Abstract
Survival can depend on the ability to change a current course of action to respond to potentially advantageous or threatening stimuli. This “reorienting” response involves the coordinated action of a right hemisphere dominant ventral frontoparietal network that interrupts and resets ongoing activity and a dorsal frontoparietal network specialized for selecting and linking stimuli and responses. At rest, each network is distinct and internally correlated, but when attention is focused, the ventral network is suppressed to prevent reorienting to distracting events. These different patterns of recruitment may reflect inputs to the ventral attention network from the locus coeruleus/norepinephrine system. While originally conceptualized as a system for redirecting attention from one object to another, recent evidence suggests a more general role in switching between networks, which may explain recent evidence of its involvement in functions such as social cognition.
Introduction
To safely navigate the environment, survive, and reproduce, animals and people must rapidly select sensory information that is relevant to their goals (e.g., routes, food, mates). They must also quickly redirect their attention and change their course of action when faced with novel, potentially threatening, or rewarding stimuli. The complex set of adjustments in response to novel and unexpected stimuli is defined here as a reorienting response. Reorienting may occur between two environmental stimuli, such as when we orient to the siren of an ambulance while reading a newspaper, or between an internally directed activity and the environment, as when the same siren interrupts a train of thought. While several autonomic and motor responses can be triggered by novel sensory stimuli through subcortical reflexes that are largely automatic and unconscious (the orienting reflex; Sokolov, 1963), more recent work indicates that this adaptive behavior involves a complex interaction between cortical systems specialized for the selection of sensory information. A dorsal frontoparietal (or dorsal attention) network enables the selection of sensory stimuli based on internal goals or expectations (goal-driven attention) and links them to appropriate motor responses. A ventral frontoparietal (or ventral attention) network detects salient and behaviorally relevant stimuli in the environment, especially when unattended (stimulus-driven attention). These systems dynamically interact during normal perception to determine where and what we attend to. In this paper, we review evidence from neuroimaging, neuropsychology, and neurophysiology on the role of these two networks, particularly the ventral network, in the reorienting response.
The Psychology of Attention to Environmental Stimuli
Psychological theories of attention are often concerned with simple behavioral goals, such as finding an object with particular features (Treisman and Gelade, 1980; Wolfe, 1994) or at a particular location (Eriksen and Hoffman, 1974; Posner, 1980) and responding to it in an appropriate manner (Hommel, 2000). This form of selection is labeled “goal-driven” or “endogenous” to emphasize the internal or top-down signals that guide perception through a dynamic interaction with sensory or bottom-up information. The biased-competition model of attention, for example, proposes that objects in a visual scene compete for access to visual short-term memory and that the competition is biased by top-down signals that promote access of behaviorally relevant objects (Desimone and Duncan, 1995). These top-down signals, characterized as working memory (e.g., Downing, 2000; but see Woodman and Luck, 2007), long-term memory (Moores et al., 2003), or action related (Craighero et al., 2002; Rosenbaum, 1991), interact with sensory (bottom-up) signals produced by objects in the visual scene, enabling the desired object to be selectively perceived and entered into memory at the expense of unimportant objects (Bundesen, 1990; Wolfe, 1994). For instance, Figure 1A shows a student who focuses on his computer desktop while writing his thesis and ignores surrounding objects and people.
Figure 1. Focusing Attention and Reorienting Attention Recruit Interacting Networks.

(Left panel) Focusing attention on an object produces sustained activations in dorsal fronto-parietal regions in the intraparietal sulcus, superior parietal lobule, and frontal eye fields, as well as visual regions in occipital cortex (yellow and orange colors) but sustained deactivations in more ventral regions in supramarginal gyrus and superior temporal gyrus (TPJ) and middle and inferior prefrontal cortex (blue and green colors). (Right panel) When an unexpected but important event evokes a reorienting of attention, both the dorsal regions and the formerly deactivated ventral regions are now transiently activated.
Adaptive behavior, however, also requires that we respond to objects that are outside the current focus of attention, i.e., that do not match current settings for selecting stimuli and responses. The object we are looking for may appear with different features than we expected or at a different location. More importantly, a new object may appear that requires a completely different course of action. While the student looks at the computer screen, a colleague may ask a question (Figure 1B), or while a monkey searches for food, a predator may appear. Moreover, we may be presented with new events requiring a response while we are engaged in “internally directed” activities that do not involve an interaction with the environment. Someone may distract us while we are considering the meaning of a sentence in the thesis we are writing, or a monkey may quickly react to the appearance of a predator while grooming or eating.
Reorienting to new objects may occur reflexively, based on their high sensory salience (Jonides and Yantis, 1988), particularly when we do not have a specific task to do (Pashler and Harris, 2001), but distinctive objects attract attention more effectively when they are also behaviorally relevant (Yantis and Egeth, 1999), either because they match our current goals or because of long-term memory associations that signal their importance, as when we hear the phone ringing or the siren of an ambulance. In fact, the degree to which a distinctive but entirely irrelevant object can attract our attention, so-called exogenous attention, is controversial (Folk et al., 1992; Gibson and Kelsey, 1998; Jonides, 1981; Posner and Cohen, 1984; Theeuwes and Burger, 1998; Yantis and Egeth, 1999). In some cases, shifts of attention to a distinctive stimulus can be part of a task goal (Bacon and Egeth, 1994), as when someone tries to detect any salient object appearing in a visual scene. In other cases, distinctive but irrelevant objects may share a specific feature with our current goal, as when we notice someone wearing a red sweater while looking for a friend with a red hat (Folk et al., 1992; Gibson and Kelsey, 1998).
A Neuroanatomical Model of Attention: Dorsal and Ventral Attention Networks
Several lines of evidence indicate that two cortico-cortical neural systems are involved in attending to environmental stimuli (Corbetta and Shulman, 2002). A dorsal frontoparietal network, whose core regions include dorsal parietal cortex, particularly intraparietal sulcus (IPS) and superior parietal lobule (SPL), and dorsal frontal cortex along the precentral sulcus, near or at the frontal eye field (FEF) (Figure 2A, blue areas), embodies the top-down control mechanism proposed by biased competition and related theories (Bundesen, 1990; Desimone and Duncan, 1995; Wolfe, 1994). The dorsal system generates and maintains endogenous signals based on current goals and preexisting information about likely contingencies and sends out top-down signals that bias the processing of appropriate stimulus features and locations in sensory cortex. This conclusion is based on evidence that the dorsal network is preactivated by the expectation of seeing an object at a particular location or with certain features (e.g., movement in a specific direction) (Corbetta et al., 2000; Hopfinger et al., 2000; Kastner et al., 1999; Shulman et al., 1999), by the preparation of a specific response (Astafiev et al., 2003; Connolly et al., 2002), or by the short-term memory of a visual scene (LaBar et al., 1999; Pessoa et al., 2002). The dorsal system is also involved in linking relevant stimuli to responses, as it is modulated when people change their motor plan for an object (Rushworth et al., 2001). Under some conditions, the preparatory activation of the dorsal frontoparietal network extends to visual cortex, presumably reflecting the top-down modulation of sensory representations (Giesbrecht et al., 2006; Hopfinger et al., 2000; Kastner et al., 1999; Serences et al., 2004; Silver et al., 2007; Sylvester et al., 2007) (Figure 1A). Accordingly, anticipatory activity may predict performance to subsequent targets (Giesbrecht et al., 2006; Pessoa and Padmala, 2005; Sapir et al., 2005; Sylvester et al., 2007). Finally, recent studies show that electrical or magnetic stimulation of FEF or IPS leads to a retinotopically specific modulation of visual areas and parallel improvement of perception at corresponding locations of the visual field (Moore and Armstrong, 2003; Ruff et al., 2006, 2007).
Figure 2. Definition of Dorsal and Ventral Networks from Activation Data and Putative Interactions.
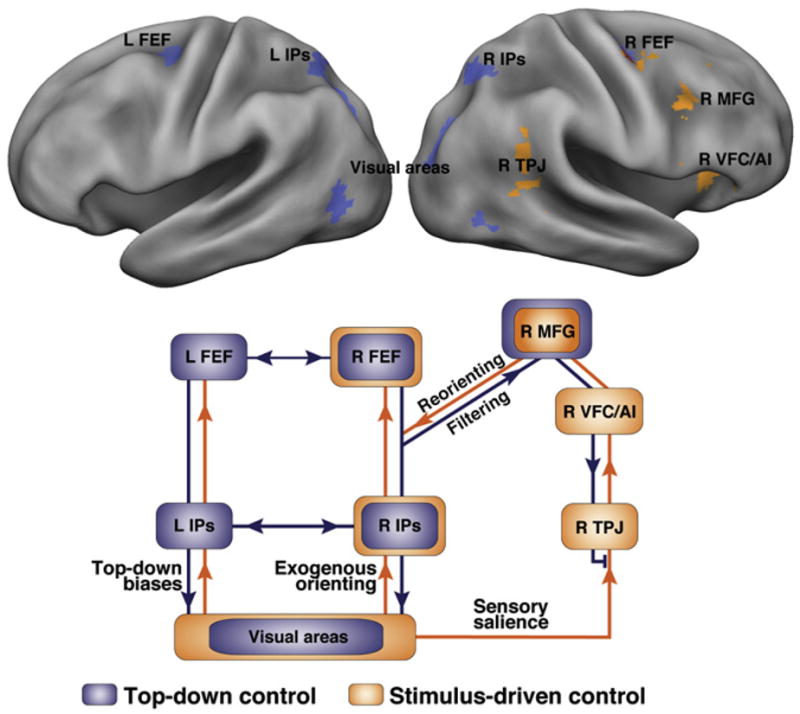
(Top panel) Results from a meta-analysis of activation data. Regions in blue are consistently activated by central cues, indicating where a peripheral object will subsequently appear or what is the feature of an upcoming object. Regions in orange are consistently activated when attention is reoriented to an unexpected but behaviorally relevant object. (Bottom panel) Model for the interaction of dorsal (blue) and ventral (orange) networks during stimulus-driven reorienting. Dorsal network regions FEF and IPS send top-down biases to visual areas and via MFG to the ventral network (filtering signal), restricting ventral activation to behaviorally important stimuli. IPS-FEF are also important for exogenous orienting. Overall, the dorsal network coordinates stimulus-response selection. Conversely, when a salient stimulus occurs during stimulus-driven reorienting, the ventral network sends a reorienting signal to the dorsal network through MFG.
