP-1) and stromelysin-1 (MMP-3), are capable of degrading intact fibrilar collagen, proteoglycans and other extracellular matrix (ECM) components (Matrisian, 1990). The failure to properly regulate these enzymes may lead to improper or excess matrix degeneration and play a role in the development of pathological tendon conditions (Matrisian, 1990; Riley, et al., 2002).
Previous studies have found a strong relationship between expression of these genes and mechanical load in tendons and ligament (Majima et al., 2000; Tsuzaki et al., 2003, Lavignino et al., 2003; Arnoczky et al., 2004; Yang et al., 2005). In rat tail tendons, for example, moderate static (Arnoczky et al., 2004) and cyclic (Lavignino et al., 2003) loading has been shown to inhibit MMP-1 expression. In rabbit menisci, cyclic tensile load inhibited MMP-1 expression (Majima et al., 2000) while cyclic hydrostatic pressure reduced MMP-3 expression (Natsu-Ume et al., 2005) compared to unloaded controls.
Few studies, however, have examined the effects of higher loads, within physiologic range, on tissue explants. A study by Flick et al. (2005) examined the effects of repetitive loading, up to 12 MPa, on avian flexor tendon viability and secretion of PGE2. They found PGE2 secretion to increase in aggressively loaded tendons relative to moderately loaded tendons, suggesting that aggressive cyclic loading may lead to an initial inflammatory response.
The goal of this study was to assess changes in gene expression levels of MMP-1 and MMP-3, in rabbit flexor tendons exposed to cyclic loads within physiologic range for an extended period of time. Tendons were cyclically loaded to peak stresses of 0 (load deprived), 2, 3, 4 or 5 MPa continuously for 18 hours. These loads are within the range measured in vivo in rabbit flexor tendons by Malaviya et al. (1998) who found tensile stresses of 1 MPa and 8 MPa during quite standing and level hopping, respectively. We hypothesized that cyclic loading at moderate loads, 2 MPa and 3 MPa, will inhibit mRNA expression of MMP-1 and MMP-3 relative to stress shielded tendons. We also hypothesized that cyclic loading to higher load, 4 and 5 MPa, would lead to an upregulation of mRNA expression relative to moderate loads.
Methods
Loading System
A custom built, in vitro tendon loading system, described elsewhere (Asundi et al. 2007), was used to simultaneously apply cyclic loads to five tendons. Briefly, the loading system consisted of six independent actuators each applying a tensional load to a single tendon. Tendons were held by two clamps. The position of the upper clamp was controlled by the actuator while the lower clamp was held stationary. Both clamps extended away from the actuator so the tendon could be fully submerged in culture media. A compact load cell, in series with the clamps continuously recorded tension applied to the tendon. Labview software (V 6.2, National Instruments, Austin, TX) was used to control the actuators and collect data.
Tendons
Six flexor digitorum profundus tendons, approximately 45 mm in length, were harvested from the hind paws of 10 New Zealand White rabbits (total of 60 tendons) under sterile conditions. The rabbits were euthanized for a separate and unrelated study. Tendons were immediately placed in CO2 Independent Media (Invitrogen, Carlsbad, CA, USA) with 10% FBS (Invitrogen, Carlsbad, CA, USA), 1% antibiotics (Invitrogen, Carlsbad, CA, USA) and 100 μg/ml of ascorbic acid (Sigma Aldrich, St. Louis, MO, USA).
Loading
The six tendons from each rabbit were randomly assigned to one of the following groups (n=10 for each group). Group 1: load deprived (0 MPa); Group 2: cyclically loaded to a peak stress of 2 MPa; Group 3: cyclically loaded to a peak stress of 3 MPa; Group 4: cyclically loaded to a peak stress of 4 MPa; Group 5: cyclically loaded to a peak stress of 5 MPa; and Group 6: a 5 mm section was cut from the tendon and snap frozen in liquid nitrogen. The Group 6 tendons were used to assess expression levels under in vivo conditions and were not included in the statistical analysis.
