Abstract
We recently reported that embryonic stem cells-conditioned medium (ES-CM) contains antiapoptotic factors that inhibit apoptosis in the cardiac myoblast, H9c2 cells. However, the mechanisms of inhibited apoptosis remain elusive. In this report, we provide evidences for novel mechanisms involved in the inhibition of apoptosis provided by ES-CM. ES-CM from mouse ES cells was generated. Apoptosis was induced after exposure with H2O2 (400μm) in H9c2 cells followed by replacement with ES-CM or culture medium. H9c2 cells treated with H2O2 were exposed to ES-CM, and ES-CM+cell survival protein phosphatidyl-inositol 3-kinase (PI-3k/Akt) inhibitor, LY294002 or extracellular signal-regulated kinase (ERK1/2), PD98050. After 24 hours, H9c2 cells treated with ES-CM demonstrated significant increase in cell survival. ES-CM significantly inhibited (p<0.05) apoptosis determined by TUNEL staining, apoptotic ELISA and caspase-3 activity. Importantly, enhanced cell survival and inhibited apoptosis with ES-CM was abolished with LY294002. In contrast, PD98050 shows no effect on ES-CM increased cell survival. Furthermore, H2O2 induced apoptosis is associated with decreased levels of phosphorylated (p) Akt activity. Following treatment with ES-CM, we observed a decrease in apoptosis with an increase in pAkt, and increased activity was attenuated with Akt inhibitor, suggesting that the Akt pathway is involved in the decreased apoptosis and cell survival provided by ES-CM. In contrast, we observed no change in ES-CM decreased apoptosis or pERK with PD98050. In conclusion, we suggest that ES-CM inhibited apoposis and is mediatd by Akt but not ERK pathway.
Keywords: stem cells, H9c2 cells, apoptosis, hydrogen peroxide, Akt, ERK
Introduction
Apoptosis is considered to play a major role in the development and progression of myocardial infarction (MI) that leads to cardiac myocyte cell loss then to heart failure (2; 3; 5; 21; 24). Cardiac myocyte cell loss in the infarcted heart is inhibited by antioxidants, angiotensin II inhibitors and expression of the antiapoptotic protein Bcl-2 (21; 24; 29)). Both adult and embryonic stem (ES) cell transplantation studies demonstrated decrease in cardiac myocyte apoptosis, limited cardiac regeneration with significant improved cardiac function (14-16; 20; 27; 28; 40; 41). Importantly, these studies suggest that autocrine or paracrine factors released in the heart following ES or adult stem cell transplantation inhibit host myocardium apoptosis (14; 15; 41). Using our cell culture model, we recently published direct evidences that factors released from ES cells contain antiapoptotic factors that inhibit H2O2 induced apoptosis in the cardiac myoblast, H9c2 cells (42).
Mechanisms of inhibited apoptosis is associated with the activation of the cell survival signaling cascades PI3K/Akt or ERK1/2 (12; 37-39). Use of antiapoptotic agents such as bradykinin, cardiotrophin-1, insulin, insulin growth factor -1 and urocortin that reduce ischemia-reperfusion injury in animals is associated with upregulation of the pPI3K/Akt and pERK1/2 pathways (17). However, the mechanisms of H9c2 cells inhibited apoptosis using factors released from ES cells are completely unknown.
Therefore, we hypothesize that factors released from ES cells inhibited apoptosis is mediated through cell survival/antiapoptotic pathways Akt and ERK. We present this data for the first time that factors released from ES cells, inhibited apoptosis and are mediated through Akt but not ERK signaling pathway.
Materials and Methods
Preparation of ES cells-conditioned medium (ES-CM)
Mouse ES cells (line CGR8) were passaged and maintained as we reported previously (42-44). In brief, mouse ES cells are maintained in Dulbecco's Minimum Essential Medium (DMEM) (Invitrogen) containing leukemia inhibitory factor (LIF), sodium pyruvate, β-mercaptoethanol, penicillin/streptomycin, glutamine, nonessential amino acids and 15% ES cells qualified fetal bovine serum (Invitrogen) (42-44). We obtained rat cardiomyocyte derived cell line H9c2 (H9c2 cells) from the American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 25 μg/ml gentamicin at 37°C in a humidified atmosphere of 5% CO2 as we reported previously (42).
