Abstract
Olfaction is a major sensory element in intraspecies recognition and communication in mice. The present study investigated scent marking behaviors of males of the highly inbred C57BL/6J (C57) strain in order to evaluate the ability of these behaviors to provide clear and consistent measures of social familiarity and response to social signals. C57 males engage in scent marking when placed in a chamber with a wire mesh partition separating them from a conspecific. Male mice (C57 or outbred CD-1 mice) showed rapid habituation of scent marking (decreased marking over trials) with repeated exposure at 24-hr intervals, to a stimulus animal of the C57 or CD-1 strains, or to an empty chamber. Subsequent exposure to a genetically different novel mouse (CD-1 after CD-1 exposure, or CD-1 after C57 exposure) or to a novel context (different shaped chamber) produced recovery of marking, while responses to a novel but genetically identical mouse (C57 after C57 exposure) or to the empty chamber did not. This finding demonstrated that male mice differentiate familiar and novel conspecifics as expressed by habituation and recovery of scent marking, but neither C57 or CD-1 mice can differentiate new vs. familiar C57 males; likely due to similarities in their odor patterns. The data also indicate that scent marking can differentiate novel from familiar contexts.
Keywords: social recognition, urine marking, familiarity, context recognition, C57BL/6J mice
1. Introduction
The ability to differentiate and respond to individuals has advantages in many social contexts, enabling animals to adjust their behavior in accord with this information [8,31,33,42]. Among mammals, including mice, urinary scent plays a significant role in olfactory social signaling [2,12,34]. Urinary odors are used both to discriminate between individual conspecifics [7,24,32], and to communicate information such as dominance and health [27,29,35,61]. Scent marking is also used by males to advertise their male quality to potential mates [26,46], and to advertise territory ownership [18,25,30]. When territory owners encounter scent from other males within their territory, they increase their own rate of scent marking to countermark the alien scent [27,45], but show no such response to their own scent marks or to these from males genetically identical to themselves [28,41–42].
The tendency for rodents to investigate conspecifics has long been used as a paradigm to evaluate social attraction and social memory [17,56–57]. This social investigation paradigm has been used for a variety of mouse models associated with neurocognitive disorders including autism [44,58], Fragile X syndrome [38,55] and Rett syndrome [50], as well as studies of the effects of neuropeptides including oxytocin [21,22] and vasopressin [49]. A habituation-dishabituation paradigm for social investigation to conspecifics can provide a measure of social memory [56–57]. In this task, an adult male is allowed to investigate a juvenile or ovariectomized female that is introduced to its cage. When the stimulus animal is removed and later re-introduced, subjects display reduced investigation compared to the first encounter [6]. A three-chamber test modeled on place preference paradigms has been shown to provide quantitative, replicable, data on investigation of social and nonsocial stimuli, demonstrating preference for social stimuli, and habituation to individual social stimuli over repeated exposures [9–10,39–40].
The present experiments aim to assess a complementary approach to analysis of social behaviors and memory, through scent marking. Conspecific communication in mice involves both the emission of olfactory social signals (scent marking) and response to such social signals through additional scent marking, often overmarking the initial scent [2,12,25,34]. The present experiments of scent marking utilized C57BL/6J (C57) mice, an inbred strain often used as a background for transgenic mice [15–16,51], in order to facilitate the potential use of this technique for genetic studies of animal models of neurocognitive disorders. Experiments 1, 2 and 3 investigated scent marking response of adult male mice to inbred (C57) or outbred (CD-1) mice, and the habituation of this response over repeated exposures. Experiment 4 examined a potential confound in these studies; scent marking and habituation of marking to an initially novel context, and recovery of marking to a novel context. Experiment 5 attempted to separate habituation to a context from habituation to a stimulus mouse, utilizing a paradigm that evaluated these changes independently.
2. Materials and methods
2.1 Subjects and rearing condition
All protocols and animal handling and treatment were approved by the Institutional Animal Care and Use Committee at the University of Hawaii. Male C57BL/6J mice bred from stock obtained from the Jackson Laboratory (Bar Harbor, ME), were used as the subjects. They were weaned at 4 weeks of age and then housed in same-sex groups (N=3–4), in polypropylene cages, 26.5 × 17 × 11.5 (H) cm, under 12L:12D cycle (lights on 06:00) in a temperature- (22±2 °C) and humidity- (60 %) controlled room at the University of Hawaii Laboratory Animal Services. For 1 week prior to the test, they were singly housed in their home cages. Male outbred CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA), singly housed in polypropylene cages for at least 3 week prior to the test. All animals were allowed free access to food and water in their home cages.
Experiment 1
Fourteen C57 males, 16 weeks of age, were used as the subjects. Fourteen CD-1 males, 16 weeks of age, were used as the stimulus animals.
Experiment 2
Fourteen CD-1 males, 16 weeks of age, were used as the subjects and also as the stimulus animals. Fourteen C57 males, 16 weeks of age, were used as the stimulus animals.
Experiment 3
Twelve C57 males 15 weeks of age were used as the subjects. Six CD-1 males aged 14 weeks were used as the stimulus animals.
Experiment 4
Twelve C57 males 15 weeks of age were used as the subjects.
Experiment 5
Twelve C57 males 15 weeks of age were used as the subjects. Twelve CD-1 males 16 weeks of age were used as the stimulus animals.
2.2 Apparatus
Testing for scent marking was conducted in a bottomless Polycarbonate cage (46 × 24 × 21 (height) cm), placed upside down on a rough paper (457 × 365 mm, Rough Newsprint paper, Bienfang) substrate. The test chamber was divided into two equal-sized compartments by a wire mesh screen, which prevented direct physical contact between animals, but allowed olfactory, visual, and auditory cues to be received.
