Abstract
Purpose
To compare the effects of conventional amplification (CA) and digital frequency compression (DFC) amplification on the speech recognition abilities of candidates for a partial-insertion cochlear implant, that is, candidates for combined electric and acoustic stimulation (EAS).
Method
The participants were 6 patients whose audiometric thresholds at 500 Hz and below were ≤60 dB HL and whose thresholds at 2000 Hz and above were ≥80 dB HL. Six tests of speech understanding were administered with CA and DFC. The Abbreviated Profile of Hearing Aid Benefit (APHAB) was also administered following use of CA and DFC.
Results
Group mean scores were not statistically different in the CA and DFC conditions. However, 2 patients received substantial benefit in DFC conditions. APHAB scores suggested increased ease of communication, but also increased aversive sound quality.
Conclusion
Results suggest that a relatively small proportion of individuals who meet EAS candidacy will receive substantial benefit from a DFC hearing aid and that a larger proportion will receive at least a small benefit when speech is presented against a background of noise. This benefit, however, comes at a cost—aversive sound quality.
Keywords: hearing aids, frequency compression, cochlear implants, amplification, electric and acoustic stimulation (EAS)
Approximately 31.5 million individuals in the United States have hearing loss (Kochkin, 2005). The most common losses are at frequencies higher than 1 kHz, for which the most common rehabilitation option is conventional, frequency-shaped amplification. The benefits of conventional amplification, however, vary considerably with the configuration and degree of the hearing loss. Several studies report little or no benefit of high-frequency amplification when auditory thresholds exceed 55–60 dB HL at or above 2000 Hz when speech materials are presented in quiet (Amos & Humes, 2001; Ching, Dillon, & Byrne, 1998; Hogan & Turner, 1998; Turner & Cummings, 1999). Although amplification of high frequencies may be of some benefit in noise (Plyler & Fleck, 2006), listeners with severe high-frequency losses remain at a significant disadvantage in all listening environments.
Listeners with greater degrees of hearing loss are at the greatest disadvantage because speech reception is negatively influenced, first, by the high presentation levels used to improve audibility (e.g., Dubno, Horwitz, & Ahlstrom, 2005, 2006; French & Steinberg, 1947; Hornsby & Ricketts, 2006; Studebaker, Sherbecoe, McDaniel, & Gwaltney, 1999) and, second, by the increased likelihood of damage to inner hair cells in the basal region of the cochlea (e.g., Moore, Huss, Vickers, Glasberg, & Alcántara, 2000). Thus, the use of conventional, frequency-shaped amplification results in the presentation of less intelligible speech to an impaired auditory system.
Combined Electric and Acoustic Stimulation (EAS)
Despite the shortcomings of traditional amplification, few alternative rehabilitation options have been available to listeners with relatively good low-frequency hearing and precipitous high-frequency hearing loss. Recently, however, studies have examined the use of a cochlear implant to provide high-frequency information through direct electrical stimulation of the spiral ganglion cells in the basal region of the cochlea. Although this treatment option involves a significant risk of damaging neural tissue in the apical region of the cochlea—causing a greater or complete loss of acoustic hearing—multiple studies have now demonstrated that an electrode array can be inserted 10 or even 20 mm into the cochlea without destroying residual low-frequency hearing (e.g., Gantz & Turner, 2004; Gantz, Turner, Gfeller, & Lowder, 2005; Kiefer et al., 2005; von Ilberg et al., 1999). A successful surgery allows a listener to perceive a combination of electric and acoustic stimulation (EAS) from the same ear.
Since the first published report of successful EAS surgery (Vonllberg et al., 1999), there have been questions regarding the efficacy of this treatment and the recipient’s ability to integrate the acoustic and electric signals. A number of cochlear implant centers have reported several cases of hearing preservation and have demonstrated that patients can integrate acoustically and electrically stimulated percepts (Gantz et al., 2005; Gantz & Turner, 2004; Gstoettner et al., 2004; Kiefer et al., 2005; Skarzynski, Lorens, & Piotrowska, 2004; von Ilberg et al., 1999). The mean monosyllabic word recognition scores of EAS listeners have been reported to be as high as 79% (Gantz et al., 2005)—much higher than the mean score of 55%–60% correct reported for conventional, unilateral cochlear implants (Helms et al., 1997).
Frequency Compression
Given that EAS involves the risk of losing hearing in the operated ear, clinicians should exhaust all possible acoustic amplification options prior to recommending surgical intervention. The remaining acoustic intervention option involves the use of frequency-compression amplification.
Until recently, research with hearing-impaired listeners has demonstrated little or no improvement in speech intelligibility with various methods of frequency compression (Beasley, Mosher, & Orchik, 1976; Bennett & Byers, 1967; Mazur, Simon, Scheinberg, & Levitt, 1979; Posen, Reed, & Braida, 1993). There has been evidence, however, of improved speech intelligibility in adults with severely limited audibility who have been fitted with newer frequency-compression devices. Parent, Chmiel, and Jerger (1997) demonstrated significant improvements in speech intelligibility and quality of life ratings for 2 out of 4 participants using frequency compression combined with amplification (AVR TranSonic). McDermott, Dorkos, Dean, and Ching (1999) also demonstrated significant improvements in speech intelligibility with the TranSonic frequency-compression hearing aid. The results, however, revealed that the majority of benefit arose from the increased low-frequency gain provided by the device relative to the listeners’ conventional hearing aids. Nevertheless, McDermott et al. (1999) were able to demonstrate a small benefit due to the frequency-compression characteristic for 2 of 4 listeners. Turner and Hurtig (1999) demonstrated significant improvements in speech intelligibility when uniform frequency compression was combined with frequency-dependent amplification and presented acoustically via headphones.
