Abstract
Objective
In line with Crow's hypothesis, altered hemispheric lateralization of language would cause the main symptoms of schizophrenia. The present experiment aimed to demonstrate the loss of the hemispheric specialization for linguistic processing in schizophrenia patients at the level of early automatic evoked potentials (N150).
Methods
A sample of 10 outpatients with schizophrenia treated with low levels of neuroleptics and 10 matched healthy control subjects were administered 3 linguistic tasks based on stimulus pair comparisons (phonological, semantic and word–picture matching tasks). Laterality scores of early evoked potentials were analyzed during 2 time windows corresponding to the N150- and N400-like components.
Results
The patients failed to develop the typical left hemispheric N150 component evoked by the first word (S1), which was consistently achieved by the healthy control group in posterior sites (p < 0.01). The effect was specific and stable for linguistic stimuli. As well, for the N150 elicited by the target stimulus (S2), the patients exhibited a lack of linguistic lateralization. In the control task (word–picture matching task), in which S2 was a picture, the 2 groups revealed very similar bilateral recognition potentials.
Conclusion
The results point to a failure of language lateralization in patients with schizophrenia, a deficit involving those linguistic networks automatically activated in the earliest phase of word recognition (N150). Consistent with the current view of schizophrenia, this finding may be related to lack of integration among specific processes and reduced interconnection of underlying linguistic networks.
Medical subject headings: schizophrenia, language, hemispheric asymmetry, evoked potentials, recognition potential, N150
Abstract
Objectif
Selon l'hypothèse de Crow, l'altération de la latéralisation hémisphérique du langage causerait les principaux symptômes de la schizophrénie. L'expérience visait à démontrer la perte de la spécialisation hémisphérique du traitement linguistique chez les patients atteints de schizophrénie au niveau des premiers potentiels évoqués automatiques (N150).
Méthodes
On a demandé à un échantillon de 10 patients externes atteints de schizophrénie et traités au moyen de neuroleptiques de faible concentration et à 10 sujets témoins en bonne santé jumelés d'exécuter 3 tâches linguistiques fondées sur des comparaisons de paires de stimuli (jumelage phonologique, sémantique et mot-image). On a analysé les scores de latéralité des premiers potentiels au cours de deux créneaux correspondant aux composantes analogues à N150 et N400.
Résultats
Les patients n'ont pas développé la composante N150 hémisphérique gauche typique évoquée par le premier mot (S1), ce que les sujets du groupe témoin en bonne santé ont réussi constamment à faire dans les sites postérieurs (p < 0,01). L'effet a été spécifique et stable dans le cas des stimuli linguistiques. En outre, dans le cas de la composante N150 suscitée par le stimulus cible (S2), on a constaté un manque de latéralisation linguistique chez les patients. Dans la tâche témoin (jumelage mot-image), où S2 était une image, on a constaté chez les deux groupes des potentiels très semblables de reconnaissance bilatérale.
Conclusion
Les résultats indiquent une défaillance de la latéralisation du langage chez les patients atteints de schizophrénie, déficit qui met en cause les réseaux linguistiques activés automatiquement au début de la reconnaissance des mots (N150). Conformément à ce qu'on pense actuellement de la schizophrénie, cette constatation pourrait être liée au manque d'intégration dans des processus spécifiques et à une réduction de l'interconnexion des réseaux linguistiques sous-jacents.
Introduction
Schizophrenia is a psychiatric disorder marked by symptoms affecting a wide range of functional domains such as perception, emotion, thinking, language, motion and volition.1,2 Among the many hypotheses and theories aimed at explaining this disorder, Crow3,4 has suggested that the genetic variance linked to cerebral specialization that followed the achievement of linguistic skills in Homo sapiens carried the risk of developing schizophrenia symptoms. Persons lacking the typical left hemisphere dominance of language would be exposed to increased risk for developing such characteristic symptoms as auditory hallucinations and delusions. Evidence of altered hemispheric lateralization in patients with schizophrenia5 has been shown with both structural6–10 and functional11,12 brain imaging methods. Crow13,14 concluded that the leading deficit in schizophrenia consists of the disruption of the hemispheric specialization for linguistic processes. A few electrophysiological and metabolic studies have yielded evidence of altered cortical lateralization to tones, syllables15,16 or words.17,18 Among the linguistic functions presumed to be affected, the most important is phonological articulation, which typically activates the left dorsolateral prefrontal cortex.19–22 Although functional magnetic resonance imaging (fMRI)17,18 cannot measure the time course of linguistic lateralization, and therefore cannot differentiate early automatic from late cognitive components, electroencephalography (EEG) and magnetoencephalography (MEG) studies on reading demonstrated that in healthy people, in addition to visual object representation, written words automatically also elicit early electrical and magnetic waves over left occipitotemporal areas. These components are usually represented by short latency peaks (i.e., between 130 and 250 ms) following word onset and have been termed recognition potentials (RPs).23–34 Most studies converge on the view that, among all RPs, the negative wave peaking at about 130–170 milliseconds and termed N150 is the earliest component that reliably distinguishes between word-like strings and other visual stimuli such as faces, objects and symbol signs (see note 1). Information provided by methods that are more precise in the space dimension, such as positron emission tomography (PET) and fMRI, have identified the brain structures involved in various processes associated with the first phases of reading. These areas include the ventral occipitotemporal cortex, fusiform gyrus, lingual gyrus and frontal operculum of the left hemisphere, as well as the superior temporal and postcentral gyri bilaterally.35 A few studies have aimed at clarifying how and where this early visual–perceptual analysis of words occurs: the surface of the left occipitotemporal cortex, and in particular the left fusiform gyrus, would represent crucial regions involved in category-specific responses to letter strings and faces.36 Dehaene and