Abstract
Two cannabinoid receptors have been identified: CB1, present in the central nervous system (CNS) and to a lesser extent in other tissues, and CB2, present outside the CNS, in peripheral organs. There is evidence for the presence of CB2-like receptors in peripheral nerve terminals. We report now that we have synthesized a CB2-specific agonist, code-named HU-308. This cannabinoid does not bind to CB1 (Ki > 10 μM), but does so efficiently to CB2 (Ki = 22.7 ± 3.9 nM); it inhibits forskolin-stimulated cyclic AMP production in CB2-transfected cells, but does so much less in CB1-transfected cells. HU-308 shows no activity in mice in a tetrad of behavioral tests, which together have been shown to be specific for tetrahydrocannabinol (THC)-type activity in the CNS mediated by CB1. However, HU-308 reduces blood pressure, blocks defecation, and elicits anti-inflammatory and peripheral analgesic activity. The hypotension, the inhibition of defecation, the anti-inflammatory and peripheral analgesic effects produced by HU-308 are blocked (or partially blocked) by the CB2 antagonist SR-144528, but not by the CB1 antagonist SR-141716A. These results demonstrate the feasibility of discovering novel nonpsychotropic cannabinoids that may lead to new therapies for hypertension, inflammation, and pain.
C;;3annabis sativa preparations have been known for millennia as therapeutic agents against various diseases (1). Their active constituent, Δ9-tetrahydrocannabinol (THC) (2), is prescribed today, under the generic name Dronabinol, against vomiting and for enhancement of appetite, mainly in AIDS patients. However, separation between the therapeutically undesirable psychotropic effects from the clinically desirable ones has not been reported with agonists that bind to cannabinoid receptors. THC, as well as the two major endocannabinoids identified so far, anandamide (3) and 2-arachidonylglycerol (2-AG) (4, 5), produce most of their effects by binding to both the CB1 and CB2 cannabinoid receptors.
The CB1 receptor is present in the central nervous system (CNS) (6, 7) and, to a lesser extent, in other tissues. Although the CB2 receptor is not present on central neurons, it is present elsewhere, mostly in peripheral tissue associated with immune functions, including macrophages and B cells (8). CB2-like receptors may be present in peripheral nerve terminals (refs. 9 and 10; for recent reviews, see refs. 11–14). Although the effects mediated by CB1, mostly in the CNS, have been thoroughly investigated, those mediated by CB2 have not been well explored.
A direct route that can clarify the CB2 effects and facilitate the introduction of new therapeutic entities is the preparation and evaluation of specific CB2 agonists. We report now the synthesis of such a specific ligand, code named HU-308, its differential binding to CB1 and CB2, its effect on cyclic AMP production, and its action on several in vivo assays known to be affected by cannabinoids.
Materials and Methods
Synthesis and Administration of HU-308.
The starting materials, 4-hydroxymyrtenyl pivalate (I), 5-(1,1-dimethylheptyl)resorcinol (II), and the intermediate (III) in the synthesis of HU-308, were prepared as previously reported (15). The synthesis of HU-308 is described in Scheme S1. The indicated structure of HU-308 (structure V), melting point 50°C, [α]D +127o (c = 2.87 mg/ml of CHCl3), was supported by NMR, GC-MS, and high-resolution MS (HRMS) data. NMR, 300 MHz (CDCl3) δ: 6.45 (2H, s, aromatic), 5.7 (1H, olefinic), 4.12 (2H, CH3O−), 4.01 (1H, benzylic), 3.7 (6H, OCH3); HRMS calculated for C27H42O3 414.3134, found 414.3114.
Scheme 1.
Synthesis of HU-308. Step a, dry p-toluenesulfonic acid in methylene chloride; step b, potassium carbonate, methanol; step c, lithium aluminum hydride.
HU-308 was dissolved in ethanol/Emulphor/saline (1:1:18) as described previously for other cannabinoids (16, 17). HU-308 was administered i.p. into mice in the behavioral, the anti-inflammatory, and the antinociceptive assays. In experiments in which blood pressure was monitored, it was administered i.v. into rats. The time schedules and details of administration are described in the legends of Figs. 2–6.
Figure 2.
