Abstract
Purpose
The authors assessed whether (a) a full-insertion cochlear implant would provide a higher level of speech understanding than bilateral low-frequency acoustic hearing, (b) contralateral acoustic hearing would add to the speech understanding provided by the implant, and (c) the level of performance achieved with electric stimulation plus contralateral acoustic hearing would be similar to performance reported in the literature for patients with a partial insertion cochlear implant.
Method
Monosyllabic word recognition as well as sentence recognition in quiet and at +10 and +5 dB was assessed. Before implantation, scores were obtained in monaural and binaural conditions. Following implantation, scores were obtained in electric-only and electric-plus-contralateral acoustic conditions.
Results
Postoperatively, all individuals achieved higher scores in the electric-only test conditions than they did in the best pre-implant test conditions. All individuals benefited from the addition of low-frequency information to the electric hearing.
Conclusion
A full-insertion cochlear implant provides better speech understanding than bilateral, low-frequency residual hearing. The combination of an implant and contralateral acoustic hearing yields comparable performance to that of patients with a partially inserted implant and bilateral, low-frequency acoustic hearing. These data suggest that a full-insertion cochlear implant is a viable treatment option for patients with low-frequency residual hearing.
Keywords: cochlear implants, hearing aids, electric and acoustic stimulation (EAS), bimodal hearing, speech perception in noise
For patients with a bilateral, precipitously sloping high-frequency hearing loss, there are four treatment options: Conventional amplification, digital frequency compression, a partial-insertion cochlear implant, and a full-insertion cochlear implant. Most patients with this type of hearing loss do not benefit from either conventional amplification (Amos & Humes, 2001; Ching, Dillon, & Byrne, 1998; Hogan & Turner, 1998; Turner & Cummings, 1999) or digital frequency compression (Gifford, Dorman, Spahr, & McKarns, in press). Thus, either a partial- or full-insertion cochlear implant remains as a treatment option. With a partially inserted (10 or 20 mm) electrode array, patients receive acoustic stimulation from residual acoustic hearing (A) in both the implanted and the nonimplanted ear and electrical stimulation (E) from the implanted ear (Gantz & Turner, 2004; Gantz, Turner, Gfeller, & Lowder, 2005; Gstoettner et al., 2004; Kiefer et al., 2005; Skarzynski, Lorens, & Piotrowska, 2004; von Ilberg et al., 1999). With a fully inserted electrode array, patients receive electric stimulation from the implanted ear and acoustic stimulation from the contra-lateral ear. In both cases, patients have the opportunity to benefit from the combination of electric and acoustic stimulation (EAS).
Initial reports of hearing preservation and speech understanding following partial insertion of an electrode array have been very encouraging. Gantz et al. (2005) studied 11 patients with a 10-mm electrode insertion and reported an average postimplant threshold shift of 9.5 dB for frequencies ≤1 kHz. One patient lost hearing postoperatively following a cytomegalovirus infection. Postimplant monosyllabic word scores with the consonant–nucleus–consonant (CNC) word lists (Peterson & Lehiste, 1962) averaged 69% correct in the combined electric and binaural acoustic condition. Using a 20-mm electrode insertion, Gstoettner et al. (2004) reported an average threshold shift of 10.5 dB for a sample of 21 patients. Hearing was preserved within 20 dB for 86% of the sample and within 10 dB (at 500 Hz) for 64% of the sample. Three patients had a total loss of hearing. In a subset of this population, Kiefer et al. (2005) reported, postimplant, monosyllabic scores of 67% correct.
A number of studies have examined the performance of patients who have a fully inserted electrode array in one ear and who have residual hearing in the non-implanted ear (e.g., Armstrong, Pegg, James, & Blamey, 1997; Ching, Incerti, & Hill, 2004; Dunn, Tyler, & Witt, 2005; Hamzavi, Pok, Gstoettner, & Baumgartner, 2004; Kong, Stickney, & Zeng, 2005; Mok, Grayden, Dowell, & Lawrence, 2006; Shallop, Arndt, & Turnacliff, 1992; Tyler et al., 2002). The amount of residual hearing varied widely across studies, and few patients had hearing thresholds similar to those in Gantz et al. (2005), Kiefer et al. (2005), or Gstoettner et al. (2004). The improvement in speech intelligibility gained from adding the residual hearing in the nonimplanted ear to the electric hearing ranged from 8% to 30% on measures of speech recognition in noise. There were, however, reports of listeners who did not demonstrate any benefit from adding acoustic hearing to the electric stimulation (Mok et al., 2006; Shallop et al., 1992); however, the listeners examined in these studies had severe to profound hearing loss in the nonimplant ear, even in the low frequency range.