colleagues37 proposed that the left fusiform gyrus corresponds to a visual word form area specialized in prelexical representation of a word as an ordered sequence of abstract letters. There is still open debate about the cortical localization of visual word form representation.38,39 Price and Devlin38 have reviewed both neuropsychological and neuroimaging data and suggest that visual word processing involves a more interconnected set of posterior regions in the left hemisphere. In addition, this mechanism would operate independently of size, font, case34,40 and position of the stimulus in the visual field.30 Several EEG studies have been performed to measure the early ERP components associated with memory and attention deficits in schizophrenia. However, to our knowledge, there are no studies specifically focusing on lateralization of automatic word processing in patients with schizophrenia, especially in view of the lack of lateralization hypothesis advanced by Crow. Based on a continuous word (new v. old words) recognition task, a study by Kayser and colleagues41 revealed a deficit in early N200 and P300 components (with a latency of 330 and 600 ms, respectively) in patients with schizophrenia compared with a healthy control group (i.e., a lack of left lateralization in the posterior regions). In a second study, using a visual word serial position test, Kayser and colleagues42 found a significant left-lateralized N100 component in patients with schizophrenia, peaking about 190 milliseconds after word onset. The only difference with respect to the control group consisted of slightly smaller N100 amplitudes. Using 2 dichotic listening tasks, Bruder and colleagues43 observed considerably smaller N100 amplitudes in patients with schizophrenia, compared with a control group, when they listened to tones rather than syllables, especially in the frontocentral sites of both hemispheres. The control group revealed greater N200 amplitude over the left, compared with right, hemisphere in the syllable but not tone task, whereas patients showed smaller N200 amplitude and did not show left lateralization of N200. In a special paradigm based on rapid stream stimulation, Martín-Loeches and colleagues44 also found limited evidence of a reduced left RP in patients with schizophrenia, but this experiment was not aimed at investigating language lateralization. Because of the peculiar paradigm, the RP component in the control group was greatly delayed with respect to N150, as it peaked about 260–290 milliseconds after stimulus onset.
In the present study, we measured both spatial aspects and the temporal dimension of early stages of word processing in patients with schizophrenia compared with healthy subjects. A paradigm that used the same word sample in different tasks allowed us to avoid the typical confounded variables (e.g., word length, frequency, semantic relevance) affecting experiments that contrast different words in different tasks.21,22,45–47 On the basis of our previous study on early linguistic ERP components,34 we examined the earliest stage of word processing in the schizophrenia patient's brain, the RP, as an index of automatic word classification, as well as a late time interval corresponding to the N400-like component presumed to represent a stage of more specific word processing able to differentiate tasks and linguistic processes.27 In line with past evidence, we expected that the control group would show greater left posterior lateralization (i.e., RP) induced by automatic skilled reading, independently of the task. In the psychiatric sample, we expected a significant reduction or lack of early posterior left activation following automatic word recognition, in line with Crow's hypothesis. To differentiate phonological-semantic from visual object processing, we also analyzed the RP evoked by pictures in the presentation of a second stimulus, which served as a control condition and for which we did not expect group differences in lateralization.
Methods
Participants
We recruited 10 outpatients (5 women, 5 men) diagnosed with chronic schizophrenia from the psychiatric medical facility of Rovigo, Italy. The patients' mean age was 36.3 (standard deviation [SD] 11.6, range 24–60) years, and they had, on average, 12.3 (SD 3.2) years of education. The diagnosis of schizophrenia was psychiatrically assessed both clinically, according to DSM-IV-TR criteria,48 and quantitatively, by means of the Scales for the Assessment of Positive and Negative Symptoms (SAPS49 and SANS,50 respectively). The diagnostic procedure classified 2 patients with disorganized, 5 with residual, 2 with paranoid and 1 with undifferentiated schizophrenia. The average duration of illness was 14.7 (SD 9.26, range 3–31) years, and all patients were taking medication at the time of testing. With regard to medication, we selected a group of patients with the lowest levels of neuroleptic medication and treatment (mean chlorpromazine daily equivalent 70 mg, range 20–130 mg) to limit effects on performance and brain responses. Thus our sample was quite rare in that patients were treated with less than one-third of the standard neuroleptic medication dosage. Ten healthy volunteers matched for sex (5 women, 5 men), age (mean 37.6, SD 14.2, range 27–65 y) and educational level (mean 14.4, SD 4.3 y) served as the control group. They had no psychiatric illness or symptoms and denied alcohol or substance abuse.
All participants were right-handed according to the Edinburgh Handedness Inventory51 and participated in the electrophysiological session after giving their written informed consent. The psychiatrists who treated the patients explained the whole experimental procedure to their patients and ensured their mental competence in understanding and giving fully informed consent to participate in the research. In addition, shortly before the beginning of the experimental session, the experimenter again verified patients' understanding and intent to enter the study. Experimental procedures were approved by the Ethics Committee of the Faculty of Psychology, University of Padova.
Apparatus and physiological recordings
EEG cortical activity was recorded by 26 tin electrodes: 19 placed on an elastic cap (Electro-Cap, Electro-Cap International Inc., Eaton, Ohio) according to the International 10–20 system52; the other 7 electrodes were applied below each eye (Io1, Io2), on the 2 external canthi (F9, F10), nasion (Nz) and mastoids (M1, M2). All cortical sites were referenced online to Cz. Data were stored with the DC-MES 32-channel system. Amplitude resolution was 0.1 μV; bandwidth ranged from DC to 30 Hz (6 dB/octave). Sampling rate was set at 100 Hz, and impedance was kept below 5 Ω.
