Abstract
Background
Tobacco smoking, driven by the addictive properties of nicotine, is the most prevalent preventable cause of death in the Western World. Accumulated evidence suggests that nicotine may increase appetitive responding for non-drug incentives in the environment.
Methods
To test this hypothesis, we conducted a randomized, double-blind, placebo-controlled, crossover study of the effect of a single dose of transdermal nicotine on reward responsiveness in 30 psychiatrically healthy non-smokers. A novel signal detection task in which correct responses were differentially rewarded in a 3:1 ratio was used to assess the extent to which participants modulated their behavior as a function of reward.
Results
Despite expected adverse effects such as nausea, nicotine significantly increased response bias toward the more frequently rewarded condition, at the expense of accuracy, independent of effects on attention or overall vigilance. Additionally, response bias on placebo was greater in participants who received nicotine in the first session, indicating that an effect of nicotine on reward responsiveness or reward-based learning persisted for at least one week.
Conclusions
These findings suggest that a single dose of nicotine enhances response to non-drug-related rewards in the environment, with lasting effects. This effect may contribute to reinforcement of early smoking behavior and development of nicotine dependence.
Keywords: Reward, Nicotine, Reward Responsiveness, Anhedonia, Non-Smokers, Drug addiction
Introduction
Unlike substances such as cocaine and heroin, euphoric effects of nicotine are mild. Despite this, nicotine is highly addictive [1]. Animal studies indicate that reinforcing properties of nicotine may be mediated through enhancement of salience of non-drug-related experiences; nicotine self-administration is associated with increased responsiveness to non-drug reward [2], while nicotine withdrawal is associated with refractoriness to reward [3]. Phasic dopamine release modulates salience attribution and motivation [4, 5], and nicotine may increase appetitive responding for non-drug incentives via activation of presynaptic nicotinic receptors (nAChR’s) on mesocorticolimbic dopaminergic neurons [1, 2]. Consistent with this hypothesis, nicotine enhances the incentive value of monetary reward in smokers following overnight abstinence [6]. Whether nicotine enhances responding for non-drug incentives in the absence of potentially confounding effects of nicotine withdrawal or chronic effects of nicotine on reward responsiveness is unknown. We conducted a randomized, double-blind, placebo-controlled crossover study of a single dose of transdermal nicotine in healthy adult non-smokers to determine if responsiveness to non-drug reward is increased in non-smokers acutely treated with nicotine. This was an ancillary study conducted in conjunction with a larger study of the effect of nicotine on cognitive performance in non-smokers [7].
Methods
The study took place at an urban community mental health clinic (Freedom Trail Clinic) and was approved by Institutional Review Boards of the Massachusetts General Hospital and the Massachusetts Department of Mental Health. All participants were assessed by a doctoral level investigator as competent to consent and provided written informed consent.
Participants
Adults, aged 18–60, were recruited via local press advertisement and were eligible to participate if they were non-smokers for ≥3 months with salivary cotinine < 10 ng/ml (Nicalert™, JANT Pharmacal Corp., Encino, CA) and expired air carbon monoxide (CO) <9 ppm (Micro Smokerlyzer III, Bedfont Scientific Ltd., Kent, U.K.). Participants were excluded if they reported a lifetime history of Axis I psychiatric diagnosis by Structured Clinical Interview for DSM-IV, head injury, first-degree relative with a schizophrenia spectrum disorder, recent exposure to investigational medications, or saliva positive for drugs or alcohol (Accutest Saliva Test™, JANT Pharmacal Corp., Encino, CA, ALCO Screen, CHEMATICS, Inc., North Webster, IN). Participants completed the Beck Depression Inventory (BDI) at baseline and were randomized by computer-generated, random number assignment with concealed allocation for order of receiving active or placebo patches. The randomization procedure was conducted by a staff member from another research team, was concealed using opaque envelopes and all study investigators, staff and subjects were blind to group allocation for the duration of the study. Participants then attended two study visits, separated by 1–2 weeks, at which they wore two 7 mg (Nicoderm CQ) nicotine or identical placebo patches (Alza Corp., Mountain View, CA). We elected to use two 7 mg patches instead of a single 14 mg patch to allow for dose reduction if participants experienced adverse effects. Following application of patches, participants had lunch and watched an affectively neutral or slightly positive movie of their choice. Tests of reward responsiveness were performed after three hours. At the end of the testing period, blood was drawn for serum nicotine concentration and patches were removed.