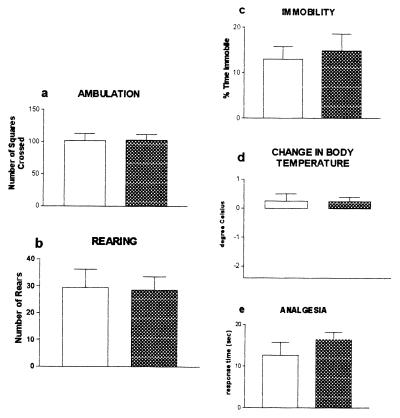
Absence of psychoactive cannabinoid effect of HU-308. Mice (female C57/BL6) were tested 2.5 hr after i.p. injection of HU-308 (40 mg/kg), in the tetrad of tests for cannabinoid activity: Ambulation (a) and rearing (b) in open field; immobility on a ring (“catalepsy”) (c); hypothermia (d); and antinociception on a hot plate (e). It should be noted that no histopathological damage was observed when mice were kept for up to 60 s on a 59°C hot plate (44). Open bars, vehicle-treated; shaded bars, HU-308.
Figure 6.
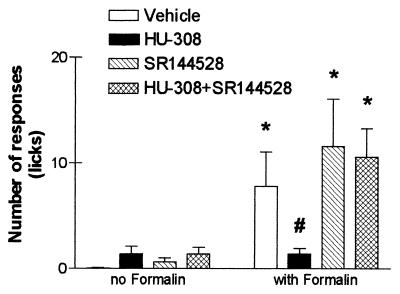
Effects of HU-308 with or without SR-144528 on formalin-induced peripheral pain. Vehicle or SR-144528 (0.5 mg/kg) was injected, i.p. 15 min before an injection of vehicle or HU-308 (50 mg/kg). Ninety min later, formalin was injected s.c. into one hind paw. Pain was assessed as the total number of licks of the injected paw, recorded every 5 min for 1 hr. Early (5 min) and late (25–60 min) phase pain were observed as described (21). However, HU-308-induced effects were observed only during the late phase. Data collected at 30 min after formalin injection are presented here. *, Significantly different from non-formalin-treated mice (P < 0.05); #, significantly different from vehicle-treated mice that had also received formalin (P < 0.05).
Receptor Binding Assays.
The CB1 binding assays were performed with synaptosomal membranes prepared from rat brains (3). The CB2 assays were performed with transfected cells (4). All assays were done in triplicate. The previously described probe [3H]HU-243 was used in a centrifugation-based ligand binding assay (3, 18). It has a Ki value of 45 ± 7 pM.
Cyclic AMP Assay.
The ability of HU-308 to inhibit forskolin-stimulated cyclic AMP production in Chinese hamster ovary (CHO) cells, stably transfected with human CB1 or CB2 receptors, was measured by a method we have used previously for the bioassay of other cannabinoids (19). The effect of HU-308 on cyclic AMP production has been expressed in percentage terms and mean values for EC50; maximal effects (Emax) and the SEM or 95% confidence limits of these values have been calculated by nonlinear regression analysis with the equation for a sigmoid concentration-response curve (Prism software from GraphPad, San Diego) (19).
Animals and Drugs.
Female Sabra mice (2 months old, Harlan–Sprague–Dawley, Jerusalem) were used in a series of tests for psychotropic effects (the “tetrad”), for assessing intestinal immotility (defecation), and in the assays for inflammation and peripheral pain. Groups of five mice were used in each experiment. Blood pressure was measured in male Sabra rats. HU-308, SR-141716A, and SR-144528 (the latter two were a generous gift of Sanofi Recherche, Paris) were dissolved in a vehicle (ethanol/Emulphor/saline = 1:1:18) and injected in volumes of 0.1 ml/10 g in mice or 0.1 ml/100 g in rats.
The experiments on animals were performed according to standards determined by the Committee on Ethics in Animal Research of the Hebrew University of Jerusalem.
The Tetrad of Pharmacological Assays in Mice.