Stimuli, tasks and procedure
The experimental paradigm has been validated in Italian subjects21,22,34 and applied to clinical populations with linguistic disorders, i.e., to dyslexic children and aphasic patients.45,46 Furthermore, it forces complex linguistic processing (word feature comparison) and allows the measure of subjects' behavioural responses (response times and error rates).
Words and pictures of objects served as visual stimuli. Line drawings of objects representing concrete and frequently used words were selected from the collection of Snodgrass and Vanderwart.53 Verbal stimuli consisted of bi-or trisyllabic Italian content words selected from a frequency dictionary of 5000 written Italian words.54 Stimuli were presented in pairs appearing one at a time on the centre of a 17-inch computer monitor, with an interstimulus interval (ISI) of 2 seconds: the first stimulus (S1) was always a word and remained on the screen for 1 second; the second (S2 or target) was either a word (phonological and semantic tasks) or a picture (word–picture matching task) that was visually presented until the subject responded by pressing a keyboard button, but in any case for not longer than 5 seconds. Pairs were administered in 3 separate blocks that corresponded to 3 linguistic tasks. During the phonological and semantic tasks, the same words were presented as S1 in a different randomized order. In the phonological task, on S2 target presentation, subjects had to decide whether word pairs rhymed (e.g., butter–cutter) or not (e.g., tooth–button). In the semantic task, they had to decide whether the target word S2 was of the same semantic category as S1 (e.g., butter–bread) or not (e.g., tooth–shoes). In the word–picture matching task used as a control in which the word (S1) was followed after a 2-second ISI by the presentation of a picture of an object, they had to decide whether the picture matched the previous word (i.e., whether the object depicted the word presented in S1). Participants pressed the button with their left index or middle finger to indicate responses. Each task included 80 trial/stimulus pairs. In all tasks, 50% match and 50% mismatch trials varied in pseudorandom order. The order of the tasks varied randomly across subjects.
Data analysis
The error rates (ERs) and response times (RTs) of each subject served as behavioural measures, and mean performance was compared between groups and tasks. The EEG was continuously recorded in the DC mode and stored for subsequent analysis. Data were off-line rereferenced to the average reference and epoched into 1-second intervals around S1 and S2. A baseline of 100 milliseconds preceding S1 was subtracted from the whole trial epoch. Single trials were corrected for vertical and horizontal eye movements and blinking artifacts. For this, we used BESA 5.1 software (MEGIS Software GmbH, Brain Electrical Source Analysis, Version 5.1, Gräfelfing, Germany) to compute ocular correction coefficients, according to Berg and Scherg's multiple source eye correction (MSEC) method.55,56 Each trial was then visually inspected for any residual artifacts, which were rejected if found. According to the MSEC method, orbitofrontal electrodes (Fp1, Fp2, F9, F10, Nz, Io1, Io2) are considered artifact-free active cortical sites.
After eye movement correction, all accepted trials were averaged for each task and subject. Starting from grand-average waveforms, we computed the global field power (GFP; sum of all squared potentials) across all channels and conditions to quantify the overall signal strength at each time point. Thus GFP represents the overall energy of the ERP signal across time and helps in detecting the time intervals with the greatest signal energy independently of location and peak negativity/positivity. On the basis of GFP (see Fig. 1A and 1C) and after visual inspection of grand-average waveforms, 2 epochs for each stimulus (S1, S2) entered separate statistical comparisons: the first interval included the average potential corresponding to the RP (i.e., the N150) in the interval of 110–130 milliseconds after stimulus onset (S1, S2); the second epoch included the mean potential measured in the interval of 100 milliseconds centred on the N400-like wave (i.e., during the interval of 350–450 ms).
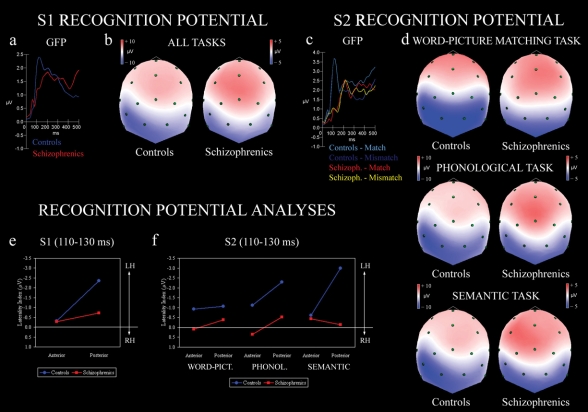
Fig. 1: Event-related potentials during (a,b) first word (S1) presentation: (a) GFP and (b) spline maps with upper posterior scalp view of all collapsed tasks. Potentials evoked during (c,d) second stimulus (S2) presentation: (c) GFP and (d) spline maps for each task. Statistical analysis of recognition potential for S1 (e) and S2 (f) stimuli in the schizophrenia (red line) and control (blue line) groups. Lateralization score measured as difference of scalp activity from electrodes of left hemisphere minus activity of right homologues, shown for anterior and posterior quadrants. Negative values indicate greater N150 over left side. GFP = global field power.
