Abstract
Control of thermoregulatory effectors by the autonomic nervous system is a critical component of rapid cold-defense responses, which are triggered by thermal information from the skin. However, the central autonomic mechanism driving thermoregulatory effector responses to skin thermal signals remains to be determined. Here, we examined the involvement of several autonomic brain regions in sympathetic thermogenic responses in brown adipose tissue (BAT) to skin cooling in urethane-chloralose-anesthetized rats by monitoring thermogenic (BAT sympathetic nerve activity (SNA) and BAT temperature), metabolic (expired CO2), and cardiovascular (arterial pressure and heart rate) parameters. Acute skin cooling, which did not reduce either rectal (core) or brain temperature, evoked increases in BAT SNA, BAT temperature, expired CO2, and heart rate. Skin cooling-evoked thermogenic, metabolic, and heart rate responses were inhibited by bilateral microinjections of bicuculline (GABAA receptor antagonist) into the preoptic area (POA), by bilateral microinjections of muscimol (GABAA receptor agonist) into the dorsomedial hypothalamic nucleus (DMH), or by microinjection of muscimol, glycine, 8-OH-DPAT (5-HT1A receptor agonist), or kynurenate (non-selective antagonist for ionotropic excitatory amino acid receptors) into the rostral raphe pallidus nucleus (rRPa), but not by bilateral muscimol injections into the lateral/dorsolateral part or ventrolateral part of the caudal periaqueductal gray. These results implicate the POA, DMH, and rRPa in the central efferent pathways for thermogenic, metabolic, and cardiac responses to skin cooling, and suggest that these pathways can be modulated by serotonergic inputs to the medullary raphe.
Keywords: GABA, glutamate, serotonin, somatosensory thermal afferent, thermoregulation
Introduction
The body temperature of homeothermic animals is maintained within a narrow range through the fine control by the nervous system. As a framework of the central thermoregulatory system, it is proposed that temperature information from thermoreceptors in the skin, several brain regions, and peripheral body core structures is collected and integrated in the preoptic area (POA), and efferent command signals leading to the defense of body temperature are sent from the POA to peripheral thermoregulatory effectors (30). However, our understanding of the afferent and efferent neuronal pathways responsible for the regulation of body temperature remains incomplete.
Earlier work using conscious animals has shown that temperature information from the skin and from the brain or body core structures provides independent signals contributing to the onset of thermoregulatory responses (21, 41). To narrowly maintain body temperature, however, rapid thermoregulatory responses to changes in environmental temperature are essential, and thermal afferent information from the skin, whose temperature is first affected in the body by environmental temperature changes, should be the most important for such rapid thermoregulatory responses. In a recent report, deep body core and brain temperatures of conscious rats were not substantially decreased during exposure to a cold environment (4°C, 2 h), while skin surface temperature dropped rapidly (6). This observation indicates that thermoreceptors in the skin, rather than in the brain or peripheral body core structures, provide the dominant temperature information necessary for rapid feedforward thermoregulatory responses to environmental temperature changes.
For the defense of body temperature, efferent signals from central thermoregulatory networks regulate metabolism in specific thermogenic tissues, as well as heat dissipation from the body surface. The former includes shivering and non-shivering thermogenesis and the latter includes sweating, salivation, panting, piloerection, and cutaneous vasoconstriction. Among them, non-shivering thermogenesis in brown adipose tissue (BAT) is well-known as a major heat source for the defense of body temperature in cold environments, especially in rodents (7), and the central mechanisms controlling BAT thermogenesis have been extensively studied (27).
BAT thermogenesis is governed by the sympathetic nervous system, and anatomical observations from transneuronal tracing after inoculation of pseudorabies virus into BAT suggest that BAT-controlling neurons in autonomically-related brain regions are organized in a hierarchical network in which more rostral regions such as the POA influence the activity of increasingly caudal structures such as the dorsomedial hypothalamic nucleus (DMH), caudal periaqueductal gray (cPAG), and rostral medullary raphe nuclei (1, 3, 8, 9, 32, 36, 50). Furthermore, chemical stimulation of neurons in the DMH, cPAG, and rostral medullary raphe nuclei results in an increase in BAT thermogenesis (9, 11, 25, 28, 29, 32, 51, 52), and the POA provides a tonic inhibition of BAT thermogenesis (10). Thus, it is possible that the tonic inhibitory signals descending from the POA determine the activity level of the neurons in the caudal regions whose stimulation leads to BAT thermogenesis. On the basis of this hypothesis for the central efferent system driving cold-defense responses, we reasoned that disinhibition or stimulation of the POA or inhibition of the DMH, cPAG, or rostral medullary raphe should reduce cold-evoked thermogenic responses in BAT. To determine whether and how these autonomically-related regions contribute to the central efferent system for thermogenic responses in cold environments, we examined the effect of chemical modulation of neurons in these autonomically-related regions on skin cooling-evoked BAT sympathetic nerve activation and BAT thermogenesis with an in vivo electrophysiological approach.
MATERIALS AND METHODS
Animals
Twenty-six male Sprague-Dawley rats (250–500 g) contributed to the present study. The animals were housed with ad libitum access to food and water in a room air-conditioned at 22–23°C with a standard 12 h light/dark cycle. All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health & Science University.
Preparation
Rats were transitioned to intravenous urethane (0.8 g/kg) and α-chloralose (80 mg/kg) anesthesia after cannulation of a femoral artery, a femoral vein, and the trachea under anesthesia with 3% isoflurane in 100% O2. To record arterial pressure and heart rate (HR), the arterial cannula was attached to a pressure transducer. To monitor skin temperature (Tskin), the trunk was shaved and a copper-constantan thermocouple (Physitemp, Clifton, NJ) was taped onto the abdominal skin. The trunk was wrapped with a plastic water jacket, which did not cover the dorsal portion of the rostral half of the back needed for access to the interscapular BAT and the vertebra to be clamped. The animal was positioned in a stereotaxic apparatus with a spinal clamp on the tenth thoracic vertebra; the incisor bar level was adjusted so that the bregma and lambda were at the same dorsal level. To monitor brain temperature (Tbrain), a needle-type copper-constantan thermocouple (0.33 mm diameter; Physitemp) was perpendicularly inserted into the basal forebrain (2.2 mm anterior to the bregma, 1.3 mm lateral (right) to the midline, 7.0–7.5 mm ventral to the brain surface). The animal was then paralyzed with D-tubocurarine (0.6 mg i.v. initial dose, supplemented with 0.3 mg every 1 h), and artificially ventilated with 100% O2 (60 cycles/min, tidal volume: 3.5 ml). Mixed expired CO2 was measured using a capnometer (Capnogard 1265, Respironics, Murrysville, PA), and rectal temperature (Trec) was monitored using a copper-constantan thermocouple inserted into the rectum. Trec was maintained at 36.5–38.0°C by perfusing the water jacket with warm or cold water. All thermocouples were connected to a thermocouple meter (TC-1000, Sable Systems, Las Vegas, NV) for temperature display and acquisition of the analogue signal.