Tasks and Procedures
A signal detection task, designed to measure shift in responding toward a differentially (more) rewarded stimulus, was administered twice, in two separate sessions, 3 hours after nicotine patch and after placebo patch application, as an objective measure of reward responsiveness [8–10]. This operationalization of response bias as a measure of reward responsiveness fits with the view that reinforcers are stimuli that increase frequency of responding [11]. For each trial, participants were asked to choose which of two stimuli (short or long mouth) was presented on a previously mouthless cartoon face by making a corresponding response on a keyboard. At each session, participants performed three blocks, each containing 50 trials of the short and 50 trials of the long mouth. Critically, the difference between mouth sizes (11.5 mm vs. 13 mm) and the stimulus exposure time (100 ms) is small, making the participants’ choice difficult, and thus allowing the development of a response bias. In line with prior studies [10], an asymmetric reinforcer ratio was used to elicit a response bias. Correct identification of one stimulus was rewarded (“Correct!! You won 5 Cents”) three times more frequently (“rich stimulus”) compared to correct identification of the other stimulus (“lean stimulus”). To expose each subject to the intended 3:1 reward ratio, only 40 correct trials (30 rich, 10 lean) were rewarded in each block. Choice of rich stimuli (long vs. short mouth) was counterbalanced between participants and across visits (e.g. if the long mouth was the rich stimulus at the first visit then the short mouth would be the rich stimulus at the second). Before the task, participants were instructed to try to win as much money as possible and that the money they win will be given to them to keep. They were specifically instructed that not all correct responses would receive a reward feedback, and that lack of feedback did not indicate inaccuracy and that they receive no feedback for errors. They were not informed, however, about the differential reward schedule. Prior studies with this paradigm in healthy subjects have shown that unequal frequency of reward to correct responses to the more frequently (rich) vs. less frequently rewarded (lean) stimulus produces a systematic preference for the response paired with the more frequent reward, which typically increases across blocks [8, 12, 13]. Performance is analyzed in terms of response bias, an index of the tendency to choose the more rewarded stimulus and an objective assessment of reward responsiveness. Control analyses are performed for accuracy, discriminability, and reaction time (RT), which provide information about overall task performance. Response bias (log b) and discriminability (log d) were computed as follows [10]:
As evident from the formula, a high response bias is observed if a participant has a high number of correct identifications for the more frequently rewarded (rich) stimulus and a low number of correct identifications for the lean stimulus. Discriminability, which assessed participants’ ability to distinguish between the two stimuli, was used as an indicator of task difficulty. Participants also completed the State Trait Anxiety Inventory (state form) and other cognitive tests reported separately [7].
Data Reduction and Analyses
Trials with RT <150 ms or >2500 ms and/or >3 standard deviations from the mean computed for each subject individually after applying a logarithmic transformation were identified as outlier responses and excluded. There were a priori criteria to exclude participants with accuracy of less than chance (50%) or total rewards of <30 in any block. Data from two participants were removed by these criteria. Data were assessed for distributional properties prior to analysis and one variable (RT) required log transformation to meet criteria for normal distribution (pre-transformation skew=1.71 ± 0.31, kurtosis = 3.70 ±0.61; post-transformation skew= 0.97±0.31, kurtosis=1.27 ±0.61). Identical split-plot repeated measures analyses of variance (ANOVA) were performed for response bias and discriminability with Treatment (nicotine, placebo) and Block (1, 2 and 3) included as within-subject factors, and Order of Drug Administration as the between-subject factor. For analyses of RT and accuracy, Stimulus Type (lean vs. rich) was included as an additional within-subject factor. In case of significant ANOVA effects, posthoc Newman-Keuls tests were performed. Greenhouse-Geisser corrections were employed when Mauchley’s test of sphericity was significant. Data are presented as mean ± standard deviation. Analyses were performed using SPSS for Windows 10 (SPSS Inc.) and Statistica.
Results
All study procedures took place between January 2005 and July 2006. Thirty-two participants completed all study procedures (i.e., a placebo and nicotine session). Data for two participants were lost (they met a priori criteria for exclusion). Data from 30 participants are presented. See Supplementary Material 1. Demographic characteristics are given in Table 1. Serum nicotine levels were higher in the nicotine condition (7.5 vs <0.5 ng/ml, t=12.6, p<0.0001).
Table 1. (N=30).