To limit the typically low signal-to-noise ratio of single electrode activity, electrodes were clustered into 4 regions of interest,21,22,45–47 and the laterality score was adopted to decrease the number of statistical variables (as a consequence, the factor “hemisphere” was dropped) and to increase the statistical power. The laterality score was computed as the difference in the mean activity of left (electrodes F9, F7, F3) minus right (electrodes F10, F8, F4) anterior quadrants; a similar lateralization was computed for posterior sites, that is, left (electrodes P3, P7, O1) minus right (electrodes P4, P8, O2) posterior quadrants. Thus the laterality score was negative when activity was left-lateralized and positive when right-lateralized.
For each time window, the analysis of variance (ANOVA) included only a between-subjects variable (group, with 2 levels: control group v. schizophrenia group) and 2 within-subjects variables (task, with 3 levels: word–picture matching v. phonological v. semantic; and region, with 2 levels: anterior v. posterior). In addition, analysis of S2 stimuli included the within-subjects response factor (with 2 levels: match v. mismatch) to verify whether match/mismatch comparisons revealed different cortical processing.
With regard to behavioural measures (mean ERs and RTs), the ANOVA included the between-subjects group factor (with 2 levels: control group v. schizophrenia group) and the within-subjects task factor (with 3 levels: word–picture matching v. phonological v. semantic).
Post hoc comparisons were computed with the Newman-Keuls test (p < 0.05), and the Greenhouse-Geisser correction was applied when necessary (df > 1). In each analysis, all main effects and interactions were computed, but only significant findings are reported here.
Results
Performance data
The group main effect (F1,18 = 10.34, p < 0.01) showed that patients were slower to respond than control subjects (mean 1209, SD 275 v. mean 882, SD 232 ms, respectively). Also, all participants were faster in word–picture matching and in phonological tasks than in the semantic task (mean 949, SD 24 and mean 1020, SD 332 v. mean 1166, SD 290 ms, respectively; task main effect F2,36 = 20.57, p < 0.001, ε = 0.91). Analysis of ERs showed only a task main effect (F2,36 = 4.20, p < 0.05, ε = 0.67), revealing that, regardless of group, both word–picture matching and phonological tasks were performed more accurately than the semantic task (mean 4.9%, SD 5.4 and mean 4.2%, SD 4.6 v. mean 7.1%, SD 6.0, respectively).
Evoked potentials
N150 (RP)
Concerning the N150 elicited by S1 (see note 2), the significant group main effect (F1,18 = 4.81, p < 0.05) showed that the N150 was more left-lateralized in the control group than in patients (mean –1.34, SD 1.74 v. mean –0.50, SD 1.27 μV, respectively), whereas the significant region main effect (F1,18 = 11.24, p < 0.01) revealed greater left lateralization in posterior than in anterior clusters (mean –1.54, SD 1.73 v. mean –0.30, SD 1.12 μV, respectively). In addition, independently of task or group, the N150 was more left-lateralized in posterior than in anterior sites (p < 0.05 for all tasks; task by region interaction F2,36 = 5.56, p < 0.01, ε = 0.90).
Figure 1B shows the spline-interpolated maps referring to the early N150 evoked by the first word (S1). Regardless of task, the control group exhibited strong left posterior negativity, corresponding to the visual word RP, whereas in the schizophrenia group, posterior negativity was more evenly distributed.
This pattern is revealed by the significant group by region interaction (F1,18 = 4.61, p < 0.05), which confirmed that groups were differently left-lateralized only over posterior locations (p < 0.01, see Fig. 1E). The control group showed greater left lateralization in posterior than in anterior sites (p < 0.01), whereas patients exhibited similar reduced lateralization in both anterior and posterior regions.
In Figure 1D, spline-interpolated maps show the N150 measured during the presentation of the target stimulus (S2), which was either a picture (word–picture matching) or a word (phonological and semantic tasks). As in S1, the control group showed overall more left-lateralized N150 than patients (mean –1.50, SD 1.89 v. mean –0.17, SD 2.19 μV, respectively; group main effect F1,18 = 10.63, p < 0.01). In detail, the control group had clear-cut left-lateralized posterior negativity corresponding to word recognition during both phonological and semantic tasks, whereas they showed bilateral posterior negativity during picture processing (Fig. 1D). In contrast, patients with schizophrenia had bilateral posterior negativity, independently of task. This pattern was confirmed by the significant 3-way group by task by region interaction (F2,36 = 3.91, p < 0.05, ε = 0.87; see Fig. 1F). Indeed, groups showed different left-lateralized asymmetry in posterior regions during both phonological (p < 0.05) and semantic (p < 0.001) tasks but no differences in lateralization during the word–picture matching task. The control group also had significantly more left-lateralized posterior N150 when compared with the anterior regions, specifically, during linguistic tasks (p < 0.05 for the phonological and p < 0.01 for the semantic task). With regard to this anteroposterior difference, the within-subjects effect sizes computed in the anterior versus posterior lateralization scores of the control group revealed a medium Cohen's d value (d = –0.60) for the phonological task, which indicates a relatively greater left lateralization in posterior compared with anterior sites, and a very large Cohen's d value (d = –1.16) for the semantic task, which indicates much greater left lateralization in posterior compared with anterior regions. Patients with schizophrenia showed no significant differences in lateralization within the anteroposterior axis.
