Abstract
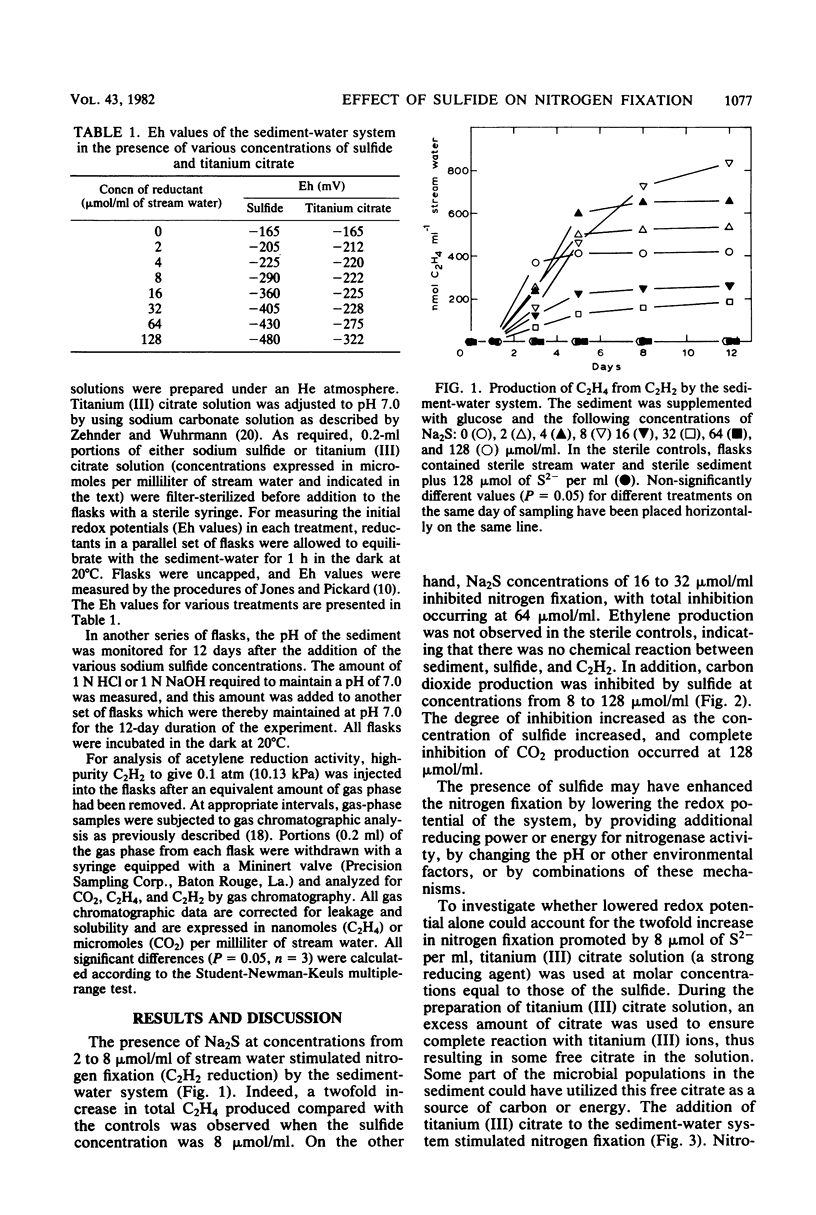
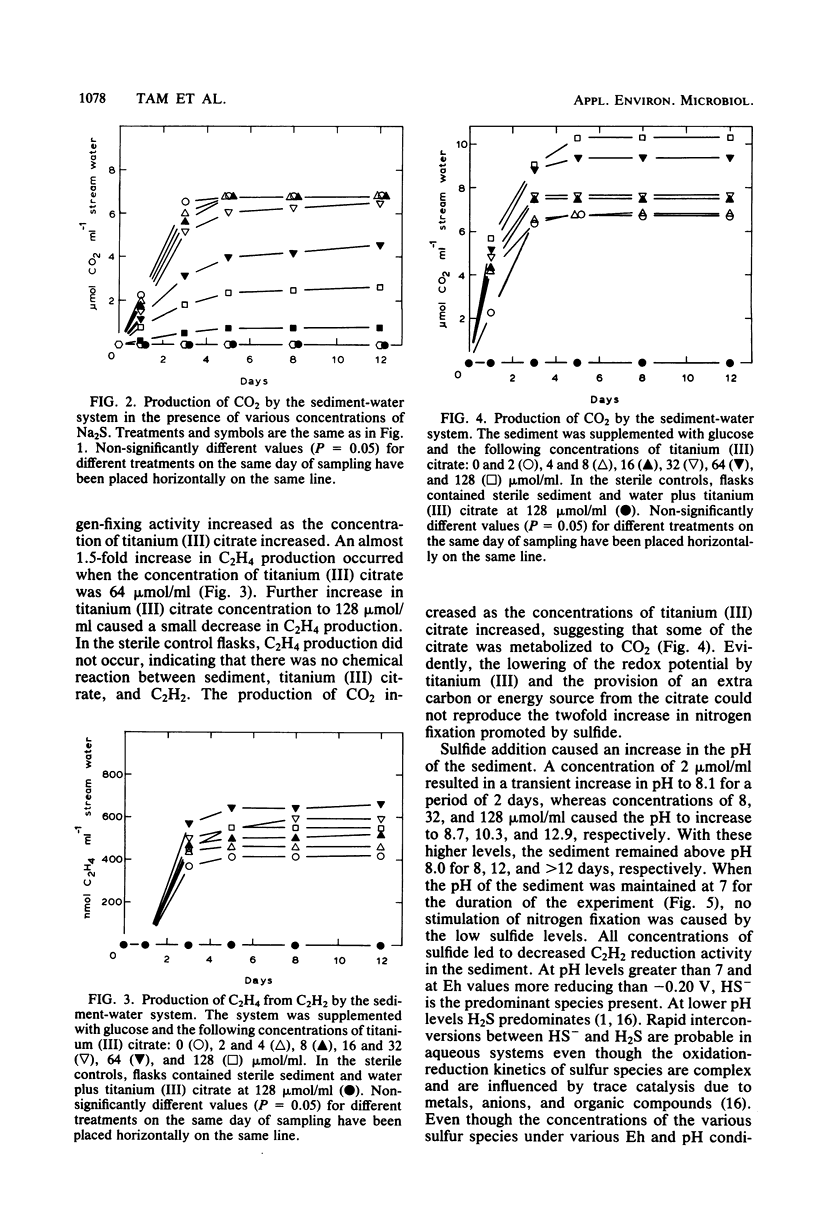
Nitrogen fixation (C2H2 reduction) in a sediment-water system was studied under anaerobic incubation conditions. Sodium sulfide at low concentrations stimulated activity, with a twofold increase in C2H4 production occurring in the presence of 8 μmol of S2− per ml of stream water. Sodium sulfide at concentrations of 16 μmol of S2− per ml or greater inhibited nitrogen fixation, with 64 μmol of S2− per ml being completely inhibitory. Sulfide at levels of 16 μmol/ml or above inhibited CO2 production, and the degree of inhibition increased with increasing concentration of sulfide. Titanium (III) citrate (used to modify Eh levels) stimulated both nitrogen fixation and CO2 production, but could not duplicate, at any concentration tested, the twofold increase in nitrogen fixation caused by 8 μmol of S2− per ml. Sulfide additions caused pH changes in the sediment, and when the sediment was adjusted and maintained at pH 7.0 all concentrations of sulfide inhibited nitrogen fixation activity. From considerations of the redox equilibria of H2, H2S, and other sulfur species at various pH values, it appeared that H2S was the toxic entity and that HS− was less toxic. The observed stimulation of activity was apparently due to a pH change coupled with the concurrent production of HS− from H2S.