In contrast, McDermott and Knight (2001) showed no significant improvement in speech intelligibility using frequency-compression technology (AVR ImpaCt) for 3 individuals with severe-to-profound hearing loss. Simpson, Hersbach, and McDermott (2005) reported no significant benefit of an experimental, nonlinear frequency compression device at the group level for 17 enrolled participants on measures of phoneme recognition; however, at the individual level, 8 participants demonstrated a significant increase in phoneme recognition. Simpson, Hersbach, and McDermott (2006) examined this same nonlinear frequency compression scheme in 7 individuals with steeply sloping hearing loss. They reported no group-level differences in speech perception with the frequency-compression scheme over appropriately fit conventional amplification. In fact, subjective comparisons between conventional hearing aids and the frequency compression scheme using the Abbreviated Profile of Hearing Aid Benefit (APHAB; Cox & Alexander, 1995) revealed that the majority of listeners preferred conventional amplification. Differences across studies were likely influenced by the nature of the frequency-compression algorithm, speech materials used for assessment, and the severity and type of audiometric configuration for the subjects tested.
The current study evaluated the performance of patients fit with a digital frequency compression (DFC) device manufactured by AVR Sonovation that is designed specifically to apply DFC only when detecting high-frequency speech signals (voiceless consonants) that would otherwise be inaudible to the listener. DFC is not applied during other segments of the speech signal. The ratio of proportional DFC is based on the slope and severity of the listener’s hearing loss and is then modified in response to the listener’s behavioral responses to sounds (e.g., Ling 5 sound test; Ling & Ling, 1978). This implementation of frequency compression—in contrast to frequency transposition—maintains the ratios between the spectral characteristics of voiced speech. For more details concerning this technology see Davis (2000, 2001).
Aim
The aim of this study was to assess whether individuals with relatively good low-frequency thresholds and very poor high-frequency thresholds—who meet EAS candidacy requirements—would benefit more from a combination of amplification and DFC than from frequency-shaped amplification alone. The goal was to determine whether DFC is a viable, nonsurgical alternative to cochlear implantation.
Method
Participants
Six participants (4 male, 2 female) with hearing loss were recruited for the study. The mean age was 71.5 years, with a range of 60–84 years. They were paid an hourly wage for their participation. All participants were experienced users of binaural amplification for at least 5 years. Research participants were chosen to match the audiologic criteria for participation in the FDA clinical trial of EAS as conducted by Cochlear Americas for the Nucleus Hybrid device. Specifically, Hybrid candidacy requires that the following criteria be met: (a) thresholds at 500 Hz and below must be ≤60 dB HL, (b) thresholds at 2000 Hz and above must be ≥80 dB HL, and (c) listeners must demonstrate moderate performance on open set speech understanding (monosyllabic word recognition ≤60% in the ear to be implanted, and no greater than 80% in the nonimplant ear). Figure 1 displays individual and mean audiometric thresholds for all participants. The bold lines in each panel outline the range of acceptable thresholds at any given frequency to meet EAS candidacy. As demonstrated in Figure 1, all participants met audibility requirements for EAS.
Figure 1.
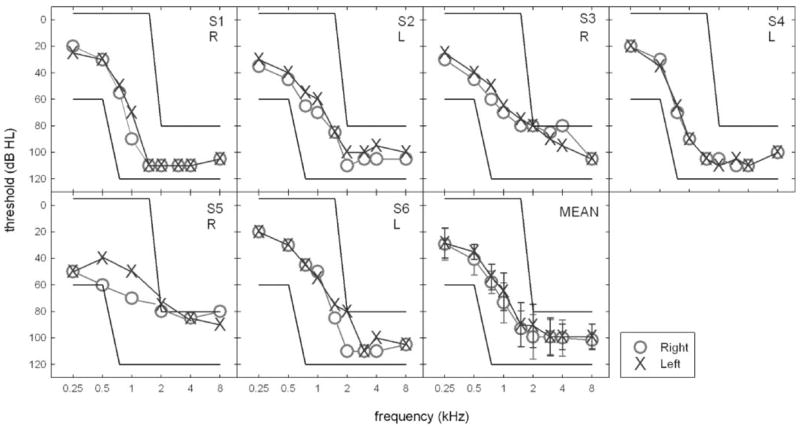
Individual and mean audiometric thresholds. The bold lines in each panel outline the range of acceptable thresholds for candidacy in the clinical trial of the Nucleus Hybrid cochlear implant device as outlined by Cochlear Americas. The ear tested for the monaural aided conditions is noted in each subject panel.
Test Materials and Design
Performance was measured before and after participants were fit binaurally with AVR Sonovation Nano Xp DFC hearing aids. All measurements were performed over three to four test sessions spanning a 5- to 8-week period. Participants were not given feedback on performance until participation in the study was concluded. Test order on all measures was randomized for each individual.
The test materials included monosyllabic words presented in quiet and sentences presented in quiet and at +10 dB signal-to-noise ratio (SNR). Word recognition and sentence recognition in quiet and in noise was tested in both binaural and monaural conditions. This was done on the recommendation of the manufacturer, who indicated that patients reported a higher level of satisfaction with binaural DFC. The monaural and binaural test conditions were randomized for each participant.