In Experiment 4, two different cages were used to provide different contexts. One was the same rectangular cage that used in other experiments, while the other was a triangle-shaped opaque plastic cage, 47.4 × 36 × 30.8 × 18 (height) cm, the top of which was covered with clear Plexiglas and one side of which was delimited by a wire mesh wall.
2.3. Procedure
All test trials were conducted during the light phase of the light/dark cycle under dimly lit conditions. Twenty min before the beginning of each trial, animals were moved in their home cage from holding room to the experimental room. Animals were placed in one compartment of the test chamber, the bottom of which was covered by a fresh sheet of rough paper. At the end of the 20-minute trial, the animals were returned to their home cage and moved back to the holding room. Between trials, the apparatus was cleaned with 15% alcohol and dried with paper towels. Inter-trial intervals were 24 hrs in length. Scent marking was evaluated on each trial.
Experiment 1
Each of 14 C57 males was confronted with an initially novel CD-1 male, in an initially novel test chamber, on each of four trials (to permit habituation). On the fifth, discrimination, trial, a novel CD-1 male was presented as the stimulus.
Experiment 2
Each of 14 CD-1 males was confronted with an initially novel C57 male in an initially novel situation on four (habituation) trials, with a novel C57 male presented on the fifth, discrimination, trial, and a novel CD-1 male presented on a sixth trial. After a 3-day interval, the same 14 CD-1 male subjects were each confronted with an initially novel CD-1 male in the familiar test situation for 3 trials. On the fourth trial in this series, an unfamiliar CD-1 male was presented.
Experiment 3
C57 males were exposed to an initially novel C57 male on each of four trials. On the fifth, discrimination, trial, each C57 male was exposed to a novel C57 male. On the sixth trial, each C57 male was exposed to a novel CD-1 male.
Experiment 4
To evaluate habituation of C57 males to an initially novel context, each C57 male was first placed in the same empty chamber (rectangular or triangle chamber cage, randomly assigned) on each of four trials. On the fifth trial, they were placed in the unfamiliar empty chamber (triangle or rectangular cage, as appropriate).
Experiment 5
To differentiate habituation to social stimuli from that to context, each C57 male was first exposed to the empty chamber on five trials, for habituation-indexed context learning. On 4 subsequent trials, they were placed in the same chamber but with the same, initially unfamiliar, CD-1 male, to evaluate habituation-indexed social learning. On trial 10th, each C57 male was again exposed to the same empty chamber. On trial 11th, the C57 males were exposed to a novel CD-1 male in this chamber.
Papers were collected at the end of the session each test day, and mouse urine on the paper substrate was fixed by Ninhydrin spray (LC-NIN-16, Criminal Research Products, LLC) to be easily quantified. After 24 hours drying, the number of scent marks was measured by overlapping a transparent grid sheet over the paper and counting the number of grid (10 × 10 mm) containing scent marks (maximum: 552 squares). To eliminate normal micturition products, pools of urine larger than four square grids that formed a larger quadrant were not included in the quantification of scent marks. Four squares in a row, however, were included. The number and total size (squares) of micturition pools and the number of fecal boli were also counted.
2.4. Statistical analysis
For all experiments, a one-way analysis of variance (ANOVA) with repeated measure was used to analyze the data. Bonferroni tests were used for post hoc comparisons of scent marks on specific days: Each successive day vs first day of a sequence with a specific, individual stimulus; first exposure to a novel stimulus vs last day of exposure to a relevant preceding stimulus; A probability level of p<.05 was adopted as the level of statistical significance for all analyses.
3. Results
3.1. Experiment 1 C57 males exposed to CD-1 males
Fig. 1a depicts the mean number of squares with scent marks for C57 males toward an initially unfamiliar CD-1 male for first 4 trials and then toward a novel CD-1 male on trial 5. ANOVA found a significant main effect of trials, F(4,52)= 11.859, p<.001. C57 males deposited substantial marking on the first trial which decreased during 4 exposures to the same CD-1 male, as shown by reduced marking on both trials 3 and 4 compared to trial 1 (Bonferroni test, p<.05). Following these 4 habituation sessions, the C57 males showed more frequent scent marking when exposed to a novel CD-1 male, compared to that on the last day of habituation (p<.05). These data indicate that C57 males showed habituation to the novel CD-1 male and the novel situation over 4 days of exposure, with recovery of scent marking when an unfamiliar CD-1 opponent was presented in the same situation.
Fig. 1.
