Abstract
Objective
Autism is characterized by impairment in communication and social interaction, by repetitive behaviours and by difficulty in adapting to novel experiences. The objective of the current investigation was to replicate and extend our previous findings showing variable circadian rhythm and significant elevations in cortisol following exposure to a novel stimulus (mock magnetic resonance imaging [MRI]).
Methods
Circadian rhythms of cortisol were estimated in 22 children with and 22 children without autism via analysis of salivary samples collected in the morning, afternoon and evening over 6 separate days. We assessed hypothalamic-pituitary-adrenal (HPA) responsiveness by examining changes in salivary cortisol in response to a mock MRI. One-half of the children were re-exposed to the MRI environment.
Results
Children with autism showed a decrease in cortisol in the morning over 6 days while maintaining higher evening values. Children with autism also showed more within-and between-subject variability in circadian rhythms. Although the cortisol values tended to be higher in some of the children with autism, a statistically significant elevation in cortisol in response to the initial mock MRI was not observed. Rather, both groups showed heightened cortisol at the arrival to the second visit to the imaging centre, suggesting an anticipatory response to the re-exposure to the mock MRI.
Conclusion
Children with autism showed dysregulation of the circadian rhythm evidenced by variability between groups, between children and within individual child comparisons. Both groups demonstrated increased salivary cortisol in anticipation of re-exposure to the perceived stressor.
Medical subject headings: autistic disorder, cortisol, circadian rhythm, stress
Abstract
Objectif
L'autisme est caractérisé par un déficit de la communication et de l'interaction sociale, par des comportements répétitifs et par la difficulté d'adaptation aux expériences nouvelles. L'étude en cours visait à reproduire et à étendre nos résultats antérieurs montrant un rythme circadien variable et des élévations importantes du cortisol à la suite d'une exposition à un stimulus nouveau (imagerie par résonance magnétique [IRM] fictive).
Méthodes
On a estimé les rythmes circadiens du cortisol chez 22 enfants sans autisme et 22 enfants atteints d'autisme en analysant des échantillons de salive prélevés le matin, l'après-midi et le soir pendant six jours. Nous avons évalué la sensibilité hypothalamus-hypophyse-surrénales (hypothalamic-pituitary-adrenal ou HPA) en étudiant des changements du cortisol salivaire en réponse à une IRM fictive. La moitié des enfants ont été exposés de nouveau à l'environnement de l'IRM.
Résultats
Chez les enfants atteints d'autisme, le cortisol baissait le matin pendant les six jours tandis que les valeurs se maintenaient à un niveau plus élevé le soir. Les rythmes circadiens des enfants atteints d'autisme ont aussi varié davantage chez un même sujet et d'un sujet à l'autre. Même si les valeurs du cortisol avaient tendance à être plus élevées chez certains des enfants atteints d'autisme, on n'a pas observé d'élévation statistiquement significative du cortisol en réponse à l'IRM fictive initiale. On a plutôt constaté une élévation du cortisol chez les deux groupes à leur arrivée à la deuxième visite au centre d'imagerie, ce qui indique une réaction d'anticipation à la réexposition à l'IRM fictive.
Conclusion
Les enfants atteints d'autisme présentaient une dysrégulation du rythme circadien démontrée par la variabilité entre les groupes, d'un enfant à l'autre et chez un même enfant. Le cortisol salivaire a augmenté chez les deux groupes en anticipation d'une réexposition au facteur de stress perçu.
Introduction
Autism is characterized by impairment in verbal and nonverbal communication and reciprocal social interaction and a markedly restricted repertoire of activities and interests.1 Although several neural correlates of social and cognitive behaviour have been identified, dysregulation of other biological systems, including the hypothalamic-pituitary-adrenal (HPA) axis, has been implicated in autism.2–7
Regulation of the HPA axis involves 3 interrelated processes: the maintenance of a diurnal rhythm, activation in response to stress or threat and the restoration of basal activity via negative feedback mechanisms. Cortisol is the primary glucocorticoid in humans. Cortisol exhibits diurnal variations peaking in the early morning hours (about 30 minutes after waking), declining rapidly in the morning, with a slower decrease in the afternoon, and reaching its lowest level in the evening. This pattern is already well developed in the third month of infancy.8,9
The HPA axis, like most biological systems, is highly regulated and dependent on the ability of the system to maintain, respond and reset itself (homeostasis). One form of dysregulation of the HPA axis is manifested by disruptions in circadian rhythms. Dysregulation of the circadian rhythms may be characterized by a change in the pattern that results in the absence, elevation or suppression of the slope. An example of this would be the flattening of the slope that has been reported in at-risk populations of children.10
Although the findings are not entirely consistent, some of the early work in children with autism shows alterations in the normal circadian patterns of cortisol.3,7,11–13 In our previous research,2 we observed no differences in the slope of the circadian decline in cortisol for children with and without autism. There were marked individual differences within the autism group. We cannot ascertain from previous research whether circadian rhythms for children with autism are inherently less predictable or whether the heterogeneity of our previous study population led to more pronounced individual differences. If circadian rhythms for autistic children are less predictable, this may represent another, less explored form of dysregulation that has been described as chaotic circadian rhythms.14 Such variance would not be detected in the slope; instead, it would need to be evaluated through repetitive sampling over several days and comparable times.