A second system, the ventral frontoparietal network, is not activated by expectations or task preparation but responds along with the dorsal network when behaviorally relevant objects (or targets) are detected (Corbetta et al., 2000). Both dorsal and ventral networks are also activated during reorienting, with enhanced responses during the detection of targets that appear at unattended locations. For example, enhanced responses are observed when subjects are cued to expect a target at one location but it unexpectedly appears at another (i.e., “invalid” targets in the Posner spatial cueing paradigm) (Arrington et al., 2000; Corbetta et al., 2000; Kincade et al., 2005; Macaluso et al., 2002; Vossel et al., 2006) or when a target appears infrequently, as in “oddball” paradigms (Bledowski et al., 2004; Braver et al., 2001; Linden et al., 1999; Marois et al., 2000; McCarthy et al., 1997; Stevens et al., 2005) (Figure 1B). Core regions of the ventral network include temporoparietal junction (TPJ) cortex (anatomically, TPJ is more strictly defined as the cortex at the intersection of the posterior end of the STS, the inferior parietal lobule, and the lateral occipital cortex), defined as the posterior sector of the superior temporal sulcus (STS) and gyrus (STG) and the ventral part of the supramarginal gyrus (SMG) and ventral frontal cortex (VFC), including parts of middle frontal gyrus (MFG), inferior frontal gyrus (IFG), frontal operculum, and anterior insula (Figure 2A, orange regions). An early theory of how the two networks interact (Corbetta and Shulman, 2002) proposed that when attention is reoriented to a new source of information (stimulus-driven reorienting), output from the ventral network interrupts (as a “circuit breaker”) ongoing selection in the dorsal network, which in turn shifts attention toward the novel object of interest.
Although both attentional networks have been most extensively investigated in vision, the available evidence indicates a supramodal function (Driver and Spence, 1998; Macaluso et al., 2002). The ventral network (right TPJ, right IFG) registers salient events in the environment not only in the visual but also in the auditory and tactile modalities (Downar et al., 2000), and similar dorsal and ventral parietal and frontal regions are modulated by reorienting to invalid targets (Arrington et al., 2000; Corbetta et al., 2000; Giessing et al., 2006; Kincade et al., 2005; Macaluso et al., 2002; Mayer et al., 2006; Vossel et al., 2006) or by oddballs (Braver et al., 2001; Kiehl et al., 2001; Linden et al., 1999; Marois et al., 2000) in different modalities.
The sections below review in more detail recent work on these networks, particularly the ventral network, including: (1) the functional-anatomical independence of each network, (2) the importance of behavioral relevance rather than sensory salience in driving the ventral network, (3) whether the output of the ventral network initiates a reorienting response and how the dorsal and ventral networks interact, (4) how the functions of the ventral network may generalize beyond perception and action to include memory and social cognition, and finally (5) the emerging link between activity in the ventral network and the output of the locus coeruleus-norepinephrine system (LC-NE), as recently outlined by neurocomputational theories (Aston-Jones and Cohen, 2005; Bouret and Sara, 2005; Dayan and Yu, 2006; Yu and Dayan, 2005).
We do not consider in this discussion the relationship between cortical and subcortical regions involved in the control of attention. There is strong evidence that subcortical structures like the superior colliculus are involved in stimulus-driven but also goal-driven attention (Bell et al., 2004; Fecteau et al., 2004; Rafal et al., 1988; Sapir et al., 1999). The pulvinar nucleus of the thalamus has been proposed as a gateway structure that funnels top-down biases from parietal areas into visual cortex (Petersen et al., 1987; Shipp, 2004).
The Dorsal and Ventral Attention Systems Form Separate Functional-Anatomical Networks
A basic question is the degree to which different regions in each putative system cohere as a functional-anatomical network. The hypothesis of two attention networks, originally based on the patterns of activation under different task conditions (Corbetta and Shulman, 2002), has been strongly supported by studies of interareal correlation of low-frequency (<0.1 Hz) fluctuations of the spontaneous (not task-evoked) BOLD signal over time, called functional connectivity by MRI (fcMRI) (Biswal et al., 1995). Several groups have reported a number of fcMRI networks (e.g., visual, auditory, somatomotor, default, attention) (Biswal et al., 1995; Fox et al., 2005b, 2006a; Fransson, 2005; Greicius et al., 2003; Mantini et al., 2007), which are related to the underlying anatomical connectivity (Vincent et al., 2007) and replay at rest the patterns of functional activation evoked by behavioral tasks (Fox et al., 2005b, 2006a; Greicius et al., 2003; Hampson et al., 2002; Vincent et al., 2007). In other words, brain regions that are commonly recruited during a task are anatomically connected and maintain in the resting state (in the absence of any stimulation) a significant degree of temporal coherence in their spontaneous activity. Furthermore, there is growing evidence that the integrity and strength of spontaneous functional connectivity are behaviorally significant (Hampson et al., 2006; Seeley et al., 2007; He et al., 2007b). For instance, breakdown of interhemispheric functional connectivity in posterior parietal cortex correlates in a group of patients with post-stroke neglect with their visuospatial deficits (He et al., 2007a; see below).
Regions that putatively belong to the dorsal and ventral attention systems, based on their consistent activation in the Posner cueing paradigm to spatial cues and unattended targets, respectively, also show significant interregional correlation at rest (Fox et al., 2006b) or during an active task with the mean task signal removed (He et al., 2007a) (see Figure 3). There is a remarkable similarity between the dorsal parietal and frontal regions identified by a meta-analysis of task-evoked activation studies (Figure 2) and those showing high resting-state correlations (Figure 3). Similar results are found for ventral frontoparietal regions coactivated during stimulus-driven orienting (Fox et al., 2006a; He et al., 2007a). Moreover, the right hemispheric bias observed in the ventral attention network in several activation studies (Arrington et al., 2000; Corbetta et al., 2000; Downar et al., 2000) is mirrored in fcMRI (Fox et al., 2006a; He et al., 2007a) (compare ventral network in Figures 2 and 3).
Figure 3. Functional Connectivity Defines Separate Dorsal and Ventral Networks.
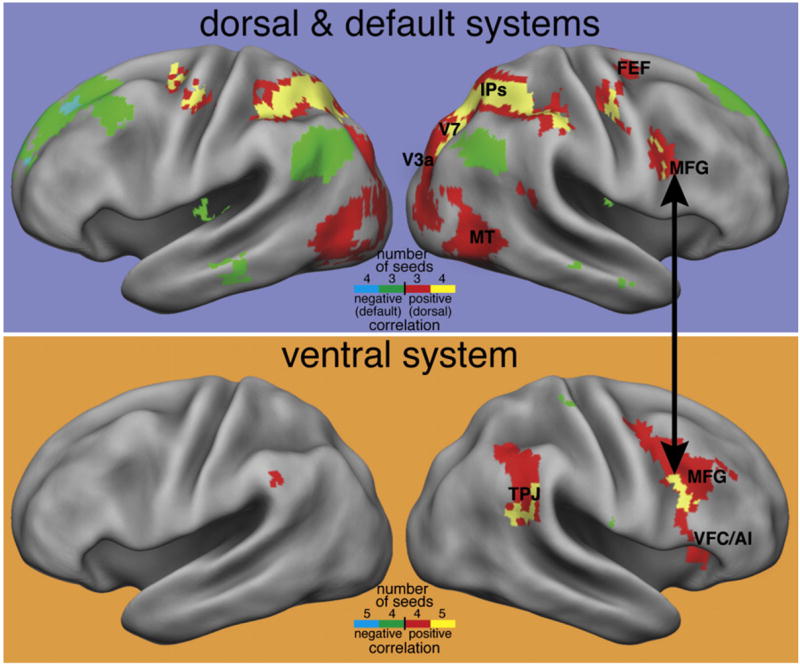
(Top panel) Four dorsal frontoparietal regions from the meta-analysis of activation studies shown in Figure 2 were used as seeds in an FC analysis of resting-state data. The map indicates regions that showed significant positive correlations with three (red) or four (yellow) of the seed regions. The dorsal network is largely reproduced in the resting-state FC maps. Regions that show significant negative correlations with three (green) or four (blue) of the seed regions are also shown and roughly reproduce the default network, possibly indicating a push-pull relationship between the two networks. (Bottom panel) Five ventral regions from Figure 2 were used as seeds for an FC analysis. Regions showing consistent positive correlations largely reproduce the ventral network, but negative correlations in default regions are not observed. The black arrow indicates that posterior MFG near the inferior frontal sulcus appears to be connected to both networks.
While segregation between dorsal and ventral attention networks is nearly complete, spontaneous activity in right posterior MFG correlates with both networks (Figure 3), indicating that right MFG may contain intermixed neuronal populations respectively connected with dorsal or ventral regions (Fox et al., 2006a). This result raises the possibility that ventral and dorsal networks do not directly interact but are principally linked through prefrontal cortex (Fox et al., 2006a). This link is also supported by results obtained in neglect subjects showing that the functional disconnection of MFG with dorsal parietal cortex is responsible for abnormal stimulus selection (see below and He et al., 2007a) (wire diagram, Figure 2B). The functional segregation of the two networks in the absence of a task may allow their flexible recruitment during active behavior. For example, while dorsal regions are active following the presentation of an instructive cue, ventral regions are not recruited or are even suppressed (Shulman et al., 2003; Todd et al., 2005). However, following the presentation of a target, both ventral and dorsal regions respond briskly (Corbetta et al., 2000; Hampshire et al., 2007; Shulman et al., 1999, 2003). In summary, the correspondence between activation and connectivity analyses provides strong evidence for separate dorsal and ventral attention networks forming distinct functional systems.
The Ventral Network Is Activated by Important Stimuli that Reorient Attention
While reorienting to an object can be driven by salience and behavioral relevance, relevance is the critical factor that determines whether an object activates the ventral network (Downar et al., 2001). The ventral network might be considered a prime candidate for mediating orienting to salient but unimportant stimuli, i.e., exogenous attention (Posner and Cohen, 1984), because under passive conditions it is highly responsive to distinctive sensory events in all modalities (Downar et al., 2000). But this hypothesis has now been tested and rejected (Kincade et al., 2005). Kincade and colleagues separated the BOLD activity produced by an uninformative but salient peripheral cue, a red square in an array of green squares, from the activity produced by discriminating a subsequent rotated T or L (Figure 4A). In control conditions, subjects were presented with a neutral display of randomly intermixed color squares or a foveal cue that oriented attention voluntarily. Exogenous cues (the red square) did not activate the ventral network (Figure 4A), even though performance was better at that location, indicating that these cues were effective in generating a shift of attention. In contrast, the dorsal network (IPS/SPL and FEF) showed stronger activation for exogenous than neutral cues (Figure 4A), although the strongest recruitment was recorded for endogenous cues (data not shown). Many other studies have measured activations in exogenous orienting paradigms that have combined activations during the cue and target periods (Kim et al., 1999; Lepsien and Pollmann, 2002; Mayer et al., 2006; Peelen et al., 2004; Rosen et al., 1999). Although these studies are more difficult to interpret, they indicate that the ventral network is not recruited by orienting to uninformative but salient cues presented before a target appears (see Peelen et al., 2004, for an exception). Similarly, de Fockert and colleagues showed that uninformative but salient distracters that attract attention did not activate the ventral system (de Fockert et al., 2004) (Figure 4B), although they did activate the dorsal system. The overall conclusion is that exogenous orienting recruits the same dorsal frontoparietal network that is responsible for directing attention based on goals or expectations.