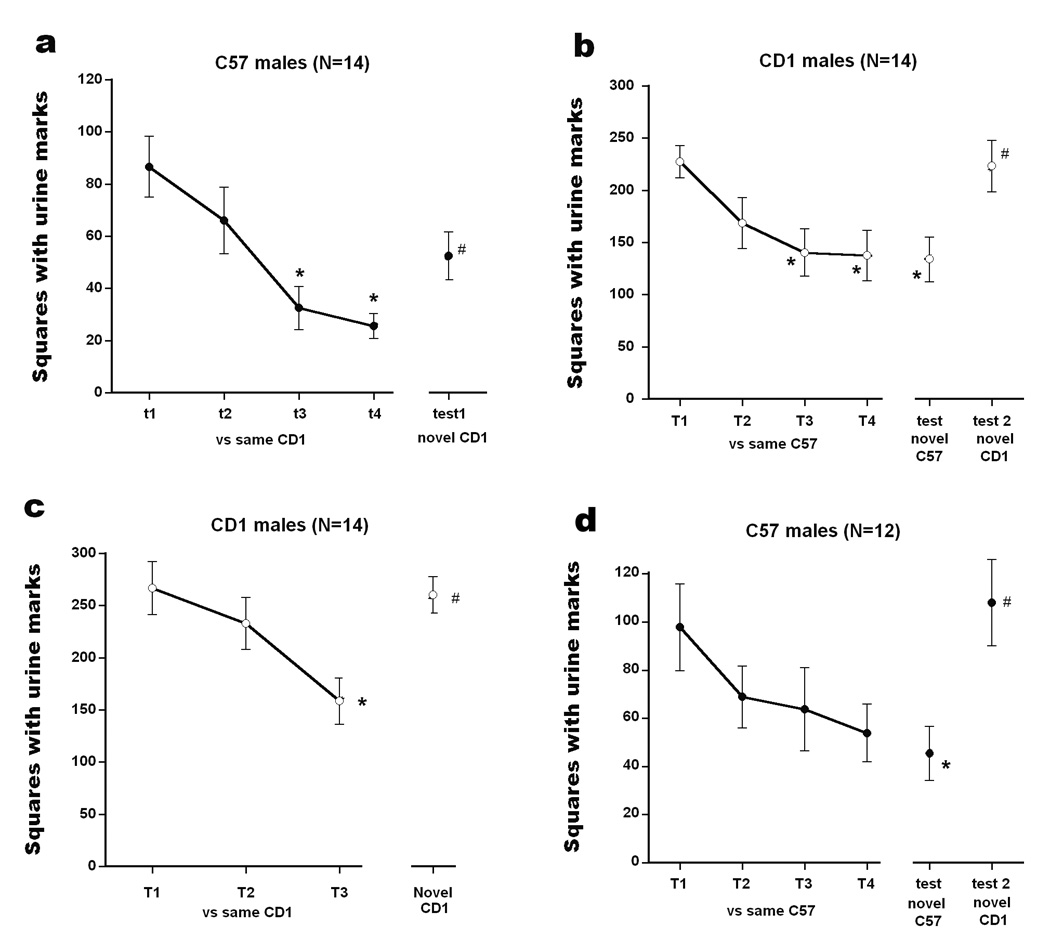
The total number of urine marked squares for (a) C57 males exposed to CD-1 males (experiment 1); (b) CD-1 males exposed to CD-1 or a C57 male (experiment 2); (c) CD-1 males exposed to CD-1 males (experiment 2); and (d) C57 males (experiment 3). For all experiments, the initial block of trials involved exposure to an initially novel stimulus male, in an initially novel situation. All tests with novel males, as marked on graphs, were done in the same, now familiar, situations as the first block of trials. The inter-trial-interval was 24 hour. Data are expressed mean ± S.E.M. Significant differences between trials compared to trial 1, *;p<.05, and to trial 4, #; p<.05 in graph (a), (b), and (d), and to trial 1, *;p<.05, and to trial 3, #; p<.05 in graph (c).
These habituation and recovery of scent marking effects were not seen for normal micturition and defecation. Table 1a presents the mean number of squares occupied by urine pools and the number of fecal boli for C57 males for each trial. ANOVA failed to show significant trial differences, for C57 males exposed to a CD-1 male: F(4,52)= 1.941, n.s.; and 1.990, n.s., for pool squares and boli, respectively;
Table 1.
The numbers of squares with urine pool and fecal boli for C57 males toward a CD-1 (a; Exp. 1) and toward a C57 (d; Exp. 3), and CD-1 males toward a C57 (b) and toward a CD-1 (c) (Exp. 2) for each trial. Test trials are shown in T5 at panel (a) in which each C57 was exposed to a novel CD-1, T5 and T6 at panel (b) in which each CD-1 was confronted with a novel C57 and a CD-1, respectively, T4 at panel (c) in which each CD-1 was confronted with a novel CD-1, and T5 and T6 at panel (d) in which each C57 was confronted with a novel C57 and CD-1, respectively.
| a | C57 vs. CD-1 |
b | CD-1 vs. C57 |
c | CD-1 vs. CD-1 |
d | C57 vs. C57 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pool size | Fecal boli | Pool size | Fecal boli | Pool size | Fecal boli | Pool size | Fecal boli | ||||
| T1 | 19.1(4.1) | 4.1(0.6) | T1 | 14.1(4.4) | 5.5(0.7) | T1 | 6.3(3.2) | 8.7(0.8) | T1 | 16.8(3.6) | 5.0(0.7) |
| T2 | 15.8(5.2) | 4.4(0.8) | T2 | 6.6(3.1) | 7.2(0.8) | T2 | 7.8(3.1) | 8.3(0.8) | T2 | 17.1(4.0) | 4.8(0.7) |
| T3 | 9.6(3.4) | 3.5(0.5) | T3 | 6.1(2.9) | 7.6(0.8) | T3 | 5.1(2.0) | 7.9(0.7) | T3 | 13.8(4.4) | 4.2(0.5) |
| T4 | 7.1(3.2) | 2.5(0.4) | T4 | 5.6(4.7) | 7.5(0.7) | T4 | 13.3(3.9) | 4.3(0.6) | |||
| T4 | 1.9(1.4) | 8.1(0.7) | |||||||||
| T5 | 14.9(4.0) | 3.3(0.7) | T5 | 11.6(5.7) | 8.2(0.7) | T5 | 14.6(4.8) | 4.0(0.7) | |||
| T6 | 1.6(1.2) | 8.1(0.5) | T6 | 7.2(4.3) | 4.0(0.5) | ||||||
Data are means (S.E.M.). There were no significant trial differences
3.2. Experiment 2 CD-1 males exposed to C57 or CD-1 males
Fig. 1b shows the mean number of scent marked squares for CD-1 males exposed to the same C57 male on the first 4 trials, to a novel C57 male on trial 5 and to a novel CD-1 male on trial 6. ANOVA found a significant main effect of trials, F(5,65)= 9.250, p<.001. CD-1 males showed substantial scent marking on the first trial, which decreased over trials, as shown by reduced marking on trial 3 and 4 compared to trial 1 (p<.05). Subsequent exposure to a novel, different C57 did not produce a change in scent marking, but exposure to a novel CD-1 male produced increased marking, as compared to the last day of habituation to the single C57 male (p<.05). These data indicate that CD-1 males showed habituation to the initially novel C57 male, in an initially novel situation. They did not show recovery of scent marking to a novel C57 male, but subsequently did increase scent marking to a novel CD-1 male in the same situation (p<.05).