This report describes the speech recognition abilities of patients with bilateral hearing losses meeting candidacy for EAS (as in Gantz et al., 2005) but who chose to receive a fully inserted, cochlear implant electrode array. At issue was (a) whether a fully inserted electrode array would provide a higher level of performance than residual, low-frequency acoustic hearing, (b) whether contralateral acoustic hearing would add to the speech understanding provided by the implant, and (c) whether the level of performance achieved with electric stimulation plus contralateral acoustic hearing would be similar to the level of performance reported in the literature for patients with a partially inserted electrode array and bilateral low-frequency acoustic hearing.
Method
Participants
Eleven individuals participated in this study (M = 72.3 years; range = 47–85 years). All participants were paid an hourly wage for their participation. Participants met the audiologic criteria for participation in the Food and Drug Administration clinical trial of EAS as conducted in the United States by Cochlear Americas for the Nucleus hybrid device: (a) thresholds at 500 Hz and below at ≤60 dB HL, (b) thresholds at 2000 Hz and above at ≥80 dB HL, (c) monosyllabic word recognition ≤60% in the ear to be implanted and no greater than 80% in the nonimplant ear. Figure 1 displays individual and mean audiometric thresholds for the contralateral or nonimplant ear for all participants. The participants’ pre-implant audiograms revealed strikingly symmetrical hearing (all interaural threshold differences—for any given frequency—were ≤15 dB). The mean thresholds for the contralateral ear at 0.25, 0.5, 0.75, and 1 kHz were 38, 53, 69, and 81 dB HL, respectively. The bold lines in each panel outline the range of acceptable thresholds at any given frequency to meet EAS candidacy as defined by Cochlear Americas. One participant (Participant 1) had a threshold at 500 Hz that was 5 dB greater than the specified criteria for the Nucleus hybrid device. Given that the test–retest variability with standard modified Hughson-Westlake audiometric test procedures (Hughson & Westlake, 1944) ranges from 5 to 10 dB for any given threshold, this individual was considered appropriate for testing in the current study.
Figure 1.
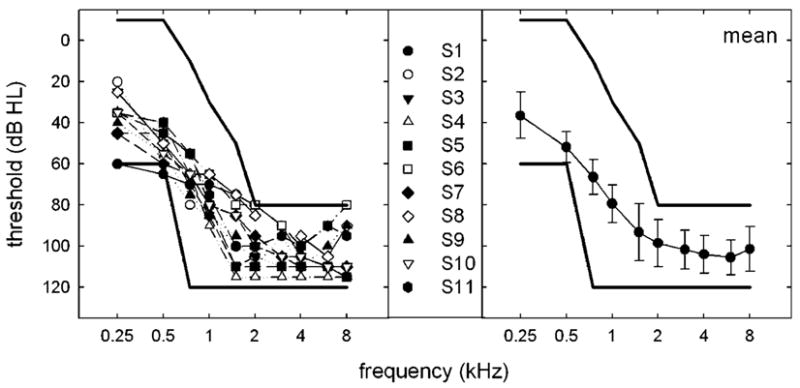
Individual and mean audiometric thresholds. The bold lines in each panel outline the range of acceptable thresholds for candidacy in the clinical trial of the Nucleus hybrid cochlear implant device as outlined by Cochlear Americas. Error bars represent ±1 SD.