Postganglionic BAT sympathetic nerve activity (SNA) was recorded from the central cut end of a nerve bundle isolated from the ventral surface of the right interscapular BAT pad after dividing it along the midline and reflecting it laterally. The nerve bundle was placed on bipolar hook electrodes under mineral oil. Nerve activity was filtered (1–300 Hz) and amplified (× 5,000–50,000) with a CyberAmp 380 (Axon Instruments, Union City, CA). BAT temperature (TBAT) was monitored with a copper-constantan thermocouple inserted into the intact left interscapular BAT pad. Physiological variables were digitized and recorded to a computer hard disk using Spike 2 software (CED, Cambridge, UK).
Experimental procedure
Protocol 1
The skin was cooled by perfusing the water jacket with ice-cold water for 150–250 sec and then rewarmed by switching to perfusion with warm water.
Protocol 2
The animals received saline vehicle and drug microinjections into the POA, dorsomedial hypothalamus, lateral/dorsolateral cPAG (l/dlcPAG), ventrolateral cPAG (vlcPAG), or rostral part of the raphe pallidus nucleus (rRPa) (33) during the BAT thermogenic response to skin cooling. Skin cooling was continued for at least 100 sec after the microinjections, and then the skin was rewarmed.
Drugs were obtained from Sigma Chemical (St. Louis, MO) dissolved in 0.9% saline and ejected through glass micropipettes (tip inner diameter, 20–30 μm) using a Picospritzer II (General Valve, Fairfield, NJ). To disinhibit local neurons, the GABAA receptor antagonist, (−)-bicuculline methiodide (0.02–2 mM, 60 nl/injection) was microinjected bilaterally into the POA (0–0.1 mm posterior to the bregma, 0.5–0.7 mm lateral to the midline, and 7.5–8.5 mm ventral to the brain surface). To inhibit local neurons, the GABAA receptor agonist, muscimol (2 mM) was bilaterally microinjected into the dorsomedial hypothalamic region consisting of the DMH and dorsal hypothalamic area (DH) (60–120 nl/injection; 3.3 mm posterior to the bregma, 0.5–0.7 mm lateral to the midline, and 7.8–8.8 mm ventral to the brain surface), into the l/dlcPAG (120 nl/injection; 7.8 mm posterior to the bregma, 0.5–0.7 mm lateral to the midline, and 4.2–4.6 mm ventral to the brain surface), or into the vlcPAG (120 nl/injection; 8.7 mm posterior to the bregma, 0.9–1.2 mm lateral to the midline, and 5.0–5.5 mm ventral to the brain surface). The midline rRPa (2.3 mm posterior to the interaural line, on the midline, and 9.5–9.7 mm ventral to the brain surface) received single microinjections (60 nl) of saline; muscimol (2 mM); glycine (0.5 M); the non-selective ionotropic excitatory amino acid receptor antagonist, kynurenic acid (0.1 M); or the 5-HT1A receptor agonist, (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT; 10 mM). Most animals received microinjections of more than one drug. In each of these cases, a recovery period of at least 1 hour was allowed between brain microinjections of different drugs, at which time responses to skin cooling and rewarming were not different from the original control values.
To mark the microinjection sites, 10–20 nl of 0.2% fluorescent microspheres (diameter, 0.1 μm; Molecular Probes, Eugene, OR) in saline was injected at the same stereotaxic coordinates through the same micropipette. After the physiological recordings, the animals were transcardially perfused with a 10% formaldehyde solution, and the brains were cryoprotected by shaking in a 30% sucrose solution overnight. After the tissue was sectioned at a thickness of 40 μm with a freezing microtome, the locations of the microinjections were identified by detecting the fluorescent microspheres under an epifluorescence microscope.
Data analysis
Spike 2 software was used to obtain a continuous measure (4-s bins) of BAT SNA amplitude by calculating the root mean square amplitude of the BAT SNA (square root of the total power in the 0–20 Hz band) from the autospectra of sequential 4-s segments of BAT SNA. Control (baseline) values of BAT SNA, TBAT, expired CO2, HR, mean arterial pressure (MAP), Trec, Tbrain, and Tskin were the averages during the 1-min period immediately prior to skin cooling.
In protocol 1, skin cooling-evoked response values for TBAT, expired CO2, HR, and Tskin were obtained at the end of skin cooling, and skin cooling-evoked response values for BAT SNA, MAP, Trec, and Tbrain were the averages during the 1-min period immediately prior to the end of skin cooling. Values for TBAT, expired CO2, HR, and Tskin during rewarming were obtained at 4 min after the start of rewarming the skin, and values for BAT SNA, MAP, Trec, and Tbrain during rewarming were the averages during the 4th min after the start of skin rewarming. Statistical analyses were performed using a repeated measures ANOVA (Instat 2.00 program; Graph Pad, San Diego, CA) to detect significant differences among the values at different time points, and a Bonferroni post hoc multiple comparisons test was then performed to detect pairwise differences.
In protocol 2, skin cooling-evoked response values for TBAT, expired CO2, and HR were taken just prior to the first of the bilateral microinjections, and skin cooling-evoked response values for BAT SNA and MAP were the averages during the 1-min period immediately prior to the first microinjection. Drug treatment effect values for TBAT, expired CO2, and HR were taken at the end of skin cooling, and treatment effect values for BAT SNA and MAP were the averages during the 1-min period immediately prior to the end of skin cooling. An unpaired Student’s t test was used to detect significant differences in post-injection changes in the variables between saline- and drug-microinjected groups.
All physiological data are presented as the means ± SEM, and statistical results with a P value of < 0.05 was considered significant.
RESULTS
Skin cooling-evoked physiological responses
We examined the effect of skin cooling on sympathetic thermogenic activity in the interscapular BAT by monitoring BAT SNA and TBAT, on metabolic rate by monitoring expired CO2, and on cardiovascular tone by monitoring HR, and MAP (n = 6). Under resting conditions in urethane-chloralose-anesthetized rats with a Trec of 37.3 ± 0.1°C and a Tskin of 38.1 ± 0.3°C, BAT SNA was typically low (Fig. 1), and resting values for TBAT, expired CO2, HR, and MAP before skin cooling are shown in Table 1. Skin cooling (duration, 199 ± 15 sec; ΔTskin, −5.2 ± 0.5°C) increased BAT SNA by 660% of baseline, TBAT by 0.9°C, expired CO2 by 0.4%, and HR by 49 bpm, and these changes were statistically significant (Fig. 1 and Table 1). Although MAP was slightly increased by skin cooling in many cases (Fig. 1), the average change was not statistically significant (Table 1). All the physiological variables increased by skin cooling were decreased to the resting levels by rewarming the skin (Fig. 1 and Table 1). Throughout the skin cooling and rewarming, changes in Trec (body core temperature) and Tbrain were very small (Table 1), and Tbrain was slightly increased by the end of skin cooling in all the experimental cases, indicating that the observed changes in BAT SNA, TBAT, expired CO2, HR, and MAP were induced by the effect of skin temperature changes rather than changes in body core parts or in the brain.