Cross-sectional area (CSA) of each tendon was measured with a load applied micrometer (Butler et al., 1984) to establish the required force in order to achieve the desired stresses. Measurements were done prior to in vitro loading. Briefly, tendons were fit into a slot 1.3 mm wide. A plunger measuring 8 mm long and 1.3 mm wide was pressed down on the tendon with a 50 g weight applying a constant pressure of 0.05 MPa for 30 seconds. CSA was calculated from the measured tendon thickness and the width of the slot. Tendons were then removed from the micrometer and the fibrocartilage zone of the tendon (Region B/C according to Okuda et al., (1987)) was secured in the proximal clamp. The distal clamp was padded with sterile gauze before securing the distal end of the tendon.
Tendons were preconditioned by loading from 1 MPa to 2 MPa for 20 cycles. This was to allow the tendons to settle in to the clamps. Gauge length was assessed after preconditioning by applying a 0.5N preload and measuring the clamp to clamp length. A slack of 2mm was provided to tendons in the load deprived group. Cyclic loading was conducted using a modified square wave form at 0.45Hz with a 50% duty cycle and a peak stress rate of 10 MPa (Figure 1). Trough stress for all loaded tendons was 0.5 MPa.
Figure 1.
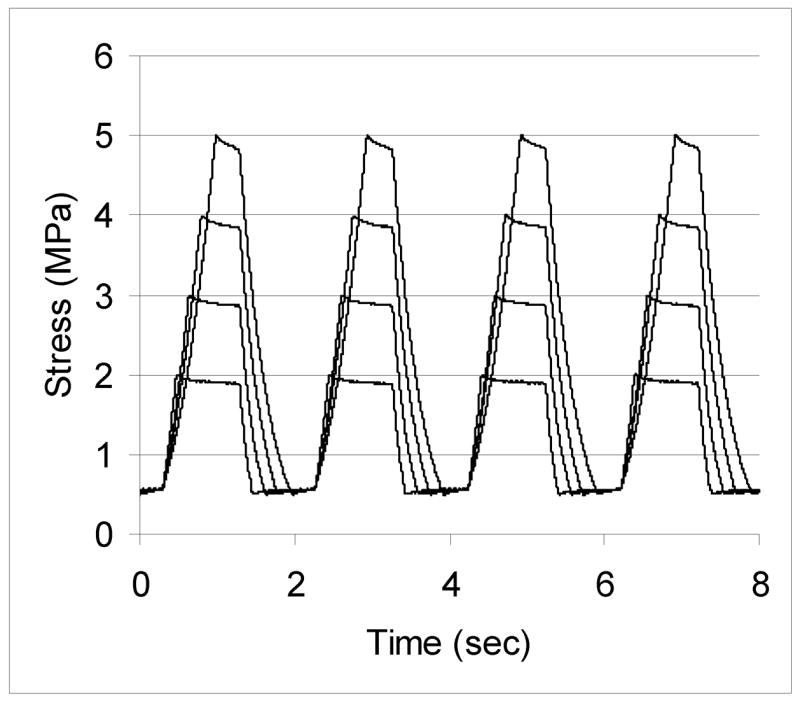
Loading profile over four cycles of load for tendons loaded between 0.5 MPa and a peak stress of 2, 3, 4 or 5 MPa.
Initial strain was defined as the strain at peak stress for the first cycle of loading. Final strain was defined as the strain at peak stress for the last cycle of loading. Creep was defined as the difference between final and initial strain.
At the end of loading, tendons were released from the clamps and a 5 mm section of the tendon was cut and frozen in liquid nitrogen. The 5 mm section was cut at least 3 mm away from either clamp to avoid possible clamp effects. Sections were stored at −70°C until processed.
RNA Extractions
Total RNA was extracted from each tendon section using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). After extraction, RNA was DNase treated and concentrated using an RNeasy Minelute cleanup Kit (Qiagen, Valencia, CA, USA). An aliquot of 385 ng of RNA was then reverse transcribed (Taqman Reverse Transcription Reagents, Applied Biosystems, Foster City, Ca, USA) to cDNA.