Monosyllabic word recognition was assessed with one of ten 50-item Consonant Nucleus Consonant (CNC) word lists (Peterson & Lehiste, 1962). CNC word lists were chosen in a pseudorandom manner for each listener. The presentation level of the words was fixed at an overall root-mean-square level of 70 dB SPL. The lists were scored for overall percentage of words and phonemes repeated correctly.
Sentence recognition was assessed using both the City University of New York (CUNY) and AzBio sentence materials. CUNY sentences were used because there is a large literature on CUNY sentence intelligibility by patients fit with cochlear implants. AzBio sentences were used because the CUNY sentences are relatively easy and commonly suffer ceiling effects when used with patients fit with a cochlear implant.
For the CUNY sentence material, a total of 24 sentences (two lists) were used in each condition. All lists were taken from the Cochlear Corporation Investigational Test Battery CD (Boothroyd, Hanin, & Hnath, 1985).
The AzBio sentence materials were drawn from an original set of 33 lists of 20 sentences recorded by 2 male and 2 female speakers. The sentences were produced in a conversational style by untrained speakers. The lists were found to be equally intelligible (89% correct) when passed through a simulation of a five-channel, cochlear implant signal processor and presented to normal-hearing listeners (Spahr & Dorman, 2004). The assignment of a list to a condition was randomized for each participant. One 20-sentence list, with an equal number of male and female speakers, was presented in quiet and one 20-sentence list was presented in noise. Sentences were scored as total words correct. Sentences were presented at 70 dB SPL in quiet and at +10 dB SNR (four-talker babble).
Verification of Hearing Aid Settings
Before testing pre-DFC performance, each participant’s own hearing aid settings were first run with a simulated speechmap fitting system using the Verifit simulated real-ear mode (S-REM) to match NAL-NL1 targets. That is, all measurements were made in the test chamber (with the 2-cc coupler) and were converted to an estimated real ear SPL. Given that all speech stimuli in the current study were presented at an overall level of 70 dB SPL, the participants’ hearing aids were required to meet prescribed targets for average-level speech at 70 dB SPL. Hearing aids that did not meet target were reprogrammed to achieve a match. This was required for 2 of the 6 participants (S1 and S4) whose preferred frequency response was below NAL-NL1 prescribed targets.
Binaural fitting of the AVR Sonovation Nano Xp hearing aids was accomplished with the SonoFit programming software provided by the manufacturer. All participants were fit with custom earmolds with venting, when possible. All but 1 participant—whose own hearing aid was an in-the-ear model—used the new earmolds with their own hearing aids as well as with the Nano Xp DFC hearing aids before data collection began. Each participant was first run on the Sonofit in situ target test for both soft and loud inputs to help determine each individual’s dynamic range in seven spectral bands. Next, each hearing aid was configured and a prescribed DFC ratio was assigned for each participant based on the severity and slope of the hearing loss. Each participant was presented with a live audio-only presentation of the Ling sounds (with use of a screen) before and after setting the DFC ratios. The degree of DFC was adjusted as needed for each individual depending on their behavioral responses to the Ling sounds as well as to conversational speech during the fitting. That is, the DFC ratio was increased until both/s/and/sh/were audible to the listener.
Following this initial fitting, the Nano Xp settings were then also verified with a simulated speechmap using the Verifit S-REM to match NAL-NL1 targets. To obtain the speechmap, DFC ratios were set to 1.0 (no DFC) to yield an accurate estimate of gain for average-level speech stimuli (70 dB SPL). Following this procedure, DFC ratios were reset to individually determined values. This constituted a first fit and each participant was instructed to wear the hearing aids for a minimum of 8 hr/day for at least 5 weeks before evaluating speech intelligibility performance with the DFC technology. Although the Nano Xp hearing aids are not equipped with data-logging capabilities, all participants reportedly adhered to at least the minimum usage requirements for the DFC devices. Each participant was informed that the hearing aids had been optimally programmed for their hearing loss; however, there was an optional visit scheduled 2 weeks after the initial fitting to address any concerns that may have arisen. Of the 6 participants enrolled in the study, 3 came back for the 2-week, post-fitting visit for adjustments of gain and/or DFC ratios. Following these adjustments, another simulated speechmap was obtained to verify that NAL-NL1 targets were still met for 70-dB SPL input. For these 3 participants, the minimum 5-week trial was restarted from the date of the adjustment. Table 1 displays the final DFC ratio assigned for each hearing aid for all 6 participants as well as the number of weeks included in the trial period, age, etiology of hearing loss, and manufacturer/model/style of their own hearing aids.
Table 1.
Participants’ ages, etiology of hearing loss, manufacturer/model/style of own hearing aids, and final digital frequency compression (DFC) ratios used for each participant’s Nano Xp hearing aid, as well as the number of weeks during which the Nano Xp hearing aids were worn before testing.
| Participant | Age (years) | Etiology | Own hearing aid | DFC ratio | No. weeks in trial |
|---|---|---|---|---|---|
| S1 | 84 | Noise exposure, presbycusis | Oticon Syncro BTEs | 5.00:5.00 | 8 |
| S2 | 67 | Unknown | Starkey 10 series intelli mid-freq circuit ITE L, Oticon Adapto BTE R | 3.75:3.75 | 6 |
| S3 | 75 | Familial | ReSound Canta 4 BTEs | 2.25:2.25 | 7 |
| S4 | 67 | Noise exposure/military service | Oticon Adapto-P BTEs | 5.00:5.00 | 8 |
| S5 | 76 | Unknown | Oticon Adapto BTEs | 2.50:2.25 | 6 |
| S6 | 60 | Noise exposure/military service | Widex Senso Diva BTEs | 4.00:4.25 | 5 |
Note. BTE = behind the ear; ITE = in the ear.