Figure 4. Ventral Network Activity Is Restricted to Task-Relevant Stimuli.
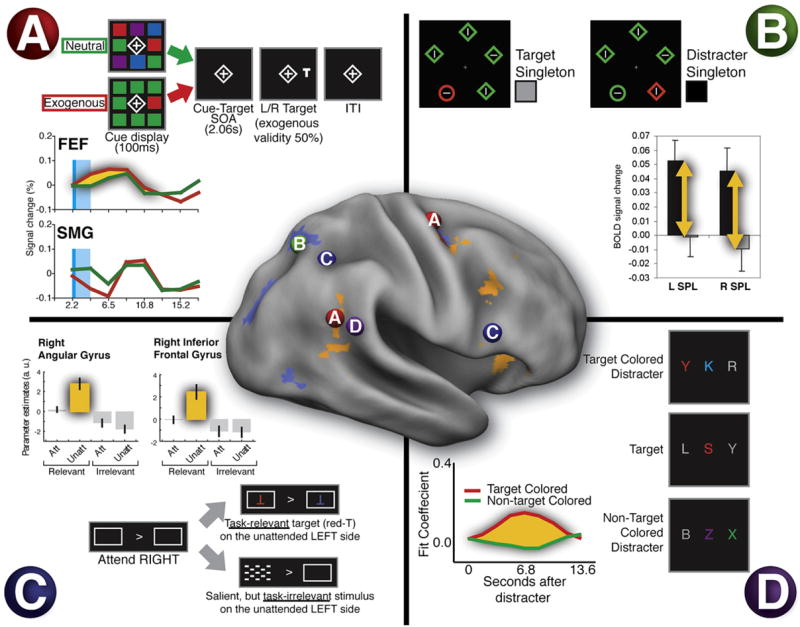
(A) Exogenous orienting does not activate TPJ. Subjects saw a color singleton (exogenous cue) or a heterogeneous colored array (neutral cue) followed by a target (cue and cue period in light blue). Behavioral performance was speeded when the target location matched the singleton location, even though the cue and target locations were random. The time course of the BOLD signal shown in the graph indicates that R FEF (see “A” in the surface-rendered brain) showed a larger response for exogenous than neutral cues. In contrast, SMG showed a small deactivation during the cue period, followed by a small activation when the cue period ended (Kincade et al., 2005).
(B) Salient irrelevant distracters influence the dorsal, not ventral, system. Subjects categorized the orientation of a line within a singleton shape (the circle). Salient but irrelevant singleton color distracters that impaired behavioral performance activated dorsal region SPL (see “B” in brain) rather than TPJ (de Fockert et al., 2004).
(C) Unattended stimuli only activate TPJ if they are task relevant, not if they are irrelevant, even though they have high sensory salience. A task-relevant unattended letter activated angular gyrus and inferior frontal gyrus (see “C” in brain), but no responses were seen to the unattended but highly salient checkerboard. The angular gyrus response may reflect the combined activation of dorsal (IPS/SPL) and TPJ regions (Indovina and Macaluso, 2007).
(D) Distracters only activate TPJ if they share features with a target, indicating a strong effect of task relevance. Subjects identified red foveal letters while ignoring irrelevant peripheral letters. Peripheral letters that matched the target color interfered with performance and activated TPJ, while non-target-colored letters had no effect (“D” in brain) (Serences et al., 2005).
Conversely, the ventral network is well activated by stimuli that are important, even if they are not very distinctive. Indovina and Macaluso (2007), for example, showed that unattended targets of low salience activated regions in both dorsal (FEF, precuneus) and ventral (IFG and anterior insula) attention networks, in line with previous results (Arrington et al., 2000; Corbetta et al., 2000; Macaluso et al., 2002), to a much greater degree than highly salient but irrelevant distracters (see Figure 4C). Finally, the ventral network is activated by irrelevant objects when they are similar to a target object. Serences et al. (2005) asked subjects to categorize red foveal letters interspersed among a rapid, successive series of colored foveal letters (rapid serial visual presentation, or RSVP) while peripheral distracter letters were occasionally presented in the target color (red) or in a nontarget color (green) (Figure 4D). This situation is analogous to when we look in a crowd for a friend wearing a red sweater and notice people wearing red but not green clothes (“contingent” orienting; Folk et al., 1992). TPJ activation was only observed for the red distracters (Figure 4D), consistent with the hypothesis that the ventral network responds mainly to stimuli thought to be behaviorally relevant (see also Downar et al., 2001).
In summary, the ventral network is not activated by orienting to distinctive but unimportant stimuli (exogenous orienting), except perhaps in the special case where subjects do not have an ongoing task, but does underlie reorienting to environmental stimuli based on their task relevance. An important conclusion from these neuroimaging studies is that the psychological distinction between exogenous and endogenous orienting (Jonides, 1981) may not map onto different neural systems. Rather, a more fundamental distinction appears to be between systems involved in orienting, both exogenous and goal-driven, i.e., the dorsal attention system, and those involved in stimulus-driven reorienting, i.e., the ventral and dorsal attention systems.
Preventing Activation of the Ventral Network by Unimportant Objects
The poor response of the ventral network to distinctive but unimportant objects when a person focuses on a task prevents shifts of attention that could interfere with task performance. Two studies have now shown that this poor response may be due to suppression of the ventral network by a sustained top-down signal.
In one study, subjects saw a rapid stream of letters (RSVP) and were instructed to look for an occasional digit (Figure 5A) (Shulman et al., 2003). Prior to the point at which the digit was detected, while subjects were still searching the letter displays, the ventral network (bilateral TPJ, R MFG, and R IFG) showed a sustained deactivation (Figure 5C, green-blue voxels). These deactivated regions overlapped regions that showed increased positive responses to unattended targets in a separate experiment, indicating that the deactivation occurred within ventral regions involved in stimulus-driven reorienting. Shulman and colleagues (Shulman et al., 2003) suggested that the suppression of activity prevented an inappropriate response to irrelevant stimuli. Because targets still triggered a robust positive response, however, activity in the ventral network appeared to have been gated by task relevance or filtered, with only targets passing the filter. Stronger filtering appeared to correlate with better performance, because the average deactivation in right TPJ was significantly larger on trials in which the subsequent target was detected than missed (Shulman et al., 2007) (Figure 5A).
Figure 5. TPJ Activity Is Suppressed during Focused Attention.
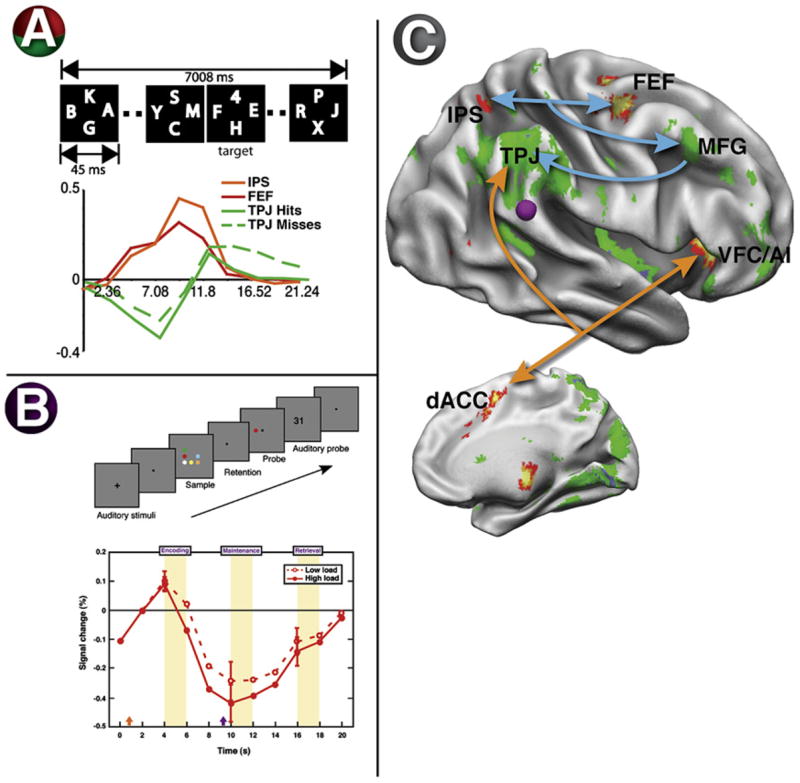
(A) Subjects searched a rapid serial visual presentation (RSVP) display for a target digit. The number of distracter frames containing only letters prior to the target frame containing the target was varied. The graph shows the time course of activity in dorsal regions IPS and FEF and ventral region TPJ under conditions in which the target appeared near the end of the trial. In TPJ, a deactivation to the letter distracters was followed by an activation when the digit was presented or the trial was terminated. Interestingly, the deactivation to the letters was significantly greater when the subsequent digit was detected than when it was missed. Conversely, IPS and FEF showed sustained activations during search (Shulman et al., 2003).
(B) Subjects encoded a visual display that they had to remember and then match to a probe display. During the retention interval, TPJ showed a deactivation (purple disk in the surface-rendered brain) that increased with the number of display items that had to be retained (Todd et al., 2005).
(C) The statistical map shows regions with sustained activity as subjects searched through letter distracters in the RSVP experiment (see panel [A]), including dorsal attention regions IPS and FEF (red/orange in surface-rendered brain) but also regions in anterior insula and anterior cingulate that form a putative task-control network (Dosenbach et al., 2006). These regions may send top-down signals (see arrows) to the ventral network, which showed sustained deactivations during search (blue/green in surface-rendered brain), restricting its input to task-relevant objects (Shulman et al., 2003).
In a second study (Todd et al., 2005), subjects remembered a set of objects in a visual display, and following a blank retention interval, decided whether any of the objects were present in a new display (Figure 5B). The larger the number of objects the subject had to remember (the memory load), the more R TPJ was deactivated during the retention interval. The authors separately showed that higher memory loads resulted in poorer detection of a novel unattended stimulus, suggesting that high memory loads suppress activity in R TPJ and prevent stimulus-driven reorienting (Todd et al., 2005). Together, these studies indicate that when subjects focus on a task, signals for task relevance (“filtering” in Figure 2B) deactivate TPJ, preventing reorienting to unimportant objects.
Source of Signals that Restrict Ventral Activation to Important Objects
The source of signals for task relevance may be the dorsal network (IPS, FEF), which shows strong anticipatory activity when people expect to see an object at a particular location or with particular features (Corbetta et al., 2000; Kastner et al., 1999). In the previous RSVP experiment, IPS and FEF were each one of the few regions in the brain that showed sustained activation to distracters prior to target detection (Shulman et al., 2003) (Figures 5A and 5C). These sustained signals may have filtered the input to the ventral network (blue arrows in Figure 5C; filtering signal in Figure 2B).