A series of four consecutive observations were performed on each mouse, following a standard procedure employed to evaluate psychoactive cannabinoid-induced effects in mice (16) with time intervals similar to those described previously by our group (17). In short, at various times after injection (see Results and Discussion), mice were tested in four assays consecutively: (i) motor activity (ambulation and rearing) in an open field (20 × 30 cm, divided into 12 squares of equal size) for 8 min; (ii) immobility (catalepsy) on a ring of 5.5 cm diameter for 4 min; (iii) rectal temperature with a telethermometer (Yellow Springs Instruments); and (iv) antinociception on a hot plate maintained at 55°C, measured as the latency (in s) until the first hind paw lick or jump from the plate (the latter response was rarely observed) with a maximum of 45 s.
Intestinal Immotility.
Immediately after injection of HU-308 (10 or 20 mg/kg), the mice were separated into individual cages and the number of fecal pellets was recorded every 15 min for 2 hr as a measure of intestinal motility. Rectal temperature was recorded as a measure of central activity.
Arachidonic Acid-Induced Ear Inflammation in the Mouse.
Ear inflammation was measured by assessing ear tissue swelling after topical application of arachidonic acid. Nonsteroidal anti-inflammatory drugs have been shown to reduce swelling in this model (20). Thus, at various times after i.p. injections (see Fig. 5) of HU-308 (50 mg/kg), arachidonic acid was applied to the inner surface of one ear (4.5 mg dissolved in 5 μl of ethanol). The opposite ear served as control (5 μl of ethanol). Ear thickness was determined (in 0.01-mm units) by using a dial thickness gauge (Mitutoyo, Japan) every 15 min for 90 min, starting immediately after arachidonic acid application.
Figure 5.
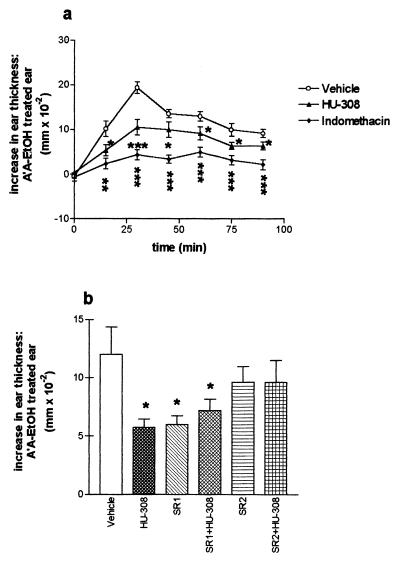
Effects of HU-308 and indomethacin on arachidonic acid (A′A)-induced swelling of the ear. The results are presented as the difference between the A′A-treated ear and the EtOH-treated ear, n = 5 for each treatment group. Mice (female Sabra) were treated with 4.5 mg of A′A (in 5 μl of EtOH), dispersed on the inner surface of one of the ears. The other ear was treated with 5 μl of EtOH. Ear swelling was assessed by measuring ear thickness with a dial thickness gauge, just before treatment, thence, every 15 min after application, for 90 min. (a) Time curve illustrating that peak swelling of the ear was achieved at about 30 min after A′A application. Vehicle, HU-308 (50 mg/kg), or indomethacin (20 mg/kg) was injected i.p. 60 min before A′A. Injection of HU-308 at 30 or 90 min before A′A yielded similar results (data not shown). (b) Effects of CB1 and CB2 receptor antagonists on the anti-inflammatory effect of HU-308. The CB1 antagonist (SR1 = SR-141716A, 5 mg/kg) or the CB2 receptor antagonist (SR2 = SR-144528, 1 mg/kg) was injected i.p., and followed 60 min afterward by HU-308 administration i.p. (50 mg/kg). A′A was administered 50 min after HU-308. *, Significantly different from vehicle-treated mice (P < 0.05); **, significantly different from vehicle-treated mice (P < 0.01); ***, significantly different from vehicle-treated mice (P < 0.001).
Peripheral Pain.
Pain mediated by the peripheral nervous system was tested in the “formalin test” for cutaneous (peripheral) pain (21–23). HU-308 or the vehicle was injected i.p. In experiments involving an antagonist, the latter was administered i.p. 15 min before HU-308. Formalin (3.2% dissolved in saline) was injected (in 20-μl volumes) s.c. in the plantar surface of the hind paw of a mouse 90 min after HU-308. Immediately after formalin administration, nociception was assessed (every 5 min for 1 hr) by the number of times the animal licked the formalin-injected paw.