Considering the relatively small number of patients and control subjects, to verify the robustness of group effects57 for lateralization of N150 in posterior sites, additional group effect sizes were computed. The N150 lateralization difference between the control group and the schizophrenia group evoked by S1 gave Cohen's d values of 1.35 and 1.16 (very large effect size) for the phonological and semantic tasks, respectively, and a Cohen's d of 0.84 (large effect size) for word–picture matching. Similarly, the N150 lateralization evoked by S2 for match/mismatch collapsed responses showed a Cohen's d of 0.95 (large effect size) for phonological, 1.41 (very large effect size) for semantic and 0.42 (small effect size, not significant in ANOVA statistics) for word–picture matching tasks.
N400-like component
Statistical analysis of the interval 350–450 milliseconds after S1 onset revealed no significant effects. Again, in this same time window, but after S2 onset, significant main effects of group (F1,18 = 5.14, p < 0.05) and response (F1,18 = 7.28, p < 0.01) showed more left-lateralized activity in the control group than in patients (mean –1.26, SD 2.42 v. mean –0.35, SD 2.28 μV, respectively) and a more left-lateralized N400-like component in match/mismatch trials (mean –1.10, SD 2.43 v. mean –0.51, SD 2.32 μV, respectively). In addition, regardless of group or task, the 2-way response by region interaction (F1,18 = 5.51, p < 0.05) showed that match conditions had significantly greater left lateralization than mismatch conditions in anterior locations (p < 0.001; mean –1.23, v. mean –0.98, μV for anterior sites and mean –0.31, v. mean –0.71, μV for posterior sites, match and mismatch, respectively.
Correlations between N150 elicited by S1 and S2
Pearson's correlation between N150 amplitudes elicited by S1 and S2 served to evaluate the stability of this component. Thus we correlated laterality scores from collapsed phonological plus semantic tasks during S1 with those achieved from the second word, S2. Statistics revealed a strong positive correlation between the amplitudes of N150 elicited by S1 and S2 (r18 = 0.90, p < 0.001): the larger the N150 left lateralization to S1, the larger the left lateralization obtained after S2 onset. This finding was also clear after computing Pearson's correlation separately for the patients and for the control group (r8 = 0.85 and r8 = 0.86, p < 0.001, respectively). In detail, the control group had reliable left-lateralized N150 following each word (mean –2.61, SD 1.46 and mean –2.65, SD 2.08 μV to S1 and S2, respectively), whereas patients showed reduced left-lateralized N150, especially during processing of the second word (mean –0.78, SD 1.46 and mean –0.33, SD 1.78 μV to S1 and S2, respectively).
Discussion
Every stimulus that orients our attention is categorized by associative areas of our visuoperceptual system. At the electrophysiological level, the first evoked component reflecting the automatic recognition of a word is the left-lateralized N150.23–27,29,30,33,34,37
Analysis carried out over the S1 interval revealed greater left lateralization of N150 in the control group, independently of the task, because in this phase only words were presented. The patients with schizophrenia showed a significant lack of asymmetry. This finding was further strengthened by S2 analysis, in which both words and pictures were visually presented. Also in this condition, the significant 3-way group by task by region interaction revealed the significantly greater left posterior amplitude of the N150 in the control group, which peaked during the semantic task, in comparison with the lack of lateralization found during picture processing (word–picture matching task). Unlike the control group, in all tasks the schizophrenia group showed a bilateral N150 to words. In addition, the high Pearson's correlation between N150 recorded in S1 and S2 for phonological and semantic tasks confirmed the reliability of the posterior left lateralization linked to word recognition in the control group and the consistent lack of posterior asymmetric activation in patients with schizophrenia.
To our knowledge, this study provides the first demonstration of the significant lack of left N150 asymmetry in schizophrenia, a fast component that cannot be detected with metabolic measures such as fMRI and PET. Indeed, although Martín-Loeches and colleagues44 studied a component functionally classified as RP in schizophrenia, this component cannot be considered an N150 for the paradigm chosen and the large delay (latency around 260–290 ms). In addition, the above authors used a Go/No-Go paradigm in which linguistic and nonlinguistic stimuli were visually presented with a rapid-stream stimulation procedure. Although that study was not aimed at examining the lateralization of the RP (the main statistics were computed on only a single electrode of the left hemisphere, PO7, out of 58 recorded sites), in line with our results there was some evidence of a reduced left RP in schizophrenia patients compared with a control group. Similarly, Kayser and colleagues41 found an interesting lack of left lateralization of the N200-P300 elicited by words in a continuous word recognition memory paradigm, a result in agreement with the present study. However, the effect described in that study was found for later components, at 330–600 milliseconds of latency, whereas the N150 (termed N1 by Kayser and colleagues) did not show statistical lateralization differences between schizophrenia and control groups, probably because the paradigm used stressed memory load (i.e., the comparison of new v. old words) and increased the related components N200 and P300 typically observed in such paradigms. Indeed, in the above study, the N200 greatly overlapped the N150 with respect to our data, in which the N200 was very small. The globally reduced N150 amplitude observed in patients with schizophrenia (see note 2) is quite common and is in line with other ERP studies on similar and later components.41,44 However, given the generalized effects involving most ERP components and EEG sites, this main group effect involved all quadrants and did not play a significant role in lateralization or anteroposterior asymmetry.
In the present study, the failure of word RP left lateralization is also consistent with previous evidence from patients with schizophrenia, attesting to both selective impairment in object recognition abilities and altered processing of the gestalt local/global properties of visual stimuli during the very early phases of visual processing.58–61 However, in comparison with other studies focusing on attention deficits, we interpret our results to be mainly related to the failure of brains affected by schizophrenia to completely recruit left hemisphere linguistic networks. We cannot entirely rule out the influence of attentional deficits in the present results, but according to our experimental design, attention should not affect lateralization. Indeed, the control condition represented by the picture presented as S2 in the word–picture matching task showed similar nonlateralized patterns in both groups, an effect that rules out several possible confounding factors, among which attention is the most important. The lack of significant group differences in ERs and the low level of neuroleptic medication also indicate that attention probably has little relevance for our findings.