Mouse ES cells at 9000 ES cells/cm2 were grown in 0.5% gelatinized petri dishes containing cell culture medium with LIF for 24 hours. Cells were then replaced with fresh cell culture medium without LIF. After 48 hours, supernatants were collected and labeled as ES-CM. ES-CM was filtered (0.22μm cellulose syringe filter) and used to determine the effects on H2O2 induced apoptosis in H9c2 cells.
Apoptotic Cell culture Model
H9c2 cells were cultured (2000 cells/well) for 24 hours. We reported previously (42) and used an optimal dose of 400μm of H2O2 (Sigma chemicals). H9c2 cells were exposed with H2O2 (400μm) for two hours followed by replacement with fresh cell culture or ES-CM or ES-CM+LY294002 (40μM) or ES-CM+PD98059 (25μM) (Calbiochem). We performed apoptotic ELISA study using different concentrations of LY294002 (20-80μM) and PD98050 (12.5-25 μM) to determine the optimal concentrations. The LY294002 (40μM) and PD98059 (25μM) concentrations used in the present study are well within the range of LY and PD (5-50μM) used by other investigators in their cell culture studies (9; 13; 34; 42). After 24 hours, control wells without H2O2 treatment were replaced with fresh cell culture medium. Cells were cultured for an another 24 hour and then counted in each of 24 wells based on their rod shape morphology. Rod-shaped cells were considered to be alive while round cells were considered to be dead. Numbers of rod shape cells in duplicate wells were counted. Mean of the duplicated wells were taken and used to analyze the data. Cell viability was also examined using trypan blue staining.
Using our cell culture model system, apoptosis was examined by TUNEL staining, apoptotic ELISA kit and caspase 3 activities. Phosphorylated P1-3K/Akt and ERK activities were measured using Superarray ELISA kits.
Trypan Blue Staining
H9c2 cells (5000 cells) were cultured in 35mm petri dish for 24 hours. Cells were treated with and without H2O2 and then replaced with cell culture medium or ES-CM or ES-CM+LY294002 or ES-CM+ PD98059 or LY294002 or PD98059 for an additional 24 hous. Cells were washed with PBS. Cells were enzymatically dissociated using trypsin/EDTA (Gibco), washed with PBS, and were incubated with 0.4% trypan blue stain (Sigma) for 5min at room temperature. Cells were counted in a hemocytometer chamber. Parallel, cells were also stained in the petridish using trypan blue staining without trypsinization. Experiments were repeated five times in duplicate. Cells stained blue (dead) and unstained (live) cells were counted. Percentages of viable cells were calculated as total number of live cells divided by total number of cells and multiplied by 100.
Tunel Staining
TUNEL staining was performed to determine apoptotic positive nuclei in H9c2 cells following exposure with H2O2 and respective treatment groups. H9c2 cells washed with PBS and fixed in 4% paraformaldehyde in phosphate buffered saline (PBS). Following fixation, cells were permeabilized with 0.1% Triton-×-100 in 0.1% sodium citrate followed by proteinase K (25μg/ml in 100 mM Tris-HCl). Apoptotic cell death detection kit (TMR red, Roche Applied Bio Sciences) based on TUNEL assay was used to detect apoptotic cells according to manufacturer's instruction. Each experiment includes negative controls by omitting TUNEL enzyme TdT reaction mixture and incubating the cells with label solution provided in the kit. Cells were mounted with Antifade Vectashield mounting medium containing DAPI (Vector Laboratories) to stain total nuclei and examined with a fluorescence microscope (Zeiss Axiovert 200). To calculate percentage apoptotic nuclei, we counted number of TUNEL staining red fluorescence nuclei divided by total DAPI positive blue fluorescence nuclei, and ×100.
Cell death Detection Enzyme-Linked Immunosorbent Assay (ELISA)
The cell death detection ELISA plus kit obtained from Roche Applied Sciences was applied to measure apoptosis based on the principle of histone-bound DNA fragments in an ELISA format. Medium was used as reported by us (42) and others (32) to examine apoptosis collected at 24hrs from different groups.