Blood Pressure Assay.
Systemic blood pressure was monitored in male rats (Sabra strain, 270–350 g). Under pentobarbital anesthesia (60 mg/kg), a cannula (P 50, Clay Adams) was implanted into the femoral artery. The arterial cannula was attached to a pressure transducer (Db23, low volume displacement; Statham, Oxnard, CA). The transducer was connected to a data acquisition system (codas software and scroller card, Dataq, Akron, Ohio), and the pressure was sampled at a rate of 1/s. Concomitantly, the jugular vein was cannulated (polyethylene 10 tip welded to polyethylene 50) for drug administration. Recordings were taken for 30 to 60 min before treatment started. Only a single bolus of HU-308 (5–40 mg/kg) with or without antagonist (SR-141716A to block CB1 receptors; SR-144528 to block CB2 receptors), was administered to each rat. The antagonists alone were also administered (Fig. 4).
Figure 4.
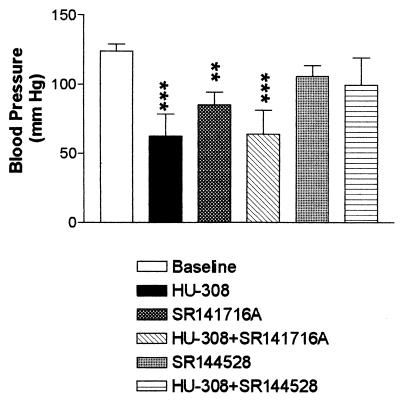
Hypotensive effects of HU-308. Anesthetized rats were cannulated. Baseline blood pressure was recorded before HU-308 was injected i.v. (30 mg/kg, n = 4) Lower doses did not have significant effects. In experiments with antagonists, SR-141716A (3 mg/kg, n = 3) or SR-144528 (1 mg/kg, n = 4), was injected 5 min before HU-308 (see text). The data represent the peak effect, occurring between 2.5 and 5 min. **, Significantly different (P < 0.01) from baseline (124 ± 5.2 mm/Hg). ***, Significantly different (P < 0.001) from baseline.
Preliminary observations had indicated that the effects of HU-308 on blood pressure dissipated well within a 30-min period after administration. Hence, measurements were performed for 30 min after bolus injections of HU-308.
Statistical Analyses.
Time curves were compared by two-way ANOVA (time × dose). Differences from vehicle treatments were compared by one-way ANOVA, followed by posthoc Newman–Keuls tests (Prism, GraphPad).
Results and Discussion
Selective agonists for a specific receptor are of interest and importance as they make possible the biochemical and pharmacological investigations of individual receptors and may serve as drug leads. Several synthetic cannabinoids have been shown to bind to the CB2 receptor with a higher affinity than they have to the CB1 receptor (for review, see ref. 24). Most of these compounds exhibit only modest selectivity (25–27). Of particular relevance to the present work are cannabinoid-type compounds, such as L-758,656 and L-759,633, in which the phenolic group is blocked as a methyl ether. One of these, L-759,656, was reported to have a CB1/CB2 affinity ratio >1000 (25); however, recent work has reported a ratio (measured with membranes from CHO cells stably transfected with human receptors) of only 414 (28). Both L-759,656 and L-759,633 are potent inhibitors of forskolin-stimulated cyclic AMP production in CB2-transfected cells (28). In vivo pharmacology of these agonists has yet to be described. An indole derivative, AM-620, has been found to have a CB1/CB2 affinity ratio of 165. It behaves as an inverse agonist at CB2 receptors and as a weak partial agonist at CB1 receptors (28). Other heterocyclic compounds that bind preferentially to CB2 have been reported in the patent literature (24).
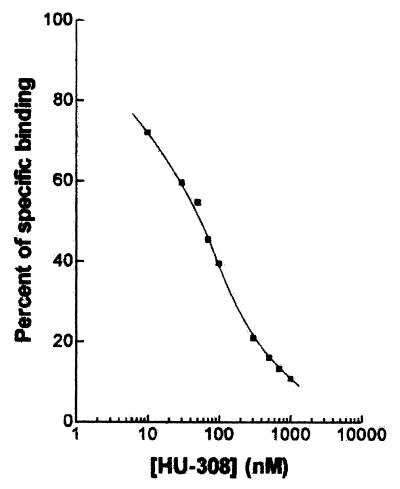
We now report the synthesis of a CB2-specific agonist, the bicyclic HU-308 (Scheme S1), with a Ki = 22.7 ± 3.9 nM (Fig. 1). It does not bind to CB1 (Ki > 10 μM).