Fig. 1.
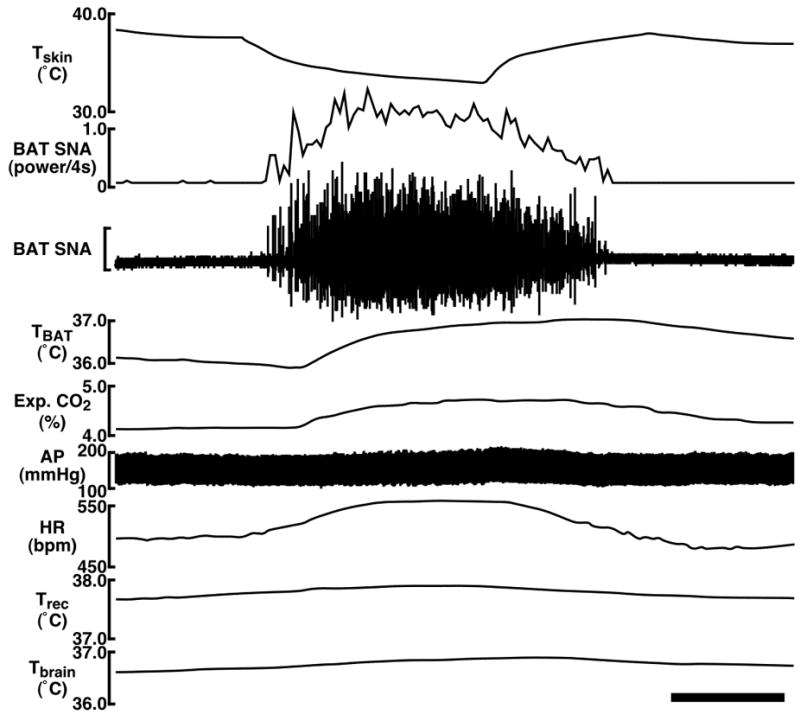
Changes in BAT SNA, TBAT, expired (Exp.) CO2, HR, arterial pressure (AP), Trec, and Tbrain in response to cooling the trunk skin. The horizontal scale bar represents 100 sec, and the vertical scale bar for the BAT SNA trace represents 100 μV. Note that Trec and Tbrain do not change substantially during the skin cooling and rewarming, indicating that the observed changes in BAT SNA, TBAT, Exp. CO2, HR, and AP were evoked by the effect of skin temperature changes rather than changes in body core parts or in the brain.
TABLE 1.
Skin cooling-evoked changes in physiological variables
| Before cooling | End of cooling | During rewarming | |
|---|---|---|---|
| BAT SNA (% of baseline) | 100 ± 26 | 760 ± 128*** | 96 ± 27††† |
| TBAT (°C) | 35.5 ± 0.3 | 36.4 ± 0.3*** | 35.8 ± 0.4*, †† |
| Expired CO2 (%) | 4.0 ± 0.1 | 4.4 ± 0.1*** | 4.0 ± 0.1††† |
| HR (bpm) | 425 ± 17 | 474 ± 22*** | 408 ± 18††† |
| MAP (mmHg) | 128 ± 5 | 131 ± 5 | 122 ± 5† |
| Tskin (°C) | 38.1 ± 0.3 | 32.9 ± 0.5*** | 38.0 ± 0.7††† |
| Trec (°C) | 37.3 ± 0.1 | 37.4 ± 0.1 | 37.3 ± 0.2†† |
| Tbrain (°C) | 36.5 ± 0.1 | 36.7 ± 0.1** | 36.6 ± 0.2† |
The data represent the mean ± SEM (n = 6) of the physiological variables in the course of a skin cooling-rewarming challenge. Values before cooling were the averages during the 1-min period immediately prior to skin cooling. Values of end of cooling were those at the end of the skin cooling period (TBAT, expired CO2, HR, and Tskin) or the averages during the 1-min period immediately prior to the end of the skin cooling (BAT SNA, MAP, Trec, and Tbrain). Values during rewarming were those at 4 min after the start of rewarming the skin (TBAT, expired CO2, HR, and Tskin) or the averages during the 4th min after the start of the skin rewarming (BAT SNA, MAP, Trec, and Tbrain). Values of BAT SNA are expressed as percentage of the mean value before cooling.
P < 0.05,
P < 0.01, and
P < 0.001 compared with the values before cooling;
P < 0.05,
P < 0.01, and
P < 0.001 compared with the values at the end of cooling (Bonferroni multiple comparisons test following a repeated measures ANOVA).
We next examined the effect on skin cooling-evoked changes in BAT SNA, TBAT, expired CO2, HR, and MAP of altering neuronal discharge in a series of brain regions potentially involved in mediating the skin cooling-evoked responses in BAT thermogenesis: the POA, DMH, l/dlcPAG, vlcPAG, and rRPa. A total of 52 drug or saline vehicle microinjections were made during the rising phase of the increase in BAT SNA evoked during skin cooling, and skin cooling was continued for at least 100 sec after microinjections. Changes in physiological variables after microinjections were examined at the end of the skin cooling episodes, whose total durations were 200–300 sec. At the time of the microinjections, skin cooling had increased BAT SNA by 752 ± 64% of baseline, TBAT by 0.3 ± 0.04°C, expired CO2 by 0.3 ± 0.02%, HR by 31 ± 3 bpm, and MAP by 3 ± 1 mmHg (n = 52). Comparison with those in Table 1 indicates that, on average, microinjections were made near the peak of the skin cooling-evoked responses.