Real Time PCR
Expression levels of MMP-1, MMP-3 and 18s were quantified by Real Time PCR (ABI-Prism 7000 Sequence Detection System) using Applied Biosystem’s SYBR Green master mix. Oligonucleotide sequences for the genes of interest were obtained from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). Primer sets for MMP-1 (5′-AGGAGCCTTCCCAAGAGGAA-3′ | 5′-CTTGTCTCTTGCATATCAGGATGATG-3), MMP-3 (5′-AGCCAATGGAAATGAAAACTCTTC-3′ | 5′-CCAGTGGATAGGCTGAGCAAA-3′), and 18s (5′-AGTGCGGGTCATAAGCTTGC-3′ | 5′-GGTGTGTACAAAGGGCAGGG-3′) were designed from the sequences using Applied Biosystem’s Primer Express software V2. Data was analyzed using the standard curve method (Applied Biosystems, ABI Prism 7700 SDS User Bulletin #2). MMP-1 and MMP-3 expression was first normalized to 18s. Gene expression fold change relative to the load-deprived (0 MPA) samples was calculated for each loaded sample (2, 3, 4 and 5 MPa).
Statistics
Gene expression fold changes were log transformed to normalize their distribution before means were compared. Differences in mean expression levels were compared between loading groups 1 through 5 by repeated measures ANOVA and significant differences were followed up with a Tukey test. All statistical analyses were performed with SAS (SAS Institute Inc., Cary, NC, USA) and significance was taken as p<0.05.
Results
Loading
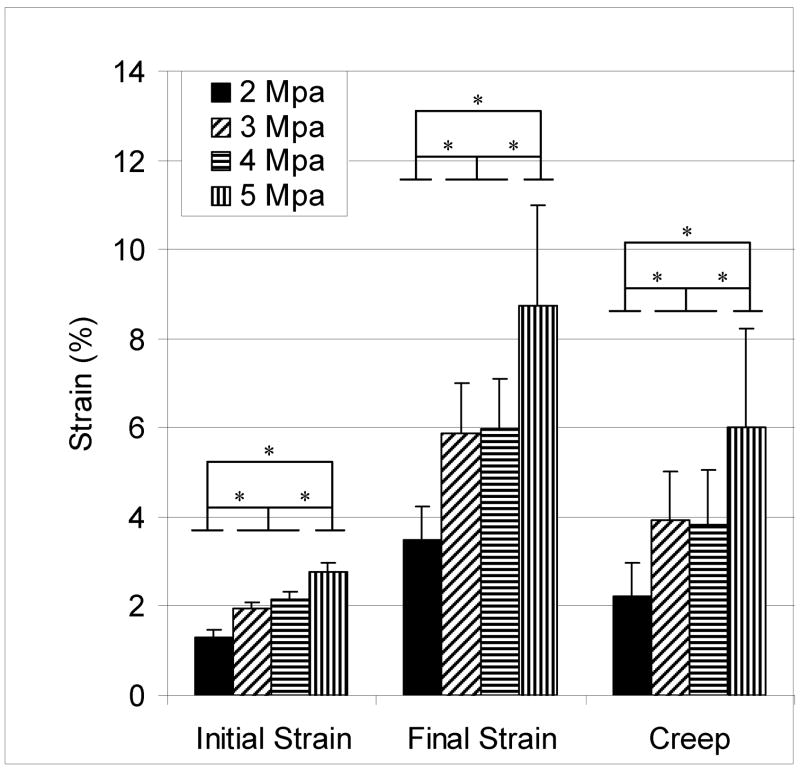
Greater peak stress led to greater initial strain (Figure 2). Initial strain was significantly greater in tendons exposed to a peak stress of 3 MPa, 4 MPa and 5 MPa compared to 2 MPa. Initial strain was also significantly greater in tendons exposed to a 5 MPa peak stress compared to a 3 and 4 MPa peak stress. Initial strain was not significantly different between tendons exposed to 3 MPa and 4 MPa. These differences between loading groups was also found for final strain and creep (Figure 2).