Fig. 1c shows the mean number of scent marked squares for the same group of CD-1 males when exposed to an initially novel CD-1 male for 3 trials in the familiar test situation, and then exposed to an unfamiliar CD-1 male on trial 4. ANOVA found a significant main effect of trials, F(3,39)= 14.164, p<.001. During the first trial of the stimulus habituation session, CD-1 males deposited substantial marking, with significantly reduced marking on trial 3 compared to trials 1 and 2 (p<.05). Subsequent exposure to a novel CD-1 male produced significantly increased marking compared to the last trial of the habituation session (p<.05). These data indicate that CD-1 males show habituation to an initially novel CD-1 male in a familiarized situation, with recovery of scent marking to a novel CD-1 male in the same situation.
Normal micturition and defecation did not change over trials (Table 1b,1c) for CD-1 males exposed to a C57 male: F(5,65)= 1.541, n.s.; and 2.137, n.s., respectively, and for CD-1 males exposed to a CD-1 male: .F(3,39)= 1.639, n.s.; and 0.215, n.s., respectively.
3.3. Experiment 3 C57 males exposed to C57 males
Fig. 1d presents the mean number of scent marked squares for C57 males exposed to an initially unfamiliar C57 male in an initially unfamiliar situation on each of 4 habituation trials; to a novel C57 male on trial 5; and to a novel CD-1 male on trial 6. ANOVA found significant differences between trials, F(5,55)= 6.664, p<.001. C57 males showed substantial marking to the unfamiliar C57 male on the first trial, with significantly decreased marking after 4 days of exposure as indexed by reduced scent marks on trial 4 compared to trial 1 (p<.05). Subsequent exposure to a novel C57 male did not change scent marking, but exposure to a novel CD-1 male significantly increased marking, as compared to fourth day of habituation (p<.05). These data indicate that C57 males display habituation to a initially unfamiliar male of the same strain in an unfamiliar situation, but do not show recovery of marking to a novel C57 male in the now-familiar test situation. However, they did increase marking to a novel CD-1 male.
Again, normal micturition and defecation did not show habituation or recovery to these stimuli. Table 1d shows the number of squares with urine pools, and fecal boli. ANOVA failed to find significant differences between trials, F(5,55)= 1.685, n.s and F(5,55)= 0.563, n.s, respectively, for pools and boli.
3.4. Experiment 4 Context learning: Habituation and recovery of scent marking to context
Fig. 2 shows the mean number of scent marked squares for C57 males exposed to the same chamber for 4 trials and subsequently exposed to a different shaped novel chamber on trial 5. The C57 male subjects showed rapid habituation to the context over 4 trials, as indexed by reduced marking on trials 3 and 4 compared to trial 1, F(4,44)= 16.307, p<.001. Subsequent exposure to the novel context induced increased marking, as compared to that seen during the fourth habituation session (p<.05). Micturition and defecation did not change over trials (Table 2): ANOVA failed to find significant differences between trials, F(4,44)= 0.311, n.s.; and 1.424, n.s., respectively, for squares with urine pools or fecal boli. These findings indicate that C57 males showed habituation to an initially unfamiliar cage chamber and dishabituation to an unfamiliar different shaped chamber. It might be noted that such habituation/dishabituation occurred despite the continued presence of a salient stimulus, the rough paper substrate, necessary for analysis of scent marks, that likely reduced the novelty of the new situation.
Fig. 2.