Effect of disinhibition of neurons in the POA on skin cooling-evoked increases in physiological variables
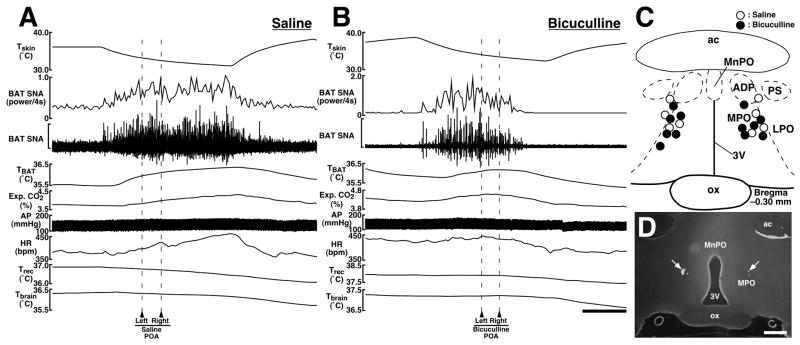
Bilateral microinjections of the GABAA receptor antagonist, bicuculline into the POA (n = 6; Fig. 2C,D) reversed the skin cooling-evoked increase in BAT SNA (Fig. 2B). By the end of skin cooling, BAT SNA was reduced by 89% of the skin cooling-evoked increase, and TBAT, expired CO2, and HR were reduced to their resting levels (Fig. 2B and Table 2). In contrast, BAT SNA, TBAT, expired CO2, HR, and MAP kept rising until the end of skin cooling after bilateral microinjections of saline into the POA (n = 4; Fig. 2A and Table 2). The changes in BAT SNA, TBAT, expired CO2, and HR after bicuculline microinjections into the POA were significantly different from those after saline microinjections into the POA (Table 2). The changes in MAP after microinjections into the POA were not statistically different between the bicuculline- and saline-microinjected groups (Table 2).
Fig. 2.
A and B: Effects of microinjections of saline (A) or bicuculline (B) into the POA on skin cooling-evoked changes in physiological variables. Microinjections were made bilaterally at the time points indicated by arrowheads and broken lines. The horizontal scale bar represents 100 sec, and vertical scale bars for BAT SNA traces represent 50 μV in (A) and 400 μV in (B). C: Location of the sites of all intra-POA microinjections for the group data shown in Table 2. Microinjection sites are plotted on a brain map adopted from an atlas (40). D: Representative view of sites of bilateral microinjections into the POA. Each injection site is clearly identified as a cluster of fluorescent beads (arrows). The scale bar represents 0.5 mm. Abbreviations: 3V, third ventricle; ac, anterior commissure; ADP, anterodorsal preoptic nucleus; LPO, lateral preoptic area; MnPO, median preoptic nucleus; MPO, medial preoptic area; ox, optic chiasm; and PS, parastrial nucleus.
TABLE 2.
Effect of microinjections into autonomic brain regions on skin cooling-evoked increases in physiological variables
| Site | Injection | n | ΔBAT SNA
(% change) |
ΔTBAT (°C) |
ΔExp. CO2 (%) |
ΔHR
(bpm) |
ΔMAP
(mmHg) |
|---|---|---|---|---|---|---|---|
| POA | Saline | 4 | +24 ± 15 | +0.3 ± 0.1 | +0.2 ± 0.1 | +23 ± 17 | +1 ± 2 |
| Bicuculline | 6 | −89 ± 4*** | −0.2 ± 0.1* | −0.2 ± 0.1* | −41 ± 10** | +9 ± 5 | |
| DMH/DH | Saline | 3 | +98 ± 11 | +0.4 ± 0.1 | +0.3 ± 0.1 | +40 ± 17 | +5 ± 0.3 |
| Muscimol | 5 | −86 ± 7*** | −0.3 ± 0.1** | −0.3 ± 0.03*** | −25 ± 5** | −7 ± 2** | |
| l/dlcPAG | Saline | 3 | +7 ± 18 | +0.3 ± 0.1 | +0.2 ± 0.1 | +9 ± 4 | +1 ± 1 |
| Muscimol | 4 | +54 ± 22 | +0.6 ± 0.2 | +0.3 ± 0.1 | +5 ± 4 | +5 ± 4 | |
| vlcPAG | Saline | 3 | +29 ± 33 | +0.3 ± 0.1 | +0.1 ± 0.04 | +2 ± 11 | +7 ± 4 |
| Muscimol | 4 | +25 ± 24 | +0.2 ± 0.3 | +0.1 ± 0.1 | +3 ± 8 | +2 ± 1 | |
| rRPa | Saline | 4 | +29 ± 9 | +0.4 ± 0.1 | +0.1 ± 0.03 | +4 ± 2 | +2 ± 2 |
| Muscimol | 4 | −105 ± 1*** | −0.3 ± 0.1** | −0.2 ± 0.04*** | −27 ± 10* | −17 ± 9 | |
| Glycine | 4 | −102 ± 1*** | −0.3 ± 0.1*** | −0.3 ± 0.1*** | −35 ± 10** | −19 ± 8* | |
| Kynurenate | 4 | −109 ± 7*** | −0.4 ± 0.2** | −0.5 ± 0.1*** | −26 ± 3*** | −7 ± 2* | |
| 8-OH-DPAT | 4 | −103 ± 0.5*** | −0.5 ± 0.3* | −0.4 ± 0.1* | −65 ± 27* | −33 ± 19 |
Microinjections were made during the rising phase of the BAT SNA response to skin cooling, and the skin cooling was continued for at least 100 sec after the microinjections. Changes in BAT SNA, TBAT, expired (Exp.) CO2, HR, and MAP after microinjections were examined at the end of skin cooling (see MATERIALS AND METHODS). The data represent the mean ± SEM, and the injection sites are listed in the order of their rostrocaudal anatomical location. Changes in BAT SNA are expressed as percentage of skin cooling-evoked increase. Positive and negative values indicate increases and decreases from the skin cooling-evoked response values just prior to the microinjections, respectively.
P < 0.05,
P < 0.01, and
P < 0.001 compared with the group in which saline was injected into the same region (unpaired Student’s t test).
Effect of inhibition of neurons in the dorsomedial hypothalamus on skin cooling-evoked increases in physiological variables
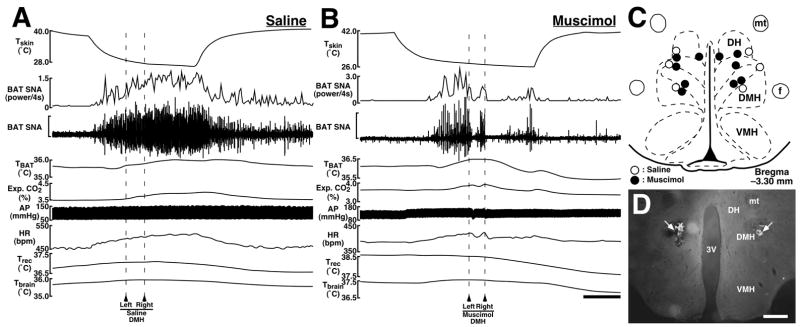
Bilateral microinjections of the GABAA receptor agonist, muscimol into the dorsomedial hypothalamic region consisting of the DMH and DH (n = 5; Fig. 3C,D) attenuated the level of BAT SNA by 86% of the increase evoked by skin cooling, and also reversed skin cooling-evoked increases in TBAT, expired CO2, HR, and MAP to the resting levels by the end of skin cooling (Fig. 3B and Table 2). When saline was microinjected into the DMH/DH, all the physiological variables continued to increase throughout the period of skin cooling (n = 3; Fig. 3A and Table 2). The post-injection changes in all the variables were significantly different between saline- and muscimol-microinjected groups (Table 2).