Perhaps the most studied aspect of the HPA axis has been the response to stress. Herman and Cullinan15 classified stimuli capable of activating the HPA system as either systemic or processive. Systemic stressors are physical, can occur independent of context and conscious awareness and usually involve a life-threatening event. Systemic stimuli that activate the HPA system are relayed to the periventricular nuclei of the hypothalamus via the brain stem. In contrast to systemic stressors, processive stimuli require the comparison of current information with past experience, are context-dependent and are assigned emotional meaning. Processive stimuli are mediated by the frontal lobes and the limbic system structures. Among the processive events that can activate the HPA axis is exposure to novelty or unpredictability. Repeated exposure to stressful stimuli or conditions leads to greater situational predictability and reduced novelty, and therefore, experience with a stressor very often leads to habituation. For some situations, experience leads to an increase in the response with subsequent exposure (sensitization). There is, however, little information on habituation and sensitization in children. Insofar as habituation appears to be yet another example of HPA regulation, we exposed a subsample of our population to a second homotypical stress experience to determine whether children with autism would demonstrate habituation or sensitization to a relatively noninvasive stressor.
Autism has often been characterized as a disorder accompanied by increased arousal, stress and sensory sensitivity. Inasmuch as the HPA axis has been shown to reflect increased levels of arousal and stress, it is not surprising that studies have been conducted on the HPA axis and autism.3–7,12,13
Previously, we investigated circadian regulation and the responsivity of this neurodendocrine system in a sample of children aged 6 to 12 years and suffering from autism, compared with a sample of children with typical development. The children with autism showed a more variable circadian rhythm as well as statistically significant elevations in cortisol after exposure to a novel stimulus, a mock magnetic resonance imaging (MRI) scanner.2 The current study was undertaken to further evaluate and expand our previous findings in a larger group of well-characterized, high-functioning children with autism. Specifically, the investigation aimed to examine the following: 1) the circadian pattern of cortisol secretion in the 2 populations, with a more extensive sampling procedure than in our previous study; 2) the between-subject variability in circadian rhythms; 3) day-to-day variability in the individual child; 4) response to stress, in an attempt to replicate our previous finding2 in children with autism of an enhanced cortisol response to first exposure to the mock MRI; 5) response to a repeat exposure to the mock MRI (habituation or sensitization); and 6) response to the real MRI environment that involved the performance of functional MRI (fMRI) tasks.
Methods
Subjects
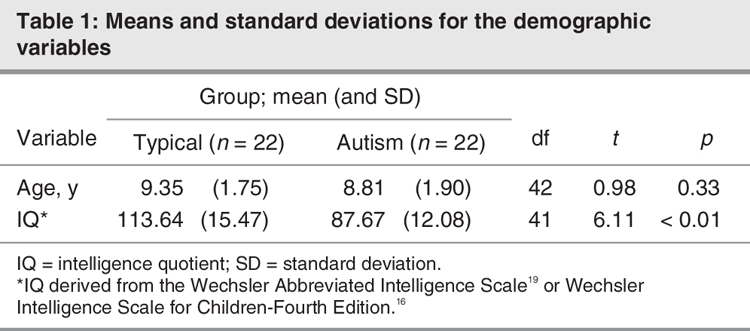
The 44 subjects were predominantly male children between 6.5 years and 12 years of age (mean age 9.08 y), 22 of whom were diagnosed with autism (1 female child) according to a strict diagnosis and 22 of whom were neurotypical children (3 female children). Table 1 provides demographic information.
Table 1
Six children with autism were recruited from the University of California Davis Medical Investigation of Neurodevelopmental Disorders (M.I.N.D.) Institute Subject Tracking System database. These children had a confirmed diagnosis based on the Autism Diagnostic Observation Schedule (ADOS)17 and the Autism Diagnostic Interview.18 The remaining 16 children were recruited from area schools or autism outreach groups. Their diagnosis of autism was based on criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders1 and established by a previous diagnosis of autism by a psychologist, psychiatrist or behavioural pediatrician; by the first author's (B.A.C.) clinical judgment; and by confirmation in the form of a total score at or above the autism threshold on the ADOS Social Communication Questionnaire17 (see below). We excluded children with autism spectrum disorders, namely, pervasive developmental disorder not otherwise specified and Asperger syndrome.