Another possible source of top-down signals is prefrontal cortex (Desimone and Duncan, 1995; Miller and Cohen, 2001). Resting-state analyses suggest that R MFG may link dorsal and ventral networks (Fox et al., 2006a), possibly funneling top-down biases from the dorsal network onto the ventral network (Figure 5C and 2B). R MFG is probably not the source because it showed sustained deactivation along with R TPJ and R IFG (Figure 5C). However, sustained increases were observed in anterior cingulate and anterior insula (Shulman et al., 2003), which have been postulated to form the core of a network for cognitive control (Dosenbach et al., 2006) (orange arrows in Figure 5C). The influence from these cortical regions may be direct through cortico-cortical interactions or indirectly via subcortical loops. In the last section, we relate the pattern of activity in TPJ, including filtering signals, to the output of the LC-NE, which receives input from the anterior cingulate and the anterior insula (Aston-Jones and Cohen, 2005; Ongur et al., 2003).
If prefrontal cortex is the source or the conduit of these modulations onto TPJ, then poor top-down control of stimulus-driven reorienting should be evident after prefrontal lesions. Chao and Knight (1995) reported that patients with unilateral dorsolateral prefrontal cortex (DLPFC) lesions showed markedly decreased performance in an auditory match-to-sample task due to irrelevant distracter tones presented during the retention interval. Loss of prefrontal inputs may have decreased top-down control over TPJ, resulting in inappropriate reorienting to distracting stimuli (see also Ro et al., 1998; Snow and Mattingley, 2006).
In summary, only environmental stimuli that are behaviorally relevant trigger the ventral network. The ventral network response is suppressed when irrelevant stimuli are presented, even if they are distinctive, reflecting a “filtering” signal that gates sensory responses by behavioral relevance. The source of the filtering signal may be the dorsal network or other parts of prefrontal cortex, either directly or indirectly via subcortical loops.
Do Signals from the Ventral Network Initiate Reorienting?
Above, we discussed the inputs to the ventral system that ensure it is mainly activated by behaviorally important stimuli. Next, we consider how the output from this system affects activity in other neural systems and behavior. One possibility is that, when an important stimulus appears outside the current focus of attention, fast-latency signals from the ventral network initiate reorienting by sending a “circuit-breaking” or interrupt signal to dorsal regions, which change the locus of attention (Corbetta and Shulman, 2002).
The dorsal network contains the neural machinery for directing attention and the eyes to sensory stimuli appearing at unexpected locations, with spatially selective responses to contralateral stimuli and responses to movements of attention or the eyes (Beauchamp et al., 2001; Corbetta et al., 1998, 1993; Nobre et al., 1997; Schluppeck et al., 2005; Sereno et al., 2001; Sweeney et al., 1996; Sylvester et al., 2007). In contrast, group-averaged studies of ventral regions (TPJ, VFC) have not found spatially selective responses during reorienting (Corbetta et al., 2002; Macaluso et al., 2002; Macaluso and Patria, 2007). Similarly, mapping studies in individuals have only reported weak spatially selective responses near or within the ventral network in parts of MFG (Hagler and Sereno, 2006; Jack et al., 2007) and superior temporal gyrus (STG) (Jack et al., 2007). The weak evidence for spatial selectivity in the ventral network suggests that spatial reorienting is not mediated solely by that network but involves joint activation of dorsal and ventral regions.
There is little evidence, however, that short-latency responses in the ventral attention network precede those in dorsal areas and trigger a reorienting response. Within dorsal parietal and frontal sites, EEG- or MEG-based estimates of visual response latency to targets for an eye movement vary between 130 and 170 ms (Evdokimidis et al., 2001; McDowell et al., 2005; Sestieri et al., 2008). Within ventral sites in TPJ and IFG, the response to targets is thought to be indexed by the P300 potential, with a latency of 300–400 ms, considerably longer than the dorsal latencies (Bledowski et al., 2004; Daffner et al., 2003; Knight et al., 1989; Menon et al., 1997; Yamaguchi and Knight, 1991a). Unfortunately, P300 and eye movement paradigms are difficult to compare. There have been a number of ERP/MEG studies of spatial reorienting, but the results are ambiguous in relation to the relative latency of dorsal and ventral parietal regions (Luck et al., 1994; Mangun and Hillyard, 1991). Invalid targets that follow a voluntary cue to shift attention increase a late-positive deflection (230–400 ms) at central, parietal, and occipital sites that might correspond to P300 (Mangun and Hillyard, 1991). At temporal electrodes ipsilateral to the target (Hopfinger and Ries, 2005), invalid targets that follow an uninformative (exogenous) cue produce a negative-going deflection in the range of 200–250 ms, preceding a separate P300. Although this latter paradigm involved noninformative cues, the ERP component was sensitive to several task-contingent factors, reflecting top-down signals (Hopfinger and Ries, 2005).
Overall, the latency of visual responses to salient behaviorally relevant visual stimuli is, if anything, shorter in dorsal parietal than in ventral parietal areas, but definitive studies have not been conducted. In awake behaving monkeys, neural responses to visual stimuli in lateral intraparietal area (LIP), the putative homolog of human IPS/SPL, show a very rapid nonselective volley (~50 ms) followed by slower oscillations (100–200 ms) that are modulated by spatial attention (Bisley et al., 2004). In more ventral parietal cortex, in correspondence with area 7A, which shows modulation by unattended stimuli (Constantinidis and Steinmetz, 2001; Robinson et al., 1995) and salient oddball stimuli during simple fixation (Constantinidis and Steinmetz, 2005), similar to the ventral attention network, average response are about 100 ms (typical range 70–200 ms; Constandidinis personal communication). No direct comparison on the same task has been carried out, however. Reorienting of attention to a behaviorally relevant and salient stimulus outside of the current focus is probably initiated in dorsal frontoparietal cortex in conjunction with subcortical structures (e.g., superior colliculus). Ventral system activity during reorienting may reflect slower adjustments necessary to complete or carry out a complex reorienting response that involves shifts in task sets, expectations, reward contingencies, and arousal.
Do Signals from the Ventral Network Influence Reorienting and the Dorsal Network?
While the latency data from electrophysiological studies are ambiguous on whether ventral network activity triggers dorsal activity during reorienting, transcranial magnetic stimulation studies (TMS) nonetheless support a key role for ventral regions in reorienting attention and detecting targets in conjunction with dorsal frontoparietal regions (IPS, FEF). An extensive discussion of TMS studies of visuospatial attention is beyond the scope of this review, but some conclusions can be drawn from the extant literature. First, interference with regions in inferior parietal cortex (TPJ, SMG, AG) disrupts visual target detection and reorienting (Chambers et al., 2004a; Ellison et al., 2004; Meister et al., 2006). Second, disruption has been demonstrated for stimulation latencies ranging from 90–120 ms (Chambers et al., 2004a) to 200–300 ms following target onset (Chambers et al., 2004a; Ellison et al., 2004; Meister et al., 2006). Early interference effects may reflect disruption of a signal that disengages attention from its current location and initiates reorienting (Chambers et al., 2004a). Third, the regions in inferior parietal cortex that show effects of TMS depend on the task: R TPJ during detection of bilateral stimuli (Meister et al., 2006), angular but not supra-marginal gyrus (SMG) during reorienting in an exogenous cueing paradigm (Chambers et al., 2004a), SMG during reorienting in an endogenous cueing paradigm (Chambers et al., 2004b), and STG during visual search (Ellison et al., 2004). Fourth, a larger set of studies has reported effects of TMS in FEF or posterior parietal cortex (PPC) on detection, search, and orienting (Fuggetta et al., 2006; Grosbras and Paus, 2002; Muggleton et al., 2003; O’Shea et al., 2004; Taylor et al., 2007; Thut et al., 2005). Overall, in agreement with the imaging evidence showing that dorsal and ventral networks are coactivated during target detection and stimulus-driven reorienting (Corbetta et al., 2002; Giessing et al., 2006; Kincade et al., 2005; Marois et al., 2000), TMS of both ventral and dorsal regions affects reorienting, detection, and search.
We have reported direct evidence for an interaction between the two networks in fMRI studies of stroke patients with spatial neglect. Spatial neglect is a syndrome characterized by a bias to attend and respond to objects on the contralesional side and is observed more frequently after right than left hemisphere strokes (Heilman et al., 1987b; Mesulam, 1999). Lesions that cause neglect are typically localized in ventral frontal or temporoparietal cortex and underlying white matter (Husain and Kennard, 1996; Karnath et al., 2004; Mort et al., 2003; Vallar and Perani, 1987). We recently demonstrated that the spatial bias of neglect depends on a physiological imbalance between left and right dorsal parietal cortex (IPS/SPL), which is caused by structural and physiological abnormalities in the ventral attention network (Corbetta et al., 2005; He et al., 2007a). The inter-hemispheric imbalance in IPS/SPL is evident both during spatial attention tasks, with a significant relationship between left-side neglect and hyperactivation of left parietal cortex, and in measures of functional connectivity at rest. For instance, Figure 6A shows BOLD time series collected from a stroke patient who suffered extensive damage to inferior frontal, perisylvian, and TPJ cortex and showed severe left-side neglect at the acute stage. The time series clearly show abnormal correlation of the resting BOLD signal between left and right IPS, which is not structurally damaged. This deficit correlates across subjects with the severity of neglect and recovers over 9 months as neglect improves (He et al., 2007a). Interestingly, the degree of functional impairment in dorsal parietal cortex correlates with the degree of impaired functional connectivity in the structurally damaged ventral network, hence demonstrating the interaction between the two networks. Notably, this interaction involved right MFG and the white matter fibers connecting this region to dorsal parietal cortex (He et al., 2007a), providing more support for the hypothesis that right MFG links ventral and dorsal systems (Figure 2B).
Figure 6. Interaction of Dorsal and Ventral Attention Networks.
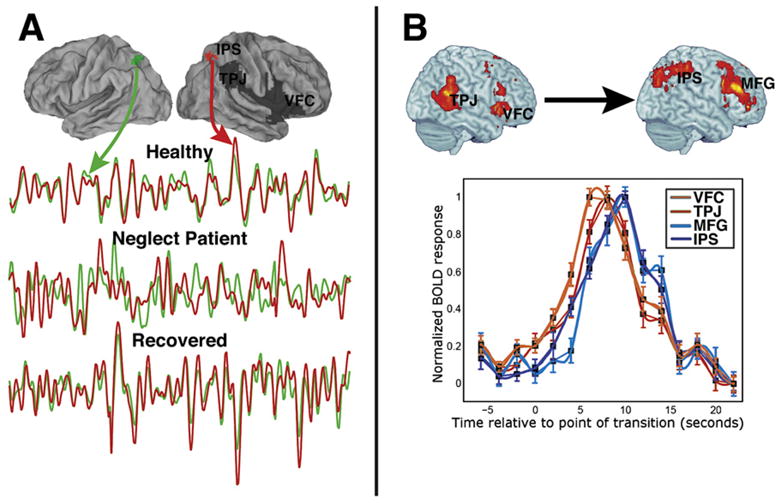
(A) The surface-rendered brains show the damaged right hemisphere regions (in dark gray) of a stroke patient with spatial neglect. The bottom graph shows the time course of BOLD activity in undamaged regions of IPS, with the green and red lines indicating, respectively, the time series for the indicated left and right IPS regions. Time courses from these regions are shown for a healthy subject, for the stroke patient immediately following the stroke, and for the same patient following recovery. While the healthy subject and the recovered stroke patient show highly correlated interhemispheric IPS activity, the same patient immediately after the stroke shows activity that is much less correlated. Therefore, damage to ventral regions, possibly including white matter tracts, impairs physiological interactions between undamaged dorsal regions (He et al., 2007a).