Fig. 3.
A and B: Effects of microinjections of saline (A) or muscimol (B) into the dorsomedial hypothalamic region consisting of the DMH and DH on skin cooling-evoked changes in physiological variables. Microinjections were made bilaterally at the time points indicated by arrowheads and broken lines. The horizontal scale bar represents 100 sec, and vertical scale bars for BAT SNA traces represent 300 μV in (A) and 50 μV in (B). C: Location of the sites of all intra-DMH/DH microinjections for the group data shown in Table 2. D: Representative view of sites of bilateral microinjections into the DMH as indicated by clusters of fluorescent beads (arrows). The scale bar represents 0.5 mm. Abbreviations: f, fornix; mt, mammillothalamic tract; and VMH, ventromedial hypothalamic nucleus.
Effect of chemical modulation of neuronal discharge in the rRPa on skin cooling-evoked increases in physiological variables
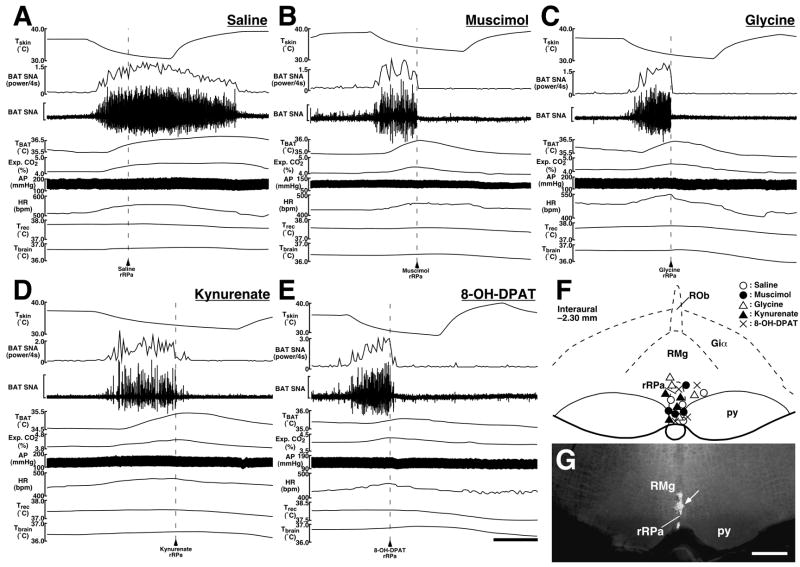
By the end of the skin cooling episode, the skin cooling-evoked increase in BAT SNA was completely reversed by a microinjection of muscimol (105% reduction; n = 4), glycine (102% reduction; n = 4), the non-selective ionotropic excitatory amino acid receptor antagonist kynurenate (109% reduction; n = 4), or the 5-HT1A receptor agonist 8-OH-DPAT (103% reduction; n = 4) into the rRPa (Fig. 4B–G and Table 2). These drug microinjections into the rRPa also decreased the levels of TBAT, expired CO2, and HR from those increased by skin cooling to the resting levels (Fig. 4B–E and Table 2). In contrast, a saline microinjection into the rRPa did not attenuate any of the physiological variables during skin cooling (n = 4; Fig. 4A and Table 2). The changes in BAT SNA, TBAT, expired CO2, and HR after the drug microinjections into the rRPa were statistically different from those after saline microinjections into the rRPa (Table 2). Although MAP was decreased after intra-rRPa microinjection of each of the drugs, and in some cases fell below the baseline level, MAP changes significantly different from the saline-injected group were obtained only with glycine and kynurenate microinjections (Table 2).
Fig. 4.
A–E: Effects of microinjections of saline (A), muscimol (B), glycine (C), kynurenate (D), or 8-OH-DPAT (E) into the rRPa on skin cooling-evoked changes in physiological variables. Microinjection was made at the time point indicated by an arrowhead and broken line. The horizontal scale bar represents 100 sec, and vertical scale bars for BAT SNA traces represent 100 μV in (A and C), 50 μV in (B), 400 μV in (D), and 40 μV in (E). F: Location of the sites of all microinjections into and around the rRPa for the group data shown in Table 2. G: Representative view of a microinjection site into the rRPa as indicated by a cluster of fluorescent beads (arrow). The scale bar represents 0.5 mm. Abbreviations: Giα, alpha part of the gigantocellular reticular nucleus; py, pyramidal tract; RMg, raphe magnus nucleus; and ROb, raphe obscurus nucleus.
Effect of inhibition of neurons in cPAG regions on skin cooling-evoked increases in physiological variables
Muscimol or saline was microinjected bilaterally into the l/dlcPAG at −7.8 mm caudal to the bregma (Fig. 5B,C) or into the vlcPAG at −8.7 mm caudal to the bregma (Fig. 5E,F), as in previous studies (11, 12). Skin cooling kept increasing BAT SNA, TBAT, expired CO2, HR, and MAP even after muscimol microinjections into either the l/dlcPAG (n = 4; Fig. 5A) or the vlcPAG (n = 4; Fig. 5D), and the changes in these variables after muscimol microinjections into the l/dlcPAG and vlcPAG were not significantly different from those after saline microinjections into the same regions (n = 3 for each region) (Table 2).
Fig. 5.