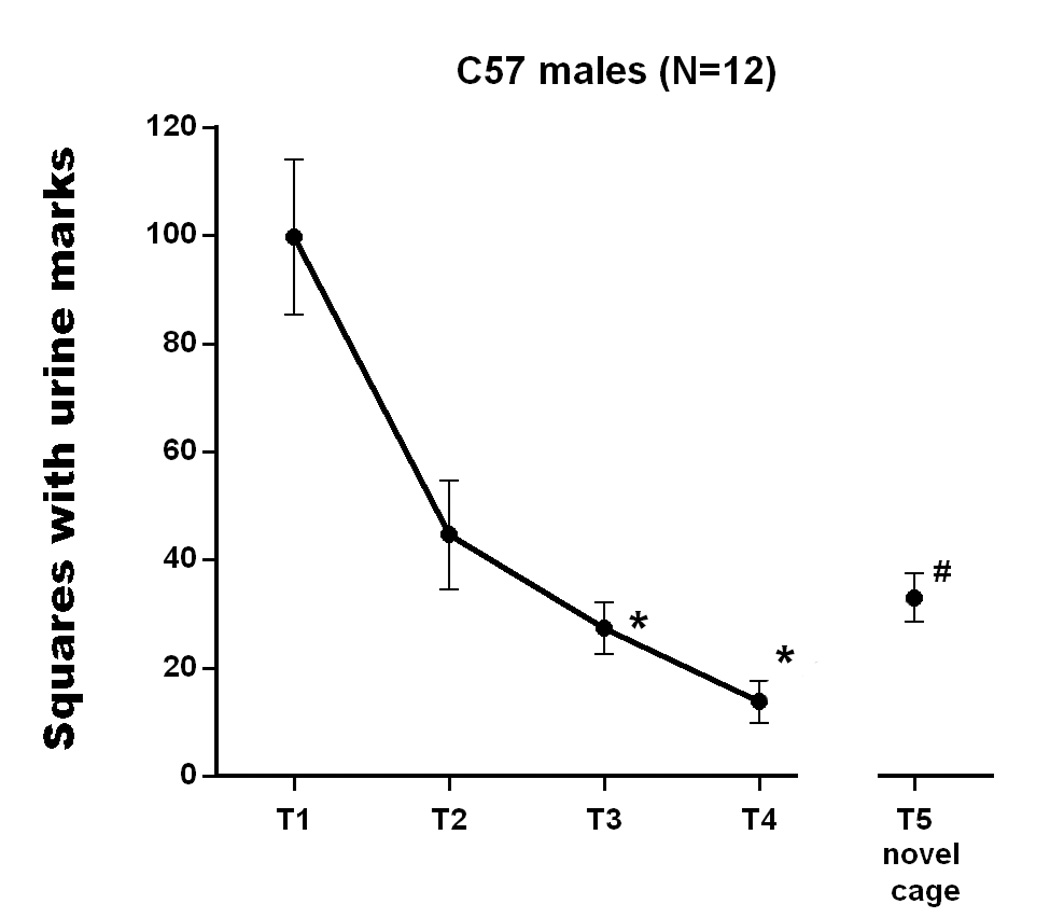
The total number of squares with urine marks in male C57 mice. During first four trials, C57 were exposed to the same empty chamber. On the fifth trial, they were exposed to another (novel) empty chamber. Inter-trial-interval was 24 hour. Data are expressed as mean ± S.E.M. Significant differences between trials compared to trial 1, * p<.05, and to trial 4, # p<.05.
Table 2.
The numbers of squares with urine pool and fecal boli for C57 males exposed to the chamber alone for each trial in Experiment 4. Test trial is shown in T5, in which each C57 was placed on a novel different-shaped chamber.
| Pool size | Fecal boli | |
|---|---|---|
| T1 | 11.9(4.0) | 3.7(0.7) |
| T2 | 8.3(2.3) | 3.1(0.6) |
| T3 | 8.3(3.5) | 2.7(0.6) |
| T4 | 7.3(2.9) | 2.3(0.5) |
| T5 | 7.5(3.9) | 2.2(0.6) |
Data are means (S.E.M.) (N=12)
3.5. Experiment 5 Differentiation of social learning from context learning
Fig. 3 presents the mean scent marked squares in each of 11 trials: 5 trials alone in an initially novel situation; 4 trials with an initially novel CD-1 male stimulus; one additional trial in the now-familiarized situation without a stimulus mouse; and a final trial with a novel CD-1 male stimulus, in the same situation. ANOVA revealed a significant trial effect, F(10,110)=9.234, p<.0001. C57 males showed decreased scent marking over the first 5 trials, as indexed by reduced marking on trials 4 and 5 compared to the first trial (p<.05). Subsequent exposure to the same chamber with an initially unfamiliar CD-1 male produced substantially increased marking, as compared to the last day of context habituation (p<.05), which also declined over repeated exposure to the same CD-1 male, as indexed by decreased marking on fourth day compared to the first day of exposure to the same CD-1 male (p<.05). Subsequent exposure to the empty chamber did not change scent marking, compared to the last day of exposure to the chamber alone (Trial 5), but exposure to a novel CD-1 male increased marking, as compared to the last day of habituation to the single CD-1 (p<.05). These results indicate that C57 males display independent habituation to context and social stimuli as expressed by scent marking. Reduced marking to the habituated chamber did not recover following trials with exposure to a stimulus male in the chamber. Exposure to a novel CD-1 male stimulus in the habituated cage did produce recovery of marking.
Fig. 3.