F= Female, M=Male, C=Caucasian, A=African American, Cigarettes per day calculated for former smokers, BDI=Beck Depression Inventory. The BDI anhedonia subscale includes: loss of pleasure, loss of interest, loss of energy and loss of interest in sex.[28]
| Baseline Characteristics | |
|---|---|
| Age | 39 (12) years |
| Gender | 14 F, 16 M |
| Race | 24 C, 6 A |
| IQ | 111 (9) |
| Expired Air CO | 0.8 (0.9) ppm |
| Unemployed | 23% (7/30) |
| Education | 16 (3) years |
| Paternal Education | 14 (5) years |
| BDI | 2.0 (2.5), range (0–9) |
| BDI Anhedonia Subscale | 0.5 (0.6), range (0–2) |
| Past Smoking | 23% (7/30) |
| Past Cigarettes per Day | 5 (7) |
Response Bias
As shown in Figure 1, nicotine increased responding to reward (main effect F1,28=8.18, p=0.008). Interestingly, while there was no overall interaction between Treatment(between session factor) and Block (within session factor) (F1.5, 43.0=0.95, p=0.37), there was a Treatment by Block by Order of Treatment Administration interaction (F1.5, 43=4.29, p=0.029). Follow-up ANOVAs, considering each visit separately, revealed a main effect of Treatment in each visit (first visit: F1, 28=4.31, p=0.047; second visit: F1, 28=4.76, p=0.038), confirming that participants (n = 15) had significantly greater response bias when receiving nicotine than when receiving placebo (n = 15) at both visits. See Figure 2. To further evaluate the triple interaction, Block by Order of Treatment Administration ANOVAs were run for response bias in nicotine and placebo conditions separately. Importantly, a Block by Order effect emerged for response bias on placebo (F1.61, 45.14=5.20, p=0.014), but not nicotine (F1.36, 38.19=0.62, p=0.48). This interaction was due to development of greater response bias across the three blocks in the placebo condition for participants who received nicotine first compared with those who received placebo first (RB3-RB1= 0.16±0.19 vs. −0.10±0.14; t38=4.30, p=0.0002).
Figure 1. Response Bias.
Nicotine treatment increased response bias in the signal detection task (main effect of Treatment: F1, 28=8.18, p=0.008) indicating increased responding for the more rewarded (rich) Stimulus Type. Data are presented as means ± standard error.
Figure 2. Response Bias at Visit One and Visit Two.
Nicotine significantly increased response bias at both visits (main effect of Treatment at visit 1: F1, 28=4.31, p=0.047; visit 2: F1, 28=4.76, p=0.038).
Because of lasting effects of nicotine on response bias observed in this study and reports of persistent effects of chronic nicotine use on cognitive function [14], analyses were repeated in a subsample of 23 participants who reported never smoking a single cigarette. The effect of nicotine on response bias was unchanged; the main effect of Treatment remained significant (F1,21=13.79, p=0.001).
Control Analyses
Accuracy
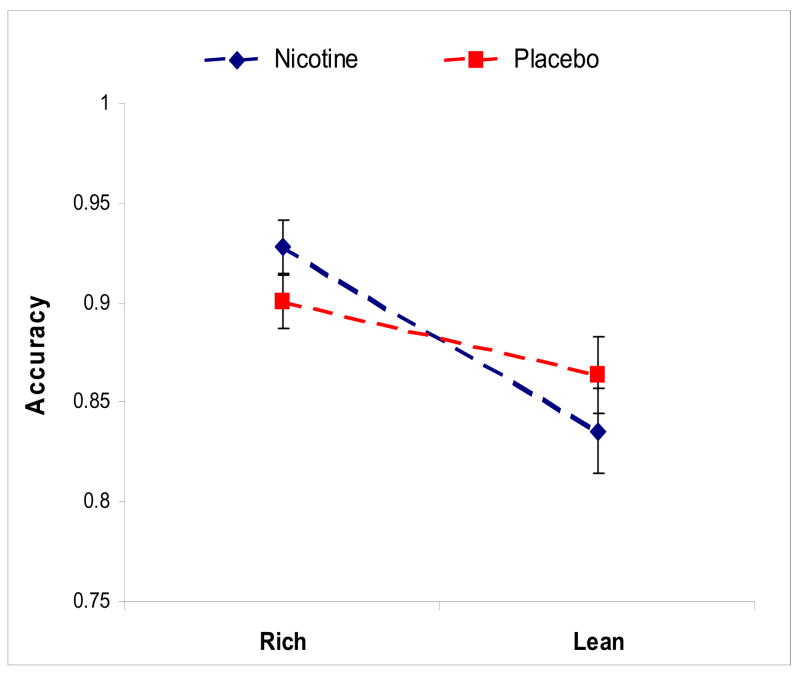
The Treatment (placebo, nicotine) by Block (1, 2 and 3) by Order of Drug Administration by Stimulus Type (lean, rich) ANOVA on accuracy scores indicated that the main effect of Treatment was not significant (F1,28 =0.0005, p= 0.98). Replicating prior studies with independent samples[8, 13], accuracy was significantly greater for the more rewarded stimulus type (Stimulus Type: F1,28=36.07, p=<0.0001), an effect that was seen in all three blocks (Newman-Keuls, ps < 0.001). In addition, a significant Stimulus Type by Block interaction (F 2,56=3.66, p=0.032) was due to lower lean accuracy in Blocks 2 (Newman-Keuls, p = 0.050) and 3 (Newman-Keuls, p < 0.060) vs Block 1. Overall, these findings indicate that the differential reinforcement schedule successfully elicited a behavioral preference toward the more frequently rewarded (rich) stimulus. Importantly, this effect was greater on nicotine, (Treatment by Stimulus Type interaction F1,28=5.08, p=0.03), indicating that when participants were on nicotine, their accuracy was greater in the rich condition and lower in the lean condition than when they were on placebo. See Figure 3.