Figure 1.
Binding of HU-308 to the CB2 cannabinoid receptor, measured by competitive inhibition of [3H]HU-243 binding in COS-7 cells transfected with plasmids containing the CB2 receptor gene (4).
In CB2-transfected cells, HU-308 inhibited forskolin-stimulated cyclic AMP production in a concentration-related manner with a mean EC50 value of 5.57 nM (1.68 and 18.5 nM; n = 5), and a mean Emax value of 108.6 ± 8.4% (n = 5). In CB1-transfected cells, the mean inhibitory effect of HU-308 was 9.9 ± 15.9% at 1 μM (n = 5) and 72.5 ± 8.5% at 10 μM (n = 4). At concentrations up to 10 μM, HU-308 had no effect on forskolin-stimulated cyclic AMP production in cells that had not been transfected with cannabinoid receptors (n = 3). These data provide strong evidence that HU-308 shares the ability of established cannabinoid receptor agonists to inhibit cyclic AMP production (19), and they confirm that it interacts significantly more readily with CB2 than with CB1 receptors.
These differences in binding and in inhibition of cyclic AMP production are reflected in the results of the pharmacological assays. In mice, a high dose of HU-308 (40 mg/kg) did not decrease the activity in an open field trial, did not cause catalepsy, did not reduce body temperature, and did not cause analgesia, measured on a hot plate when tested 10, 30 (data not shown), or 150 min after i.p. administration (Fig. 2). Such effects are considered to be mediated by the CB1 receptor (12, 14, 16).
The binding data for compounds that bind to CB2 reported so far (24–28), as well as for HU-308 in the present paper, indicate that major differences exist between the structure–activity relationships (SAR) for binding to CB1 and CB2. Although a free phenolic group in cannabinoid-type compounds is a basic structural feature for CB1 binding and cannabimimetic activity (29), it is not required for CB2 binding. The data published so far do not allow a detailed SAR analysis; however, the results with cannabinoid-type as well as with heterocyclic compounds (24, 27) indicate that very significant differences exist.
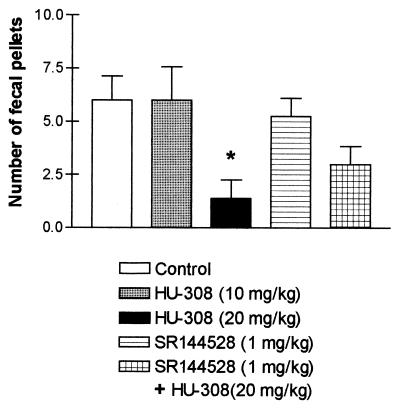
Inhibition of gastrointestinal activity has been observed after administration of Δ9-THC (see refs. 30 and 31 and refs. therein) and of anandamide (32, 33). This effect has been assumed to be CB1-mediated, because the specific CB1 antagonist SR-141716A blocked the effect (33). A previous report from our laboratory (32), however, suggested that inhibition of intestinal motility may also have a CB2-mediated component. The results presented now strongly support this assumption. HU-308 caused complete inhibition of intestinal mobility at 20 mg/kg (Fig. 3), which was partially blocked by SR-144528. Hence this gastrointestinal effect may be mediated, in part at least, by the peripheral CB2 receptor.
Figure 3.
Intestinal immotility after HU-308 administration with or without SR-144528. Mice (female Sabra, 9–10 weeks old) were randomly divided into five groups of five. Each mouse was injected (i.p.) twice with a 45-min interval. The data presented reflect the number of fecal pellets voided over 105 min, after the second injection. In addition, 50 and 100 mg/kg of HU-308 caused an effect comparable to that recorded with 20 mg/kg (data not shown). *, Significantly less than controls (P < 0.05).