Figure 2.
Mean (± SD) initial strain, final strain and creep in tendons cyclically loaded to a peak stress of 2, 3, 4, 5 MPa for 18 hours. Cyclic loading to higher peak stresses led to greater initial strain, final strain and creep. (n = 10, *p<0.05)
Gene Expression
Gene expression levels for fresh frozen controls (Group 6) were 0.1% (0.0%) and 0.9% (0.2%) of the expression level in load deprived samples for MMP-1 and MMP-3, respectively.
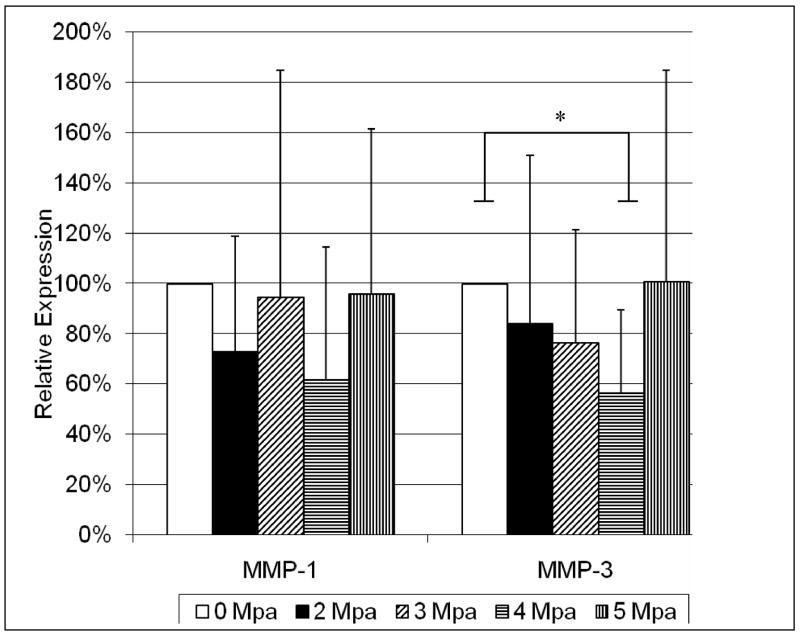
Cyclic loading reduced MMP-1 expression relative to stress shielding; however, expression levels were not significantly different for any loading group (Figure 3). MMP-3 mRNA expression in tendons cyclically loaded to a peak stress of 4 MPa was significantly reduced (0.57, p = 0.04) in comparison to stress shielded tendons (Figure 3). (Note: Figure 3 presents the mean fold change for each loading group, which over represents increases (e.g. the average of a 2 fold increase and a 0.5 fold decrease is 1.25, not 1). Mean fold changes are presented instead of mean log transformed values however as they are easier to interpret.)
Figure 3.
Mean (±SD) expression levels in tendons statically loaded to 2, 3, 4, or 6 MPa relative to 0 MPa [load deprived tendon]. MMP-1 expression was not significantly different between loading groups. Cyclic loading to a peak stress of 4 MPa resulted in a significant decrease (0.57) in MMP-3 expression relative to stress shielded tendons. (n=10, *p=0.04).
Discussion
In this study we examined the effects of 18 hours of cyclic loading within physiologic range on the expression of MMP-1 and MMP-3 mRNA in tendon explants. Contrary to our hypothesis, no significant changes were found in MMP-1 expression with cyclic loading. Cyclic loading to a peak stress of 4 MPa led to significant inhibition of MMP-3 relative to stress shielded controls; however no significant differences were found between moderate and higher loads.