Results
Differences between conditions were evaluated with a repeated-measures analysis of variance using the Bonferroni correction for multiple comparisons.
Monaural Versus Binaural Stimulation
Figure 2 displays the means and standard deviations for all scores obtained in monaural- and binaural-aided conditions. For conventional amplification, there were no statistically significant effects of monaural versus binaural presentation: for CNC words (40% vs. 41% correct), F(1, 5) = 1.000, p = .363; for CNC phonemes (58% vs. 57% correct), F(1, 5) = 1.106, p = .341; for CUNY in quiet (83% vs. 86% correct), F(1, 5) = 0.593, p = .476; for CUNYat +10 dB SNR (70% vs. 81% correct), F(1, 5) = 2.092, p = .208; for AzBio in quiet (50% vs. 60% correct), F(1, 5) = 8.013, p = .037; and for AzBio at +10 dB SNR (40% vs. 46% correct), F(1, 5) = 1.949, p = .222.
Figure 2.
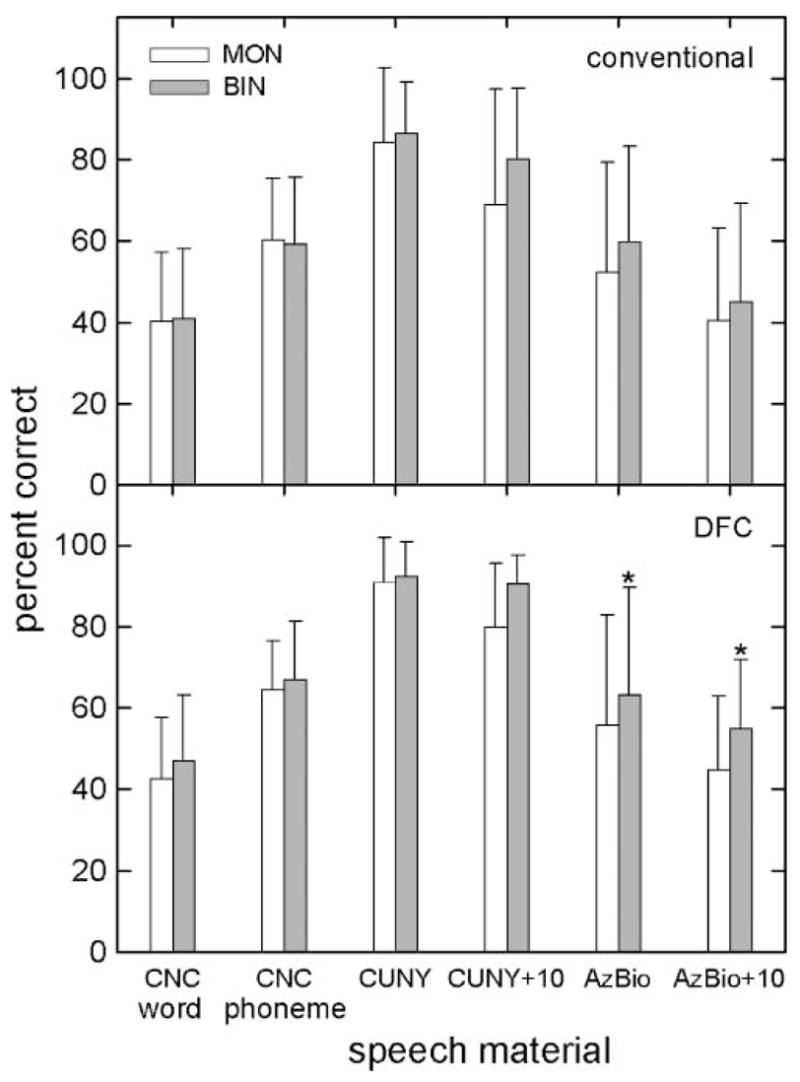
Means and standard deviations on all measures tested in both monaural (MON; unfilled bars) and binaural (BIN; filled bars) conditions for conventional (upper panel) and digital frequency compression (DFC; lower panel) amplification. Conditions that differ significantly are indicated by an asterisk. CNC = Consonant Nucleus Consonant; CUNY = City University of New York.
For DFC amplification, there were two significant effects of listening condition—for AzBio sentences in quiet and at +10 dB SNR. Mean scores and F ratios were as follows for the various test materials: for CNC words (42% vs. 48% correct), F(1, 5) = 3.824, p = .108; for CNC phonemes (64% vs. 66% correct), F(1, 5) = 1.915, p = .225; for CUNY in quiet (79% vs. 90% correct), F(1, 5) = 0.772, p = .420; for CUNYat +10 dB SNR (80% vs. 90% correct), F(1, 5) = 8.335, p = .034; for AzBio in quiet (56% vs. 63% correct), F(1, 5) = 32.961, p = .002; and for AzBio at +10 dB SNR (46% vs. 56% correct), F(1, 5) = 25.103, p = .004.