Cannabinoids are well known for their cardiovascular activity (for review, see ref. 34). In an outstanding series of papers, Kunos and his group have shown that activation of peripheral CB1 receptors contributes to hemorrhagic and endotoxin-induced hypotension, and that anandamide and 2-AG, produced by macrophages and platelets, respectively, may be mediators of this effect. The hypotension in hemorrhaged rats was prevented by the CB1 antagonist SR-141716A (35, 36). Recently, the same group found that anandamide-induced mesenteric vasodilation is mediated by an endothelially located SR-141716A-sensitive “anandamide receptor,” distinct from the CB1 cannabinoid receptor, and that activation of such a receptor by an endocannabinoid, possibly anandamide, contributes to endotoxin-induced mesenteric vasodilation in vivo (37). The highly potent synthetic cannabinoid HU-210, as well as 2-AG, had no mesenteric vasodilator activity (37). Furthermore, it was shown that mesenteric vasodilation by anandamide apparently has two components: one mediated by an SR-141716-sensitive non-CB1 receptor (located on the endothelium), and the other by an SR-141716A-resistant direct action on vascular smooth muscle (37).
We have reported that the production of 2-AG is enhanced in normal, but not in endothelium-denuded, rat aorta on stimulation with carbachol, an acetylcholine receptor agonist (38). 2-AG potently reduces blood pressure in rats and may represent an endothelium-derived hypotensive factor (38).
The observations reported now further complicate this already complex picture. We have found that HU-308 (in doses above 30 mg/kg) reduces blood pressure when administered to rats (Fig. 4), and that this cardiovascular effect is blocked by the CB2 antagonist SR-144528, but not by the CB1 antagonist SR-141716A. SR-144528 alone has no effect, whereas SR-141716A reduces blood pressure (Fig. 4). The effect of SR-141716A is not unusual; in several studies, it has been reported that SR-141716A produces effects that are similar to those of cannabinoid agonists (39). The effect of HU-308 was unexpected. Contrary to our results, it has previously been shown that Win-55212–2, a highly potent cannabinoid, with Ki of 2.0 nM for CB1 and 0.3 nM for CB2 (40), causes no hypotension in CB1 knockout mice (41). Its hypotensive effect in normal mice is completely blocked by the CB1 antagonist SR-141716A (42), indicating that with Win-55212–2, all the reduction of blood pressure was mediated by CB1. The difference may be caused by the existence of subtypes of CB2 receptors, and HU-308 and Win-55212–2 may act on different subtypes. However, there are no data yet to support such an assumption. In any case, the hypotensive effect caused by HU-308 is produced through a mechanism that differs from the previously described CB1-mediated (or the anandamide receptor-mediated) hypotension produced by endocannabinoids, and apparently acts through CB2 or a CB2-like receptor. Whatever the molecular basis of the presently reported observations, they may serve as a starting point for novel hypotensive drugs; HU-308 causes no psychotropic effects, as established by the lack of effect in the tetrad of assays described above. Therefore, we expect that HU-308 will not cause major undesirable effects in humans; most cannabinoids, other than the psychotropic ones, do not produce significant side effects.
HU-308 and indomethacin, at a dose of 50 and 20 mg/kg, respectively, and injected between 30 and 90 min before the application of arachidonic acid, induced significant reduction of arachidonic acid-induced ear swelling (Fig. 5). However, the anti-inflammatory effect produced by indomethacin was greater than that produced by HU-308. The CB1 antagonist SR-141716A (5 mg/kg), administered 15 min before HU-308, did not prevent the anti-inflammatory effect of HU-308. Rather, it was SR-141716A itself that reduced arachidonic acid-induced ear swelling. As mentioned above, SR-141716A may act as agonist (39). By contrast, the CB2 receptor antagonist SR-144528 (0.5 mg/kg) did not itself induce an anti-inflammatory effect; however, it reduced the anti-inflammatory effect of HU-308 (Fig. 5).
Calignano et al. (22) and Jaggar et al. (23) have shown that anandamide attenuates the early or the late phase, respectively, of pain behavior caused by formalin-induced chemical damage. This effect is produced by interaction with CB1 (or CB1-like) receptors, located on the peripheral endings of sensory neurons involved in pain transmission. Palmitoylethanolamide, which, like anandamide, is present in the skin, also exhibits peripheral antinociceptive activity during the late phase of pain behavior (22, 23), although it does not bind to either CB1 or CB2 (40). Its analgesic activity is blocked by the specific CB2 antagonist SR-144528, though not by the specific CB1 antagonist SR-141716A. Hence, the existence of a CB2-like receptor was postulated (22).