The typically developing children were recruited through the Subject Tracking System, area schools, fliers and recreational centres. After initial contact, potential subjects were screened via parent interview for the absence of neurodevelopmental disorders. Subjects with a history of serious physical illness (e.g., endocrine, cardiovascular or neurologic disorders) were excluded from enrolment in the study. The groups were balanced on age but not on intellectual functioning (see Table 1). Informed written consent was obtained from parents and verbal assent was obtained from all research subjects before they were included in the study, on the diagnostic visit. The Institutional Review Board of the University of California, Davis, approved the study.
Diagnostic and inclusion measures
Testing was completed after informed consent procedures were completed on the first visit.
The Wechsler Abbreviated Intelligence Scale (WASI)19 is a measure of general intelligence used to obtain an estimated IQ. It was administered to most of the subjects (n = 39) unless a full-scale IQ score from a comprehensive measure was available (n = 5).16,20 Participants needed to have an IQ of 80 or higher to be included in the study.
The ADOS17 comprises semistructured, interactive activities conducted with a child and designed to assess specific current behaviours indicative of autism. The ADOS provides an algorithm with cut-offs for autism and autism spectrum disorders.17
The Social Communication Questionnaire21 was used as a screening tool to ensure the absence of autism symptoms in the typically developing control children.
Study design
We used saliva sampling, a noninvasive method, to avoid stressors associated with drawing blood or other means of collecting biological specimens.22 For the autism group, it was deemed particularly important to minimize novelty (both environmental and social) in the collection of at-home samples. Further, with cortisol used as a measure of stress responsiveness, a saliva sample was collected on the afternoon of each at-home sample day for comparison with estimates of HPA responsiveness in the laboratory. We examined HPA responsiveness to potentially stressful procedures by exposing the children for 20 minutes to a mock MRI scanner, referred to as Mock 1. This manipulation included exposure to a novel stimulus, mild restraint and noise that could result in the activation of the HPA axis. To evaluate negative feedback, we obtained cortisol measures for an extended period of time after the termination of the stressor. Next, about one-half of the participants (14 with autism, 14 neurotypical) were brought back to the imaging centre within roughly 2 months for a repeat exposure to both the mock MRI (Mock 2) and a real MRI (fMRI) scan to evaluate habituation.
Procedures
Cortisol sampling methods
A total of 22 salivary samples were collected from each research subject; these included 18 samples collected at home to obtain the cortisol diurnal rhythm and 4 samples collected in the laboratory to evaluate the subject's response to stress. In addition, about one-half of the participants took part in the Mock 2 and fMRI portion of the study, from whom we obtained an additional 5 samples.
Measurement of cortisol in saliva has been determined to be a valid, reliable and noninvasive technique for estimating HPA activity.22 A detailed presentation of methods used to collect saliva samples was previously reported.2 In short, research assistants instructed parents on the saliva sampling procedure for in-home collection, including the accurate collection and labelling of samples and completion of diary forms. We asked parents to report any incidents of illness; none were recorded.
Tubes were stored in a container fitted with a Trackcap (Aprex, Union City, Calif.). The participant was given Trident Original Sugarless chewing gum, which served as a saliva stimulant, then asked to deposit saliva into a prelabelled tube by spitting. The home samples were temporarily stored in the home refrigerator until the end of the second week, at which time they were taken to the laboratory.
TrackCap
A potential complication was the danger of parental noncompliance with collection of multiple salivary samples. As described previously,2 we complemented the standardized parental instruction, tube labelling and sample diary by using objective recording of sampling dates and times. The empty collection tubes were contained within a bottle fitted with a TrackCap. A TrackCap contains microelectronics that record the date and time at which the bottle is opened. To evaluate the accuracy of recording and adherence to the sampling protocol, we computed Pearson correlations between the parent report sampling times and the Trackcap report times. For any disagreements of more than 30 minutes, we scrutinized the log and the cortisol values for accuracy. A discrepancy was observed in less than 1% of the samples. Preliminary analysis showed that the exclusion of these data points did not alter the results; thus, the samples remained in the analysis.
Circadian rhythmicity
Basal levels of salivary cortisol were collected for 6 diurnal cycles. Within 48 hours of the final saliva sample, the test tube kits were collected by the research assistant, placed in a cooler and brought to the endocrine laboratory.
Home-based samples were collected by parents 3 times daily for 3 consecutive days over 2 consecutive weeks, resulting in a total of 18 samples (week 1 Mon, Tues, Wed; week 2 Mon, Tues, Wed). The samples were collected at about the same time of day for each participant. The morning sample was collected within a half-hour of waking and before the participant ate, drank or brushed teeth. The afternoon sample was collected between 1300 and 1600 hours, with the participant avoiding eating for a minimum of 1 hour before sampling. The evening sample was collected within a half-hour of bedtime, with the participant avoiding eating or brushing teeth for an hour before.