Table 1 displays demographic information, including age, sex, processor type and strategy, implant ear, duration of experience with the implant, and hearing aid device in the nonimplanted ear. Given that all 11 participants had symmetrical hearing before receiving the implant, the choice for the implant ear was determined individually by each patient. At the time of testing, all participants had at least 5 months’ experience with electric stimulation (range = 5 months–3 years) and at least 5 years’ experience with amplification prior to implantation. Ten of the 11 participants reported wearing both their implant and hearing aid for the majority of their waking hours since their initial implant activation. One of the participants, S5, did not wear a hearing aid for the first 3 months following initial activation of the implant because of reported high levels of speech understanding with the implant alone. S5 did, however, report that the combination of the hearing aid and cochlear implant has greatly increased his ease of listening and subjective speech understanding. At the time of testing, participant S5—who was 6 months post initial activation—had 2 months’ experience with bimodal hearing.
Table 1.
Individual participant demographics.
| Participant | Age | Gender | Implant processor and strategy | Implant ear | Months’ experience with implant | Hearing aid type |
|---|---|---|---|---|---|---|
| 1 | 55 | Male | Freedom, ACE | Right | 5 | Widex Senso Diva, BTE |
| 2 | 78 | Male | Auria, HiRes | Right | 8 | Phonak Claro BTE |
| 3 | 77 | Male | Freedom, ACE | Right | 6 | Widex Senso C18, BTE |
| 4 | 85 | Female | Auria, HiRes | Right | 6 | Widex Senso Diva, BTE |
| 5 | 84 | Male | Auria, HiRes | Right | 6 | Oticon Syncro P, BTE |
| 6 | 80 | Male | Freedom, ACE | Right | 5 | Starkey Sequel II, BTE |
| 7 | 67 | Male | ESPrit 3G, ACE | Right | 18 | Oticon Syncro P, BTE |
| 8 | 47 | Female | ESPrit 3G, ACE | Right | 39 | Beltone D71 Polara, BTE |
| 9 | 70 | Male | ESPrit 3G, ACE | Left | 28 | Unitron US 80-PP, BTE |
| 10 | 77 | Male | Auria, HiRes | Left | 12 | Oticon Syncro P, BTE |
| 11 | 75 | Male | Auria, HiRes | Right | 7 | Unitron UE 10-H, BTE |
Test Materials and Design
Postimplant performance was measured in the following conditions: aided contralateral acoustic alone (A), electric alone (E; nonimplant ear occluded with an EAR foam plug as well as a circumaural headphone), and combined electric and contralateral–aided acoustic (bimodal). All measurements were performed over 2–5 test sessions, depending on whether the research participant was required to travel to the testing site. Test order on all measures as well as mode of stimulation was randomized for each participant.
Pre- and postimplant CNC scores were available from all 11 participants. Postimplant scores on all measures of speech understanding were also obtained from all 11 participants. Only 6 participants, however, received the entire test battery at both a pre- and a postimplant evaluation.1 The 6 participants for whom we obtained the entire test battery in both pre- and postimplant conditions were examined prospectively. The remaining 5 participants were chosen retrospectively on the basis of their pre-implant audiograms.
The test battery included measures of monosyllabic word recognition and sentence recognition in quiet and in noise (+10 dB and +5 dB signal-to-noise ratio [SNR]). Signals were presented at 70 dB SPL via a loudspeaker placed at a distance of 1 m in front of the listener. Prior to testing, each participant’s hearing aid settings were electroacoustically verified with a simulated speechmap fitting system to match NAL–NL1 targets using the Verifit simulated real-ear mode—that is, all measurements were made in the test chamber (with the 2-cc coupler) and were converted to an estimated real-ear SPL. Given that all speech stimuli in the current study were presented at an overall level of 70 dB SPL, the participants’ hearing aids were only required to meet prescribed targets for the average-level speech signal, which is also presented at 70 dB SPL.
CNC words
All patients were tested with a 50-item CNC word list (Peterson & Lehiste, 1962). The three standard practice words (duck, bomb, and June) were presented with each list for all test conditions.
Sentence material
Lists of 20 sentences from the multiple-talker, AzBio sentence corpus (Spahr & Dorman, 2005) were used as test material. The sentences were spoken by male and female speakers in a conversational speaking style. The sentences ranged in length from four to seven words. Sentences were scored as words correct. The sentences were presented in quiet and at +10 and +5 dB SNR. The noise was a 4-talker babble from an Auditec CD. The noise started 100 ms before the onset of the signal and ended 100 ms after the end of the signal. Five practice sentences were presented with each list for all test conditions.