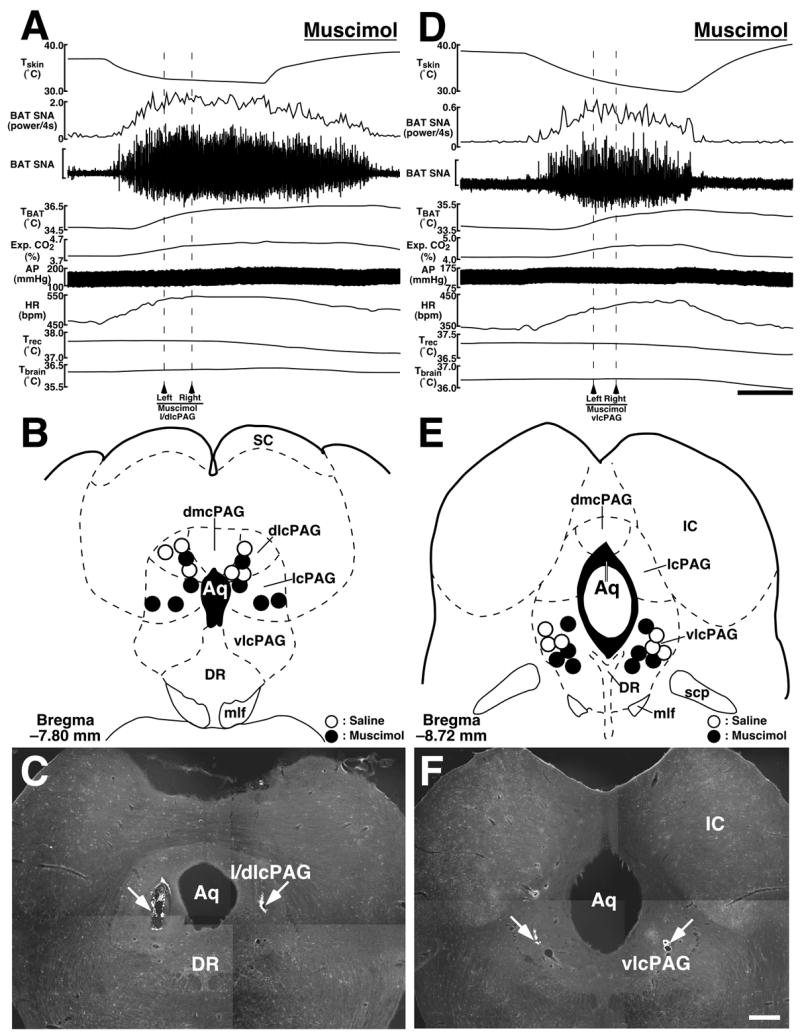
A and D: Effects of muscimol microinjections into the l/dlcPAG (A) or vlcPAG (D) on skin cooling-evoked changes in physiological variables. Microinjections were made bilaterally at the time points indicated by arrowheads and broken lines. The horizontal scale bar represents 100 sec, and vertical scale bars for BAT SNA traces represent 150 μV in (A) and 15 μV in (D). B and E: Location of the sites of all microinjections into the l/dlcPAG (B) and vlcPAG (E) for the group data shown in Table 2. C and F: Representative views of sites of bilateral microinjections into the l/dlcPAG (C) and vlcPAG (F) as indicated by clusters of fluorescent beads (arrows). The scale bar represents 0.5 mm. Abbreviations: Aq, aqueduct; dmcPAG, dorsomedial caudal periaqueductal gray; DR, dorsal raphe nucleus; IC, inferior colliculus; mlf, medial longitudinal fasciculus; SC, superior colliculus; and scp, superior cerebellar peduncle.
DISCUSSION
In the central thermoregulatory network, the POA plays a pivotal role as a site of integration of thermal information from the skin, several brain regions, and peripheral body core structures. Inhibition of the POA leads to increases in body core and brain temperatures (20, 38, 39) and transection caudal to the POA induces BAT thermogenesis (10). These previous findings suggest that the POA provides a tonic inhibition to caudal brain regions involved in the control of thermoregulatory effectors, including BAT, a major thermogenic organ especially in rodents (7). Furthermore, it is hypothesized that temperature information coming from the skin through somatosensory afferent pathways modulates the tonic activity of caudally projecting inhibitory neurons in the POA, which might be those previously characterized as warm-sensitive neurons (30). In the present study, microinjections of a GABAA receptor antagonist into the POA attenuated the skin cooling-evoked increases in sympathetically regulated thermogenic activity in BAT and whole-body metabolic rate as indicated by expired CO2. This result indicates that GABAergic neurotransmission in the POA is critical for the skin cooling-evoked stimulation of BAT thermogenesis, and also raises a possibility that skin-derived cold signals elicit a GABAergic attenuation of the tonic inhibitory activity of POA projection neurons, thereby leading to an increase in BAT thermogenesis (Fig. 6). The inhibitory effect of bicuculline in the POA on skin cooling-evoked BAT thermogenesis in the present study is consistent with recent reports that bicuculline into the POA (a) eliminated the skin cooling-evoked increase in oxygen consumption (38), an indicator of overall metabolic rate and (b) induced intense hypothermia in free-moving rats under cold ambient temperature (20), probably due to reduced thermogenesis and/or increased heat dissipation.
Fig. 6.
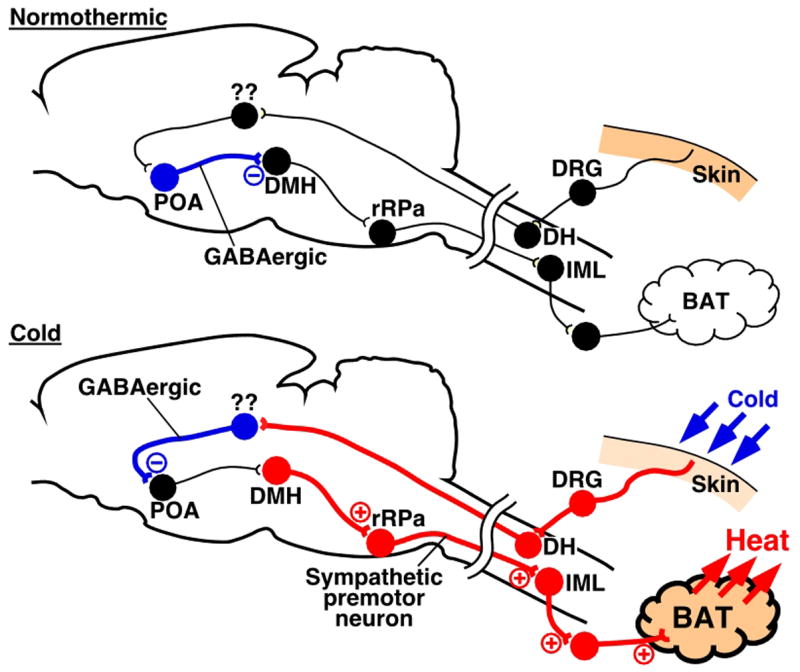
A current model of the neural pathways mediating skin cooling-evoked BAT thermogenesis. In a normothermic environment (Normothermic), GABAergic neurons in the POA tonically inhibit neurons in the DMH. In a cold environment (Cold), signals from somatosensory nerves in the skin activate GABAergic neurons in unknown regions through afferent pathways. The activated GABAergic neurons in the afferent pathway suppress the tonic firing of the GABAergic neurons in the POA and, thereby, disinhibited DMH neurons which activate BAT-controlling sympathetic premotor neurons in the rRPa, leading, in turn, to stimulation of the BAT-controlling sympathetic output system. For detailed discussion, see text. Blue, red, and black circles denote cell bodies of activated inhibitory neurons, activated excitatory neurons, and suppressed or resting neurons, respectively. Abbreviations: DH, dorsal horn; DRG, dorsal root ganglion; and IML, intermediolateral cell column.