Figure 3. Accuracy.
A main effect of Stimulus Type (F1,28=36.07, p=<0.0001) indicates greater accuracy for the more rewarded (rich) stimulus. A Treatment by Stimulus Type interaction (F1,28=5.08, p=0.03) indicated that, compared to placebo, nicotine was associated with greater accuracy for the rich stimulus but lower accuracy for the lean stimulus.
Discriminability
There was no main effect of Block on discriminability, indicating no change over the course of the test. There was a trend for an effect of nicotine on discriminability (log d’ nicotine = 0.98 ± 0.34; log d’ placebo = 0.93 ± 0.28) (main effect of Treatment: F1,28 =3.12, p=0.09).
Reaction Time
As expected, RT was faster for the rich stimulus (rich: 441.9 ±103.2 ms vs. lean: 467.8 ±118.7 ms; main effect of Stimulus Type: F1,28=36.6, p=1.6E−6) and in the nicotine condition (main effect of Treatment F1,28=4.75, p=0.04). See Figure 4.
Figure 4. Reaction Time.
A significant main effect of both Stimulus Type (F1,28=36.6, p=1.6E−6) and Treatment (F1,28=4.75, p=0.04) indicated that RT was faster for the more rewarded (rich) stimulus and in the nicotine condition vs placebo.
Adverse Events
State anxiety was higher in the nicotine condition (STAI=33.5±9.3 vs. 29.7±06.5, t =−2.5, p=0.02). Thirteen participants experienced nausea and 4 required dose reduction to 7 mg. Other adverse effects included skin irritation (n=12), dizziness (n=9), headache (n=8) and palpitations (n=1). No significant correlation emerged between number and severity of side effects and change in response bias between blocks 1 and 3.
Discussion
This is the first study to demonstrate that nicotine can enhance responding for a positive response cue and monetary reward in non-nicotine dependent as well as nicotine-naïve humans. These data suggest that nicotine increases salience of rewarding stimuli in the environment, a property that may contribute to initial development and maintenance of nicotine dependence. Cigarette smoking takes place in the context of many daily activities, and nicotine may increase the salience of environmental reinforcers in these situations. If attempts at smoking cessation are associated with loss of salience of numerous everyday pleasurable experiences then this may make smoking abstinence more difficult to sustain, triggering relapse.
Striatal dopamine and acetylcholine release are believed to play an important role in probabilistic reward-based learning and attention, signaling behavioral significance of environmental events and influencing decision making or choice [15–19]. The elimination of cholinergic neurons in the striatum results in impairments in reward- related learning [20], and phasic activation of both striatal dopaminergic and cholinergic neurons has been demonstrated in response to rewarding stimuli [16]. Data from animal models investigating the effects of d-amphetamine on responding for conditioned reinforcers [21–23] support a role for phasic dopaminergic activation in enhancing response to reward-related stimuli. Based on these animal data, we postulate that effect of nicotine to enhance responding for positive response cues may be mediated via dopamine or acetylcholine-dependent mechanisms in mesocorticolimbic regions. Future studies will be required to test this hypothesis.
Nicotine did not significantly modulate discriminability on the task, indicating that effects on response bias were not mediated by improvement in attention or general task performance. Furthermore, if the effect of nicotine on response bias described were primarily due to improvement in attention and overall vigilance, we would expect improved accuracy for both types of stimuli presented in the task rather than differentially increased responding for the stimulus associated with the more frequent reward at the expense of accuracy for the lean stimulus, as observed. In addition, reaction time was faster on nicotine due to an expected improvement in motor speed. However, the signal detection task measures the tendency to choose the more rewarded response and is independent of speed.