The results reported now throw further light on the involvement of the CB2 receptor in peripheral antinociception. HU-308 apparently acts through the CB2 (or a CB2-like) receptor as it binds to CB2, but not to CB1. Thus, in our studies, HU-308 reduced peripheral pain during the late phase of pain behavior (Fig. 6), which was prevented by SR-144528, the CB2 antagonist, but not by SR-141716A, the CB1 antagonist. This observation is in agreement with reports on the presence of CB2-like receptors on peripheral nerve terminals (9, 10), and with the report of Zimmer et al. (43) on a residual analgesic effect of THC in CB1 knockout mice. The Zimmer data indicate that THC may act in part through CB2, or a CB2-like receptor. Whatever the exact mechanism of the activity of HU-308 on pain transmission, our results indicate that cannabinoids may serve as peripheral analgesics that have no central effects.
In summary, we have synthesized a CB2-specific agonist, code-named HU-308. This cannabinoid does not bind to CB1 (Ki > 10 μM), but does so efficiently to CB2 (Ki = 22.7 ± 3.9 nM); it inhibits forskolin-stimulated cyclic AMP production in CB2-transfected cells, but much less so in CB1-transfected cells. It shows no activity in a tetrad of behavioral tests in mice, which, together, have been shown to be specific for THC-type activity in the CNS. However, HU-308 reduces blood pressure, blocks defecation, and elicits anti-inflammatory and peripheral analgesic activity. The hypotension, the inhibition of defecation, the anti-inflammatory activity, and the peripheral analgesic activity produced by HU-308 are blocked (or partially blocked) by the CB2 antagonist SR-144528, but not by the CB1 antagonist SR-141716A. These results demonstrate the feasibility of discovering novel nonpsychotropic cannabinoids that may lead to new therapies for hypertension, inflammation, and pain.
Acknowledgments
We thank Judith Schleyer for excellent technical assistance with the blood pressure assays, Dr. U. Meiri for setting up and processing the data of the blood pressure assay, Prof. Z. Vogel for the supply of the transfected cells for the CB2 binding, and the Israel Science Foundation and the U.S. National Institute on Drug Abuse for partial financial support (to R.M. and R.P.).
Abbreviations
- 2-AG
2-arachidonylglycerol
- CNS
central nervous system
- THC
tetrahydrocannabinol
References
- 1.Mechoulam R. In: Cannabinoids as Therapeutic Agents. Mechoulam R, editor. Boca Raton, FL: CRC; 1986. pp. 1–19. [Google Scholar]
- 2.Gaoni Y, Mechoulam R. J Am Chem Soc. 1964;86:1646. [Google Scholar]
- 3.Devane W A, Hanuš L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 4.Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski N E, Schatz A R, Gopher A, Almog S, Martin B R, Compton D R, et al. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 6.Herkenham M, Lynn A B, Little M D, Johnson M R, Melvin L S, de Costa B R, Rice K C. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlett A C. Neurobiol Dis. 1998;5:405–416. doi: 10.1006/nbdi.1998.0215. [DOI] [PubMed] [Google Scholar]
- 8.Munro S, Thomas K L, Abu-Shaar M. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 9.Griffin G, Fernando S R, Ross R A, McKay N G, Ashford M L, Shire D, Huffman J W, Yu S, Lainton J A, Pertwee R G. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- 10.Pertwee R G. Life Sci. 1999;65:597–605. doi: 10.1016/s0024-3205(99)00282-9. [DOI] [PubMed] [Google Scholar]
- 11.Axelrod J, Felder C C. Neurochem Res. 1998;23:575–581. doi: 10.1023/a:1022418217479. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee R G. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 13.Breivogel C S, Childers S R. Neurobiol Dis. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- 14.Mechoulam R, Fride E, Di Marzo V. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 15.Mechoulam R, Lander N, Breuer A, Zahalka J. Tetrahedron: Asymmetry. 1990;1:315–319. [Google Scholar]