Stress
As in our previous investigation,2 this paradigm was conducted at the UC Davis Imaging Research Center (IRC), which houses an MRI simulator (mock scanner). The mock MRI was used as a moderate stressor that involves mild restraint, novelty and exposure to the computer-simulated unpleasant noises generated by the MRI scanner. The children were requested to lie still in the scanner for about 20 minutes while hearing a series of simulated sounds. All sessions occurred between 1300 and 1600 hours (for procedural details, see Corbett and colleagues2). At the time of the Mock 1 visit, 4 saliva samples were collected as follows: on arrival at the IRC, 20 minutes postexposure to the stressor, 40 minutes postexposure and 2 hours postexposure (sampled at home).
Mock scanner visit 2 and fMRI
As noted, just over one-half of the participants (n = 28) in the study returned to the IRC for a second visit (Mock 2) and a real MRI scan. For various reasons (e.g., time constraints, not wanting their child exposed to a real MRI), some families chose not to complete this portion of the study. The previous mock procedures were employed. In addition, samples were collected at the beginning of a real MRI scanning session, (about 60 min after arrival) and at the end of the scan (about 120 min after arrival). The children performed 2 fMRI tasks of emotion perception while in the scanner (data not presented in this manuscript). Cortisol was assayed to determine whether there was a different response to the mock MRI when compared with the real MRI.
Cortisol storage and assays
Once collected, salivary samples were stored at –20°C. Prior to assay, the saliva samples were thawed and centrifuged at 6000 rpm for 10 minutes to separate aqueous components from mucins and other suspended particles. Cortisol assays were performed with coated-tube radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles, Calif.). Assay procedures were modified as follows to accommodate overall lower levels of cortisol in human saliva relative to plasma: First, standards were diluted to concentrations ranging from 2.76 to 345 nmol/L. Second, sample volume was increased to 200 μl. Third, incubation times were extended to 3 hours. Serial dilution of samples indicated that the modified assay displayed a linearity of 0.98 and a least detectable dose of 0.548 nmol/L. Cross-reactivity with other naturally occurring steroids was minimal (e.g., corticosterone = 0.94%; cortisone = 0.98%). Intra-and interassay coefficients of variation were 4.36% and 6.66%, respectively.
Statistical analysis
We used a linear mixed model to assess the entire time course of the samples, including the pattern of daily cortisol variation, changes over time in this pattern, the response to stress and differences in these patterns between the autism and typical groups. Because the cortisol measurements were positive and skewed toward large values, the natural logarithm of cortisol was used in all analyses.
Fixed effects were estimated within each diagnostic group as follows: circadian morning, afternoon and evening levels on the first day; a separate linear trend for each time of day across the subsequent 5 days of home samples; and, for every sample, time during the mock and real MRIs. A likelihood ratio test was conducted, comparing this model with a larger model in which each time point had its own fixed effect, to determine whether the linear trend was adequate to capture the average circadian pattern in each group. Random effects were included for each child's morning, afternoon and evening level. Separate variances for the morning, afternoon and evening random effects (assessing the between-child variance structure) and for the residual error (assessing the within-child variance) were estimated in each group.
We used the Wald test23 to determine the statistical significance of fixed effects. Comparisons were made between groups at each of the 27 time points. Additionally, within each group, the following were estimated and tested for statistical significance: the morning, afternoon and evening time trend across the first 6 days; the difference between the afternoon level on the sixth day and the level at arrival immediately preceding each mock MRI; the difference between the level immediately preceding each mock MRI and the levels at 20 and 40 minutes after exposure to the mock MRI; and the difference between the level immediately preceding the first mock MRI and the level immediately preceding the second mock MRI.
To test whether the between-and within-child variances differed by diagnosis, we computed alternative models that differed from the primary model (described above) only in the variance structure. In the first such alternative, both diagnostic groups were constrained to have the same between-child variance structure, but the within-child (residual) variance was allowed to differ between groups. In the second alternative, the groups shared the same within-child variance but were allowed to differ in their between-child variance structure. These constrained models were compared with the primary model by the likelihood ratio test.
Finally, to ensure that there were no statistically significant differences between the participants who participated in the whole study and those who did not return for a second mock visit, we conducted general linear model multivariate analysis.
Results
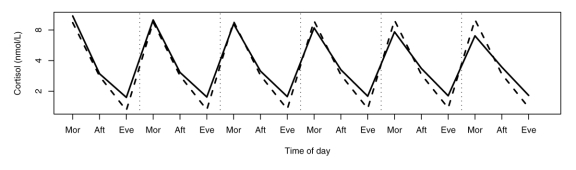
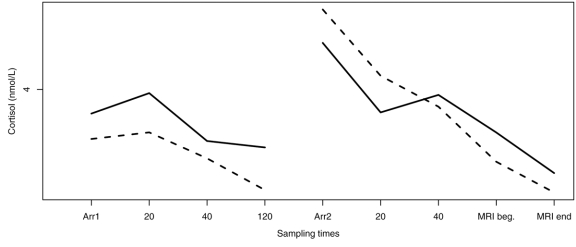
Cortisol measurements are displayed by diagnostic group and time point in Figure 1 (home samples) and Figure 2 (imaging centre samples) and Table 2. No evidence was found that a more complex model was needed to capture the group patterns (χ224 = 30, p = 0.18).