(B) The surface-rendered brains show ventral (left) and dorsal (right) regions that are activated when the completion of a symphonic movement is detected. The time courses indicate that ventral activations (red lines) preceded the dorsal activations (blue lines), while a Granger Causality analysis of these regions indicated that ventral activity predicted dorsal activity (Sridharan et al., 2007).
Finally, a recent paper used a Granger Causality analysis to show an influence of ventral activity on dorsal activity when healthy subjects passively listened to a movement from a symphony (Sridharan et al., 2007) (Figure 6B), consistent with an interaction between the networks. Completion of the movement activated both networks, but the ventral activation preceded the dorsal activation (Sridharan et al., 2007). The authors suggested that the ventral network activity marked an event boundary and influenced dorsal activity during a subsequent updating of working memory.
In summary, TMS, neuroimaging, and lesion evidence support the hypothesis that ventral and dorsal networks are both necessary and interact when attention is reoriented to behaviorally relevant environmental stimuli.
Reorienting Perceptual and Response Processes to Environmental Stimuli
Although many of the studies that have been discussed involved spatial reorienting to environmental stimuli, we emphasized in the introduction that the ventral network mediates a broader set of changes in response to an environmental stimulus. Unfortunately, these broader changes involve many processes that can be difficult to isolate. For example, an early indication that the ventral network was recruited under circumstances other than spatial reorienting came from studies using the oddball paradigm, in which subjects detect a target presented infrequently (10%–20%, “oddball”) in a stream of frequent “standard” objects. Enhanced responses to oddballs are observed in a set of regions that includes most consistently the temporoparietal junction and the lateral prefrontal cortex but also dorsal regions in parietal and frontal cortex involved in shifting attention. Because the oddball is usually defined by a different feature(s) than the standard, rather than by a different location (see Marois et al., 2000, for a comparison of the two cases), the enhancement to the oddball is not related to a spatial shift of attention.
But the oddball paradigm combines a range of processes, making the fMRI activations difficult to interpret. For example, a spatial or feature cue in a typical visual attention task may indicate what object should be attended (e.g., “attend to the red letter”) (Broadbent, 1971; Bundesen, 1990) but not how the object should be categorized or responded to (e.g., “if the letter is a vowel, press the left key”), restricting the relevant processes to those involved in stimulus selection (Logan and Gordon, 2001). In the oddball paradigm, however, the oddball/standard distinction indicates what response should be made, adding processes involved in categorizing the oddball, selecting a response (whether overt or covert, go or no-go) based on the current stimulus-response mapping, making the response, and generating signals related to performance monitoring.
Several other studies suggest that the ventral network marks transitions when one behavior is interrupted or terminated and a new behavior begins, including transitions at event boundaries (for a general discussion of event boundaries, see Zacks et al., 2007). A similar phenomenon appears to occur during the transition between a period of rest and a task block involving many trials (task onset) or the transition from the task block to rest (task offset). Both block onsets and offsets robustly and transiently activate R TPJ and VFC, but also other regions, including dorsal prefrontal cortex and the dorsal attention network (Dosenbach et al., 2006; Fox et al., 2005a; Konishi et al., 2001) (Figure 7A). Even within a single trial, coactivation of dorsal and ventral frontoparietal areas at task offset may index a readjustment or interruption of ongoing task sets (Shulman et al., 2002). Interestingly, in this latter study, the transient signal at task transition occurred both in dorsal frontoparietal areas that were engaged prior to the transition, but only at the transition point in the ventral network. One interpretation is that the ventral network signals the task transition and/or provides a reset signal. As discussed below, it is possible that these cortical reset signals are related to similar signals identified in the LC-NE system, which putatively allows for a shift of cortical architecture at task boundaries (Bouret and Sara, 2005).
Figure 7. Common Activation of TPJ during Reorienting, Task Transitions, and Social Cognition.
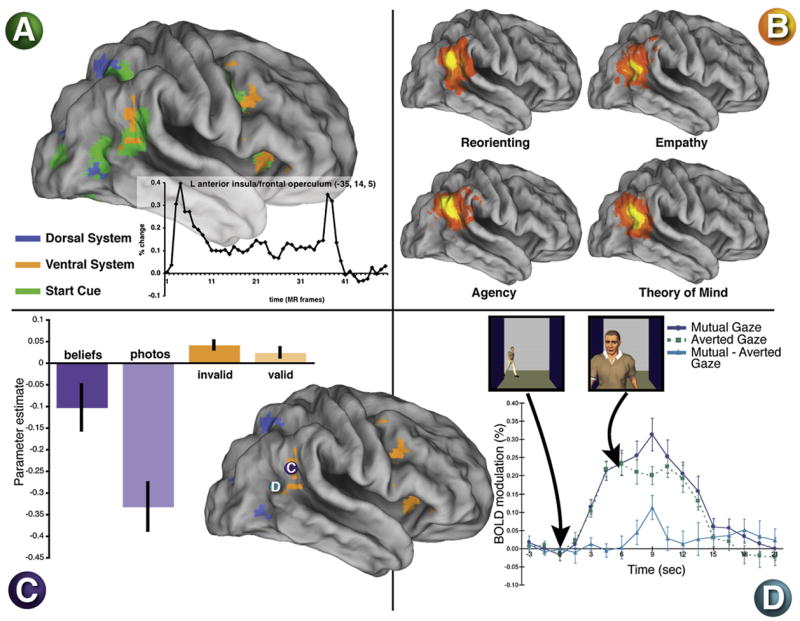
(A) Regions in green show transient activity at the transition between a rest period and the onset of an extended block of trials (see time course in inset, indicating that both onsets and offsets are often observed). “Start cue” activity is observed within some ventral and dorsal regions, indicating the involvement of both networks in task transitions (Dosenbach et al., 2006).
(B) A meta-analysis of activations across studies measuring reorienting and various aspects of social cognition. Largely similar TPJ activity is observed across paradigms, with perhaps a more posterior extension of activity in the social cognition paradigms (Decety and Lamm, 2007).
(C) A within-subject comparison of reorienting and ToM paradigms revealed that both activated very similar TPJ regions (Mitchell, 2007). The bar graph shows the magnitude of the TPJ activation in the two paradigms. A large deactivation was observed in the control “false-photograph” condition with a significantly smaller deactivation in the experimental ToM “false-beliefs” condition. The reorienting paradigm yielded event-related activations that were larger during trials with invalid than valid cues. The blue and orange regions are taken from the meta-analysis of the dorsal and ventral networks in Figure 2.
(D) Gaze perception activates superior temporal regions. The graph shows the time course of activity when another person makes or averts eye contact with the observer. Mutual gaze enhances the activation (Pelphrey et al., 2004).
Overall, the above studies suggest that, whenever environmental stimuli call for a change in a maintained task, ventral (and dorsal) attention networks are modulated at the transition point. Interestingly, the ventral network is not recruited when people regularly switch from one task to another over short time periods (e.g., task-switching paradigms). This form of task control appears to involve a separate set of dorsal parietal and frontal regions (Brass and von Cramon, 2004; Braver et al., 2003; Kimberg et al., 2000; Rushworth et al., 2002).
Reorienting from ‘Internally Directed’ Processes to Environmental Objects
Stimulus-driven reorienting has mainly been discussed in the context of changing the control of behavior from one environmental input to another, but similar reorienting mechanisms may also be involved in shifting from a broad range of “internally directed” processes in order to deal with environmental events, as when interrupting memory retrieval (“did I lock the car door?”) to respond to a sudden stimulus (“is that my cell phone ringing?”). We hypothesize that the ventral attention network may play a central role in this function.
Important aspects of internally directed processing, such as introspection, self-referential thoughts, or projecting oneself into a situation (e.g., envisioning or planning one’s future or remembering one’s past as in episodic memory) are thought to involve the so-called “default” network (Raichle et al., 2001). This network of cortical regions is strongly deactivated during a wide range of demanding cognitive tasks relative to a passive resting or viewing state (Binder et al., 1999; Mazoyer et al., 2001; Shulman et al., 1997). It has been proposed that these regions mediate a number of “default” processes to which the brain returns in the absence of a task (Raichle et al., 2001). A similar set of regions show high temporal correlation in resting-state fcMRI (Fox et al., 2005b; Greicius et al., 2003).
Some authors have proposed that default and dorsal attention networks represent two fundamental axes of functional organization in the brain, with the dorsal attention network controlling environmentally directed processes (e.g., perception and action) and the default network controlling internally directed processes (e.g., memory, introspection) (Fox et al., 2005b; Golland et al., 2007). This hypothesis is based on the observation that goal-directed tasks activate the dorsal attention network and deactivate the default network. Moreover, several fcMRI studies have reported that default activity is negatively correlated with the dorsal network (see top panel of Figure 3; (Fox et al., 2006a; Fransson, 2005; but see Golland et al., 2007; Nir et al., 2006). Finally, during natural vision, the posterior part of the brain is entirely occupied either by regions that are positively correlated with the dorsal attention network or the default network (Golland et al., 2007; Nir et al., 2006).
The hypothesis that the ventral network may function as a system to switch (reorient) between internally and externally directed activities is based on two sets of observations. First, the ventral network is largely segregated in terms of functional connectivity from both dorsal attention and default networks (see Figure 3; Fox et al., 2006a). The independence in the resting state of the ventral network from both dorsal and default networks may allow a flexible interaction during externally or internally directed behavior. Second, although both ventral attention and default systems may deactivate during goal-oriented behavior, the deactivations depend on different factors. During a perceptual task, in which subjects monitored an RSVP stream for a single target (Shulman et al., 2003), the TPJ component of the ventral network was deactivated only while subjects searched the stream for the target (Figure 5A), but the angular gyrus component of the default network was deactivated as long as the RSVP stream remained on the screen. In other words, TPJ was deactivated by the attentional component, while the angular gyrus was deactivated by the sensory component of the task. Moreover, the presentation of the attended target activated TPJ but not angular gyrus (see Golland et al., 2007, for a different dissociation between anterior and posterior portions of IPL). In contrast, when subjects searched their episodic memory for an item (an internally directed task), both sets of regions were still deactivated, but only the angular gyrus was then activated by a positive match in memory (Shannon and Buckner, 2004; Wheeler and Buckner, 2004). The similarity of response profile when looking for a target in the environment or in memory raises the possibility that the ventral attention network plays a similar role in both processes. In both cases, filtering of the ventral attention network is necessary to protect the system from involuntarily reorienting to environmental stimuli when resources are allocated to perceptual, memory, or self-referential processing.