Because binaural stimulation was as effective or was more effective than monaural stimulation, the next section describes differences between conventional amplification and DFC amplification for only the binaural conditions.
Conventional Amplification (CA) Versus DFC
Group mean scores are shown in Figure 3 for binaural test conditions. There were no statistically significant differences between conventional amplification (CA) and DFC for any of the measures tested. The mean score for CNC words was 41% correct for CA and 48% correct for DFC, F(1, 5) = 2.8, p = .16. The mean score for CNC phonemes was 57% correct for CA and 66% correct for DFC, F(1, 5) = 2.1, p = .21. The mean score for CUNY sentences in quiet was 86% correct for CA and 90% correct for DFC, F(1, 5) = 5.7, p = .06. The mean score for CUNY sentences at +10 dB SNR was 81% correct for CA and 90% correct for DFC, F(1, 5) = 4.2, p = .10. The mean score for AzBio sentences in quiet was 60% correct for CA and 63% correct for DFC, F(1, 5) = 1.0, p = .36. The mean score for AzBio sentences in noise was 46% correct for CA and 56% correct for DFC, F(1, 5) = 6.1, p = .06.
Figure 3.
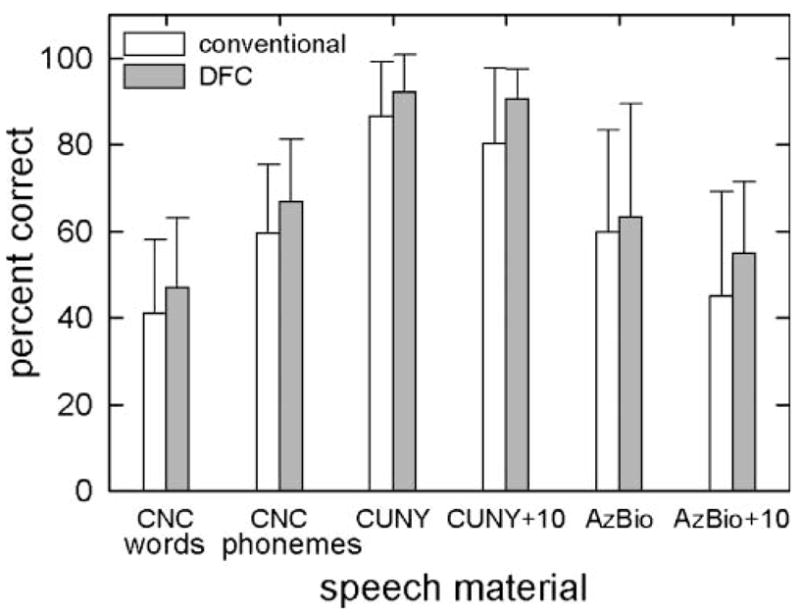
Means and standard deviations on all measures tested in binaural listening conditions for conventional (unfilled bars) and DFC (filled bars) amplification.
Individual patient performance as a function of test material and device is shown in Figure 4. S2 had higher scores with the DFC device than the CA device in all test conditions. Performance gains varied between 10% and 31% and averaged 22%. S5 also benefited in test conditions for which there were no ceiling effects. Performance gains varied between 4% and 14% and averaged 12%. For the 4 other patients, performance in quiet was generally no better with the DFC device than with the CA device. In noise, however, 3 of the remaining 4 patients showed small gains in performance with the DFC device. The gains varied between 2% and 15%.
Figure 4.
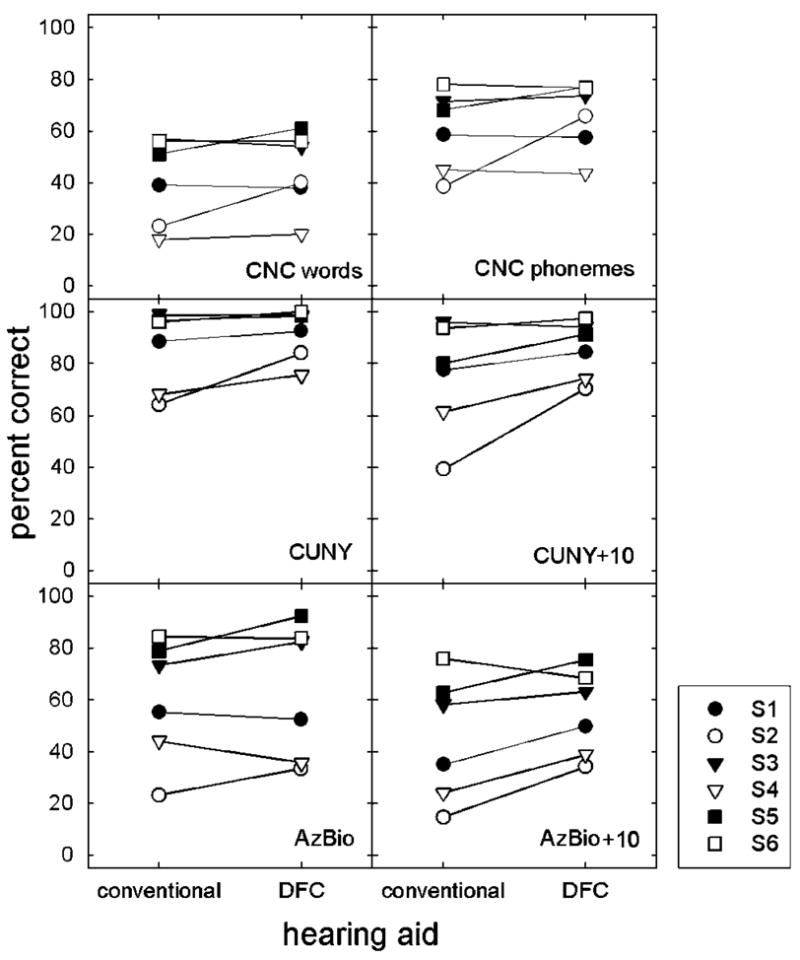
Individual scores as a function of test condition for six measures of performance.