Importantly, a carryover effect of nicotine on reward responsiveness was observed. This is consistent with a finding in animals in which nicotine self-administration was associated with increased reward responsiveness during active use and for 36 days after removal of nicotine availability [2]. These results suggest that a single nicotine dose may have lasting effects on reward sensitivity in humans, potentially through its effects on incentive or motivational salience, which may contribute to repeated use and the development of dependence.
The study was conducted in non-smokers to avoid confounding effects of nicotine use on reward responsiveness [2, 24]. Although the observed effect of nicotine on reward responsivity was small, it is possible that even a small enhancement in sensitivity to rewarding environmental stimuli may be sufficient to reinforce smoking behavior in vulnerable individuals. Additionally, nicotine delivered by smoke would have faster pharmacokinetics and may have a greater effect on responsivity to reward than that observed for transdermal nicotine. There are limitations to our study that require specific mention. Participants had quite low levels of depressive symptoms as indicated by low BDI scores, limiting our ability to assess clinical correlates of response bias development. The findings of this study may however have relevance for those with disorders of hedonic tone such as anxiety, depressive disorders or schizophrenia who also have high rates of smoking [25–27]. These individuals may be particularly vulnerable to become nicotine dependent if they experience a reduction in anhedonia when they smoke. Future studies investigating generalizability of the present findings to those with disorders of hedonic tone are warranted.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes on Drug Abuse and the Stanley Medical Research Institute. The authors would like to thank James O’Shea and Kyle Ratner for technical assistance and Drs. Nancy Rigotti and Maurizio Fava for critical review of the manuscript.
Financial Disclosures and Potential Conflict of Interest
Dr. Evins has received grant support from Janssen Pharmaceuticals, AstraZenica and GlaxoSmithKline and has a collaborative agreement with GSK as a PI in a Collaborative Drug Discovery Group for Nicotine Dependence funded by NIDA through a U01 mechanism.
Dr. Goff has received research funding from the following: Janssen Pharmaceuticals, Pfizer Inc., Bristol Meyer Squibb, GlaxoSmithKline and Cephalon. In addition, he has received honoraria from Eli Lilly and Company, Janssen Pharmaceuticals, Pfizer Inc and Bristol Myer Squibb. He is a member of the DSMB for Wyeth and serves on the advisory board of Janssen Pharmaceuticals, Pfizer Inc., Bristol Meyer Squibb, GlaxoSmithKline, Merck, Solvay Pharmaceuticals and AstraZeneca Pharmaceuticals.
Dr. Pizzagalli was supported by NIMH Research Grant R01MH68376 and has received research support from GlaxoSmithKline and Merck & Co., Inc. GSK is licensed to use the Nicoderm CQ trademark, and the transdermal preparation of nicotine used in this study was Nicoderm CQ.
Footnotes
Dr. Barr and Ms. Culhane have no relevant financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 2.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 3.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–69. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 6.Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology (Berl) 2006;189(3):355–67. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- 7.Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The Effects of Transdermal Nicotine on Cognition in Non-Smokers with Schizophrenia and Non-Psychiatric Controls. Neuropsychopharmacology (in press) 2007 doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- 8.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2001;42(5):691–8. [PubMed] [Google Scholar]
- 10.Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28(3):366–75. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- 11.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22(11):521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy DC. Behavior detection theory: some implications for applied human research. In: Nevin JA, Davison MC, Commons M, editors. Signal Detection: Mechanisms, Models and Applications. Lawrence Erlbaum; Hillsdale, NJ: 1991. [Google Scholar]
- 13.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60(10):1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25(3):313–9. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 15.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 16.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43(1):133–43. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36(2):285–98. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 18.Daw ND, Doya K. The computational neurobiology of learning and reward. Curr Opin Neurobiol. 2006;16(2):199–204. doi: 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci U S A. 2003;100(13):7965–70. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84(3):405–12. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90(3):390–7. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- 23.Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology (Berl) 1991;104(3):377–85. doi: 10.1007/BF02246039. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res. 2001;139(3):278–86. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- 25.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Jama. 2000;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 26.Hughes J. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 27.de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L. Initiation of daily smoking and nicotine dependence in schizophrenia and mood disorders. Schizophr Res. 2002;56(1–2):47–54. doi: 10.1016/s0920-9964(01)00217-1. [DOI] [PubMed] [Google Scholar]
- 28.Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model's prediction of anhedonia's specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119(3):243–50. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.