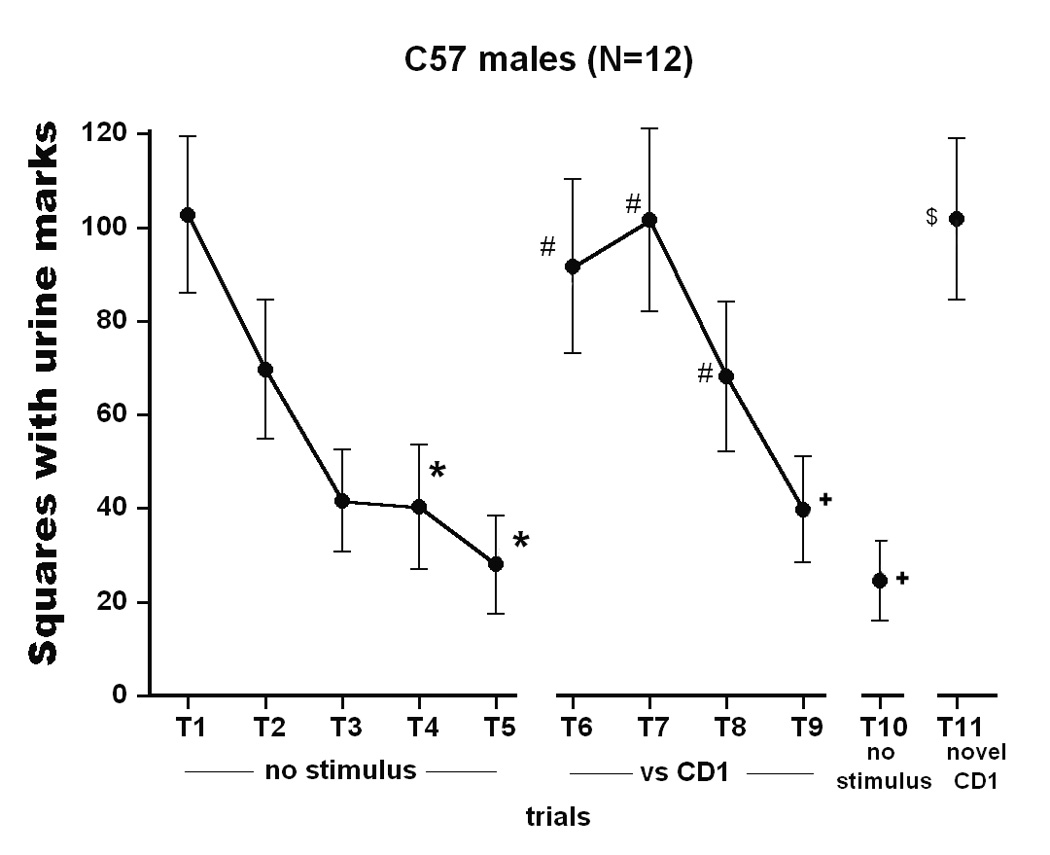
The total number of squares with urine marks in male C57BL/6J mice. During first five trials, C57 were exposed to the empty chamber. For subsequent 4 trials, they were exposed to the same chamber but with a single CD-1 male. On trial 10, they were exposed to the test chamber alone again, and then on trial 11, they were exposed to a novel CD-1. Inter-trial-interval was 24 hour. Data are expressed as mean ± S.E.M. Significant differences between trials compared to trial 1, * p<.05, to trial 6, # p<.05, and to trial 11, $ p<.05.
Micturition and defecation, indexed by pooling and number of boli, did not respond to these habituation and recovery phenomena (Table 3). ANOVA failed to find trial differences in these measures F(10,110)= 1.761, n.s.; and 1.728, n.s., respectively.
Table 3.
The numbers of squares with urine pool and fecal boli for C57 males in Experiment 5. For first 5 trials, C57 were exposed to the chamber alone and for second 4 trials, they were exposed to a CD-1, and on trial 10 exposed alone again, and on trial 11 exposed to a novel CD-1.
| Pool size | Fecal boli | |
|---|---|---|
| T1 | 10.4(3.8) | 3.2(0.7) |
| T2 | 13.9(2.5) | 4.3(0.6) |
| T3 | 8.3(3.2) | 3.3(0.6) |
| T4 | 5.4(2.9) | 3.0(0.6) |
| T5 | 5.6(2.5) | 2.5(0.6) |
| T6 | 1.8(1.0) | 2.8(0.7) |
| T7 | 3.1(2.1) | 3.8(0.7) |
| T8 | 12.8(5.7) | 3.3(0.6) |
| T9 | 5.8(2.6) | 2.5(0.4) |
| T10 | 6.3(3.3) | 4.5(0.8) |
| T11 | 3.5(1.9) | 2.9(0.7) |
Data are means (S.E.M.) (N=12)
4. Discussion
Major urinary proteins (MUPs), one source of odor complexity in rodents, are a class of non-volatile polymorphic proteins [31–33], which produce genetically individual scents [33,41–42]. The major histocompatibility complex (MHC) encodes highly polymorphic glycoproteins [62–63]. This is another source of variation in the urinary scents produced by a range of animals including mice [43,63], rats [13,53] and humans [59]. They act through a complex mixture of volatile and nonvolatile metabolites bound and released by urinary proteins or peptides [52–54]. Volatiles from urine also vary according to social status, diet, and animals’ bacterial gut flora [11,48,65], while MUPs and MHC peptides are hard-wired into the genome and stable throughout the life of the animal [31,53,64].
The present experiments demonstrated that isolated C57 and CD-1 male mice deposited scent marks in the presence of novel conspecifics (Exp. 1, 2, and 3); C57 males also mark to a novel context (Exp. 4 and 5: CD-1 mice not tested). Repeated exposure to the same stimulus mouse in an initially novel chamber (Exp. 1, 2 and 3) or to an initially novel chamber alone (Exp. 4 and 5) produced habituation as indexed by decreased scent marking over trials, and by significant reductions in scent marking after several trials, compared to the first encounter with that stimulus. The Exp. 4 and 5 context findings are consistent with reports by Maruniak et al. [37] of strong habituation of scent marking with repeated exposure to the test chamber alone. In addition to Exp. 1, 2, and 3, all showing habituation when both the stimulus mouse and the context were novel, a situation in which the relative effects of stimulus and cue experience are confounded, Exp. 5 demonstrated that even in a previously habituated context, C57 mice showed habituation to a CD-1 male. Together with the Exp. 4 and 5 findings of habituation to the test chamber without a mouse stimulus, this indicates that habituation of scent marking independently occurs to both chamber context and social stimuli. Habituation of scent marking to conspecifics has been demonstrated for mice cohabitating for 96 hrs in test cages [37]. The present results (Exp. 5) are in agreement with this finding, but demonstrate rapid, independent, habituation to chamber context and to social stimuli.