Results
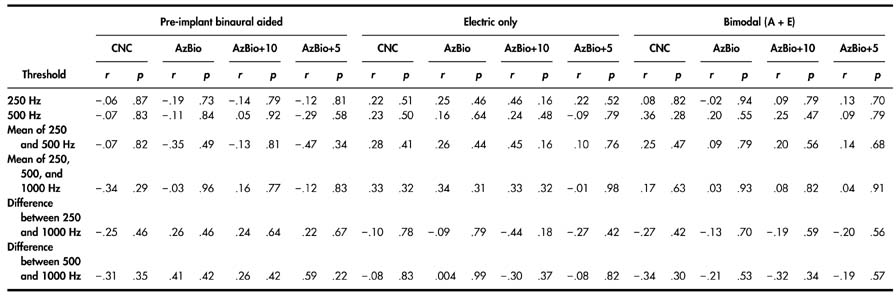
Percent correct scores for all participants in all test conditions are shown in Table 2. Mean scores are shown in Figure 2. Statistical analyses are reported below. A Bonferroni correction of α/4 was used to set the level of significance (.0125) for each of the four repeated measure analyses of variance (ANOVAs).
Table 2.
Individual participant scores on all measures of speech understanding for the following conditions: pre-implant A (pre-A), contralateral A (contra A), pre-implant binaural A (pre-bin A), E alone, and bimodal (A+E).
Note. Conditions for which pre-implant measures could not be obtained are represented by an em dash. CNC = consonant–nucleus–consonant; A = residual acoustic hearing; E = electrical stimulation; AzBio = AzBio sentence corpus (Spahr & Dorman, 2005); SNR = signal-to-noise ratio.
Figure 2.
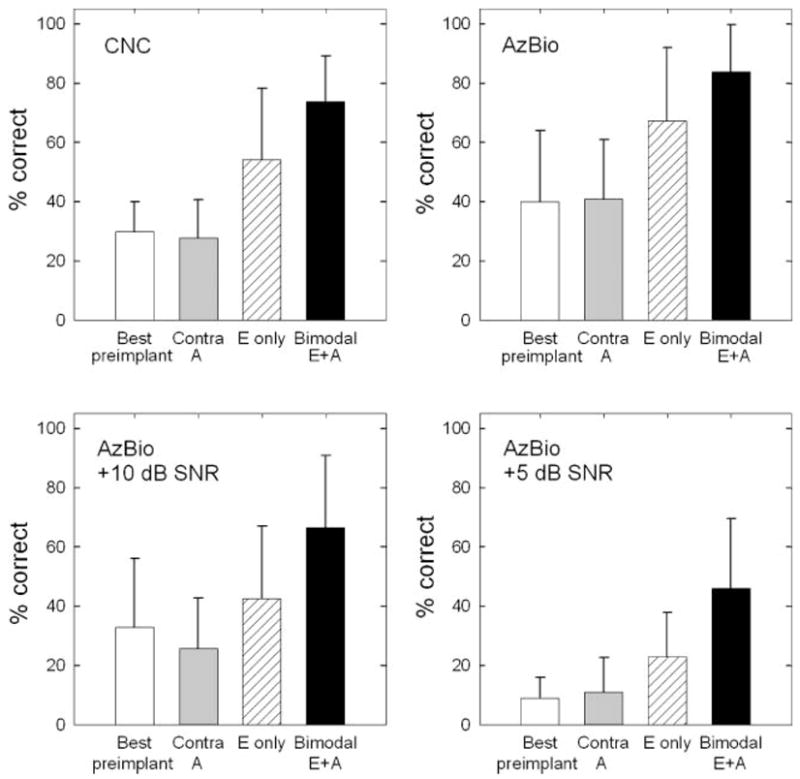
Means and standard deviations for four tested conditions. The four panels represent monosyllabic word recognition (consonant–nucleus–consonant [CNC]), AzBio sentence recognition in quiet, AzBio sentence recognition at +10 dB SNR, and AzBio sentence recognition at +5 dB SNR. A = residual acoustic hearing; E = electrical stimulation; CNC = consonant–nucleus–consonant; AzBio = AzBio sentence corpus (Spahr & Dorman, 2005); Contra A = contralateral A; SNR = signal-to-noise ratio. Error bars represent XXX.