Reorienting during Theory of Mind Cognition
An intriguing development of the last few years is that activation of right TPJ, the posterior core of the ventral attention network, has been reported during “theory of mind” (ToM) cognition, i.e., reasoning about other people’s mental states (Fletcher et al., 1995; Gallagher and Frith, 2003). ToM cognition involves a close interaction between perceptual processes and those involved in self-projection (Buckner and Carroll, 2007). Subjects may judge the intentions of a person they are viewing in a movie or judge a person’s intentions based on a written description.
A recent study reported that ToM activations, measured by comparing responses to false-belief stories and control stories involving outdated photographs, colocalized with activations from reorienting to invalid targets in a Posner cueing task (Mitchell, 2007) (Figure 7C). Figure 7B shows the close correspondence between activations during attentional reorienting and social cognition in R TPJ from a recent meta-analysis (Decety and Lamm, 2007), although there was a tendency for the social cognition activations to extend slightly more posteriorly, perhaps into the default system proper.
Colocalization of activations from ToM and reorienting paradigms does not necessarily imply a common process. First, the colocalization, while impressive, is only approximate. In addition to the fact that fMRI activity averages over large cell populations, there may be a slightly more posterior distribution for ToM activations. To our knowledge, the VFC component of the ventral attention network has not been reported in studies of social cognition. Instead, social cognition paradigms often activate, in addition to TPJ, foci in posterior cingulate and medial prefrontal cortex that belong to the default network, which we argued above is distinct from the ventral attention network. Perhaps a slightly more posterior location for the TPJ focus in some ToM paradigms reflects connectivity with these default regions. Second, colocalization may mask subtle but systematic differences in the voxelwise distributions of the activations (Downing et al., 2007). Demonstrating that two voxelwise patterns or distributions are not identical, however, begs the question of why both patterns occur in the same cortical tissue. Although in principle the two distributions could reflect completely unrelated functions that are juxtaposed, i.e., a specialized ToM module (Saxe and Powell, 2006) and a node within a reorienting network (Corbetta and Shulman, 2002), the close anatomical correspondence may suggest a less arbitrary relationship.
If activations from reorienting and ToM are not completely unrelated, why might they be linked? First, colocalization might reflect factors that are poorly controlled in either or both paradigms. For example, ToM paradigms generally involve blocks or trials in which subjects comprehend animations, movie sequences, or stories over an extended period. The cognitive or working memory loads of the experimental and control stories in these ToM paradigms have not been explicitly controlled. In several studies, the selective activation of right TPJ during ToM conditions as compared to control conditions actually reflected a lesser deactivation (e.g., Figure 7C from Mitchell, 2007). Because greater memory loads produce stronger TPJ deactivations (Todd et al., 2005), differential TPJ activity in experimental and control conditions of ToM paradigms could reflect overall differences in memory load or task complexity. The consistency of R TPJ activations in ToM and reorienting experiments across very different paradigms, however, suggests that any single methodological factor may not explain the colocalization.
Second, colocalization might reflect cognitive processes that are present in both paradigms. For example, both reorienting and ToM paradigms often involve breaches of expectation (e.g., invalid cues [Arrington et al., 2000; Corbetta et al., 2000; Macaluso et al., 2002] or false-belief stories [Gallagher and Frith, 2003; Vogeley et al., 2001]), which appear to modulate the ventral network. Decety and Lamm (2007) suggest that many aspects of social cognition involve a comparison of “internal predictions with actual external events,” explaining the ubiquitous presence of R TPJ activity. However, some ToM studies have included controls for this factor (Saxe et al., 2004), and some ToM and reorienting studies have not involved manipulations of expectation (Saxe and Powell, 2006; Serences et al., 2005).
Another possibility along these lines is that TPJ activity during ToM tasks reflects signals linked to shifts in eye gaze or for perception or imagery of gaze. Several studies have shown that posterior STS is activated during the perception of gaze shifts (Allison et al., 2000; Pelphrey et al., 2003, 2004). Within a social context, activation from viewing-gaze shifts are larger when they occur toward the viewer (mutual gaze) than when they occur away from the viewer (averted gaze) (Figure 7D). This error signal may reflect a mismatch between our expectation and the observed direction of another person’s gaze (similar to an invalidly cued target) or an error signal in the inferred state of mind of the other person (a ToM signal). In general, strong evolutionary reasons link mechanisms for shifting attention to the development of social mechanisms for conspecific interactions in old and new world monkeys to mechanisms to infer others’ intention or ToM in higher apes and humans (Tomasello et al., 2001). Gaze-related activations in STS, however, may not colocalize with those for reorienting or ToM (these functions have not been assessed within the same experiment) (see Gobbini et al., 2007, for a recent meta-analysis).
Finally, in ToM experiments, subjects continually shift between a simulation or judgment of the other person’s mind or viewpoint and processing of perceptual evidence from their own viewpoint that supports the simulation or judgment. Interestingly, recent evidence indicates that disruption of TPJ activity either by seizure activity or electrical stimulation can engender a number of hallucinatory misperceptions that involve a mismatch between the perception of the surrounding environment and one’s own body. For example, subjects may feel as if they see their body from the outside or as if the perception of their own body is not aligned with the body’s visual representation and surrounding environment (reviewed in Blanke and Arzy, 2005). These changes in body self-perception can be manipulated experimentally (Lenggenhager et al., 2007) and produce right TPJ activity (Arzy et al., 2006). These findings have been interpreted by considering TPJ cortex a site of multimodal integration of visuospatial, vestibular, and body-related signals and that the alignment of these signals generates and maintains one’s own sense of body or bodily self (Blanke and Arzy, 2005). While the relationship between reorienting signals in the ventral attention network and sense of body remains to be explored, an intriguing hypothesis is that similar environmental and bodily representations and their comparison may be co-opted for ToM interactions and that attention signals in TPJ may be important to switch between internal, bodily, or self-perspective and external, environmental, or other’s viewpoint, a key ingredient of ToM.
The Role of Expectation in Reorienting
Many of the conditions that activate the ventral network involve violating an expectation. For example, because people prepare for expected objects, an unexpected target object is often an unattended object, evoking “stimulus-driven reorienting.” Similarly, event boundaries, which appear to activate the ventral network, may be determined by monitoring whether the sensory input departs from a current model of ongoing behavior (Zacks et al., 2007). Discrepancies or breaches of expectation indicate that a new behavior has occurred, marking an event boundary and requiring the model to be updated. But activations to unexpected stimuli may also reflect processes that are either entirely separate from reorienting or modulate reorienting. Important objects that violate an expectation may also increase arousal, dishabituate neuronal responses in sensory and associative areas in paradigms in which expectations are driven by stimulus frequency (e.g., oddball paradigms), or produce error signals that drive learning, reward, or affective mechanisms. While, in some cases, violations of expectation may be an essential feature of the process that drives ventral network activation, it will also be important in future work to explicitly manipulate stimulus-driven reorienting independently from expectation.
Several neuromodulators have been linked to the detection of unexpected events, including dopamine and norepinephrine (NE) (Dayan and Yu, 2006). Although dopaminergic responses to unexpected stimuli are often discussed in the context of reward (Schultz, 1998; Schultz et al., 1997) some authors have proposed that they more generally facilitate a shift of attention to unexpected and behaviorally important stimuli (Horvitz, 2000; Redgrave et al., 1999; Zink et al., 2003). This putative function is very similar to that proposed for the ventral attention network, but there is no evidence of a significant dopaminergic projection to TPJ. In contrast, there is evidence in monkey for a strong noradrenergic innervation of inferior parietal cortex and superior temporal gyrus, possible homologs of human TPJ (Foote and Morrison, 1987; Morrison and Foote, 1986). Therefore, we next consider the functional relationship between the ventral attention network and activity in the locus coeruleus (LC), the primary source of NE.
Links between Ventral Attention Network and Locus Coeruleus-Norepinephrine System
The LC-NE system is a monoaminergic neuromodulatory system that originates from a small nucleus in the dorsal pons, the locus coeruleus, projecting diffusely to the brainstem, cerebellum, diencephalon, and neocortex. Several neurocomputational theories of the LC-NE system activity (Aston-Jones and Cohen, 2005; Bouret and Sara, 2005; Dayan and Yu, 2006; Yu and Dayan, 2005) bear striking resemblance to some of the ideas put forward in this review regarding the role of the ventral attention network.
LC neurons exhibit both tonic and phasic activity modes. Tonic activity is low in an unaroused state that facilitates sleep and disengagement from the environment (Aston-Jones and Bloom, 1981; Rajkowski et al., 1994), moderate when the organism is engaged in a focused task of high utility and filters out irrelevant stimuli (Usher et al., 1999), and high when the organism is not committed to a task, is exploring the environment, and there is uncertainty concerning the proper relationship between stimuli and responses (Aston-Jones et al., 1997) (i.e., unexpected uncertainty). Although these transitions in tonic firing of LC neurons occur over seconds or minutes, decrements of tonic LC activity have been observed on a shorter timescale in the period between a warning cue instructing the onset of a trial and a rewarded target stimulus (Bouret and Sara, 2005). Aston-Jones and Cohen have proposed that LC-NE tonic signals enable transitions between behavioral states (sleep, focused alert, exploratory) and that the decrement of tonic activity from an exploratory state to a specific task state reflects the higher utility associated with the detection of upcoming target stimuli. Accordingly, transitions between different tonic levels are enabled by cortical inputs from prefrontal regions (anterior cingulate, orbitofrontal cortex) that heavily project to LC and are sensitive to task context and reward information.
The second component of LC discharge is the phasic response observed to target stimuli, which is most strongly generated in the moderate tonic task-focused mode. Interestingly, phasic responses of LC neurons share many similarities with the P300 target-related cortical evoked potential, which was previously discussed in relation to the timing of the response in the ventral attention network (Aston-Jones and Cohen, 2005; Nieuwenhuis et al., 2005). Two different yet related theories have been proposed to explain the putative function of the LC phasic response to targets. According to Aston-Jones and Cohen, the phasic response enhances the gain of neural responses in the complex neural matrix involving sensory, decision, and motor regions and therefore speeds up behavioral responses. Importantly, the LC phasic response is thought to be triggered by pre-frontal inputs only after the sensory evidence for a target has exceeded a decision threshold in the relevant cortical network, i.e., it is a relatively late postdecision signal that restricts LC activity to target stimuli (Clayton et al., 2004; Rajkowski et al., 2004), consistent with the relatively late P300 response to target detection. Alternatively, the phasic signal has been conceptualized as an “interrupt” signal (Dayan and Yu, 2006) or as a “network reset” signal (Bouret and Sara, 2005) that allows the flexible configuration of a target network once a target is detected. Bouret and Sara note that this interpretation is consistent with the role that norepinephrine plays in much simpler organisms. For instance, in the stomatogastric nervous system of crustacea, synchronized activity from a small number of neuromodulatory cells can construct ex novo a functional network from neurons otherwise belonging to a different functional network (Marder and Thirumalai, 2002; Meyrand et al., 1994; Simmers et al., 1995). The phylogenetic stability of norepinephrine systems from crustaceans to humans is a powerful argument for stability of function. The Aston-Jones/Cohen theory of the phasic LC-NE signal is not necessarily inconsistent with this idea, because the authors note that the phasic signal effectively reconfigures the target cortical network from a multilayer to a single-layer network following a decision phase but does not capture the “network reset” idea.
