Abstract
Objective
Telomere shortening has been observed in many human diseases, including atherosclerosis, cancer, aging syndromes, Alzheimer disease and vascular dementia. The present study aimed to investigate the mean telomere lengths of patients with schizophrenia.
Methods
We analyzed the lengths of telomeric DNA, comparing 2 groups of patients with schizophrenia (34 good responders and 34 poor responders). A control group of 76 healthy volunteers was also included. Blood samples were obtained, and telomere length was measured by Southern blot analysis on the mean length of terminal restriction fragment (TRF).
Results
Compared with the control group, a significant amount of telomere shortening was found in peripheral blood leukocytes from patients with schizophrenia who experienced poor response to antipsychotics (p < 0.001).
Conclusion
Shortened telomere length in chronic schizophrenia may be a trait marker caused by oxidative stress, and the ensuing cellular dysfunction may be a factor contributing to the progressive deterioration in treatment-resistant schizophrenia.
Medical subject headings: telomere; DNA restriction enzymes; blotting, Southern; schizophrenia
Abstract
Objectif
On a observé un raccourcissement des télomères dans beaucoup de maladies humaines, y compris l'athérosclérose, le cancer, les syndromes du vieillissement, la maladie d'Alzheimer et la démence vasculaire. L'étude visait à analyser la longueur moyenne des télomères chez les patients atteints de schizophrénie.
Méthodes
Nous avons analysé la longueur de l'ADN télomérique en comparant deux groupes de patients atteints de schizophrénie (34 qui réagissaient bien et 34 qui réagissaient mal). L'étude comportait aussi un groupe témoin de 76 volontaires en bonne santé. On a prélevé des échantillons de sang et mesuré la longueur des télomères par transfert de Southern portant sur la longueur moyenne du fragment de restriction terminal (FRT).
Résultats
Comparativement au groupe témoin, on a constaté un raccourcissement important des télomères dans les leucocytes du sang périphérique des patients atteints de schizophrénie qui réagissaient mal aux antipsychotiques (p < 0,001).
Conclusion
Le raccourcissement des télomères dans les cas de schizophrénie chronique peut constituer un marqueur caractéristique causé par le stress oxydatif, et le dysfonctionnement cellulaire qui en découle peut contribuer à la détérioration progressive dans les cas de schizophrénie résistant au traitement.
Introduction
Telomeres, the special structures at the ends of human chromosomes, are composed of repetitive DNA and DNA-binding proteins. They serve as caps with several functions, including protecting the ends of chromosomes, preventing chromosome fusion, facilitating chromosome segregation, distinguishing a chromosome end from a double strand break in the genomic DNA and maintaining genome stability.1 Because of the limiting nature of linear DNA replication mechanisms, the telomeric DNA shortens in each round during cell division.2,3 The telomeres in fibroblasts of children are longer than those in older adults (~9 kilobases [kb] v. ~4–6 kb).4 However, in the human germ line, telomeres are 10–20 kb,5 and it is thought that the lengths are maintained by the balanced interaction between the enzyme telomerase and various telomere-associated proteins.6 In contrast to the germ line and early embryonic cells, most somatic cells do not express telomerase.7 It is thought that normal cells respond to critically shortened telomeres, which are presumably dysfunctional, by undergoing cellular senescence.8 Human cells senesce when the telomeres reach an average length of 4–7 kb.4 Telomere shortening has been observed in many studies including tissues with high cell turnover and in the pathogenesis of several diseases. Aging-related telomere shortening has been detected in cell and tissue types such as fibroblasts,4 leucocytes,9 vascular tissues10 and liver11 and kidney12 tissue. Shortened telomeres have been reported in studies of human diseases that include atherosclerosis,13 cancer,14 aging syndromes,15 vascular dementia16 and Alzheimer disease.17
In the present study, we investigated the relation between mean telomere length and treatment response of inpatients with chronic schizophrenia.