Caspase 3 Activity Assay
One million H9c2 cells were cultured in 150 mm2 petri dishes for 2-3 days. Cells were treated with and without H2O2 and then replaced with cell culture medium or ES-CM or ES-CM+LY294002 (40μM) or ES-CM+PD98059 (25μM) for an additional 24 hour. Caspase-3 activity was measured as we reported previously using a caspase 3 colorimetric activity assay kit from BioVision (CITY, CA). Cells were dissociated enzymatically with trypsin/EDTA (Invitrogen), and then collected in the cell lysis buffer provided in the kit. Cell lysate was prepared by incubating the cells on ice for 30 min and then centrifuged. Supernatant was isolated and used for protein concentration and caspase 3 activity. Protein concentrations were measured in the supernatant using a Bio-Rad assay. Caspase-3 activity was performed as per manufacturer's instructions provided in the kit, colorimetric reaction was developed, and measured at 405 nm in a microtiter plate reader.
Phosphorylated Akt and ERK Activity
H9c2 cells (7500 cells/well) were cultured in 96 well plates for 24 hours. Cells were treated with and without H2O2 and then replaced with cell culture medium or ES-CM or ES-CM+LY294002 (40μM) or ES-CM+PD98059 (25μM) for an additional 24 hour. Phosphorylated and total, Akt and ERK activities were examined using commercially available kit (Case kit Akt and ERK, Superarray Biosciences, Frederick, MD, USA). In brief, each treatment group was prepared in two sets of wells in duplicate. One set of cells in duplicate was incubated with the phosphor-Akt or ERK specific antibody to determine phosphorylated Akt and ERK. The other set of cells in duplicate was incubated with the pan-Akt or ERK specific antibody to determine total Akt and ERK. Appropriate controls such as cells with no treatment or the cells with no primary but with secondary antibodies were also included. Cell culture medium was removed and fixed with 100μl of 4% cell fixing buffer for 20min at room temperature. Blocking buffer was removed and washings were performed. Cells were incubated with a primary antibody followed by secondary antibody incubation as per manufacturer's instructions. Reaction was developed, and absorbance was measured at 450nm using an ELISA plate reader.
Furthermore, relative cell number was calculated to determine phosphorylated and total protein activity per cell number. The solution was removed from the 96 well plates, washed then incubated with 100μl of cell staining buffer for 30 min at room temperature. Washing were repeated and incubated with 1%SDS for 1 hr at room temperature. Absorbance was read at 595nm using an ELISA plate reader. To calculate the protein activity per cell number, the antibody absorbance reading at OD450 was divided by cell number OD595. Final relative extent of target protein phosphorylation for Akt and ERK was calculated from the normalized phosphospecific antibody OD450:OD595 ratio to the pan specific antibody OD450:OD595 ratio for the same experimental conditions.
Western Blot Analysis
Following treatments in different groups, H9c2 cells were washed with PBS, enzymatically dissociated with the use of trypsin/EDTA (Gibco), and finally collected in modified RIPA buffer [50 mM Tris-HCl, pH 7.4, NP-40 (1%), sodium deoxycholate 0.25%, 150 mM NaCl/ 1 mM EDTA/ 2 mM sodium orthovandate/ 5 mM sodium fluoride/ 1mM PMSF and mammalian protease inhibitor cocktail (Sigma)]. Cells were allowed to lyse following incubation for 30 min on ice. The cell lysates were centrifuged for 15 min at 14,000×g at 4°C. Protein concentration was measured in the supernatant using a Bio-Rad protein assay. Samples containing equal amounts of proteins were subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membranes (Bio-Rad). The blots were incubated with the primary antibody against phosphorylated Akt and ERK (Santa cruz) and then with secondary antibody horseradish peroxidase antimouse/rabbit IgG followed by detection with the chemiluminescence system. Same western blots were stripped using stripping solution and then incubated with the primary antibody against total Akt and ERK (Santa cruz). Following washings, blots were incubated with secondary antibody horseradish peroxidase anti-mouse/rabbit IgG followed by detection with the chemiluminescence system.
Data Analysis
Significance of differences between values was assessed using the Excel program t-test. All values were expressed as mean ± SE. Statistical significance was assigned when p<0.05.