CNC words (n = 11)
A one-way, repeated measures ANOVA was completed with five listening conditions (pre-implant A, contralateral A, pre-implant binaural, E only, and bimodal) as the variables of interest. As expected, statistical analysis revealed a significant effect of listening condition on the CNC percent correct score, F(4, 10) = 41.99, p = .0000001. A post hoc, all pairwise multiple comparison analysis (Bonferroni) revealed that bimodal hearing (M = 75%) yielded significantly higher performance than all other conditions tested. The E-alone scores (M = 57%) were significantly higher than the best pre-implant aided scores (M = 28%). None of the aided acoustic conditions were significantly different from one another.
Sentences in quiet (n = 6)
A one-way, repeated measures ANOVA revealed a significant effect of listening condition on sentence recognition in quiet, F(4, 5) = 31.37, p = .0000001. Post hoc analysis using the Bonferroni correction found performance in the bimodal hearing condition (M = 83%) to be significantly greater than in all conditions except E alone (M = 69%). As with CNC words, the E-alone scores (M = 69%) were significantly higher than the best pre-implant, binaural-aided scores (M = 40%). None of the aided acoustic conditions were significantly different from one another.
Sentences at +10dB SNR (n = 6)
Statistical analysis with a one-way, repeated measures ANOVA revealed a significant effect of listening condition on sentence recognition in noise at +10 dB SNR, F(4, 5) = 1.51, p = .000001. Post hoc analysis using the Bonferroni multiple comparison correction revealed that performance in the bimodal condition (M = 62%) was significantly higher than all other conditions. The E-alone scores (M = 39%) were significantly higher than pre-implant A (M = 21%) and contralateral A (M = 21%) scores but were not significantly different from the best pre-implant, binaural-aided condition (M = 32%). None of the aided acoustic conditions were significantly different from one another.
Sentences at +5dB SNR (n = 5)
A one-way, repeated measures ANOVA found a significant effect of listening condition on sentence recognition in noise at +5 dB SNR, F(4, 4) = 11.60, p = .00013. Post hoc analysis using the Bonferroni correction revealed that bimodal scores (M = 38%) were significantly higher than scores in all other conditions except E-alone (M = 23%). E-alone performance was not higher than the best pre-implant, binaural-aided performance (M = 9%)—although the trend for greater performance was certainly evident. As with the other test materials, none of the aided acoustic conditions were significantly different from one another.
Correlations
Correlations were computed between measures of contralateral auditory threshold and performance measures in all listening conditions (see Table 3). Recall that pre-implant audiograms were highly symmetrical across ears for all 11 participants. Given that the contralateral acoustic thresholds are what these individuals are able to use with the electric stimulation, it was those thresholds that were chosen for correlation analysis. The measures of auditory threshold were thresholds at 250 Hz and 500 Hz; the mean threshold for 250 Hz and 500 Hz; the mean threshold for 250, 500, and 1 kHz; the difference in threshold at 250 and 1000 Hz (i.e., the slope of the hearing loss); and the difference in threshold at 500 Hz and 1 kHz. There were no significant correlations among these measures of auditory threshold and any measure of speech recognition for any of the listening conditions tested. Ching et al. (2004) also examined correlations among hearing thresholds at 250, 500, and 1000 Hz in the contralateral ear and (a) bimodal performance as well as (b) the difference score between bimodal and E-alone performance. No significant correlations between (a) hearing thresholds and (b) bimodal performance or the difference scores were found for any of the measures.
Table 3.
Correlations for six thresholds.
Discussion
The aims of this research were to determine (a) whether a fully inserted electrode array would provide a higher level of performance than residual, low-frequency hearing, (b) whether contralateral acoustic hearing would add to the speech understanding provided by the implant, and (c) whether the level of performance achieved with electric stimulation plus contralateral acoustic hearing was similar to the level of performance reported in the literature for patients with a partially inserted electrode array and bilateral low-frequency acoustic hearing. The results indicated that E-alone scores were significantly higher than the pre-implant binaural scores for all measures except sentence recognition at +5 dB SNR. Thus, most generally, a fully inserted electrode array provides a higher level of performance than does residual low-frequency hearing.