Our results are in agreement with past electrophysiological and metabolic studies in patients with schizophrenia, which document their altered hemispheric laterality in different tasks and stimuli.11,12,15–18 However, not all quoted studies used words as stimuli (but, rather, syllables and tones, for example), or they used words but employed methods not able to detect the time course of early lexical recognition processes (i.e., fMRI and PET). In the present study, we used words as more ecologic stimuli capable of engaging the large linguistic networks that are also necessary during the early phases of word classification. Statistical analysis of the first epoch of S1 revealed that the groups showed different lateralization over posterior locations, regardless of task, whereas they perfectly overlapped in anterior sites. S2 analyses, in which pictures were also presented (the word–picture matching task) and match/mismatch conditions were included, demonstrated the different processes engaged by tasks in control subjects and patients with schizophrenia, thus pointing to the linguistic specificity of the asymmetry failure. That is, whereas the word–picture matching task (with a picture for S2) elicited cortical activation spread across hemispheres in both groups, the phonological and semantic judgments evoked significant greater left posterior activity in the control group but not in patients. Unlike patients, the control group showed additional linguistic specialization, the semantic task being more left-lateralized in posterior than in anterior regions, and the phonological task being clearly left-lateralized in both anterior and posterior sites (see Cohen's d values above). Thus the N150 from S2 processing points to a singular condition in which complete processing of the first word (S1) primes and drives analysis of the following word (S2), probably to optimize the linguistic processing required by the task. This is an interesting result, considering that at this time interval subjects have not yet finished reading all the words. Patients with schizophrenia exhibited an abnormal pattern in this automatic word recognition, which is typically characterized by left posterior activation, and revealed a specific functional impairment of linguistic networks involved in early processing of single words.
Past studies suggest that cognitive functions are associated with an altered brain structure in patients with schizophrenia.62 Butler and Javitt60 recently reviewed several studies of early visual processing in schizophrenia and supported the idea of patients' dysfunction in automatic phases of stimulus processing. For patients with schizophrenia, the present results show the lack of hemispheric specialization of linguistic networks that are presumed to be strengthened and specialized during the developmental years in which language skills become automated. The impaired integration of specific features of word processing, linked to a reduced marker of early automatic word classification in the left hemisphere, did not prevent our patients from performing relatively well on the whole linguistic task. Indeed, ERs did not differ between groups, and analysis of the late interval (350–450 ms following S2 onset) revealed that both groups had greater left negativity for match/mismatch conditions, independently of task. In line with this, past studies show that patients with schizophrenia have relatively spared performance on basic linguistic neuropsychological tests (e.g., token test63 or vocabulary test64), compared with other cognitive functions, but that they fail to assemble different types of information, which requires more complex left-lateralized strategies, for instance, also involving planning and working memory (e.g., verbal/semantic fluency tasks65,66). Thus results of the present study show that the hemispheric hierarchy of linguistic networks is disrupted in schizophrenia. The associated impairment would not involve the ability to use information correctly (linguistic basic functions are not affected); rather, in line with current views on this psychotic disorder,1 it occurs at a higher level of integration of language with other cognitive processes. According to Crow,3,4 the main symptoms of schizophrenia (hallucinations and thought disorders) arise from the lack of linguistic left hemisphere dominance. This deficit leads to confusion or loss of integration of information coming from the 2 hemispheres and ultimately results in confusion between thought and speech. Recent neurobiological models of language are in line with Crow's view of the relevance of linguistic brain centres such as Broca's area in organizing behaviour and complex hierarchical actions and plans.67 This latter observation, together with the present results, provides a first explanation of the still not well-understood relation between language and behaviour, as well as of the dramatic consequences of the disintegration of action, cognition and consciousness that is typical of schizophrenia.
Acknowledgments
Thanks are due to Prof. B. Rockstroh, who assisted with critical reading of the manuscript. This study was supported by a MIUR (Ministero dell'Istruzione, dell'Università e della Ricerca) grant (PRIN 2006110284 to A.A.), by University of Padova project CPDA047438 to A.A. and an “Assegno di Ricerca” #CPDR068897.
Footnotes
Notes
1. To avoid confusion, it is important to distinguish between the term “recognition potential,” which indicates a large family of ERP components associated with the recognition of a stimulus presented in various modes, and the N150, which specifically marks the recognition of a written linguistic stimulus.
2. The ANOVA of the N150 evoked by S1, made by adding the within-subjects hemisphere factor instead of lateralization scores, revealed a significant group main effect (F1,18 = 7.93, p < 0.01) with greater overall negativity in the control group than in the patients (–0.75 [SD 2.76] v. –0.17 [SD 1.66] μV, respectively). The interactions 2-way group by hemisphere (F1,18 = 4.81, p < 0.05) and region by hemisphere (F1,18 = 11.24, p < 0.01) and the 3-way task by region by hemisphere (F2,36 = 5.56, p < 0.01) and group by region by hemisphere (F1,18 = 4.61, p < 0.05) were the exact equivalents, respectively, of group and region main effects and task by region and group by region interactions obtained with laterality scores (see Evoked potentials above for comparison). Thus, to avoid redundancy and confusion, only the latter analysis is reported. The overall difference found between groups does not deserve special mention because a reduction of ERPs is commonly found in schizophrenia.41,44
Contributors: Drs. Spironelli and Angrilli designed the study, analyzed the data and wrote the article. Drs. Angrilli and Stegagno acquired the data. All authors reviewed the article and gave final approval for its publication.