Results
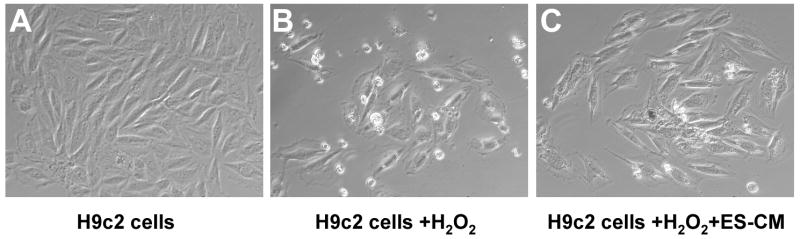
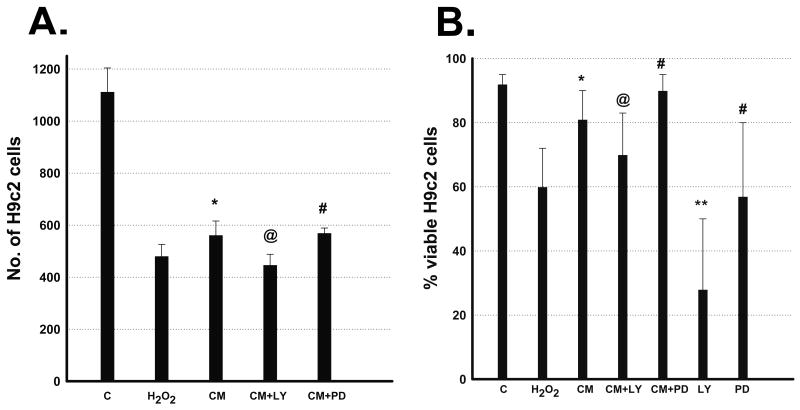
Cell death induced by 2 hour exposure of H2O2 (400μM) in H9c2 cells replaced with fresh cell culture medium or ES-CM demonstrated morphological changes as shown in Figure 1. Quantitative data in Figure 2A, shows decrease in cell survival (p<0.05) following treatment with H2O2 and this decrease was significantly improved with ES-CM. Addition of Akt inhibitor, LY294002 in ES-CM blocks the protective effects of ES-CM, suggesting ES-CM mediated cell survival involves Akt pathway. In contrast, ES-CM treated with ERK inhibitor, PD98059 did not block the cell survival effects (Figure 2A). Furthermore, we determined cell survival using additional trypan blue staining. We demonstrated a decrease in cell survival following treatment with H2O2 and this decrease was inhibited with ES-CM treatment group (Figure 2B, p<0.05). ES-CM+LY294002 abolished the protective effects of ES-CM (Figure 2B, p<0.05). In contrast, PD98059 shows no effect on ES-CM protected cell proliferation (Figure 2B). Moreover, LY294002 alone further inhibit H2O2 decreased cell survival however no such effect was observed with PD98059.
Figure 1.
Effects of ES-CM on H9c2 cell viability after exposure to H2O2. Representative photomicrographs of untreated H9c2 cells in control medium for 24 hours (A). H9c2 cells were exposed to 400μm H2O2 for two hours and then placed in normal cell culture control medium for 24 hours (B). H9c2 cells were exposed to H2O2 for two hours and then placed in ES-CM medium for 24 hours (20×).
Figure 2.
Effects of ES-CM, Akt inhibitor (LY 294002, 40μM) and ERK inhibitor (PD98050, 25μM) on H9c2 cell survival and proliferation after exposure to H2O2. Left Panel A, histogram shows quantitative number of H9c2 cells. Data are from the set of 7-9 independent experiments. Right panel B, histogram shows quantitative percent cell viable using trypan blue method (see methods). Data are from the set of 8-10 independent experiments. *p<0.05 vs H2O2, @p<0.05 vs ES-CM, **p<0.05 vs CM+LY, #p<non-significant (NS) vs CM and CM+PD.