Bimodal performance was significantly higher than E-alone performance for monosyllabic word recognition and sentence recognition at +10 dB SNR. No significant difference was found between bimodal and E-alone performance on sentence recognition in quiet or at +5 dB SNR. However, in both quiet and at +5 dB, the mean scores in the bimodal conditions were 14–15 percentage points higher than the mean scores in the E-alone conditions. Thus, information from the contralateral ear can provide significant benefit even when that information is from a limited frequency domain and when auditory thresholds in that domain are elevated. Participants with an EAS-like hearing loss should be encouraged to wear appropriate amplification in the nonimplanted ear even if they believe they receive little benefit from amplification.
In the introductory paragraphs, we described the monosyllabic word scores of patients fit with electrode arrays inserted 10 and 20 mm into the cochlea. Gantz et al. (2005) studied participants fit with a 10-mm electrode who presented with slightly better (by approximately 10 dB) average thresholds at 250 and 500 Hz than did the patients tested in this report and found an average CNC score of 69% correct in the combined electric and binaural acoustic condition. Gantz et al. (2005) also noted that a subset of that group demonstrated an average CNC score of 79% correct in the electric-plus-bilateral acoustic condition. Kiefer et al. (2005) reported a mean monosyllabic word score of 62% correct in the combined EAS condition for a sample with a 20-mm insertion. These patients had poorer pre-implant monosyllabic word scores and poorer pre-implant residual acoustic hearing than the patients in the current study. The mean bimodal CNC score of 75% correct for the participants in the current study, which combined the percepts from a fully inserted electrode array and low-frequency residual hearing from the nonimplanted ear, is comparable to the mean scores reported for 10- and 20-mm partial electrode insertions.
We found no correlation between the amount of residual hearing in the contralateral ear and performance in any condition with acoustic stimulation. This indicates that differences in auditory thresholds over the range studied here do not influence the usefulness of low-frequency information for the purpose of speech understanding. In other words, the degree of low-frequency residual hearing in the nonimplant ear is not correlated with bimodal speech perception performance. This finding is reinforced by the performance of a patient, not included in this test sample, who received a full insertion—Nucleus 24 perimodiolar electrode array—without completely destroying low-frequency residual hearing. Post-implant thresholds in the implanted ear were 40 dB HL at 250 Hz, 75 dB HL at 500 Hz, and 110 dB HL at 1 kHz. For all tests of speech understanding, the participant received as much benefit from acoustic hearing as did any participant in the sample reported here. If we assume that frequency resolution at 500 Hz was very poor (given the 75-dB HL threshold, per Glasberg & Moore, 1986), then it would appear that the benefit of low-frequency residual hearing is derived from information below 500 Hz.
Summary and Conclusion
Individuals with auditory thresholds meeting EAS candidacy received significant benefit from a fully inserted cochlear implant on tests of word recognition in quiet and on tests of sentence recognition in quiet and in noise. In the bimodal conditions, scores were 2–4 times better than scores in pre-implant, acoustic-only conditions. Scores in the bimodal condition were also generally better than in the E-alone condition. Thus, a limited range of low-frequency acoustic hearing with elevated thresholds can provide significant additional benefit to the speech understanding achieved from electric stimulation alone. The level of performance achieved by patients in the current study was similar to that of patients with partial insertion implants and bilateral low-frequency hearing (e.g., Gantz et al., 2005; Kiefer et al., 2005). Only after large patient populations have been tested with devices inserted over the range of 10–20 mm will it be possible to determine whether one of several surgical interventions is the best treatment for patients with severe to profound high-frequency hearing loss at 1–8 kHz and mild to moderate hearing loss at 500 Hz and below.
Acknowledgments
This work was supported by Grant DC006538 from the National Institute on Deafness and Other Communication Disorders (NIDCD), awarded to René H. Gifford, and by Grant RO1 DC00654-15 from NIDCD, awarded to Michael F. Dorman. A portion of the results was presented at the 2005 Conference on Implantable Auditory Prostheses in Pacific Grove, California; the 2005 Fifth Asia Pacific Symposium on Cochlear Implant and Related Sciences in Hong Kong; and the 2006 Hearing Preservation Workshop in Raleigh, North Carolina. We would like to thank Chris Turner and Helen Cullington for providing suggestions that greatly improved this article.