Fig. 1: Group patterns of the home cortisol measurements. Mor = Morning; Aft = Afternoon; Eve = Evening.
Fig. 2: Group patterns of the imaging centre measurements. Cortisol sampling times: Arr1 = first arrival; 20, 40 and 120 = minutes poststressor; Arr2 = second arrival; 20, 40 = minutes poststressor; MRI beg. = beginning of real MRI; MRI end = end of MRI scan.
Table 2
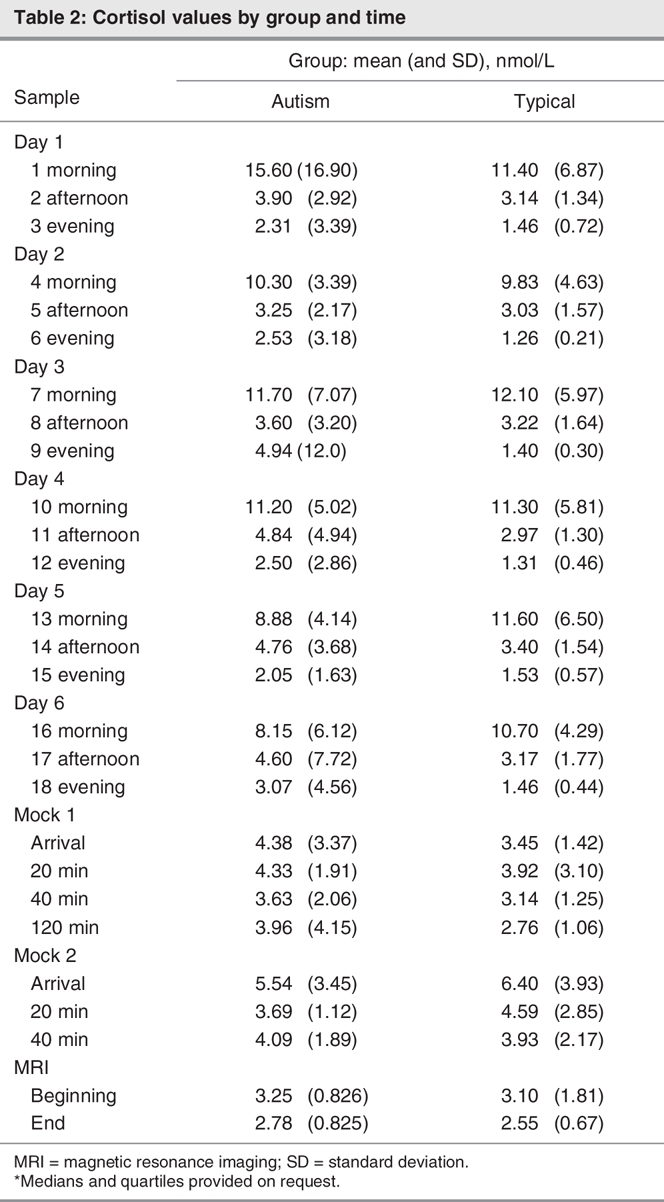
The contrasts between the group mean effects are summarized in Table 3. The evening cortisol values, averaged over 6 days, differed between the groups (p = 0.021). The children with autism showed consistently higher cortisol levels during the second through fifth evenings when compared with the typically developing children (all p < = 0.04). According to the multiple regression analysis, neither sampling times (p > 0.05) nor amount of sleep (p > 0.05) predicted cortisol values across the groups. Subsequent analysis revealed no main effect or association between age and cortisol values (p > 0.05).
Table 3
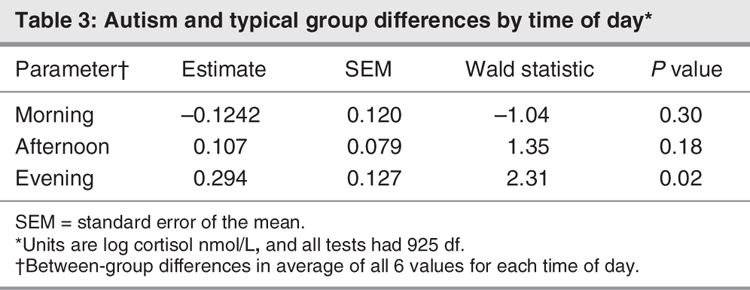
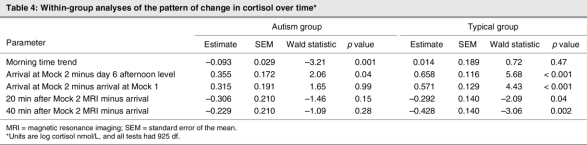
The within-group mean patterns of change over time are summarized in Table 4. In the autism group, a morning time trend was observed (p = 0.001), characterized by a gradual decrease in the morning values of salivary cortisol over the 6 days of sampling. Although some of the children showed the same trend as in the first study, the children with autism, as a group, did not demonstrate statistically significant elevation in cortisol following exposure to the first mock MRI (p > 0.05). This group showed higher cortisol at arrival immediately preceding the second mock MRI than on the afternoon of day 6 (p < 0.04), but there were no significant differences between levels in the first and second mock exposures (on arrival, at 20 min, or at 40 min; all p > 0.1).