Methods
We recruited 68 inpatients with schizophrenia from Kai-Suan Psychiatric Hospital, Kaohsiung (a major psychiatric centre in Taiwan). The study was approved by the Institutional Review Board at the Kai-Suan Psychiatric Hospital and conducted in accordance with the Declaration of Helsinki. The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)18 was used for the diagnosis. All patients met the criteria for a diagnosis of schizophrenia at admission, determined by 2 experienced psychiatrists. Subjects were physically healthy, and all laboratory parameters were within normal limits. They had no DSM-IV criteria of alcohol or substance abuse or neurodegenerative disorder on axis I. They did not present signs of disorientation to time, place or person, nor did they show any sign of impairment in immediate, recent or long-term memory. These patients were capable of performing routine daily activities, participating in occupational and recreational therapy and independently managing their personal affairs, such as laundry or money spending. We obtained their informed consent after the procedures had been fully explained. The average age of patients was 38 (range 19–59) years. We evaluated their clinical state at baseline and recorded findings based on the Brief Psychiatric Rating Scale (BPRS)19 and Global Assessment of Functioning scores (GAF).18 The patients were classified into 2 groups by a median split of GAF scores.20 The good responders comprised 34 patients who scored higher than 40 on the GAF (mean 48.4, standard deviation [SD] 6.6), and the poor responders comprised 34 patients who scored less than or equal to 40 (mean 31.2, SD 6.2). Age-matched healthy volunteers were recruited as control subjects. Control subjects had no psychiatric history, were not taking any psychotropic medication and did not have any first-degree relative with schizophrenia.
Telomere length measurement (terminal restriction fragment [TRF] assay) was performed according to the standard protocol, as previously described.10 Leukocyte DNA was extracted from peripheral blood samples. Five micrograms of DNA were digested overnight with Hinf I restriction endonuclease (Takara Bio Inc., Otsu, Shiga, Japan) to produce a terminal restriction fragment, an approximate simulation of the telomeric DNA. Equal quantities of digested DNA were loaded on a 0.6% agarose gel at 33 V for 4–5 hours in 1 × tris-acetate-EDTA buffer (40 mM Tris-acetate, 1 mM ethylenediaminetetraacetic acid). After electrophoresis, the DNA was denatured directly on the gel by treatment with denaturing and neutralizing buffers and then transferred to nylon membranes (Hybond-N+; Amersham, Little Chalfont, UK) for Southern blotting. The membranes containing transferred DNA were hybridized with a biotin-labelled oligonucleotide probe specific for the telomeric DNA repeat sequence (TTAGGG)4. Hybridization was performed at 37°C for 16–18 hours in a hybridization buffer (DIG Easy Hyb; Roche Applied Science, Mannheim, Germany). Unbound probe was removed by 2 washes in 0.15 M sodium chloride/0.015 M sodium citrate at 37°C and 2 washes with 15 mM sodium chloride/1.5 mM sodium citrate. Telomeric smears were detected with a Biotin Luminescent Detection Kit (Roche Applied Science). The mean TRF lengths were analyzed with the Photo-Capt, Version 99.03 software (Photo-Print IP-008-SD; Vilber Lourmat, Marne-la Vallée, France) by integrating the signal intensity of the TRF smear on the film as a function of its mean molecular weight, which was determined based on the standard biotin-labelled Hind III–digested markers of known molecular weight.
The differences in mean TRF between good responders, poor responders and control subjects, with consideration of possible confounders, were assessed by analysis of covariance followed by Tukey's multiple comparison procedure. All statistical analyses were performed with SAS statistical software for Windows (SAS 9.1, SAS Institute Inc., NC).