Footnotes
Of the 6 participants tested both pre- and postimplant on measures of sentence recognition, 1 participant was unable to complete sentence testing at +5 dB SNR because of time constraints.
References
- Amos NE, Humes LE. The contribution of high frequencies to speech recognition in sensorineural hearing loss. In: Breebaart DJ, Houtsma AJM, Kohlrausch A, Prijs VF, Schoonhoven R, editors. Physiological and psychophysical bases of auditory function. Maastricht, the Netherlands: Shaker; 2001. pp. 437–444. [Google Scholar]
- Armstrong M, Pegg P, James C, Blamey P. Speech perception in noise with implant and hearing aid. American Journal of Otolaryngology. 1997;18:S140–S141. [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Cochlear implantation. Medlearn Matters: Medicare Learning Network. 2005 July 6; (No. MM3796). Retrieved June 6, 2007, from www.cms.hhs.gov/MLNMattersArticles.
- Ching TY, Dillon H, Byrne D. Speech recognition of hearing-impaired listeners: Predictions from audibility and the limited role of high-frequency amplification. The Journal of the Acoustical Society of America. 1998;103:1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear and Hearing. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. Journal of Speech, Languange, and Hearing Research. 2005;48:668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngologica. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Spahr AJ, McKarns SA. The effect of digital frequency compression (DFC) on speech and melody intelligibility in candidates for a partial-insertion cochlear implant. Journal of Speech, Language, and Hearing Research. doi: 10.1044/1092-4388(2007/083). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. The Journal of the Acoustical Society of America. 1986;79:1020–1033. doi: 10.1121/1.393374. [DOI] [PubMed] [Google Scholar]
- Gstoettner W, Kiefer J, Baumgartner WD, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngologica. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- Hamzavi J, Pok SM, Gstoettner W, Baumgartner WD. Speech perception with a cochlear implant used in conjunction with a hearing aid in the opposite ear. International Journal of Audiology. 2004;43:61–65. [PubMed] [Google Scholar]
- Hogan CA, Turner CW. High-frequency audibility: Benefits for hearing-impaired listeners. The Journal of the Acoustical Society of America. 1998;104:432–441. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- Hughson W, Westlake H. Manual for program outline for rehabilitation of aural casualties both military and civilian. Transactions of the American Academy of Ophthalmology and Otolaryngology Supplement. 1944;48:1–15. [Google Scholar]
- Kiefer J, Pok M, Adunka O, Stuerzebecher E, Baumgartner W, Schmidt M, et al. Combined electric and acoustic stimulation of the auditory system: Results of a clinical study. Audiology and Neurotology. 2005;10:134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. The Journal of the Acoustical Society of America. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell RC, Lawrence D. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. Journal of Speech, Language, and Hearing Research. 2006;49:338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Shallop JK, Arndt PL, Turnacliff KA. Expanded indications for cochlear implantation: Perceptual results in seven adults with residual hearing. Journal of Speech Language Pathology and Audiology. 1992;16:141–148. [Google Scholar]
- Skarzynski H, Lorens A, Piotrowska A. Preservation of low-frequency hearing in partial deafness cochlear implantation. International Congress Series. 2004;1273:239–242. [Google Scholar]
- Spahr AJ, Dorman MF. Effects of minimum stimulation settings for the Med El Tempo+ speech processor on speech understanding. Ear and Hearing. 2005;26:2S–6S. doi: 10.1097/00003446-200508001-00002. [DOI] [PubMed] [Google Scholar]
- Turner CW, Cummings KJ. Speech audibility for listeners with high-frequency hearing loss. American Journal of Audiology. 1999;8:47–56. doi: 10.1044/1059-0889(1999/002). [DOI] [PubMed] [Google Scholar]
- Tyler RS, Parkinson AJ, Wilson BS, Witt S, Preece JP, Noble W. Patients utilizing a hearing aid and a cochlear implant: Speech perception and localization. Ear and Hearing. 2002;23:98–105. doi: 10.1097/00003446-200204000-00003. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfennigdorff T, Hartmann R, Stuerzebecher E, Klinke R. Electric-acoustic stimulation of the auditory system: New technology for severe hearing loss. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]