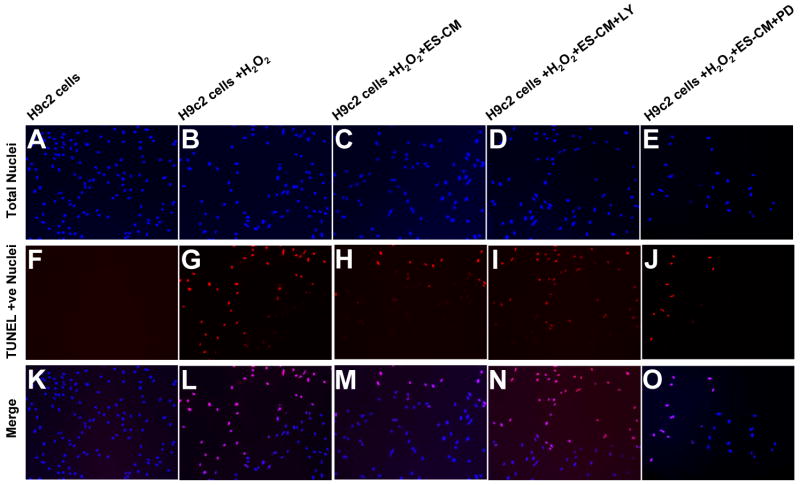
Using TUNEL staining, we determined the effect of ES-CM and ES-CM+LY294002 and ES-CM+ PD98059 on H2O2 induced apoptosis in H9c2 cells. Figure 3A-E, demonstrates the presence of total nuclei stained with 4′-6-Diamidino-2-phenylindole (DAPI) and Figure 3F-J shows TUNEL stained nuclei. Merged nuclei are shown in Figure 3K-O. Treatment of H9c2 cells with H2O2 for 24 hours increases ∼22% apoptosis (Figure 3A, p<0.05) compared with control media group. The % of apoptotic nuclei induced by H2O2 was significantly reduced (p<0.05) following treatment with ES-CM (Figure 4A). Addition of the LY294002 in ES-CM, blocks the ES-CM inhibited apoptosis (Figure 4A, p<0.05), returning apoptotic levels to nonsignificant compared with the cells seen in H2O2 treated group. In contrast, PD98059 demonstrated no effect on reduced apoptosis (Figure 4A). Quantitative apoptosis measured by the ELISA assay was significantly reduced with ES-CM and reduced apoptosis was blocked with LY294002 at concentration of 40 and 80 μm but not with 20μm (Figure 4C, p<0.05). Moreover, PD98059 at the concentration of 12.5 and 25μm did not block ES-CM inhibited apoptosis (Figure 4D). Furthermore, we determined caspase-3 activity as this is an important indicator of apoptosis and plays a major role in stress induced apoptosis in H9c2 cells. Our data shows that caspase-3 activity was higher in H2O2 induced apoptosis in H9c2 cells and this increase was significantly inhibited by ES-CM (Figure 4B, p<0.05). Inhibited caspase 3 activity with ES-CM was blocked with Akt but not with ERK inhibitor (Figure 4B), suggesting the role of Akt pathway.
Figure 3.
Effects of ES-CM, Akt inhibitor (LY 294002, 40μM) and ERK inhibitor (PD98050, 25μM) on H9c2 cells apoptosis after exposure to H2O2. Representative photomicrographs of total nuclei stained with DAPI in BLUE (A-E), TUNEL stained nuclei in RED (F-J) and Merge (K-O) (20×).
Figure 4.
Effects of ES-CM, Akt inhibitor (LY 294002, 40μM) and ERK inhibitor (PD98050, 25μM) on TUNEL positive nuclei (panel A), caspase 3 activity (panel B) and cell death ELISA (panel C and D) after exposure to H2O2. Left top panel A, histogram shows LY 294002 but not PD98050 inhibits ES-CM reduced apoptosis confirmed with TUNEL positive apoptotic nuclei (n=4-6 fields/well in each condition). Data are from the set of 5-6 independent duplicate experiments. Right top panel B, histogram shows LY 294002 but not PD98050 blocks ES-CM reduced caspase 3 activity. Data are from the set of 8-10 independent experiments. Left bottom panel C, histogram shows Akt inhibitor, LY294002 inhibits ES-CM decreased apoptosis measured by quantitative apoptotic ELISA (see methods). Data are from the set of 8-10 independent experiments. Right bottom panel D, histogram shows ERK inhibitor, PD98050 shows no effect on ES-CM decreased apoptosis measured by quantitative apoptotic ELISA (see methods). Data are from the set of 8-10 independent experiments. *p<0.05 vs H2O2, @p<0.05 vs ES-CM, #p<NS vs ES-CM.
Next, we determined pAkt and pERK activities in this cell culture model and the effects of LY294002 and PD98050. Figure 5A shows significant decreased levels of pAkt in the H2O2 treatment compared with control media group. Treatment with ES-CM increases pAkt levels (Figure 5A, p<0.05). Addition of LY294002 (40μm) in ES-CM demonstrated inhibition of increased phosphorylated levels (Figure 5A, p<0.05). In contrast, pERK were unchanged with and without H2O2 treatment as well as following treatments with ES-CM and ES-CM+PD98059 compared with control media group (Figure 5B). Furthermore, we confirmed our ELISA data using additional westernblot analysis. Figure 5C, shows treatment with ES-CM increases pAkt levels (Figure 5A, p<0.05). Addition of LY294002, (40μm) in ES-CM demonstrated inhibition of increased pAkt levels. In contrast, pERK were unchanged in all the groups (Figure 5D).