When either C57 or CD-1 males were exposed to a different CD-1 stimulus mouse following habituation to an initial mouse stimulus (Exp. 1, 2 and 3), or to a different chamber following habituation to an initial chamber (Exp. 4 and 5), they showed increased scent marking. This increased scent marking was not elicited by change of stimuli per se, since in Exp. 5 exposure to an empty chamber (trial 10) following repeated exposure to social stimuli (trials from 6 to 9) did not produce increased marking. This suggests that increased marking depends on recognition of the novelty of the stimulus or situation. Indeed, even following long-term habituation to the situation and to social stimuli, a novel social stimuli was capable of inducing a major increase in marking response (Trial 11, Exp. 5).
A particularly interesting finding (Exp. 1 and 2) is that both C57 and CD-1 males failed to show enhanced marking when exposed to a novel male of the C57 inbred strain following repeated exposure to an initially novel C57 stimulus. This contrast to the enhanced marking to a novel CD-1 male after habituation to a CD-1 male is consistent with a view that social recognition in mice is based on genetic differences in patterns of MUPs and MHC peptides expressed in mouse urine [31–32,41,62]. Since the genetically determined scent of urine is the same within males of highly inbred strains [31,41–42], mice cannot recognize a change in individual C57 males. As this phenomenon is seen with both C57 and CD-1 males, while both are responsive to new CD-1 stimuli after habituation to a previous CD-1 male, it is clear that it is not the olfactory apparatus of the C57 or CD-1 male that is compromised, but the olfactory differentiability of C57 males.
One such method uses a habituation-dishabituation paradigm based on investigatory behaviors of mice to other conspecifics [60; for rats, 56]. Adult mice will spontaneously investigate younger (juvenile) mice or ovariectomized females [6]. If the interval between trials of exposure to a given animal is less than 40 min, mice display habituation [49,57]. One report using group-housed mice showed long-lasting (at least 7 days) social memory in this paradigm [36].
An additional set of measures of social preference, social learning and memory involve a three-chamber test with a central chamber connected to two identical side chambers in which stimuli can be presented [e.g. 9,39,47]. A strange conspecific (social stimulus) is confined in a small cage in one chamber of the apparatus, while the other choice chamber can include relevant comparison stimuli such as an empty cage (a non-conspecific novel object), or a cage containing a previously encountered conspecific. Appropriate comparisons permit interpretations of social approach or sociality (conspecific > non-conspecific novel object); or preference for social novelty (novel conspecific > familiar conspecific). The use of different stimulus pairs such as own vs other cage bedding or male vs. female, or young vs old conspecifics can provide information about the olfactory abilities, social habituation, preference for conspecifics of different ages and sex, etc. of the subject [10,40].
While these tasks have produced extremely valuable information on a host of sociality-related motivations and behaviors, scent marking takes a different, and complementary, approach. Scent marking is an important feature of mouse communication, in nature. It includes behaviors initiated by the focal animal (marking to a novel situation), as well as responses of the focal animal to scent marks initiated by others, providing a broader view of the interactive nature of mouse social behaviors. It also demonstrates consistent and significant habituation effects extending over at least 24 hours (the present intertrial intervals) as well as dishabituation when scent from a novel mouse is presented. After single housing, subjects also showed scent marking with habituation/dishabituation to nonsocial stimuli, again extending over 24 hrs, providing a stable measure of context learning. Although the scent marking response to the novel context was not as large as that to a novel mouse, this likely reflects the continued presence of rough paper flooring in the novel context [5,20].
C57 mice are used as a common background strain for knockout mice relevant to disorders such as autism that include deficits in social communication, social motivation, and amicable relationships [1,3,15–16,51]. The present study demonstrated that C57 as well as CD-1 mice display good social recognition as indexed by scent marking behavior. As olfaction is the primary sensory modality of mice, and mice use scent marks to communicate with conspecifics [12,19,45], scent marking may provide a particularly relevant situation for evaluation of social communication in mice, including specific behaviors that may be responsive to factors related to social psychopathologies [8,23].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: Identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard DC, Arakawa H, Crawley JN, Blanchard RJ. Social behaviors in wild and laboratory mice with a special emphasis on th C57BL/6J inbred strain. In: Crusio WE, Sluyter F, Gerlai RT, editors. Handbook of Behavioral Genetics of the Mouse. Elsevier; In press. [Google Scholar]
- 4.Blanchard RJ, O’Donnell V, Blanchard DC. Attack and defensive behaviors in the albino mouse (Mus musculus) Agg Behav. 1979;5:341–352. [Google Scholar]
- 5.Blanchard RJ, Yang M, Li CI, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neurosci Biobehav Rev. 2001;25:587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- 6.Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 7.Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- 8.Brennan PA, Kendrick LM. Mammalian social odours: attraction and individual recognition. Phil Trans R Soc B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Brown RE. What is the role of the immune system in determining individually distinct body odours? Int J Imunopharmacol. 1995;17:655–661. doi: 10.1016/0192-0561(95)00052-4. [DOI] [PubMed] [Google Scholar]
- 12.Brown RE, McDonald DW. Social odours in mammals. Vol. 1 & 2. Oxford: Clarendon Press; 1985. [Google Scholar]
- 13.Brown RE, Singh PB, Roser B. The major histocompatibility complex and the chemosensory recognition of individuality in rats. Physiol Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN, Schleidt WM, Contrera JF. Does social environment decrease propensity to fight in male mice? Behav Biol. 1975;15:73–83. doi: 10.1016/s0091-6773(75)92105-7. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Payler R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacol. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley-Liss; 2000. [Google Scholar]