- 16.Martin B R, Compton D R, Thomas B F, Prescott W R, Little P J, Razdan R K, Johnson M R, Melvin L S, Mechoulam R, Ward S J. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 17.Fride E, Mechoulam R. Eur J Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- 18.Devane W A, Breuer A, Sheskin T, Jarbe T U C, Eisen M, Mechoulam R. J Med Chem. 1992;35:2065–2069. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 19.Ross R A, Gibson T M, Stevenson L A, Saha B, Crocker P, Razdan R K, Pertwee R G. Br J Pharmacol. 1999;128:735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young J M, Spires D A, Bedord C J, Wagner B, Ballaron S J, De Young L M. J Invest Dermatol. 1984;82:367–371. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]
- 21.Tjolson A, Berge O G, Hunskaar S, Rosland J H, Hole K. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 22.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature (London) 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 23.Jaggar S I, Hasnie F S, Sellaturay S, Rice A S C. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 24.Barth F. Exp Opin Ther Patents. 1998;8:301–313. [Google Scholar]
- 25.Gareau Y, Dufresne C, Gallant M, Rochette C, Sawyer N, Slipetz D M, Tremblay N, Weech P K, Metters K M, Labelle M. Bioorgan Med Chem Lett. 1996;6:189–194. [Google Scholar]
- 26.Huffman J W, Yu S, Showalter V, Abood M E, Wiley J L, Compton D R, Martin B R, Bramblett R D, Reggio P H. J Med Chem. 1996;39:3875–3877. doi: 10.1021/jm960394y. [DOI] [PubMed] [Google Scholar]
- 27.Gallant M, Dufresne C, Gareau Y, Guay D, Leblanc Y, Prasit P, Rochette C, Sawyer N, Slipetz D M, Tremblay N, et al. Biorg Med Chem Lett. 1996;6:2263–2268. [Google Scholar]
- 28.Ross R A, Brockie H C, Stevenson L A, Murphy V L, Templeton F, Makriyannis A, Pertwee R G. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechoulam R, Devane W A, Glaser R. In: Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Murphy L, Bartke A, editors. Boca Raton, FL: CRC; 1992. pp. 1–33. [Google Scholar]
- 30.Shook J E, Burks T F. J Pharmacol Exp Ther. 1989;249:444–449. [PubMed] [Google Scholar]
- 31.Krowicki Z K, Moerschbaecher J M, Winsauer P J, Digavalli S V, Hornby P J. Eur J Pharmacol. 1999;371:187–196. doi: 10.1016/s0014-2999(99)00165-x. [DOI] [PubMed] [Google Scholar]
- 32.Fride E. Brain Res. 1995;697:83–90. doi: 10.1016/0006-8993(95)00790-w. [DOI] [PubMed] [Google Scholar]
- 33.Calignano A, La Rana G, Makriyannis A, Lin S Y, Beltramo M, Piomelli D. Eur J Pharmacol. 1997;340:R7–R8. [PubMed] [Google Scholar]
- 34.Wagner J A, Varga K, Kunos G. J Mol Med. 1998;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- 35.Wagner J A, Varga K, Ellis E F, Rzigalinski B A, Martin B R, Kunos G. Nature (London) 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 36.Varga K, Wagner J A, Bridgen D T, Kunos G. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 37.Wagner J A, Varga K, Jarai Z, Kunos G. Hypertension. 1999;33(Part II):429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 38.Mechoulam R, Fride E, Ben-Shabat S, Meiri U, Horowitz M. Eur J Pharmacol. 1998;362:R1–R3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura-Palacios E M, Moerschbaecher J M, Barker L A. CNS Drug Rev. 1999;5:43–58. [Google Scholar]
- 40.Showalter V M, Compton D R, Martin B R, Abood M E. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- 41.Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, Bohme G A, Imperato A, Pedrazzini T, Roques B P, et al. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 42.Lake K D, Compton D R, Varga K, Martin B R, Kunos G. J Pharmacol Exp Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- 43.Zimmer A, Zimmer A M, Hohmann A G, Herkenham M, Bonner T I. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ankier S I. Eur J Pharmacol. 1974;27:1–4. doi: 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]