Table 4
For the typically developing children, a difference was also observed between the arrival immediately preceding the second mock MRI and the afternoon value on the sixth day (p < 0.001). The typical group also showed a significant difference between arrival levels for the first and second mock scan (p < 0.001). Further, at 20 minutes poststressor (p < 0.05) and at 40 minutes poststressor (p < 0.01), cortisol was lower than on arrival. No significant differences were observed for the fMRI cortisol values. No significant differences were observed when we compared the subjects who participated in the whole study with those who participated in the Mock 1 visit alone (F4,35 = 0.483, p > 0.05).
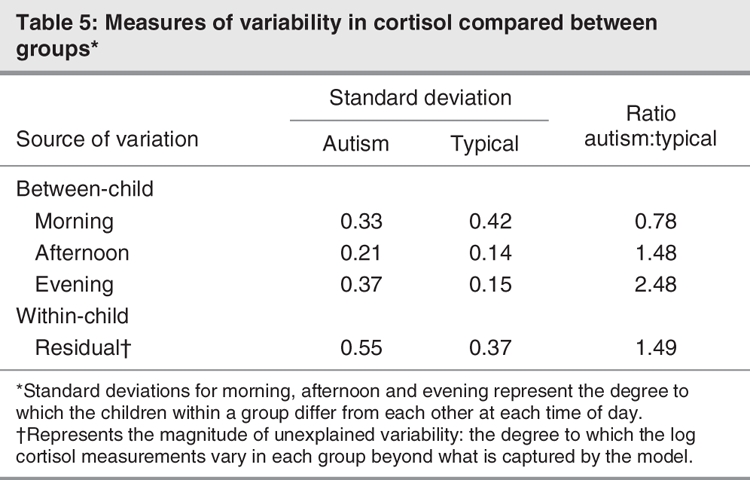
Both the between-child (χ26 = 22, p = 0.001) and within-child (χ21 = 72, p < 0.001) variance components differed between the groups. The standard deviations of the between-and within-child random effects are displayed in Table 5.
Table 5
Discussion
In the current experiment, our purpose was to investigate the neuroendocrine activity of children with high-functioning autism in comparison with typically developing children. The primary aims were to examine the following: 1) the circadian pattern of cortisol secretion, with a more extensive sampling procedure; 2) between-subject variability in circadian rhythms; 3) day-to-day variability of the individual child; 4) response to stress to attempt to replicate our previous finding2 in children with autism of an enhanced cortisol response to first exposure to the mock MRI; 5) response to a repeat exposure to the mock MRI; and 6) response to the real MRI environment.
As in our previous report, both the neurotypical children and the children with autism showed expected normal peak-to-trough rhythms. However, there were significant overall differences in the diurnal variation between the groups. The circadian pattern shown by the neurotypical children revealed no significant change over the 6 days of sampling. By contrast, over the course of the sampling, children with autism showed a gradual decrease in the morning values. Although we do not have a ready explanation for this finding, several factors have been shown to affect morning cortisol levels, including day of the week,24 wake-up time and sleep difficulties.25 None of these factors provide an adequate explanation of the findings. All the children provided samples on the same days, and the change occurred on successive days regardless of day of the week. Sample collection times in the morning and evening did not show the same gradual change across days and were determined by each child's own sleep pattern. Finally, there were no discernable between-group differences in sleep patterns or sleep duration. It thus appears that the standard explanations do not account for the trend for morning values to be decreased. It is possible that our methods, which included a time-bound sampling regimen, provided an additional zeitgeiber and, over days, altered the circadian rhythm for children in the autism group. In other words, children with autism may be more susceptible to the influence of zeitgeibers, an interpretation that supports the notion of greater circadian variability and less regulated responses in children with autism.
The more unexpected finding was that the evening values for the children with autism tended to be consistently elevated in comparison with the neurotypical group. The elevated evening values could reflect a greater responsivity to the events of the day. The decrease over time in the peak morning values in combination with the elevation in the evening results in a diminished peak-to-trough difference. It is important to point out that, in older children and adolescents with depression, reported alterations in hormones have included hypersecretion of evening cortisol.26–28
In regards to the third aim, we replicated our previous finding2 of a significant between-group difference in variation in cortisol. These findings imply a greater degree of individual differences among children with autism, which may reflect the significant variability observed in many areas of metabolic, neurologic and behavioural functioning in these children. Most studies assessing cortisol do not consider the possibility that variation within the individual child may be important in evaluating diurnal cortisol.29 Therefore, the data were simultaneously modelled and analyzed for both between-and within-child variation in diurnal cortisol. The results of both analyses were significant. Thus, in addition to the systematic differences between children with autism and control children noted above, diurnal variations in cortisol are more inconsistent in autistic than in control children. Yehuda14 described depression patients as showing a less rhythmic and more “chaotic” circadian pattern. We propose that consistency in circadian rhythms is another means of assessing HPA regulation and one that appears to characterize differences in HPA activity in children with autism.