Several recent findings suggest that the DMH is one of the most probable candidates for regions receiving a tonic inhibitory input from the POA in the regulation of BAT thermogenesis. POA neurons provide a dense direct projection to the DMH (35, 44, 47), and blockade of GABAA receptors in the DMH increases BAT SNA and BAT thermogenesis (9, 52). Furthermore, our recent anatomical observations revealed that GABAergic axon terminals derived from POA neurons are closely associated with DMH neurons projecting directly to the BAT-controlling sympathetic premotor region in the medullary raphe (see below) (35). In the present study, microinjections into the DMH/DH of muscimol, an agent widely used to inhibit virtually all mammalian neurons by virtue of its GABAA agonist actions (22), largely reduced skin cooling-evoked increases in BAT thermogenic activity and metabolic rate, indicating that DMH neurons are involved in the central mechanism for skin cooling-evoked sympathetic thermogenesis in BAT. These lines of evidence support a model in which the activity of the DMH neurons involved in the control of BAT is regulated by a tonic inhibitory input from GABAergic neurons in the POA and cold signals from the skin activate these DMH neurons by attenuating the tonic inhibitory input from the POA (Fig. 6).
Previous retrograde tracing studies have shown that many neurons distributed over the DMH and DH directly project to the medullary raphe region consisting of the rRPa and its surrounding raphe magnus nucleus (15, 18, 35, 42), in which sympathetic premotor neurons controlling BAT are localized (27, 32, 34). In the present study, inhibition of neurons in and around the rRPa with muscimol or glycine completely blocked skin cooling-evoked increases in BAT thermogenic activity and metabolic rate, indicating a critical role of medullary raphe neurons, probably BAT-controlling sympathetic premotor neurons, in thermogenic responses to cold environments. Consistent with this notion, neurons in these raphe regions have been shown to express Fos in response to cold exposure of rats (5, 8, 29, 32), and suppression of neuronal activity in the raphe pallidus nucleus with muscimol caused hypothermia in conscious rats under a normothermic condition (53). We have previously reported that Fos expression in the medullary raphe region in response to cold exposure is observed mostly in neurons expressing vesicular glutamate transporter 3 (VGLUT3) and that most of VGLUT3-expressing, putatively glutamatergic, neurons in the medullary raphe region oligosynaptically innervate the interscapular BAT as evidenced by transsynaptic retrograde tracing with pseudorabies virus (32). Furthermore, VGLUT3-expressing medullary raphe neurons were shown to directly project to sympathetic preganglionic neurons in the thoracic spinal cord (32, 46). These findings strongly support the hypothesis that BAT-controlling sympathetic premotor neurons, most of which express VGLUT3, in the medullary raphe region are situated strategically to mediate the skin cooling-evoked activation of thermogenic effectors: they may receive a direct excitatory input from the DMH and they control BAT thermogenesis through their direct projection to sympathetic preganglionic neurons controlling BAT (Fig. 6).
The present result that blockade of ionotropic excitatory amino acid receptors in the rRPa with kynurenate abolished skin cooling-evoked increases in BAT thermogenic activity and metabolic rate indicates that activation of BAT by cold stimuli requires excitatory amino acidergic, probably glutamatergic, excitation of neurons in the rRPa, likely BAT-controlling sympathetic premotor neurons. Although DMH neurons could be the source of this glutamatergic excitatory input (Fig. 6), the activity of BAT-controlling sympathetic premotor neurons in the medullary raphe region is also strongly regulated by tonic GABAergic inputs. The latter is based on the fact that blockade of GABAA receptors in the medullary raphe region increases BAT SNA (28, 29) and is supported by the observation that many GABAergic axon terminals are closely apposed to VGLUT3-expressing neurons in the medullary raphe region (34). Thus, it is possible that tonic GABAergic inputs suppress the activity of sympathetic premotor neurons controlling BAT under conditions that do not require thermogenesis, and cold-evoked excitatory inputs to the premotor neurons overcomes this tonic GABAergic inhibition. Another possibility, however, is that a reduction in the level of tonic inhibition to BAT-controlling sympathetic premotor neurons in the medullary raphe region contributes to the stimulation of BAT thermogenesis during skin cooling. In this case, a tonic or cold activated kynurenate-sensitive glutamatergic excitatory input to the sympathetic premotor neurons would be required for activation of BAT thermogenesis.
Activation of 5-HT1A receptors in the rRPa with 8-OH-DPAT inhibited skin cooling-evoked increases in BAT thermogenic activity and metabolic rate in the present study, similar to the effect on BAT thermogenesis evoked by intravenous administration of leptin (26). These results suggest that the sympathetic thermogenic efferent pathways can be modulated by serotonergic inputs to the medullary raphe through this serotonin receptor. Systemic administration of 5-HT1A receptor agonists induces hypothermia in rats (16), mice (central injection) (13), and humans (4) and also decreases BAT thermogenesis evoked in conscious rats by a cold environment (37). In light of the present result, the hypothermic effects of systemic 5-HT1A receptor agonists can, at least partly, be attributed to their action on 5-HT1A receptors located in the medullary raphe region.
Activation of the 5-HT1A receptor is considered to lead to inhibition of neuronal functions through negative coupling to adenylate cyclase via the G-protein, Gi (2), and histochemical studies have suggested that the 5-HT1A receptor is principally localized in somatodendritic portions of serotonergic neurons especially in midbrain raphe nuclei (45, 48), suggesting that it is an inhibitory somatodendritic autoreceptor. In the medullary raphe region, however, 5-HT1A receptor immunoreactivity was found in both serotonergic and non-serotonergic neuronal cell bodies as well as in a substantial population of neurons projecting to the intermediolateral cell column in the thoracic spinal cord (14). These observations support the potential localization of 5-HT1A receptors on the cell bodies of BAT-controlling sympathetic premotor neurons in the medullary raphe region and suggest that activation of these 5-HT1A receptors by serotonergic inputs could attenuate the activity of BAT-controlling premotor neurons through their postsynaptic action, leading to inhibition of BAT thermogenesis.
Bilateral microinjections of muscimol into the l/dlcPAG and vlcPAG did not show any significant effects on skin cooling-evoked physiological responses in the present study. In contrast, previous studies have suggested that these cPAG regions are involved in the central thermoregulatory mechanism: stimulation of the vlcPAG induced BAT thermogenesis (11) and DMH neurons projecting to the vlcPAG expressed Fos during cold exposure (49). In a recent study (12), unilateral muscimol microinjection into the l/dlcPAG decreased body temperature in conscious rats and attenuated an increase in body core temperature evoked by disinhibition of DMH neurons. Although, in the present study, we used a relatively high dose of muscimol and our microinjections were positioned bilaterally in the identical l/dlcPAG and vlcPAG sites, the cold-evoked stimulation of BAT SNA was unaffected. We conclude that these regions of the cPAG may mediate DMH-evoked activation of other (i.e., non-BAT) thermoregulatory effectors such as cutaneous vasoconstriction or that influences of the DMH on cPAG neurons may control BAT responses to non-thermal stimuli such as those evoking stress-induced thermogenesis.