Figure 5.

Effects of ES-CM, Akt inhibitor (LY 294002, 40μM) and ERK inhibitor (PD98050, 25μM) on phosphorylated Akt (pAkt) and ERK (pERK) activity of H9c2 cells after exposure to H2O2. Left panel A. Histogram shows quantitative phosphorylated Akt examined by case ELISA kit. Data are from the set of 5-6 independent experiments. *p<0.05 vs Control, **p<0.05 vs H2O2, #p<NS vs ES-CM. Right panel B. Histogram shows quantitative phosphorylated ERK examined by case ELISA kit. Data are from the set of 5-6 independent experiments. #p<NS vs H2O2 and ES-CM. Left panel C. shows representative western blot analysis of phosphorylated Akt, and Right panel D, shows western blot analysis of phosphorylated ERK from different groups. The same stripped membrane shows total Akt and ERK (bottom portion in panel C and D). The data are from the sets of three-four independent experiments.
Discussion
Cardiac myocyte apoptosis and necrosis are present in the myocardial infarction that leads to cardiac remodeling and eventually chronic heart failure (2; 4; 5; 21). Oxidative stress is reported to be a key mediator of apoptosis in the infarcted and reperfused hearts (21; 23). Using a cell culture model system, many investigators have shown that H2O2 induces stress mediated apoptosis in isolated adult cardiomyocytes as well as in H9c2 cells (21-23; 37). We demonstrate in this study that treatment of H9c2 cells with H2O2 induces significant cell death. Using three independent methods such as TUNEL staining, cell death ELISA, and caspase 3 activity, we present data that establishes the cell death observed in the H9c2 cells is apoptotic. We also demonstrated that ES-CM inhibit H2O2 induced apoptosis in H9c2 cells. These findings confirm our recently published observations (42) using this oxidative stress induced apoptosis model. We have also shown that four major anti-apoptotic factors including osteopontin, clusterin, cystatin-c and TIMP-1 were present in the ES-CM that play a role to inhibit H9c2 cells induced apoptosis (42). These factors have been shown to be antiapoptotic in cancer cells, neuroblastoma cells, PC12 cells, H9c2 cells and endothelial cells (19; 26; 30; 35; 47). However, the mechanisms of ES-CM inhibited H9c2 cells apoptosis remain elusive.
PI-3k/Akt and ERK pathways are commonly involved in the stress induced apoptosis in vitro and in vivo models (1; 11; 18; 25; 31). In fact, Akt pathway is activated in the H2O2 induced apoptosis in H9c2 cells (18), HL-1 cardiomyocytes (10) as well as in vivo models of ischemia-reperfusion injury (2; 21). In the present study, we demonstrated that pAkt promotes cell survival in the oxidative stress-induced apoptosis in H9c2 cells following treatment with ES-CM. Specifically, we noted reduced levels of apoptosis in H9c2 cells treated with ES-CM and compared with significant increased levels of pAkt. In contrast, increased levels of H9c2 cells apoptosis were associated with the decreased levels of pAkt (Figure 4 and 5A). Therefore, we suggest the dynamics of pAkt were consistent with its role as a negative regulator of H9c2 cell apoptosis in this model. Furthermore, Akt inhibitor blocks the antiapoptotic effects of ES-CM on H9c2 cells apoptosis confirmed with TUNEL staining, apoptotic ELISA and caspase 3 activities, suggesting that Akt activation is important and required for the pro-survival function of ES-CM under oxidative stress conditions. Our data is consistent with the recent findings that Akt pathway mediates angiopoietin-1 (45) and ghrelin (6) inhibited stress induced apoptosis in H9c2 cells and insulin like growth factor-1 (IGF-1) inhibited stress induced apoptosis in dorsal root ganglion cells (25). Importantly, this has been shown that serum deprivation induces apoptosis in different cell culture models(7; 36). Using different inhibitors of apoptosis, this has been demonstrated that apoptosis is mediated through pAkt and pERK pathway(7; 8; 36). However, future studies are warranted to develop a serum deprivation induced apoptotic H9c2 cell culture model to determine the effects of ES-CM on apoptosis as well as their mechanisms.