Regarding the response to the mock MRI, we did not fully replicate our original findings of an increase in salivary cortisol in the children with autism. Although the directionality was similar and the values were generally higher in children with autism than in the typically developing children, they were not statistically different in the current study. It may be that a lack of consistency in the response to this stressor is another manifestation of the increased variability in HPA activity exhibited by children with autism. It is also possible that the different results across the 2 studies reflect slight differences in the subject population. Our previous study contained a smaller, less homogeneous sample of somewhat lower-functioning children with autism and a mean IQ of 77, whereas the current group had a mean IQ of 88. It should be noted, however, that a statistical relation between IQ and cortisol values was not found in either study.
Even though the children with autism did not consistently demonstrate an initial cortisol increase following exposure to the initial stressor, they showed an elevated cortisol response at the arrival for the second visit to the mock MRI when compared with their afternoon value on the sixth day. Further, the typically developing children also showed robust elevation in cortisol on their second arrival at the imaging centre, despite a lack of initial cortisol increase. As can be seen in Figure 2, the enhanced cortisol response on arrival at the laboratory in both groups strongly suggests an anticipatory response that fits a profile of expectancy relating to the re-exposure to the mock MRI. Paradoxically, exposure to the actual stressor did not augment the HPA response evident on arrival. In fact, cortisol values declined significantly by 20 minutes postarrival even though the time between arrival and collection of the 20-minute sample was spent in the mock scanner.
The increased stress response to the second arrival was not predicted by behavioural observations of the children or their cortisol values at the first visit in the current study. Previous investigations that have examined response to arrival in the laboratory10,30 usually do not re-expose the children to a potential stressor, and thus the anticipatory stress response has not been evaluated. It will be important for future studies to attempt to replicate these findings in other populations of children, using different exposure paradigms.
Investigations that do include a repeat exposure to an environmental or biological agent suggest that the enhanced or diminished cortisol response cannot entirely be explained by factors such as novelty. Rather it is likely mediated by a host of interrelated psychological and metabolic variables such that, on re-exposure to a stressor, cognitive and emotional factors influence responsiveness.31,32 These context-dependent processive stimuli that have been assigned emotional meaning support the notion that novelty may not be the primary mediator of the HPA response. Still, in children it is unclear what cognitive appraisal domains may be related that contribute to or ameliorate a response to stressful situations.
We have attempted to thoroughly investigate circadian rhythmicity, variability and response to stress; nonetheless, we have a few limitations to acknowledge. First, although the children with autism had IQ scores within the average range, there was a significant difference in their IQ when compared with the typically developing children, which may be a potential contributing factor in the group differences in cortisol values. A large subsample of the children who participated in the study were brought back to the MRI for a subsequent visit. Although most participant families were contacted, a few chose not to return for a second mock and real MRI scan. It may be that some unique factors exist in those who did not want to return to the stressor by choice. Nevertheless, there were no observed differences, and comparison of response to the mock MRI did not differ between children who did or did not return for the final day of testing.
In summary, the finding that children with autism and typical development demonstrate an increase in endocrine activity, ostensibly in anticipation of re-exposure to a noxious event, warrants additional investigation of factors of expectancy that must be considered in developmental models of stress. Most notably, the current study reveals clear dysregulation of the circadian rhythm in autism characterized by gradual decrease over the course of the sampling in the morning and by elevated evening values. The greater within-child variation suggests clear disturbances in the limbic HPA axis that cannot be accounted for by mere between-child heterogeneity but points, rather, to fundamental dysregulation and increased susceptibility to external factors such as zeitgeibers.
Acknowledgments
Funding was provided by the NIH Career Development Award (5K08NMHO72958), University of California, Department of Psychiatry Tupin Award, and a M.I.N.D. Institute Investigator Initiated Pilot Grant to Blythe A. Corbett. The authors thank the children and families who participated in this multiphase study and methodically assisted in the collection of home samples. We express our gratitude to staff of the Endocrine Core of the California National Primate Research Center, Christine Brennan, Alison Bort and Nicole Maninger, who ran the cortisol assays for the project. We are appreciative of our research assistants Patricio Ayala, Kathryn Shickman and Maryam Abdullah, who assisted with subject enrolment, parent training and data entry. The authors also thank Ryan Keating and Devyn Wells for database entry and verification.