Results
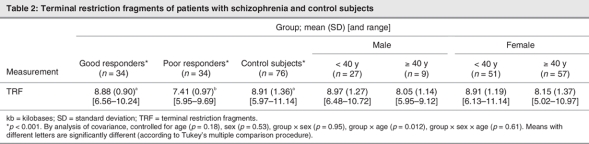
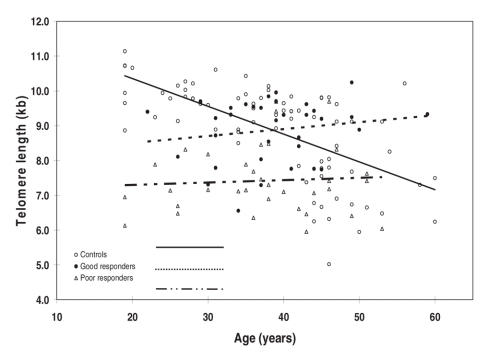
The demographic and clinical characteristics of the sample are presented in Table 1. There were no statistically significant differences in age (p = 0.86) or sex (p = 0.29) among these 3 groups, nor were there significant differences between schizophrenia subgroups in age of onset (p = 0.35), illness duration (p = 0.88) and medication dosage in chlorpromazine equivalents (p = 0.22). Figure 1 shows TRFs of a representative Southern blot for samples obtained from schizophrenia patients. Average values of mean telomere lengths for the 3 groups were significantly different after controlling for age and sex (Table 2). Individuals in the poor responders group had the shortest mean TRF length (7.41, SD 0.97, range 5.95–9.69 kb), compared with the good responders group (8.88, SD 0.90, range 6.56–10.24 kb) and the control subjects (8.91, SD 1.36, range 5.97–11.14 kb) (Table 2). The mean telomere length of the poor responders was significantly shorter than that of the control subjects (Tukey's multiple comparison procedure, p < 0.001, Table 2). In addition, comparison between good responders and poor responders also showed significant difference (Tukey's multiple comparison procedure, p < 0.001, Table 2). There was no difference between the good responders and the control subjects (Tukey's multiple comparison procedure, p > 0.05, Table 2). Interestingly, the TRF length was found to be inversely associated with age only in the control subjects; we found no association in the patient groups (Fig. 2). Mean telomere length in the control samples showed a net decrease with age of 79 base pairs (bp) yearly, assuming a constant rate of TRF loss with aging (t = 11.95–0.079 × A, R2 = 0.413, p < 0.001; t = TRF in kb, A = age in years).21 However, the same trend did not show in either good responders (p = 0.35) or poor responders (p = 0.72).
Table 1
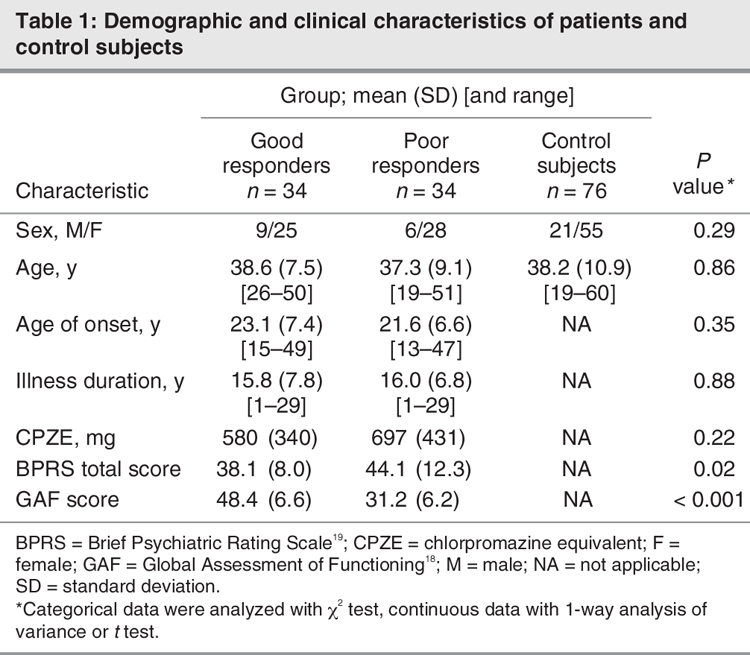
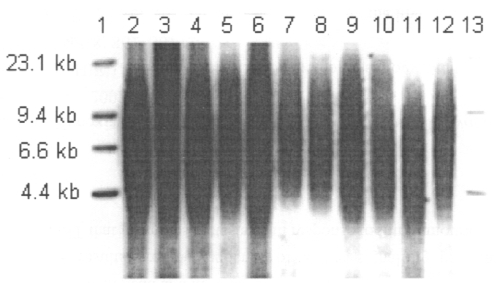
Fig. 1: An example of Southern blot for telomere lengths of leukocytes from patients with schizophrenia. Lanes 1 and 13 are λ DNA/Hind III molecular weight markers shown for comparison.