Of interest is that the 6 participants who all met audiologic criteria for EAS demonstrated a varied range of speech recognition performance. A recent paper by Gifford, Dorman, Spahr, and Bacon (2007) detailed the speech recognition performance as well as psychophysical measures of frequency selectivity, temporal resolution, and nonlinear cochlear processing in 17 individuals meeting audiologic candidacy for EAS. They reported that that EAS-qualifying individuals have a wide range of speech recognition abilities and that speech recognition was not correlated with any of the psychophysical measures of auditory function.
Subjective Analysis of DFC
All participants were asked to complete an APHAB questionnaire (Cox, 1997; Cox & Alexander, 1995) before and after fitting with the Nano Xp DFC hearing aids. The APHAB is broken into four subsets to scale the percentage of problems in situations pertaining to ease of communication (EC), background noise (BN), reverberation (RV), and aversiveness (AV). Figure 5 shows the mean ratings for conventional hearing aids and DFC hearing aids, as well as the unaided condition. Although 4 of 6 patients reported decreases in the percentage of problems associated with DFC hearing aids relative to the conventional hearing aids on the EC, BN, and RV scales, these trends did not reach statistical significance. On the AV scale, there was a trend—albeit not significant—for a greater perception of aversiveness with the DFC hearing aids (66% problems) as compared to a conventional hearing aid (34% problems). Most participants reported that while they could appreciate the increased audibility of higher frequency sounds, such as birds chirping, running water, dish and silverware noise, and higher frequency consonants, it was difficult to adapt to these sounds (see also Sakamoto, Goto, Tateno, & Kimitaka, 2000). For those listeners who had expressed considerable difficulty adapting to the DFC—particularly for non-speech events such as page turning during reading and the contact of eating utensils on dinner plates—a non-DFC program was placed in Program 2. Though the participants were instructed to use Program 1 for the majority of their waking hours, they were advised to use Program 2 for those particularly troublesome situations.
Figure 5.
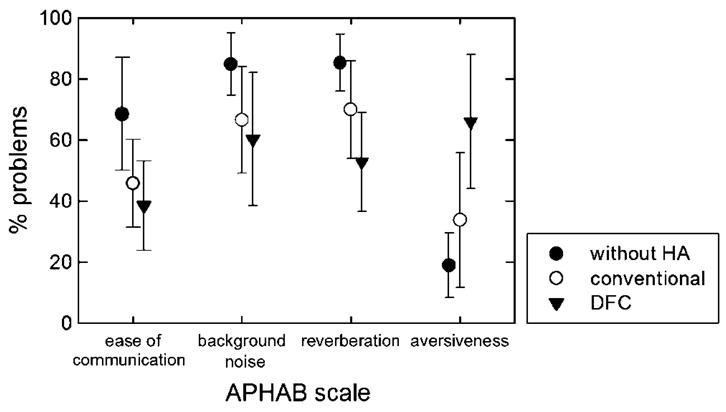
Mean subjective ratings expressed in percentage of problems, as a function of hearing aid (HA) condition, for four scales on the Abbreviated Profile of Hearing Aid Benefit (APHAB) questionnaire.
One potential bias in the subjective results was that 3 of the participants (S1, S3, and S6) had directional microphones on their own hearing aids, but not on the DFC hearing aids. Although this would not have influenced the speech perception findings with the use of a single speaker placed at 0° azimuth, it could have potentially influenced participants’ subjective ratings of the devices (and the resultant comparison). In spite of this potential bias, the mean percentage of problems with the DFC hearing aids was actually lower than the CA hearing aids on all measures but AV.
Discussion
At a group level, listeners with auditory thresholds meeting EAS candidacy did not receive significant benefit from a combination of amplification and DFC over frequency-shaped amplification alone on measures of word recognition or sentence recognition in quiet or noise. It is likely that a 6-participant sample was not large enough to reach statistical significance at the group level; however, 2 patients clearly benefited from DFC and 3 other patients showed small improvements in noise. Subjective ratings revealed a tendency for increased ease of communication with the DFC hearing aids but also a tendency for the DFC aids to be viewed as more aversive. Overall, the current results suggest that a small proportion of individuals who meet EAS candidacy can receive substantial benefit from DFC. These results also suggest that a larger portion of patients will receive at least a small DFC-related benefit when speech is presented against a background of noise. This small benefit, however, comes at a price—aversive sound quality.