Footnotes
Contributors: Drs. Corbett, Mendoza and Levine designed the study. Dr. Mendoza and Ms. Carmean acquired the data, which Drs. Corbett, Mendoza, Wegelin and Levine analyzed. All authors wrote the article; Drs. Corbett, Mendoza, Wegelin and Levine reviewed it. All authors gave final approval for publication.
This manuscript is dedicated to the memory and inspiration of our dear friend and colleague, Seymour “Gig” Levine.
Competing interests: None declared.
Correspondence to: Dr. B.A. Corbett, University of California, Davis, Department of Psychiatry and Behavioral Sciences, M.I.N.D. Institute, 2825 50th St., Sacramento CA 95817; blythe.corbett@ucdmc.ucdavis.edu
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 2.Corbett BA, Mendoza S, Abdullah M, et al. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology 2006;31:59-68. [DOI] [PubMed]
- 3.Hill SD, Wagner EA, Shedlarski JG Jr, et al. Diurnal cortisol and temperature variation of normal and autistic children. Dev Psychobiol 1977;10:579-83. [DOI] [PubMed]
- 4.Jansen LM, Gispen-de Wied CC, Van der Gaag RJ, et al. Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, multiple complex developmental disorder. Psychoneuroendocrinology 2000;25:753-64. [DOI] [PubMed]
- 5.Jansen LM, Gispen-de Wied CC, van der Gaag RJ, et al. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology 2003;28:582-90. [DOI] [PubMed]
- 6.Jensen JB, Realmuto GM, Garfinkel BD. The dexamethasone suppression test in infantile autism. J Am Acad Child Psychiatry 1985;24:263-5. [DOI] [PubMed]
- 7.Tordjman S, Anderson GM, McBride PA, et al. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry 1997;38:705-15. [DOI] [PubMed]
- 8.Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch Dis Child 1983;58:454-6. [DOI] [PMC free article] [PubMed]
- 9.Vermes I, Dohanics J, Toth G, et al. Maturation of the circadian rhythm of the adrenocortical functions in human neonates and infants. Horm Res 1980;12:237-44. [DOI] [PubMed]
- 10.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol 2001;13:515-38. [DOI] [PubMed]
- 11.Aihara R, Hashimoto T. [Neuroendocrinologic studies on autism] [article in Japanese]. No To Hattatsu 1989;21:154-62. [PubMed]
- 12.Hoshino Y, Yokoyama F, Watanabe M, et al. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Jpn J Psychiatry Neurol 1987;41:227-35. [DOI] [PubMed]
- 13.Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. J Autism Dev Disord 1992;22:433-47. [DOI] [PubMed]
- 14.Yehuda R, Teicher MH, Trestman RL, et al. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 1996;40:79-88. [DOI] [PubMed]
- 15.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997;20:78-84. [DOI] [PubMed]
- 16.Lord C, Rutter M., DiLavore P., et al. Autism Diagnostic Observation Schedule-WPS. Los Angeles: Western Psychological Services; 1999.
- 17.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659-85. [DOI] [PubMed]
- 18.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. New York: Psychological Corporation; 2003.
- 19.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio (TX): Psychological Corporation; 1999.
- 20.Roid GH. Stanford-Binet Intelligence Scales. 5th ed. Itasca (IL): Riverside Publishing; 2003.
- 21.Berument SK, Rutter M, Lord C, et al. Autism screening questionnaire: diagnostic validity. Br J Psychiatry 1999;175:444-51. [DOI] [PubMed]
- 22.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994;19:313-33. [DOI] [PubMed]
- 23.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Amer Math Soc 1943;54:426-82.
- 24.Bruce J, Davis EP, Gunnar MR. Individual differences in children's cortisol response to the beginning of a new school year. Psychoneuroendocrinology 2002;27:635-50. [DOI] [PubMed]
- 25.Silva ML, Mallozi MC, Ferrari GF. Salivary cortisol to assess the hypothalamic-pituitary-adrenal axis in healthy children under 3 years old. J Pediatr (Rio J) 2007;83:121-6. [DOI] [PubMed]
- 26.Goodyer IM, Herbert J, Altham PM, et al. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med 1996;26:245-56. [DOI] [PubMed]
- 27.Halligan SL, Herbert J, Goodyer I, et al. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry 2007;62:40-6. [DOI] [PubMed]
- 28.Halligan SL, Herbert J, Goodyer IM, et al. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biol Psychiatry 2004;55:376-81. [DOI] [PubMed]
- 29.Hruschka DJ, Kohrt BA, Worthman CM. Estimating between-and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology 2005;30:698-714. [DOI] [PubMed]
- 30.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 2002;27:199-220. [DOI] [PubMed]
- 31.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav 2004;82:751-62. [DOI] [PubMed]
- 32.Khan S, Liberzon I, Abelson JL. Effect of repeat exposure on neuroendocrine and symptom responses to pentagastrin. Psychiatry Res 2004;126:189-95. [DOI] [PubMed]