We propose a functional relationship between signals of the LC/NE system and activity in the ventral attention network, both in relation to behavioral transitions (tonic signals) and target detection (phasic response). The decrease in tonic LC activity during the transition from an exploratory state to a task-focused state may parallel the deactivation of TPJ, relative to rest, when subjects engage in a demanding task (Shulman et al., 2003; Todd et al., 2005) (Figure 8). The Aston-Jones/Cohen theory maintains that a decrease in the tonic level of LC activity promotes engagement on the current task and filtering of distracters, similar to the hypothesis that the ventral attention network is deactivated under demanding conditions, reflecting a top-down “filtering” signal that restricts the network response to a narrow range of task-relevant stimuli (targets or contingent distracters). Conversely, the hypothesized broad sensitivity to environmental stimuli during the high tonic activity/exploratory LC mode in the Aston-Jones/Cohen model may correspond to the ability of any salient stimulus to activate TPJ during passive viewing or no-task states (Downar et al., 2000). We reviewed evidence above that the sources of top-down filtering signals into the ventral network are either the dorsal network through prefrontal cortex (Figures 5C and 2B) or prefrontal regions (anterior cingulate, frontal operculum; Figure 5C) directly or indirectly through their projection via LC. On the output side, LC-NE neurons densely projects to inferior parietal cortex and superior temporal gyrus, possible homologs of human TPJ (Foote and Morrison, 1987; Morrison and Foote, 1986). Therefore, as shown in Figure 8, the deactivation of the ventral attention network during focused attention may be partly caused by a decrement of tonic activity in the LC/NE system.
Figure 8. Relationship between Activity in TPJ and Locus Coeruleus/Noradrenergic System.
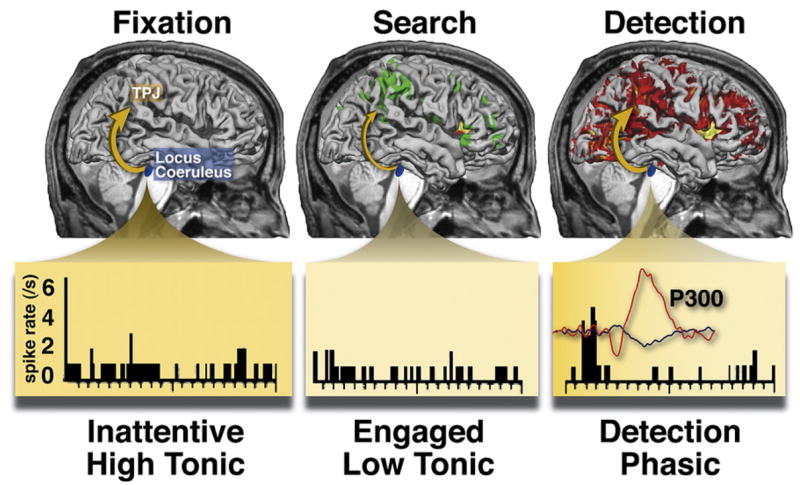
The surface-rendered brains show fMRI BOLD activations and deactivations relative to when subjects are fixating in an otherwise blank field (i.e., the baseline, left panel), when searching through letter distracters of an RSVP display (middle panel, ventral network is deactivated, dorsal network is activated), and when detecting a digit target in the display (right panel, both networks are activated along with other regions) (Shulman et al., 2003). The bottom panel shows spiking activity in monkey locus coeruleus neurons during analogous periods: an inattentive period in which a task is poorly performed and tonic activity is high, an attentive period in which the task is performed well and tonic activity is decreased, and target detection, which produces a phasic increase in activity (Usher et al., 1999). The inset trace shows event-related potentials recorded from the scalp of a human when a target is detected in a completely separate experiment, with the large positive deflection indicating the P300.
There is also a striking similarity between the target-related response in the ventral network, P300 potentials, and the phasic response in the LC (Table 1). All three (ventral network, P300, LC neurons) show enhanced responses to behaviorally relevant stimuli (targets) in multiple modalities, relative to distracters, and an enhanced response to low-frequency targets. Detection of unattended targets (i.e., “invalid” targets in the Posner cueing paradigm) enhances both TPJ activity and the amplitude of a late positive potential that may correspond to P300 (Mangun and Hillyard, 1991), while stimuli of high emotional valence modulate P300 and LC activity. On the response output side, TPJ activity, P300, and LC activity are relatively independent of response parameters (Astafiev et al., 2006; Clayton et al., 2004; McCarthy and Donchin, 1981). Finally, both P300 and LC activity can be anatomically linked to TPJ. Lesions of different parts of the ventral attention network affect different components of P300, with TPJ damage decreasing both target- and novel-evoked P300 components and prefrontal lesions affecting the novelty response (Yamaguchi and Knight, 1991b; Verleger et al., 1994; Daffner et al., 2000). A recent study showed that oddball target responses in TPJ and prefrontal cortex were abolished by propranolol, a β-adrenergic blocker drug (Strange and Dolan, 2007).
Table 1.
Ventral Attention Network/P300/LC Activity
| Inputs | TPJ/VFC | P300 | LC Phasic |
|---|---|---|---|
| Target > passive | + | + | + |
| Multimodal response (visual, auditory, tactile) | + | + | + |
| Stimulus probability (low > high) | + | + | + |
| Orienting to unattended stimuli (invalid > valid) | + | +(IIN) | ? |
| Orienting to contingent distracters (relevant > irrelevant) | + | +(IIN) | ? |
|
| |||
| Output | |||
|
| |||
| Independence from motor/response parameters | + | + | + |
These physiological similarities point to similar functions. The hypothesis that the ventral attention network is involved in reorienting from one task state to another, either in the environment or between internally and externally directed activities, is very close to the network-reset hypothesis of Bouret and Sarah. A network reset or interrupt hypothesis captures the sensitivity of the ventral attention network to task transitions or unexpected events that may require the dorsal network to be reconfigured (as in Figures 6B and 7A). Under these conditions, activity in the dorsal network reflects the reconfiguration of task processes (stimulus and motor representations) in response to the new contingency, while activity in the ventral network facilitates rather than initiates this reset or reconfiguration process. We have already discussed that activity in the ventral network, as indexed by the P300, may not be sufficiently fast to initiate a reorienting response. A similar argument applies to the LC-NE system, which has a relatively long latency to a stimulus (~100–150 ms) and a slow transmission of its output to the cortex (~50–100 ms). In the context of a nonlinear dynamic system, the highly synchronized LC-NE activation of the ventral network may allow the dorsal network to switch to or settle into another state more appropriate for the new environmental situation (Serences and Yantis, 2006).
While the adaptive-gain theory of Aston-Jones and Cohen is concerned with the role of LC/NE activity in categorization and responding to attended targets, an interrupt/reset/reorienting framework includes other situations discussed above, such as stimulus-driven shifts of attention, transitions between rest and an extended task period, and detection of event boundaries.
The disruption of a reset signal may impair shifting between objects or events in the environment and thus underlie nonlateralized attentional impairments after damage of ventral frontal and temporoparietal cortex (Husain and Rorden, 2003), such as poorer detection or identification of targets in both visual fields (Duncan et al., 1999; He et al., 2007a; Peers et al., 2005), problems with vigilance (Heilman et al., 1987b; Robertson, 2001; Wilkins et al., 1987), and an extended “attentional blink” (Husain et al., 1997; Shapiro et al., 2002). Moreveor, impaired interactions between the ventral and dorsal attention network (Corbetta et al., 2005; He et al., 2007a) produce activity imbalances in parietal spatial maps that result in a tonic attentional bias toward the ipsilesional field. Transient increases in vigilance improve spatial attention and perception (Robertson, 2001; Robertson et al., 1998), presumably through an augmentation of LC-NE output that leads to a more normal interaction between the two networks.
Future Directions
This review of the function of the ventral attention network suggests several novel avenues for future investigation. It is important to know the timing of the activation of ventral and dorsal networks on timescales that are closer to the underlying neural signals and whether temporal codes such as synchronization and coherence link widely separate neuronal populations during selection and behavioral reorienting. The recent combination of fMRI and EEG/MEG methods, as well as the integration of TMS/fMRI and EEG, should provide important information on timing and causal interactions between areas. Also, the evolutionary precursors of the ventral attention network and its right hemisphere lateralization could be uncovered by neuroimaging and single-unit studies of primates. An ongoing and critical issue is the relationship between different attentional functions and neuromodulatory systems, especially noradrenaline, acetylcholine, and dopamine, for which there is already strong evidence of a role in attention and learning. Finally, further exploration into human pathologies, both focal (e.g., stroke) and nonfocal (e.g., traumatic brain injuries, attention-deficit disorders), using cognitive neuroscience models of attention, may lead to a better theory of these debilitating conditions.