ERK is an important MAPK family protein that plays a critical role in the cell survival, proliferation and differentiation in many cell types including cardiac myocytes (11; 24; 37; 46). We explored the role of ERK protein in the ES-CM mediated cell protection in H9c2 cells. In the present study, we demonstrate there was no significant difference in the levels of phosphorylated ERK with and without H2O2 treatment. We also show that ES-CM was unable to upregulate ERK activity. Moreover, ERK inhibitor PD98050 shows no effect on ES-CM inhibited apoptosis and ERK phosphorylation. Based on this data, we suggest that ERK pathway is not involved in the inhibition of apoptosis with ES-CM under oxidative stress induced circumstances.
In conclusion, our data first time suggests that ES-CM is a cell protective for H2O2 induced apoptosis in the H9c2 cells mediated through Akt but not ERK pathway. Our data is in the agreement of previous published data on the IGF-II inhibition of mammary epithelial cells apoptosis that involves Akt but not the ERK pathway (33). Moreover, we have shown that ES-CM contains four different antiapoptotic proteins(42). Therefore, the current study opens new avenues to understand the effects of each individual antiapoptotic protein using various apoptotic in vitro and in vivo models to inhibit apoptosis and their mechanisms.
Acknowledgments
We acknowledge support provided by AHA SDG 0430227N, 1R21HL085795-01A1 and 1R01HL090646-01to (Dr. Singla).
Reference List
- 1.Aki T, Yamaguchi K, Fujimiya T, Mizukami Y. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene. 2003;22:8529–8535. doi: 10.1038/sj.onc.1207197. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93 3:8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Kajstura J. Myocyte cell death in the diseased heart. Circ Res. 1998;82:1231–1233. doi: 10.1161/01.res.82.11.1231. [DOI] [PubMed] [Google Scholar]
- 4.Anversa P, Kajstura J, Olivetti G. Myocyte death in heart failure. Curr Opin Cardiol. 1996;11:245–251. doi: 10.1097/00001573-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Anversa P, Olivetti G, Leri A, Liu Y, Kajstura J. Myocyte cell death and ventricular remodeling. Curr Opin Nephrol Hypertens. 1997;6:169–176. doi: 10.1097/00041552-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafe F, Caldarera CM, Guarnieri C. H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Lett. 2003;536:85–91. doi: 10.1016/s0014-5793(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 8.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem. 2005;280:21997–22005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- 9.Choi IJ, Kim JS, Kim JM, Jung HC, Song IS. Effect of inhibition of extracellular signal-regulated kinase 1 and 2 pathway on apoptosis and bcl-2 expression in Helicobacter pylori-infected AGS cells. Infect Immun. 2003;71:830–837. doi: 10.1128/IAI.71.2.830-837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicconi S, Ventura N, Pastore D, Bonini P, Di NP, Lauro R, Marlier LN. Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress model. J Cell Physiol. 2003;195:27–37. doi: 10.1002/jcp.10219. [DOI] [PubMed] [Google Scholar]
- 11.Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H3524–H3531. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 16.Haider HK, Ashraf M. Bone marrow stem cell transplantation for cardiac repair. Am J Physiol Heart Circ Physiol. 2005;288:H2557–H2567. doi: 10.1152/ajpheart.01215.2004. [DOI] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Hong F, Kwon SJ, Jhun BS, Kim SS, Ha J, Kim SJ, Sohn NW, Kang C, Kang I. Insulin-like growth factor-1 protects H9c2 cardiac myoblasts from oxidative stress-induced apoptosis via phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Life Sci. 2001;68:1095–1105. doi: 10.1016/s0024-3205(00)01012-2. [DOI] [PubMed] [Google Scholar]
- 19.Khan SA, Lopez-Chua CA, Zhang J, Fisher LW, Sorensen ES, Denhardt DT. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J Cell Biochem. 2002;85:728–736. doi: 10.1002/jcb.10170. [DOI] [PubMed] [Google Scholar]
- 20.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22:1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. J Lab Clin Med. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann N Y Acad Sci. 1999;874:156–168. doi: 10.1111/j.1749-6632.1999.tb09233.x. [DOI] [PubMed] [Google Scholar]