Table 2
Fig. 2: Association between mean telomere restriction fragment and age in control subjects (open circles), good responders with schizophrenia (black circles) and poor responders with schizophrenia (triangles). The linear regression lines are given for the control subjects, good responders and poor responders, respectively. The slopes of the 3 groups are different by the test of homogeneity of slopes from the analysis of covariance (p < 0.001). Good responders slope β = 0.020, F1,32 = 0.920, p = 0.35. Poor responders slope β = 0.007, F1,32 = 0.13, p = 0.72. Control subjects slope β = –0.080, F1,74 = 52.0, p < 0.001.
Discussion
Our results indicate that telomere length is shortened significantly in a subgroup of chronic schizophrenia patients who respond poorly to treatment. This is the first study, to our knowledge, to examine telomere lengths from peripheral blood leukocytes of patients with chronic schizophrenia.
We observed that the telomere lengths of the poor responders were the shortest among the 3 groups studied. On average, the mean telomere lengths of the poor responders were 1.5 kb shorter than those of age-matched control subjects. Additionally, a net reduction with age of 79 bp yearly was found in the control group in this study. The observation is in accordance with published data that documented a loss of 31–84 bp yearly.9,21,22
Several studies, including ours, suggest that there is an inverse association between telomere length and age in healthy people. However, reduction of telomere lengths in patients with schizophrenia is not inversely associated with age. As well, no inverse association between telomere length and age was found in patients with probable Alzheimer dementia or in patients with stroke and related risk factors.23
It was hypothesized that telomere erosion might serve as a biological clock that could count mitotic time and account for cell replicative senescence in culture.24 Loss of replicative capacity leads to cell growth arrest, which occurs not only after accumulated doubling populations with telomere shortening in culture but also as a consequence of subcytotoxic stress, such as mild chronic oxidative stress.25 Telomere shortening intensified by oxidative stress has been seen in induced conditions of cell cultures, such as chronic hyperoxia,26 as well as in fibroblasts from patients with Fanconi anemia27 and in peripheral blood leukocytes from patients with respiratory chain disorders,28 both of which diseases result in increased oxidative stress.
Several studies have demonstrated that reduction in antioxidant capacity and rise in oxygen free radicals might contribute to oxidative stress in schizophrenia.29–31 Recently, it has been shown that oxidative stress might cause mitochondrial dysfunction and altered brain metabolism in schizophrenia,32 which raises a possibility that increased oxidative stress, as shown by our observation of short telomeres, might exist in poor responders with chronic schizophrenia. This was supported by a report showing that oxidative DNA damage was 10 times greater in postmortem hippocampi of elderly patients with “poor-outcome” schizophrenia.33 Further studies are needed to establish the status and role of oxidative stress in poor responders with chronic schizophrenia and in the pathogenesis of schizophrenia.
In conclusion, we found that the treatment-resistant patients with chronic schizophrenia have significantly shortened telomeres, which may have been caused by oxidative stress that led to ensuing cellular dysfunction. The findings also point to shortened telomere lengths as a trait marker for chronic schizophrenia patients with poor response to treatment.
Acknowledgments
Thanks to Dr. Hay-Yan Jack Wang for critical reading and editing and to Mr. Chien-Ming Chen for computer art work for figures.
Footnotes
Contributors: Drs. Yu and Cho contributed equally to the work. Drs. Yu and Cho designed the study. Dr. Yu acquired the data, which Drs. Chang, Lin and Cho analyzed. Drs. Yu, Lin and Cho wrote the article, which Drs. Chang and Cho reviewed. All authors gave final approval for publication.
Competing interests: None declared.