- 17.Dantzer R, Bluthe R-M, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 18.Desjardins C, Maruniak JA, Bronson FH. Social rank in the house mouse: differentiation revealed by ultraviolet visualisation of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 19.Drickamer LC. Pheromones: behavioral and biomedical aspects. In: Balthazart J, editor. Advances in comparative and environmental physiology. Berlin: Springer Verlag; 1989. pp. 269–348. [Google Scholar]
- 20.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferkin MH, Li HZ. A battery of olfactory-based screens for phenotyping the social and sexual behaviors of mice. Physiol Behav. 2005;85:489–499. doi: 10.1016/j.physbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Gheusi G, Goodall G, Dantzer R. Individually distinctive odours represent individual conspecifics in rats. Anim Behav. 1997;53:935–944. [Google Scholar]
- 25.Gosling LM. A reassessment of the function of scent marking in territories. Z Tierpsychol. 1982;60:89–118. [Google Scholar]
- 26.Gosling LM, Atkinson NW, Dunn S, Collins SA. The response of subordinate male mice to scent marks varies in relation to their own competitive ability. Anim Behav. 1996;52:1185–1191. [Google Scholar]
- 27.Humphries RE, Robertson DHL, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mice. Anim Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- 28.Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty 1: communication between males. Anim Behav. 1990;40:209–222. [Google Scholar]
- 29.Hurst JL. The priming effects of urine substrate marks on interactions between male house mice, Mus musculus domesticus Schwarz and Schwarz. Anim Behav. 1993;45:55–81. [Google Scholar]
- 30.Hurst JL, Rich TJ. Scent marks as competitive signals of mate quality. In: Johnston RE, Müller-Schwarze D, Sorenson D, editors. Advances in chemical communication in vertebrates. New York: Plenum Press; 1999. pp. 209–226. [Google Scholar]
- 31.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 32.Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signaling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- 33.Hurst JL, Thom MD, Nevison CM, Humphries RE, Beynon RJ. MHC odours are not required or sufficient for recognition of individual scent owners. Proc Biol Sci. 2005;272:715–724. doi: 10.1098/rspb.2004.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston RE. Chemical communication in rodents: From pheromones to individual recognition. J Mammal. 2003;84:1141–1162. [Google Scholar]
- 35.Kavaliers M, Choleris E, Pfaff DW. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev. 2005;29:1347–1359. doi: 10.1016/j.neubiorev.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus- demendent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav. 1974;12:1035–1039. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- 38.Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 40.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. The consequences of inbreeding for recognizing competitors. Proc Biol Sci. 2000;267:687–694. doi: 10.1098/rspb.2000.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in mouse scent marks is involatile. Proc Biol Sci. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penn D, Potts WK. Untrained mice discriminate MHC-determined odors. Physiol Behav. 1998;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. [DOI] [PubMed] [Google Scholar]
- 44.Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- 46.Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim Behav. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- 47.Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Schellinck HM, Slotnick BM, Brown RE. Odors of individuality originating from the major histocompatibility complex are masked by diet cues in the urine of rats. Anim Learn Behav. 1997;25:193–199. [Google Scholar]
- 49.Sekiguchi R, Wolterink G, van Ree JM. Analysis of the influence of vasopressin neuropeptides on social recognition of rats. Eur Neuropsychopharmacol. 1991;1:123–126. doi: 10.1016/0924-977x(91)90713-5. [DOI] [PubMed] [Google Scholar]
- 50.Shahbazian MD, Young JI, Yuva-Paylor LA, Spencer CM, Antalffy BA, Noebels JL, Armstrong DL, Paylor R, Zoghbi HY. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 51.Silver LM. Mouse Genetics. Oxford: Oxyford University Press; 1995. [Google Scholar]
- 52.Singer AG, Tsuchiya H, Wellington JL, Beauchamp GK, Yamazaki K. Chemistry of odortypes in mice- fractionation and bioassay. J Chem Ecol. 1993;19:569–579. doi: 10.1007/BF00994326. [DOI] [PubMed] [Google Scholar]
- 53.Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh PR, Brown RE, Roser B. MHC antigens in urine as olfactory recognition cues. Nature. 1987;327:161–164. doi: 10.1038/327161a0. [DOI] [PubMed] [Google Scholar]
- 55.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 56.Thor DH, Holloway WR. Persistence of social investigatory behavior in the male rat: evidence for long-term memory of initial copulatory experience. Anim Learn Behav. 1981;9:561–565. [Google Scholar]
- 57.Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–1006. [Google Scholar]
- 58.Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Wedekind C, Furi S. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc Biol Sci. 1997;264:1471–1479. doi: 10.1098/rspb.1997.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacol (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- 61.Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behav Ecol. 2003;15:338–344. [Google Scholar]
- 62.Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamazaki K, Yamaguchi M, Baranoski L, Bard J, Boyse EA, Thomas L. Recognition among mice: evidence from the use of a Y-maze differentially scented by congenic mice of different major histocompatibility types. J Exp Med. 1979;150:755–760. doi: 10.1084/jem.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamazaki K, Beauchamp GK, Matsuzaki O, Kupniewski D, Bard J, Thomas L, Boyse EA. Influence of a genetic difference confined to mutation of H-2K on the incidence of pregnancy block in mice. Proc Natl Acad Sci USA. 1986;83:740–741. doi: 10.1073/pnas.83.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamazaki K, Boyse EA, Bard J, Curran M, Kim D, Ross SR, Beauchamp GK. Presence of mouse mammary tumor virus specifically alters the body odors of mice. Proc Natl Acad Sci USA. 2002;99:5612–5615. doi: 10.1073/pnas.082093099. [DOI] [PMC free article] [PubMed] [Google Scholar]