Collagenase-1 (MMP-1) is involved in the degradation of type-1 collagen by cleaving a single locus in the collagen triple helix creating ¾ and ¼ fragments which are then further degraded by other proteinases (Riley et al., 2002). Type I collagen is a very stable molecule, resistant to proteolytic cleavage (Matrisian, 1990). As one of the few enzymes capable of cleaving intact fibrilar collagen, the proper regulation of MMP-1 expression is critical in maintaining a healthy extracellular matrix.
The lack of inhibition of MMP-1 seen in our study is an unexpected finding as inhibition of MMP-1 expression with mechanical load has been demonstrated in other models (Majima et al., 2000; Lavignino et al., 2003; Arnoczky et al., 2004). In rat tail tendons, cyclic stretching to a peak strain of 6% at 0.017 Hz or to a peak strain of 1% at 1Hz, for 24 hours was enough to inhibit MMP-1 expression relative to unloaded controls (Lavignino et al., 2003). Static loads of 0.77 MPa, 1.38 MPa and 2.6 MPa have also been found to decrease expression of MMP-1 in rat tail tendons (Arnoczky et al., 2004). A decrease in expression of MMP-1 was also seen in medial collateral ligaments exposed to a cyclic tensile stress of 4 MPa, resulting in strains between 2 and 6%, at 0.5 Hz for 4 hours (Majima et al., 2000). Even though the range of stresses applied in our model included the stresses used in these studies we were unable to replicate their findings of decreased MMP-1 expression with loading relative to unloaded controls.
A possible explanation for the lack of inhibition may be uneven load distribution across tendon fibrils. A recent study examined crimp pattern extinction in tendons through optical coherence tomography (Hanson et al., 2002). Fascicles from rat tail tendons were subjected to 0.5 percent strain increments and imaged at each increment. They found banding disappeared sooner at the surface than along the center axis indicating a greater load was carried by the surface fibrils compared to center fibrils. As rabbit flexor tendons are considerably larger in diameter (1 – 1.5 mm) than rat tail tendon fascicles (200–400 μm), cyclic loading to peak stresses up to 5 MPa may not have recruited enough of the center fibrils to significantly inhibit MMP-1 expression.
In addition, unlike rat tail tendons or rabbit knee ligaments, flexor tendons contain a large fibrocartilage region. While this region was excluded from the section of tendon examined for mRNA expression, its presence may have affected how loads were distributed through the tendon.
MMP-3 expression was significantly reduced with cyclic loading to a peak stress of 4 MPa indicating it may be more sensitive to load than MMP-1. MMP-3 has broad substrate specificity, degrading proteoglycans, laminin, fibronectin and the globular portions of the basement membrane collagens. In addition to degrading various matrix molecules, MMP-3 can ‘super-activate’ MMP-1, increasing its collagenase activity up to eightfold, making it an important component in the degradation of the ECM (Matrisian, 1990). Physical stimulation has been shown to affect MMP-3 expression. In rat intervertebral disks, MMP-3 expression was found to be sensitive to magnitude and frequency, with higher magnitudes and frequencies resulting in greater expression (MacLean et. al., 2004, MacLean et al., 2005). In cell culture models, cyclic stretching has been shown to up-regulate MMP-3 expression in chondrocytes (Honda et al., 2000) and tendon cells (Tzusaki et al., 2003). Mechanical load has also been found to have an inhibitory effect. Natsu-Ume et al. (2005) exposed rabbit meniscal explants to cyclic hydrostatic pressure and found reduced MMP-3 mRNA expression levels in tissues cultured under loading compared to non-loading conditions.
This study demonstrates that MMP-3 expression is also inhibited through cyclic load in tendon explants. This inhibition is load dependent with lower peak stresses of 2 and 3 MPa decreasing expression but not significantly. Expression was significantly inhibited in tendons cyclically loaded to a peak stress of 4 MPa, however when peak stress was increased to 5 MPa, expression was no longer significantly lower compared to stress shielded tendons. This would suggest a possible “U” shape relationship between load and MMP-3 expression. Stress shielding and high cyclic loads may lead to an upregulation of expression, while moderate loads reduce such expression.