Competing interests: None declared.
Correspondence to: Prof. A. Angrilli, Department of General Psychology, University of Padova, Via Venezia 8, 35131 Padova, Italy; fax 39 049 827 6600; alessandro.angrilli@unipd.it
References
- 1.Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 1997;275: 1586-93. [DOI] [PubMed]
- 2.Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev 2000;31:106-12. [DOI] [PubMed]
- 3.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci 1997;20:339-43. [DOI] [PubMed]
- 4.Crow TJ. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev 2000;31:118-29. [DOI] [PubMed]
- 5.Flor-Henry P. Cerebral basis of psychopathology. Boston: John Wright; 1983.
- 6.Crow TJ, Ball J, Bloom SR, et al. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry 1989;46:1145-50. [DOI] [PubMed]
- 7.McDonald B, Highley JR, Walker MA, et al. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a post-mortem study. Am J Psychiatry 2000;157:40-7. [DOI] [PubMed]
- 8.Kubicki M, Westin C-F, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry 2002;159:813-20. [DOI] [PMC free article] [PubMed]
- 9.Cullen TJ, Walker MA, Eastwood SL, et al. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry 2006;188:26-31. [DOI] [PubMed]
- 10.McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry 2002;59: 321-31. [DOI] [PubMed]
- 11.Weiss EM, Hofer A, Golaszewsky S, et al. Brain activation patterns during a verbal fluency test — a functional MRI study in healthy volunteers and patients with schizophrenia. Schizophr Res 2004;70:287-91. [DOI] [PubMed]
- 12.Weiss EM, Hofer A, Golaszewsky S, et al. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Res 2006;146:185-90. [DOI] [PubMed]
- 13.Crow TJ. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom). Laterality 2004;9:233-42.
- 14.Crow TJ. Cerebral asymmetry and the lateralization of language: core deficits in schizophrenia as pointers to the genetic predisposition. Curr Opin Psychiatry 2004;17:97-106.
- 15.Rockstroh B, Clementz BA, Pantev C, et al. Failure of dominant left-hemispheric activation to right-ear stimulation in schizophrenia. Neuroreport 1998;9:3819-22. [DOI] [PubMed]
- 16.Rockstroh B, Kissler J, Mohr B, et al. Altered hemispheric asymmetry of auditory magnetic fields to tones and syllables in schizophrenia. Biol Psychiatry 2001;49:694-703. [DOI] [PubMed]
- 17.Ngan ETC, Vouloumanos A, Cairo TA, et al. Abnormal processing of speech during oddball target detection in schizophrenia. Neuroimage 2003;20:889-97. [DOI] [PubMed]
- 18.Sommer IEC, Ramsey NF, Mandl RCW, et al. Language lateralization in female patients with schizophrenia: an fMRI study. Schizophr Res 2003;60:183-90. [DOI] [PubMed]
- 19.Zatorre RJ, Evans AC, Meyer E, et al. Lateralization of phonetic and pitch discrimination in speech processing. Science 1992;256:846-9. [DOI] [PubMed]
- 20.Démonet J, Wise R, Frackowiak R. Language functions explored in normal subjects by positron emission tomography. Hum Brain Mapp 1994;1:39-47.
- 21.Angrilli A, Dobel C, Rockstroh B, et al. EEG brain mapping of phonological and semantic tasks in Italian and German languages. Clin Neurophysiol 2000;111:706-16. [DOI] [PubMed]
- 22.Spironelli C, Angrilli A. Language lateralization in phonological, semantic and orthographic tasks: a slow evoked potential study. Behav Brain Res 2006;175:296-304. [DOI] [PubMed]
- 23.Dehaene S. Electrophysiological evidence for category-specific word processing in the normal human brain. Neuroreport 1995;6:2153-7. [DOI] [PubMed]
- 24.Puce A, Allison T, Asgari M, et al. Differential sensitivity of human visual cortex to faces, letterstrings, and texture — a functional magnetic resonance imaging study. J Neurosci 1996;16:5205-15. [DOI] [PMC free article] [PubMed]
- 25.Salmelin R, Service E, Kiesilä P, et al. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann Neurol 1996;40:157-62. [DOI] [PubMed]
- 26.Schendan HE, Ganis G, Kutas M. Neurophysiological evidence for visual perceptual categorization of words and faces within 150 ms. Psychophysiology 1998;35:240-51. [PubMed]
- 27.Bentin S, Muochetant-Rostaing Y, Giard MH, et al. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. J Cogn Neurosci 1999;11:235-60. [DOI] [PubMed]
- 28.Martín-Loeches M, Hinojosa JA, Gómez-Jarabo G, et al. The recognition potential: an ERP index of lexical access. Brain Lang 1999;70:364-84. [DOI] [PubMed]
- 29.Tarkiainen A, Helenius P, Hansen PC, et al. Dynamics of letter string perception in the human occipitotemporal cortex. Brain 1999;122:2119-31. [DOI] [PubMed]
- 30.Cohen L, Dehaene S, Naccache L, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 2000;123: 291-307. [DOI] [PubMed]
- 31.Liu Y, Perfetti CA. The time course of brain activity in reading English and Chinese: an ERP study of Chinese bilinguals. Hum Brain Mapp 2003;18:167-75. [DOI] [PMC free article] [PubMed]
- 32.Rossion B, Joyce CA, Cottrell GW, et al. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage 2003;20:1609-24. [DOI] [PubMed]