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connell W. E., Patrick W. H., Jr Sulfate Reduction in Soil: Effects of Redox Potential and pH. Science. 1968 Jan 5;159(3810):86–87. doi: 10.1126/science.159.3810.86. [DOI] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. A., Pickard M. D. Effect of titanium (III) citrate as reducing agent on growth of rumen bacteria. Appl Environ Microbiol. 1980 Jun;39(6):1144–1147. doi: 10.1128/aem.39.6.1144-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg W. W., Cadle R. D., Allen E. R., Lazrus A. L., Martell E. A. The sulfur cycle. Science. 1972 Feb 11;175(4022):587–596. doi: 10.1126/science.175.4022.587. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam T. Y., Knowles R. Effects of sulfide and acetylene on nitrous oxide reduction by soil and by Pseudomonas aeruginosa. Can J Microbiol. 1979 Oct;25(10):1133–1138. doi: 10.1139/m79-176. [DOI] [PubMed] [Google Scholar]
- Tam T. Y., Mayfield C. I., Inniss W. E. Nitrogen fixation and methane metabolism in a stream sediment-water system amended with leaf material. Can J Microbiol. 1981 May;27(5):511–516. doi: 10.1139/m81-076. [DOI] [PubMed] [Google Scholar]
- Wolin M. J., Miller T. L. Molybdate and sulfide inhibit H2 and increase formate production from glucose by Ruminococcus albus. Arch Microbiol. 1980 Feb;124(2-3):137–142. doi: 10.1007/BF00427718. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]