It is instructive to ask, following the termination of this study, whether the patients (a) chose to continue to wearing their own CA hearing aids, (b) chose to purchase DFC aids, or (c) chose to receive a cochlear implant. Participants 3 and 6 (with CNC scores of 56% correct) continued to use their own hearing aids and expressed no interest in a cochlear implant. Participant 5 (with a CNC score of 60% correct) bought two Nano Xp DFC hearing aids and expressed no interest in a cochlear implant. Participants 2 and 4 (CNC scores of 40% and 20% correct, respectively) were awaiting EAS surgery at the time of this writing. Participant 2 planned to purchase a DFC hearing aid for use with EAS in her nonimplant ear. Thus, only 2 out of the 6 participants in the study purchased (or planed to purchase) a device.
To make an informed decision about whether to invest in DFC hearing aids or a cochlear implant, it is necessary to have comparative data on the level of performance that can be achieved with each of the devices. The mean CNC score with DFC for our patient sample was 44% correct. Gantz et al. (2005) reported mean CNC scores of 69%–79% correct (depending on the sample and length of time with the implant) for patients implanted with a 10-mm array and who used combined EAS. Both the current patient sample as well as those reported in Gantz et al. met audiometric criteria for EAS and both had similar CNC scores with conventional amplification. In particular, the average pre-implant binaural aided CNC score of Gantz et al.’s participant group was approximately 31%, with a range of 18%–59%. These data are strikingly similar to the average CNC score with CA for the current study population, which was an average of 41% and a range of 18%–56%. This comparison between the two data sets suggests that both the current participants and those examined by Gantz et al. had similar starting points for their binaural aided word recognition. The much higher postoperative average CNC score for Nucleus Hybrid patients (Gantz et al., 2005) provides a strong indication that a cochlear implant will provide better performance than DFC for EAS-qualifying patients.1
Acknowledgments
This work was supported by National Institute on Deafness and Other Comunication Disorders Grants DC006538 and RO1 DC00654-15. We thank AVR Sonovation Inc., and in particular Wendy Davis and Barak Dar (formerly of AVR Sonovation) for providing the time, training, and information necessary for fitting the hearing aids in the study. We also thank Robert G. Turner and Chris Turner for their helpful comments and suggestions on previous versions of this article. A portion of the reported findings was presented in August 2006 at the International Hearing Aid Research Conference in Lake Tahoe, CA.
Footnotes
One of our patients (S1) received a conventional, full insertion cochlear implant following completion of this project (Advanced Bionics 90k internal device with Auria speech processor). His pre-implant CNC score improved from 38% with DFC to 72% correct in the implant plus aided contralateral condition (or bimodal hearing). This change in score is almost double that of the largest change observed for any patient in the CA versus DFC conditions and is higher than any patient’s score with a DFC aid. AzBio sentence recognition improved from 56% with the DFC hearing aids to 95% in the bimodal condition. AzBio sentence recognition at +10 dB SNR improved from 52% correct with DFC to 89% in the bimodal condition. Thus, although the current study can only provide a direct comparison for 1 patient, there are reasons to believe, given the results of Gantz et al. (2005) and others, that a conventional hearing aid in combination with a long- or a short-electrode implant is likely to provide more benefit to speech understanding than either conventional or DFC hearing aids for patients with good low-frequency hearing and precipitous high-frequency hearing loss.
References
- Amos NE, Humes LE. The contribution of high frequencies to speech recognition in sensorineural hearing loss. In: Breebaart DJ, Houtsma AJM, Kohlrausch A, Prijs VF, Schoonhoven R, editors. Physiological and psychophysical bases of auditory function. Maastricht, the Netherlands: Shaker; 2001. pp. 437–444. [Google Scholar]
- Beasley DS, Mosher NL, Orchik DJ. Use of frequency-shifted/time-compressed speech with hearing impaired children. Audiology. 1976;15:395–406. doi: 10.3109/00206097609071799. [DOI] [PubMed] [Google Scholar]
- Bennett DN, Byers VW. Increased intelligibility in the hypacusic by slow-play frequency transportation. Journal of Auditory Research. 1967;7:107–118. [Google Scholar]
- Boothroyd A, Hanin L, Hnath T. Speech and Hearing Science Report. New York: City University New York; 1985. A sentence test of speech perception: Reliability, set equivalence, and short term learning [Internal Report RCI 10] [Google Scholar]
- Ching TY, Dillon H, Byrne D. Speech recognition of hearing-impaired listeners: Predictions from audibility and the limited role of high-frequency amplification. Journal of the Acoustical Society of America. 1998;103:1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- Cox RM. Administration and application of the APHAB. Hearing Journal. 1997;50:32–48. [Google Scholar]
- Cox RM, Alexander GC. The Abbreviated Profile of Hearing Aid Benefit (APHAB) Ear and Hearing. 1995;16:176–186. doi: 10.1097/00003446-199504000-00005. [DOI] [PubMed] [Google Scholar]
- Davis WE. Interview with Wendy Davis M.S., AVR Sonovation. 2000 July 26; Retrieved June 14, 2005, from http://www.audiologyonline.com/interview/interview_detail.asp?interview_id=9.