In the present study, HR and MAP were also monitored. While skin cooling had only a slight effect on MAP, skin cooling significantly increased HR. By increasing cardiac output, such a cooling-evoked increase in HR could provide cardiac support for the large increase in BAT blood flow, facilitating the oxygen supply to BAT and the distribution of BAT heat production to the whole body. The skin cooling-evoked HR increase was strongly inhibited by all the drug microinjections into the POA, DMH, and rRPa, in parallel with the inhibitory effects of the same injections on skin cooling-evoked BAT sympathetic nerve activation. This observation indicates that thermogenic and cardiac responses to skin cooling use similar efferent pathways. This notion is consistent with HR increase evoked by inhibition of the POA or stimulation of the DMH or rRPa, as shown in previous studies (9, 17, 25, 29, 38, 42, 43, 52). However, it is still unknown whether or not the thermal efferent signaling pathways for BAT thermogenesis and cardiac responses share the same population of neurons in those relaying regions.
The central efferent pathways for skin cooling-evoked BAT thermogenesis that have been proposed in the present study (Fig. 6) show similarity to the putative fever-inducing central pathways, which have been described in our previous publications (27, 31, 35). This suggests that fever is a host defense strategy accomplished by taking over the central thermoregulatory system. In the model of fever-inducing neuronal pathways, tonic GABAergic inputs from pyrogen-receptive POA neurons control the activity of sympathetic premotor neurons in the medullary raphe region as well as DMH neurons, based on the anatomical findings that pyrogen-receptive POA neurons directly project to both the DMH and medullary raphe region (33, 35). It remains to be determined whether these pyrogen-receptive POA neurons also play a role in skin cooling-evoked thermogenesis. However, pathways independent of the DMH might transmit ancillary signals from the POA to the medullary raphe region, since the inhibition of skin cooling-evoked BAT sympathetic nerve activation by muscimol injections into the DMH was incomplete (86%) and it seems unlikely to be a dose effect in light of a complete (105%) suppression of the cooling-evoked nerve activation by a smaller dose of muscimol microinjected into the rRPa.
The present study focused on the central efferent pathways mediating thermogenic, metabolic, and cardiovascular responses to environmental temperature changes. Skin cooling evoked significant increases in BAT SNA and thermogenesis, as reported previously (24), as well as in expired CO2 and HR with no substantial changes in Tbrain and Trec, indicating that the observed increases in the thermogenic, metabolic, and cardiac parameters were induced by thermal afferent signals from the skin rather than the effect of temperature changes in body core parts or in the brain. Indeed, cooling of the brain, specifically the POA, is known to induce BAT thermogenesis (19). However, brain temperature of conscious rats is not substantially reduced under a cold environment (4°C, 2 h), while skin surface temperature drops rapidly (6). In addition, cooling the skin of anesthetized (the present study) and conscious animals (23) with core and brain temperatures kept constant in a normothermic range increases metabolic rate and thermogenesis. Therefore, information from the skin thermoreceptors, rather than local temperature in the POA, provides the dominant afferent signal leading to feedforward thermoregulatory responses to a physiological range of environmental temperature changes. Sensing temperature in deep body core and brain regions including the POA might be important for defending body temperature under severe conditions, for example, in extremely cold environments or during surgery under general anesthesia. The information obtained in the present study contributes to our understanding of the central mechanisms within the fundamental thermoregulatory network maintaining body temperature in homeothermic animals.
Acknowledgments
This work was supported by NIH grants (NS40987 and DK57838). K.N. is a fellow for research abroad supported by the Japan Society for the Promotion of Science.
References
- 1.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 2.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacol. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 4.Blier P, Seletti B, Gilbert F, Young SN, Benkelfat C. Serotonin 1A receptor activation and hypothermia in humans: lack of evidence for a presynaptic mediation. Neuropsychopharmacol. 2002;27:301–308. doi: 10.1016/S0893-133X(02)00318-4. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B, Taché Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 6.Bratincsák A, Palkovits M. Evidence that peripheral rather than intracranial thermal signals induce thermoregulation. Neuroscience. 2005;135:525–532. doi: 10.1016/j.neuroscience.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 8.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 9.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512:883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XM, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett. 2002;331:17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 12.de Menezes RCA, Zaretsky DV, Fontes MAP, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res. 2006;1092:129–137. doi: 10.1016/j.brainres.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin GM, De Souza RJ, Green AR. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT): a model of presynaptic 5-HT1 function. Neuropharmacol. 1985;24:1187–1194. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 14.Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT1A receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- 15.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars α demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 16.Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MAP, Dampney RAL. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R824–R832. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- 18.Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphé pallidus in the rat. Exp Brain Res. 1987;66:500–506. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- 19.Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn J Physiol. 1984;34:939–943. doi: 10.2170/jjphysiol.34.939. [DOI] [PubMed] [Google Scholar]
- 20.Ishiwata T, Saito T, Hasegawa H, Yazawa T, Kotani Y, Otokawa M, Aihara Y. Changes of body temperature and thermoregulatory responses of freely moving rats during GABAergic pharmacological stimulation to the preoptic area and anterior hypothalamus in several ambient temperatures. Brain Res. 2005;1048:32–40. doi: 10.1016/j.brainres.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Jessen C. Independent clamps of peripheral and central temperatures and their effects on heat production in the goat. J Physiol. 1981;311:11–22. doi: 10.1113/jphysiol.1981.sp013570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston GAR. GABAA receptor pharmacology. Pharmacol Ther. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnen G, Jessen C. The metabolic response to skin temperature. Pflügers Arch. 1988;412:402–408. doi: 10.1007/BF01907559. [DOI] [PubMed] [Google Scholar]
- 24.Kurosawa M. Reflex changes in thermogenesis in the interscapular brown adipose tissue in response to thermal stimulation of the skin via sympathetic efferent nerves in anesthetized rats. J Auton Nerv Syst. 1991;33:15–24. doi: 10.1016/0165-1838(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 25.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R832–R837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- 27.Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- 30.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K. Fever-inducing sympathetic neural pathways. J Therm Biol. 2004;29:339–344. [Google Scholar]
- 32.Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005;51:1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 37.Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 38.Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R306–R313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- 39.Osborne PG, Onoe H, Watanabe Y. GABAergic system inducing hyperthermia in the rat preoptic area: its independence of prostaglandin E2 system. Brain Res. 1994;661:237–242. doi: 10.1016/0006-8993(94)91200-9. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 41.Sakurada S, Shido O, Fujikake K, Nagasaka T. Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Jpn J Physiol. 1993;43:659–667. doi: 10.2170/jjphysiol.43.659. [DOI] [PubMed] [Google Scholar]
- 42.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 43.Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 45.Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct Immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 46.Stornetta RL, Rosin DL, Simmons JR, Mcquiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and γ-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- 47.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 48.Vergé D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, Nakamura K, Matsumura K, Kanosue K, König M, Thiel HJ, Boldogköi Z, Toth I, Roth J, Gerstberger R, Hübschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimatsu H, Egawa M, Bray GA. Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate. Brain Res. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- 52.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 53.Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABAA receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]