- 33.Brem S, Lang-Dullenkopf A, Maurer U, et al. Neurophysiological signs of rapidly emerging visual expertise for symbol strings. Neuroreport 2005;16:45-8. [DOI] [PubMed]
- 34.Spironelli C, Angrilli A. Influence of phonological, semantic and orthographic tasks on the early linguistic components N150 and N350. Int J Psychophysiol 2007;64:190-8. [DOI] [PubMed]
- 35.Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A 1998;95:914-21. [DOI] [PMC free article] [PubMed]
- 36.Allison T, Puce A, McCarthy G. Category-sensitive excitatory and inhibitory processes in human extrastriate cortex. J Neurophysiol 2002;88:2864-8. [DOI] [PubMed]
- 37.Dehaene S, Le Clec'H G, Poline JB, et al. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport 2002;13:321-5. [DOI] [PubMed]
- 38.Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage 2003;19:473-81. [DOI] [PubMed]
- 39.Price C J, Winterburn D, Giraud AL, et al. Cortical localisation of the visual and auditory word form areas: a reconsideration of the evidence. Brain Lang 2003;86:272-86. [DOI] [PubMed]
- 40.McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci 2003;7: 293-9. [DOI] [PubMed]
- 41.Kayser J, Bruder GE, Friedman D, et al. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. Int J Psychophysiol 1999;34:249-65. [DOI] [PubMed]
- 42.Kayser J, Tenke CE, Gates NA, et al. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology 2006;43:237-52. [DOI] [PubMed]
- 43.Bruder G, Kayser J, Tenke C, et al. Left temporal lobe dysfunction in schizophrenia. Arch Gen Psychiatry 1999;56:267-76. [DOI] [PubMed]
- 44.Martín-Loeches M, Muñoz F, Casado P, et al. An electrophysiological (ERP) component, the recognition potential, in the assessment of brain semantic networks in patients with schizophrenia. Schizophr Res 2004;71:393-404. [DOI] [PubMed]
- 45.Angrilli A, Elbert T, Cusumano S, et al. Temporal dynamics of linguistic processes are reorganized in aphasics' cortex: an EEG mapping study. Neuroimage 2003;20:657-66. [DOI] [PubMed]
- 46.Penolazzi B, Spironelli C, Vio C, et al. Altered hemispheric asymmetry during word processing in dyslexic children: an event-related potential study. Neuroreport 2006;17:429-33. [DOI] [PubMed]
- 47.Spironelli C, Penolazzi B, Vio C, et al. Inverted EEG theta lateralization in dyslexic children during phonological processing. Neuropsychologia 2006;44:2814-21. [DOI] [PubMed]
- 48.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revised. Washington: The Association; 2000.
- 49.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). The University of Iowa, Iowa City, IA; 1984.
- 50.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). The University of Iowa, Iowa City, IA; 1984.
- 51.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97-113. [DOI] [PubMed]
- 52.Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 2001;112:713-9. [DOI] [PubMed]
- 53.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity and visual complexity. J Exp Psychol (Hum Learn) 1980;6:174-215. [DOI] [PubMed]
- 54.Bortolini V, Tagliavini C, Zampolli A. Lessico di frequenza della lingua italiana contemporanea [Lexical frequency in contemporary Italian]. Milano: Aldo Garzanti Editore SpA; 1972.
- 55.Berg P, Scherg M. Dipoles models of eye movements and blinks. Electroencephalogr Clin Neurophysiol 1991;79:36-44. [DOI] [PubMed]
- 56.Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol 1994;90:229-41. [DOI] [PubMed]
- 57.Cohen J. Statistical power analysis for behavioral sciences. 2nd ed. Hillsdale (NJ): Erlbaum; 1988.
- 58.Ferman TJ, Primeau M, Delis D, et al. Global-local processing in schizophrenia: hemispheric asymmetry and symptom-specific interference. J Int Neuropsychol Soc 1999;5:442-51. [DOI] [PubMed]
- 59.Doniger GM, Foxe JJ, Murray MM, et al. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry 2002;59:1011-20. [DOI] [PubMed]
- 60.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry 2005;18:151-7. [DOI] [PMC free article] [PubMed]
- 61.Johnson SC, Lowery N, Kohler C, et al. Global-local visual processing in schizophrenia: evidence for an early visual processing deficit. Biol Psychiatry 2005;58:937-46. [DOI] [PubMed]
- 62.Antonova E, Sharma T, Morris R, et al. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res 2004;70:117-45. [DOI] [PubMed]
- 63.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000;157:549-59. [DOI] [PubMed]
- 64.Moritz S, Andresen B, Perro C, et al. Neurocognitive performance in first-episode and chronic schizophrenic patients. Eur Arch Psychiatry Clin Neurosci 2002;252:33-7. [DOI] [PubMed]
- 65.Bokat CE, Goldberg TE. Letter and category fluency in schizophrenic patients: a meta-analysis. Schizophr Res 2003;64:73-8. [DOI] [PubMed]
- 66.Phillips TJ, James CD, Crow TJ, et al. Semantic fluency is impaired but phonemic and design fluency are preserved in early-onset schizophrenia. Schizophr Res 2004;70:215-22. [DOI] [PubMed]
- 67.Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron 2006;50:963-74. [DOI] [PubMed]