Acknowledgments
This work was supported by the J.S. McDonnell Foundation, the National Institute of Neurological Disorders and Stroke (R01 NS48013), the National Institute of Mental Health (R01 MH71920-06), and a Marie Curie Chair European Union (MEXC-CT-2004-006783). We thank Jan De Fockert, Emiliano Macaluso, John Serences, René Marois, Vinod Menon, Devarajan Sridharan, Nikos Dosenbach, Steve Petersen, Jean Decety, Jason Mitchell, Kevin A. Pelphrey, and Gary Aston-Jones for generously providing illustration of their results. We would like also to thank James Bisley, Christos Constantidinis, Ron Mangun, and Joe Hopfinger for helpful discussions.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Arzy S, Thut G, Mohr C, Michel CM, Blanke O. Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci. 2006;26:8074–8081. doi: 10.1523/JNEUROSCI.0745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporoparietal junction are independent of response selection. Eur J Neurosci. 2006;23:591–596. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bell AH, Fecteau JH, Munoz DP. Using auditory and visual stimuli to investigate the behavioral and neuronal consequences of reflexive covert orienting. J Neurophysiol. 2004;91:2172–2184. doi: 10.1152/jn.01080.2003. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporoparietal junction. Neuroscientist. 2005;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DE. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Decision and Stress. London: Academic Press; 1971. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bundesen C. A theory of visual attention. Psychol Rev. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nat Neurosci. 2004a;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Stokes MG, Mattingley JB. Modality-specific control of strategic spatial attention in parietal cortex. Neuron. 2004b;44:925–930. doi: 10.1016/j.neuron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. Neuroreport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple stimulus displays: II responses are suppressed at the cued location. Cereb Cortex. 2001;11:592–597. doi: 10.1093/cercor/11.7.592. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Posterior parietal cortex automatically encodes the location of salient stimuli. J Neurosci. 2005;25:233–238. doi: 10.1523/JNEUROSCI.3379-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Craighero L, Bello A, Fadiga L, Rizzolatti G. Hand action preparation influences the responses to hand pictures. Neuropsychologia. 2002;40:492–502. doi: 10.1016/s0028-3932(01)00134-8. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LF, Weitzman AM, Faust R, Rentz DM, Budson AE, Holcomb PJ. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J Cogn Neurosci. 2003;15:294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Dayan P, Yu AJ. Phasic norepinephrine: a neural interrupt signal for unexpected events. Network. 2006;17:335–350. doi: 10.1080/09548980601004024. [DOI] [PubMed] [Google Scholar]
- de Fockert J, Rees G, Frith CD, Lavie N. Neural correlates of attentional capture in visual search. J Cogn Neurosci. 2004;16:751–759. doi: 10.1162/089892904970762. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychol Sci. 2000;11:467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Downing PE, Wiggett AJ, Peelen MV. Functional magnetic resonance imaging investigation of overlapping lateral occipitotemporal activations using multi-voxel pattern analysis. J Neurosci. 2007;27:226–233. doi: 10.1523/JNEUROSCI.3619-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Spence C. Cross-modal links in spatial attention. Philos Trans R Soc Lond B Biol Sci. 1998;353:1319–1331. doi: 10.1098/rstb.1998.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. J Exp Psychol Gen. 1999;128:450–478. doi: 10.1037//0096-3445.128.4.450. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127:2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Hoffman JE. Selective attention: noise suppression or signal enhancement? Bull Psychon Soc. 1974;4:587–589. [Google Scholar]
- Evdokimidis I, Smyrnis N, Constantinidis TS, Gourtzelidis P, Papageorgiou C. Frontal-parietal activation differences observed before the execution of remembered saccades: an event-related potentials study. Brain Res Cogn Brain Res. 2001;12:89–99. doi: 10.1016/s0926-6410(01)00037-4. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92:1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–96. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005a;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005b;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006a;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006b;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Walsh V, Kiss M, Eimer M. Cortico-cortical interactions in spatial attention: A combined ERP/TMS study. J Neurophysiol. 2006;95:3277–3280. doi: 10.1152/jn.01273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gibson BS, Kelsey EM. Stimulus-driven attentional capture is contingent on attentional set for displaywide visual features. J Exp Psychol Hum Percept Perform. 1998;24:699–706. doi: 10.1037//0096-1523.24.3.699. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Weissman DH, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Res. 2006;1080:63–72. doi: 10.1016/j.brainres.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rosler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a nework analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J Neurosci. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007a;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol. 2007b;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Goldberg ME. Attention: behaviour and neural mechanisms. In: Plum F, Mountcastle VB, Geiger ST, editors. The Handbook of Physiology Section 1: The Nervous System Volume V Higher Functions of the Brain Part 2. Bethesda, MD: American Physiological Society; 1987b. pp. 461–481. [Google Scholar]
- Hommel B. The prepared reflex: Automaticity and control in stimulus-response translation. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance 18. Cambridge, MA: MIT Press; 2000. pp. 247–273. [Google Scholar]
- Hopfinger JB, Ries AJ. Automatic versus contingent mechanisms of sensory-driven neural biasing and reflexive attention. J Cogn Neurosci. 2005;17:1341–1352. doi: 10.1162/0898929055002445. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. J Neurol. 1996;243:652–657. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Indovina I, Macaluso E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb Cortex. 2007;17:1701–1711. doi: 10.1093/cercor/bhl081. [DOI] [PubMed] [Google Scholar]
- Jack AI, Patel GH, Astafiev SV, Snyder AZ, Akbudak E, Shulman GL, Corbetta M. Changing human visual field organization from early visual to extra-occipital cortex. PLoS ONE. 2007;2:e452. doi: 10.1371/journal.pone.0000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J. Voluntary vs. automatic control over the mind’s eye’s movement. In: Posner MI, Marin O, editors. Attention and Performance. XI. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1981. pp. 187–205. [Google Scholar]
- Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Perception and Psychophysics. 1988;43:346–354. doi: 10.3758/bf03208805. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D’Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Res. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroan-atomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. J Cogn Neurosci. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Patria F. Spatial re-orienting of visual attention along the horizontal or the vertical axis. Exp Brain Res. 2007;180:23–34. doi: 10.1007/s00221-006-0841-8. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Supramodal effects of covert spatial orienting triggered by visual or tactile events. J Cogn Neurosci. 2002;14:389–401. doi: 10.1162/089892902317361912. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Harrington D, Adair JC, Lee R. The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage. 2006;30:938–949. doi: 10.1016/j.neuroimage.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- Meister IG, Wienemann M, Buelte D, Grunewald C, Sparing R, Dambeck N, Boroojerdi B. Hemiextinction induced by transcranial magnetic stimulation over the right temporoparietal junction. Neuroscience. 2006;142:119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport. 1997;8:3029–3037. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M. Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J Neurosci. 1994;14:630–644. doi: 10.1523/JNEUROSCI.14-02-00630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporoparietal junction is not selective for theory-of-mind. Cereb Cortex. 2007;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moores E, Laiti L, Chelazzi L. Associative knowledge controls deployment of visual selective attention. Nat Neurosci. 2003;6:182–189. doi: 10.1038/nn996. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic and tectal visual structures in old and new world monkeys. J Comp Neurol. 1986;243:117–128. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiack RSJ, Frith CD. Functional localization of the system for visuo-spatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16:1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Pashler H, Harris CR. Spontaneous allocation of visual attention: Dominant role of uniqueness. Psychon Bull Rev. 2001;8:747–752. doi: 10.3758/bf03196213. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, Driver J, Antoun N, Duncan J. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;5:1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J Neurosci. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Padmala S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proc Natl Acad Sci USA. 2005;102:5612–5617. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bowhuis D, editors. Attention and Performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Rafal RD, Posner MI, Friedman JH, Inhoff AW, Bernstein E. Orienting of visual attention in progressive supranuclear palsy. Brain. 1988;111:267–280. doi: 10.1093/brain/111.2.267. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. Inaugural Article: A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull. 1994;35:607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Ro T, Cohen A, Ivry RB, Rafal RD. Response channel activation and the temporoparietal junction. Brain Cogn. 1998;37:461–476. doi: 10.1006/brcg.1998.1008. [DOI] [PubMed] [Google Scholar]
- Robertson IH. Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. Neuroimage. 2001;14:S85–S90. doi: 10.1006/nimg.2001.0838. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–172. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Bowman EM, Kertzman C. Covert orienting of attention in Macaques. II contributions of parietal cortex. J Neurophysiol. 1995;74:698–721. doi: 10.1152/jn.1995.74.2.698. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human Motor Control. San Diego: Academic Press; 1991. [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS fMRI. Cereb Cortex. 2007;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J Neurosci. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci. 1999;2:1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Sapir A, d’Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol. 2004;92:3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Pizzella V, Cianflone F, Romani GL, Corbetta M. Sequential activation of human oculomotor centers during planning of visually guided eye movements: a combined fMRI-MEG study. Frontiers in Human Neuroscience. 2008;1 doi: 10.3389/neuro.09/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Hillstrom AP, Husain M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Curr Biol. 2002;12:1320–1325. doi: 10.1016/s0960-9822(02)01040-0. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Tansy AP, Kincade M, Petersen SE, McAvoy MP, Corbetta M. Reactivation of networks involved in preparatory states. Cereb Cortex. 2002;12:590–600. doi: 10.1093/cercor/12.6.590. [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, d’Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. J Neurophysiol. 2007;97:229–237. doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmers J, Meyrand P, Moulins M. Modulation and dynamic specification of motor rythm-generating circuits in crustacea. J Physiol (Paris) 1995;89:195–208. doi: 10.1016/0928-4257(96)83636-9. [DOI] [PubMed] [Google Scholar]
- Snow JC, Mattingley JB. Goal-driven selective attention in patients with right hemisphere lesions: how intact is the ipsilesional field? Brain. 2006;129:168–181. doi: 10.1093/brain/awh690. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the Conditioned Reflex. New York: McMillan; 1963. [Google Scholar]
- Sridharan D, Levitin DJ, Chafe CH, Berger J, Menon V. Neural dynamics of event segmentation in music: converging evidence for dissociable ventral and dorsal networks. Neuron. 2007;55:521–532. doi: 10.1016/j.neuron.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Calhoun VD, Kiehl KA. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. Neuroimage. 2005;26:782–792. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of oddball responses in humans. Behav Brain Funct. 2007;3:29. doi: 10.1186/1744-9081-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintum MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography dtudy of voluntary saccadic eye movement and spatial working memory. J Neurophysiol. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M. Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. J Neurosci. 2007;27:14424–14433. doi: 10.1523/JNEUROSCI.3759-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Burger R. Attentional control during visual search: the effect of irrelevant singletons. J Exp Psychol Hum Percept Perform. 1998;24:1342–1353. doi: 10.1037//0096-1523.24.5.1342. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Pascual-Leone A. Dorsal posterior parietal rTMS affects voluntary orienting of visuospatial attention. Cereb Cortex. 2005;15:628–638. doi: 10.1093/cercor/bhh164. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporoparietal junction activity and induces inattentional blindness. Psychol Sci. 2005;16:965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Fogelman T. Ontogeny ofgaze following in chimpazee, Pan troglodytes, and rhesus macaque, Macaca mulatta. Anim Behav. 2001;61:335–343. [Google Scholar]
- Treisman AM, Gelade G. A feature-integration of theory of attention. Cognit Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of spatial neglect in humans. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. Amsterdam: Elsevier Science Publishers; 1987. pp. 235–258. [Google Scholar]
- Verleger R, Heide W, Butt C, Kompf D. Reduction of P3b in patients with temporoparietal lesions. Brain Res Cogn Brian Res. 1994;2:103–116. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage. 2006;32:1257–1264. doi: 10.1016/j.neuroimage.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Do the contents of visual working memory automatically influence attentional selection during visual search? J Exp Psychol Hum Percept Perform. 2007;33:363–377. doi: 10.1037/0096-1523.33.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci. 1991a;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somato-sensory stimuli. Electroencephalogr Clin Neurophysiol. 1991b;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]
- Yantis S, Egeth HE. On the distinction betweeen visual salience and stimulus-driven attentional capture. J Exp Psychol Hum Percept Perform. 1999;25:661–676. doi: 10.1037//0096-1523.25.3.661. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR. Event perception: a mind-brain perspective. Psychol Bull. 2007;133:273–293. doi: 10.1037/0033-2909.133.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