A limitation of our study was that it only examined mRNA expression. The matrix metalloproteinases are tightly regulated after expression, requiring additional activation and may be inhibitied by other proteins (Matrisian, 1990). Examination of protein levels and enzyme activity should be conducted to confirm the findings in this study. Another limitation of the study was high variability between samples. Statistical analysis of the results indicated there were no significant differences in expression levels of MMP-1 between any of the loading groups. However, the variability in gene expression levels was high (C.V. = 278%), so that even if there was a trend associated with loading, the findings would probably not have achieved statistical significance. Various factors may have contributed to increasing variability including the RNA extraction and reverse transcription process, tissue cross sectional area, location of the tissue between the clamps, distribution of fibro-cartilage within the tendon and the location of the tissue sample used.
In conclusion we demonstrate that MMP-3 expression was modulated by cyclic load and is sensitive to load magnitude. The dose-response relationship with load appears to be “U” shaped. MMP-1 mRNA expression was not significantly modulated by cyclic load in this model.
Acknowledgments
Funding for this study was provided by the Centers for Disease and Prevention, National Institute for Occupational Safety and Health Training Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnoczky S, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22(2):328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 2.Asundi K, Kursa K, Lotz J, Rempel D. In vitro system for applying cyclic loads to connective tissues under force or displacement control. Ann Biomed Eng. 2007 doi: 10.1007/s10439-007-9295-9. In press. [DOI] [PubMed] [Google Scholar]
- 3.Butler D, Grood E, Noyes FR, Zernicke RF, Brackett K. Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J Biomechanics. 1984;17(8):579–596. doi: 10.1016/0021-9290(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 4.Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech. 2006;21(1):99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Hanson K, Weiss J, Barton J. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72–77. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- 6.Honda K, Ohno S, Tanimoto K, Ijuin C, Tanaka N, Doi T, Kato Y, Tanne K. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79(9):601–609. doi: 10.1078/0171-9335-00089. [DOI] [PubMed] [Google Scholar]
- 7.Jozsa L, Kannus P. Human Kinetics. Champaign; Illinois: 1997. Human Tendons: Anatomy, Physiology and Pathology. [Google Scholar]
- 8.Kannus P. Tendons—a source of major concern in competitive and recreational athletes. Scand J Med Sci Sports. 1997;7:53–4. [PubMed] [Google Scholar]
- 9.Lavignino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile stress on the inhibition of MMP-1 mRNA expression in tendon cells: An in vitro study. Connect Tissue Res. 2003;44:181–87. doi: 10.1080/03008200390215881. [DOI] [PubMed] [Google Scholar]
- 10.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23(5):1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Majima T, Marchuk LL, Shrive NG, Frank CB, Hart DA. In-vitro cyclic tensile loading of an immobilized and mobilized ligament autograft selectively inhibits mRNA levels for collagenase (MMP-1) J Orthop Sci. 2000;5(5):503–510. doi: 10.1007/s007760070030. [DOI] [PubMed] [Google Scholar]
- 13.Malaviya P, Butler DL, Korvick DL, Proch FS. In vivo tendon forces correlate with the activity level and remain bounded: evidence in a rabbit flexor tendon model. J Biomechanics. 1998;31:1043–1049. doi: 10.1016/s0021-9290(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 14.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. TIG. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council and the Institute of Medicine. Commission on Behavioral and Social Sciences and Education. Washington, DC: National Academy Press; 2001. Musculoskeletal Disorders and the Workplace: Low Back and Upper Extremities. Panel on Musculoskeletal Disorders and the Workplace; pp. 196–199. [Google Scholar]
- 16.Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10(4):396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 17.Okuda Y, Gorski JP, An KN, Amadio PC. Biochemical, histological and biomechanical analyses of canine tendon. J Orthop Res. 1987;5:60–68. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- 18.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodeling in tendon pathology. Matrix Biology. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 19.Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89(3):556–562. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]