Correspondence to: Dr. C.-L. Cho, Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, 804, Taiwan; clcho@mail.nsysu.edu.tw
References
- 1.Greider CW. Telomere length regulation. Annu Rev Biochem 1996;65:337-65. [DOI] [PubMed]
- 2.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol 1972;239:197-201. [DOI] [PubMed]
- 3.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymatic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 1973;41:181-90. [DOI] [PubMed]
- 4.Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblast. Nature 1990;345:458-60. [DOI] [PubMed]
- 5.Allsopp RC, Vaziri H, Patterson C, et al. Telomeric length predicts replicative capacity of human fibroblast. Proc Natl Acad Sci U S A 1992; 89:10114-8. [DOI] [PMC free article] [PubMed]
- 6.Blackburn EH. Telomere states and cell fates. Nature 2000;408:53-6. [DOI] [PubMed]
- 7.Kim NW, Platyszek M, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancers. Science 1994;266:2011-5. [DOI] [PubMed]
- 8.Campisi J, Dimri GP, Hara GP. Control of replicative senescence. In: Schneider E, Rowe J, editors. Handbook of the biology of aging. New York: Academic Press; 1996. p.121-49.
- 9.Frenck RW Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A 1998;95:5607-10. [DOI] [PMC free article] [PubMed]
- 10.Chang E, Harley CB. Telomeric length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 1995;92:11190-4. [DOI] [PMC free article] [PubMed]
- 11.Aikata H, Takaishi H, Kawakami Y, et al. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res 2000;256:578-82. [DOI] [PubMed]
- 12.Melk A, Ramassar V, Helms LM, et al. Telomere shortening in kidneys with age. J Am Soc Nephrol 2000;11:444-53. [DOI] [PubMed]
- 13.Samani NJ, Boultby R, Butler R, et al. Telomere shortening in atherosclerosis. Lancet 2001;358:472-3. [DOI] [PubMed]
- 14.Meeker AK, De Marzo AM. Recent advances in telomere biology: implications for human cancer. Curr Opin Oncol 2004;16:32-8. [DOI] [PubMed]
- 15.Klapper W, Parwaresch R, Krupp G. Telomere biology in human aging and aging syndromes. Mech Ageing Dev 2001;122:695-712. [DOI] [PubMed]
- 16.Saretzki G, von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci 2002;959:24-9. [DOI] [PubMed]
- 17.Panossian LA, Porter VR, Valenzuela HF, et al. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging 2003;24:77-84. [DOI] [PubMed]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 19.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep 1962;10:790-812.
- 20.Tibbo P, Joffe K, Chue P, et al. Global assessment of functioning following assertive community treatment in Edmonton, Alberta: a longitudinal study. Can J Psychiatry 2001;46:144-8. [DOI] [PubMed]
- 21.Iwama H, Ohyashiki K, Ohyashiki JH, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet 1998;102:397-402. [DOI] [PubMed]
- 22.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 2004; 101: 17312-5. [DOI] [PMC free article] [PubMed]
- 23.von Zglinicki T, Serra V, Loernz M, et al. Short telomeres in patients with vascular dementia: An indicator of low antioxidative capacity and possible risk factor? Lab Invest 2000;80:1739-47. [DOI] [PubMed]
- 24.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965;37:614-36. [DOI] [PubMed]
- 25.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 2000;35:927-45. [DOI] [PubMed]
- 26.von Zglinicki T, Saretzki G, Docke W, et al. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 1995;220:186-93. [DOI] [PubMed]
- 27.Adelfalk C, Lorenz M, Serra V, et al. Accelerated telomere shortening in Fanconi anemia fibroblasts — a longitudinal study. FEBS Lett 2001;506:22-6. [DOI] [PubMed]
- 28.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum Mol Genet 1997;6:905-8. [DOI] [PubMed]
- 29.Yao JK, Reddy R, McElhinny LG, et al. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res 1998;32:1-8. [DOI] [PubMed]
- 30.Khan MM, Evans DR, Gunna V, et al. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res 2002;58:1-10. [DOI] [PubMed]
- 31.Ranjekar PK, Hinge A, Hegde MV, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res 2003;121:109-22. [DOI] [PubMed]
- 32.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 2004;9:684-97. [DOI] [PubMed]
- 33.Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry 2004;12:167-75. [PubMed]