- 23.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 25.Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- 27.Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31:513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 28.Li RK, Weisel RD, Mickle DA, Jia ZQ, Kim EJ, Sakai T, Tomita S, Schwartz L, Iwanochko M, Husain M, Cusimano RJ, Burns RJ, Yau TM. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J Thorac Cardiovasc Surg. 2000;119:62–68. doi: 10.1016/s0022-5223(00)70218-2. [DOI] [PubMed] [Google Scholar]
- 29.Limana F, Urbanek K, Chimenti S, Quaini F, Leri A, Kajstura J, Nadal-Ginard B, Izumo S, Anversa P. bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci U S A. 2002;99:6257–6262. doi: 10.1073/pnas.092672899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem. 2003;278:40364–40372. doi: 10.1074/jbc.M302999200. [DOI] [PubMed] [Google Scholar]
- 31.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Matylevitch NP, Schuschereba ST, Mata JR, Gilligan GR, Lawlor DF, Goodwin CW, Bowman PD. Apoptosis and accidental cell death in cultured human keratinocytes after thermal injury. Am J Pathol. 1998;153:567–577. doi: 10.1016/S0002-9440(10)65599-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorehead RA, Fata JE, Johnson MB, Khokha R. Inhibition of mammary epithelial apoptosis and sustained phosphorylation of Akt/PKB in MMTV-IGF-II transgenic mice. Cell Death Differ. 2001;8:16–29. doi: 10.1038/sj.cdd.4400762. [DOI] [PubMed] [Google Scholar]
- 34.Nauc V, De Lamirande E, Leclerc P, Gagnon C. Inhibitors of phosphoinositide 3-kinase, LY294002 and wortmannin, affect sperm capacitation and associated phosphorylation of proteins differently: Ca2+-dependent divergences. J Androl. 2004;25:573–585. doi: 10.1002/j.1939-4640.2004.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama K, Konishi A, Nishio C, raki-Yoshida K, Hatanaka H, Kojima M, Ohmiya Y, Yamada M, Koshimizu H. Expression of cystatin C prevents oxidative stress-induced death in PC12 cells. Brain Res Bull. 2005;67:94–99. doi: 10.1016/j.brainresbull.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Ogata Y, Takahashi M, Ueno S, Takeuchi K, Okada T, Mano H, Ookawara S, Ozawa K, Berk BC, Ikeda U, Shimada K, Kobayashi E. Antiapoptotic effect of endothelin-1 in rat cardiomyocytes in vitro. Hypertension. 2003;41:1156–1163. doi: 10.1161/01.HYP.0000064342.30653.24. [DOI] [PubMed] [Google Scholar]
- 37.Park C, So HS, Kim SJ, Youn MJ, Moon BS, Shin SH, Lee I, Moon SK, Park R. Samul extract protects against the H2O2-induced apoptosis of H9c2 cardiomyoblasts via activation of extracellular regulated kinases (Erk) 1/2. Am J Chin Med. 2006;34:695–706. doi: 10.1142/S0192415X06004211. [DOI] [PubMed] [Google Scholar]
- 38.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther. 2006;318:186–194. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- 39.Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta -adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001;33:1791–1803. doi: 10.1006/jmcc.2001.1442. [DOI] [PubMed] [Google Scholar]
- 40.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Singla DK, Lyons GE, Kamp TJ. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1308–H1314. doi: 10.1152/ajpheart.01277.2006. [DOI] [PubMed] [Google Scholar]
- 42.Singla DK, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis of H9c2 cells. Am J Physiol Heart Circ Physiol. 2007;293:H1590–H1595. doi: 10.1152/ajpheart.00431.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 44.Singla DK, Sun B. Transforming growth factor-beta2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun. 2005;332:135–141. doi: 10.1016/j.bbrc.2005.04.098. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Cui M, Sun L, Jia Z, Bai Y, Ma K, Chen F, Zhou C. Angiopoietin-1 protects H9c2 cells from H2O2-induced apoptosis through AKT signaling. Biochem Biophys Res Commun. 2007;359:685–690. doi: 10.1016/j.bbrc.2007.05.172. [DOI] [PubMed] [Google Scholar]
- 46.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe J. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You KH, Ji YM, Kwon OY. Clusterin overexpression is responsible for the anti-apoptosis effect in a mouse neuroblastoma cell line, B103. Z Naturforsch [C] 2003;58:148–151. doi: 10.1515/znc-2003-1-226. [DOI] [PubMed] [Google Scholar]