- Davis WE. Proportional frequency compression in hearing instruments. Hearing Review. 2001;8(2):34–39. [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Word recognition in noise at higher-than-normal levels: Decreases in scores and increases in masking. Journal of the Acoustical Society of America. 2005;118:914–922. doi: 10.1121/1.1953107. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Spectral and threshold effects on recognition of speech at higher-than-normal levels. Journal of the Acoustical Society of America. 2006;120:310–320. doi: 10.1121/1.2206508. [DOI] [PubMed] [Google Scholar]
- French NR, Steinberg JC. Factors governing the intelligibility of speech sounds. The Journal of the Acoustical Society of America. 1947;19:90–119. [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngologica. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Spahr AJ, Bacon SP. Auditory function and speech understanding in listeners who qualify for EAS surgery. Ear and Hearing. 2007;28(Suppl):117S–118S. doi: 10.1097/AUD.0b013e3180315455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstoettner W, Kiefer J, Baumgartner WD, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngologica. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- Helms J, Müller J, Schön F, Möser L, Arnold W, Janssen T, et al. Evaluation of performance with the COMBI 40 cochlear implant in adults: A multicentric clinical study. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties. 1997;59:23–35. doi: 10.1159/000276901. [DOI] [PubMed] [Google Scholar]
- Hogan CA, Turner CW. High-frequency audibility: Benefits for hearing-impaired listeners. Journal of the Acoustical Society of America. 1998;104:432–441. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- Hornsby BWY, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding. II. Sloping hearing loss. Journal of the Acoustical Society of America. 2006;119:1752–1763. doi: 10.1121/1.2161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J, Pok M, Adunka O, Stürzebecher E, Baumgartner W, Schmidt M, et al. Combined electric and acoustic stimulation of the auditory system: Results of a clinical study. Audiology & Neurotology. 2005;10(3):134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Kochkin SG. MarkeTrak VII: Hearing loss population tops 31 million. Hearing Review. 2005;12(7):16–29. [Google Scholar]
- Ling D, Ling AH. Aural habilitation. Washington, DC: AG Bell Association for the Deaf; 1978. [Google Scholar]
- Mazur M, Simon H, Scheinberg J, Levitt H. Moderate frequency compression for the moderately hearing impaired. Journal of the Acoustical Society of America. 1979;62:1273–1278. doi: 10.1121/1.381652. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, Dorkos VP, Dean MR, Ching TY. Improvements in speech perception with use of the AVR TranSonic frequency-transposing hearing aid. Journal of Speech, Languange, and Hearing Research. 1999;42:1323–1335. doi: 10.1044/jslhr.4206.1323. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, Knight MR. Preliminary results with the AVR ImpaCt frequency-transposing hearing aid. Journal of the American Academy of Audiology. 2001;12:121–127. [PubMed] [Google Scholar]
- Moore BCJ, Huss M, Vickers DA, Glasberg BR, Alcántara JI. A test for the diagnosis of dead regions in the cochlea. British Journal of Audiology. 2000;34:205–224. doi: 10.3109/03005364000000131. [DOI] [PubMed] [Google Scholar]
- Parent TB, Chmiel R, Jerger J. Comparison of performance with frequency transposition hearing aids and conventional hearing aids. Journal of the American Academy of Audiology. 1997;8:355–365. [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Plyler PN, Fleck EL. The effects of high-frequency amplification on the objective and subjective performance of hearing instrument users with varying degrees of high-frequency hearing loss. Journal of Speech, Language, and Hearing Research. 2006;49:616–627. doi: 10.1044/1092-4388(2006/044). [DOI] [PubMed] [Google Scholar]
- Posen MP, Reed CM, Braida LD. Intelligibility of frequency-lowered speech produced by a channel vocoder. Journal of Rehabilitation Research and Development. 1993;30:26–38. [PubMed] [Google Scholar]
- Sakamoto S, Goto K, Tateno M, Kimitaka K. Frequency compression hearing aid for severe-to-profound hearing impairments. Auris Nasus Larynx. 2000;27:327–334. doi: 10.1016/s0385-8146(00)00066-3. [DOI] [PubMed] [Google Scholar]
- Simpson A, Hersbach AA, McDermott HJ. Improvements in speech perception with an experimental nonlinear frequency compression hearing device. International Journal of Audiology. 2005;44:281–292. doi: 10.1080/14992020500060636. [DOI] [PubMed] [Google Scholar]
- Simpson A, Hersbach AA, McDermott HJ. Frequency-compression outcomes in listeners with steeply sloping audiograms. International Journal of Audiology. 2006;45:619–629. doi: 10.1080/14992020600825508. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, Piotrowska A. Preservation of low-frequency hearing in partial deafness cochlear implantation. International Congress Series. 2004;1273:239–242. [Google Scholar]
- Spahr AJ, Dorman MF. Performance of subjects fit with the Advanced Bionics CII and Nucleus 3G cochlear implant devices. Archives of Otolaryngology–Head and Neck Surgery. 2004;130:624–628. doi: 10.1001/archotol.130.5.624. [DOI] [PubMed] [Google Scholar]
- Studebaker G, Sherbecoe R, McDaniel D, Gwaltney C. Monosyllabic word recognition at higher-than-normal speech and noise levels. Journal of the Acoustical Society of America. 1999;105:2431–2444. doi: 10.1121/1.426848. [DOI] [PubMed] [Google Scholar]
- Turner CW, Cummings KJ. Speech audibility for listeners with high-frequency hearing loss. American Journal of Audiology. 1999;8:47–56. doi: 10.1044/1059-0889(1999/002). [DOI] [PubMed] [Google Scholar]
- Turner CW, Hurtig RR. Proportional frequency compression of speech for listeners with sensorineural hearing loss. Journal of the Acoustical Society of America. 1999;106:877–886. doi: 10.1121/1.427103. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecker E, Klinke R. Electric-acoustic stimulation of the auditory system. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]


