Abstract
Hematopoietic progenitor cells arising from bone marrow (BM) are known to contribute to the formation and expansion of tumor vasculature. However, whether different subsets of these cells have different roles in this process is unclear. To investigate the roles of BM-derived progenitor cell subpopulations in the formation of tumor vasculature in a Ewing’s sarcoma model, we used a functional assay based on endothelial cell and pericyte differentiation in vivo. Fluorescence-activated cell sorting of human cord blood/BM or mouse BM from gfp-transgenic mice was used to isolate human CD34+/CD38−, CD34+/CD45+, and CD34−/CD45+, and mouse Sca1+/Gr1+, Sca1−/Gr1+, VEGFR1+ and VEGFR2+ cells. Each of these progenitor subpopulations was separately injected intravenously into nude mice bearing Ewing’s sarcoma tumors. Tumors were resected one week later, and analyzed using immunohistochemistry and confocal microscopy for the presence of migrated progenitor cells expressing endothelial, pericyte or inflammatory cell surface markers. We demonstrated two distinct patterns of stem cell infiltration. Human CD34+/CD45+ and CD34+/CD38− and murine VEGFR2+ and Sca1+/Gr1+ cells migrated to Ewing’s tumors, co-localized with the tumor vascular network and differentiated into cells expressing either endothelial markers (mouse CD31 or human vascular endothelial cadherin), or the pericyte markers desmin and α-smooth muscle actin. By contrast, human CD34−/CD45+ and mouse Sca1−/Gr1+ cells migrated predominantly to sites outside of the tumor vasculature, and differentiated into monocytes/macrophages expressing F4/80 or CD14. Our data indicate that only specific BM stem/progenitor subpopulations participate in Ewing’s sarcoma tumor vasculogenesis.
Keywords: bone marrow cells, pericytes, endothelial cells, vasculogenesis, Ewing’s sarcoma
Introduction
The creation and expansion of a functional vascular network is known to be essential for tumor growth. Aggressive malignancies, such as Ewing’s sarcoma, depend on angiogenesis and vasculogenesis in order to support the rapid rate of tumor cell proliferation (1). Therefore understanding how tumor vessels expand to support the tumor’s nutritional and oxygen needs may identify novel therapeutic targets to interfere with this process. Endothelial progenitor cells (EPCs) have been shown to participate in postnatal vasculogenesis (2). However, defining a set of markers that uniquely identifies these cells has generated much controversy. No clear designation exists for when an EPC becomes a mature endothelial cell, and a universally accepted functional assay for these cells is also lacking. As a result, several types of assays have been used to show that diverse cell types possess EPC-like capacity. Traditionally, EPCs were believed to express CD34, AC133 and vascular endothelial growth factor receptor-2 (VEGFR2) (2–4). However, it has also been argued that the majority of EPCs are derived from monocytes/macrophages, which are CD34− (5). A specific subset of CD14+ monocytes, those that express VEGFR2, were described to have endothelial differentiation capacity (6). CD34+/VEGFR3+ progenitor cells were reported to play a role in lymphangiogenesis (7). Even mesenchymal stem cells have been shown to form endothelial cells in the presence of VEGF and serum in vitro (8).
Direct stem/progenitor cell incorporation into the endothelial lining of functional tumor neovessels has been reported (9). In addition, recent investigations have demonstrated that bone marrow (BM) cells may have other functions within the tumor microenvironment. PDGFR-β-expressing pericyte progenitor cells (PPPs) were shown to differentiate into NG2+, desmin+, alpha smooth muscle actin+ pericytes within the tumor vasculature (10). De Palma et al. reported that Tie-2-expressing monocytes homed to tumors in mice and promoted angiogenesis in a paracrine fashion (11, 12). Similarly, Gr+/CD11b+ myeloid immune suppressor cells homing to tumors were found not only to directly incorporate into tumor endothelium, but also promote tumor angiogenesis and growth indirectly via release of matrix metalloproteinase 9 (13).
The extent of bone marrow cell incorporation into tumors may be highly variable and depend on the specific tumor model or the quantity/type of bone marrow cells injected (9). Few studies have investigated more than one stem/progenitor cell subpopulation. To clarify the roles of various types of EPCs in tumor vasculogenesis, we investigated the participation of different BM-derived progenitor cell subpopulations in a murine model of Ewing’s sarcoma tumors. We chose this model because we previously showed that Ewing’s tumors recruit BM-derived progenitor cells, which participate in tumor vessel formation (1, 14). Here we demonstrate that CD34+/CD38−, CD34+/CD45+, CD34−/CD45+, VEGFR2+ (Flk1+), VEGFR1+ (Flt1+), Sca1+/Gr1+ and Sca1−/Gr1+ stem/progenitor cells all migrate to Ewing’s sarcoma tumors. Of these progenitor cell types, CD34+/CD45+, CD34+/CD38−, VEGFR2+ and Sca1+/Gr1+ cells establish residence within the expanding tumor vascular network and differentiate into endothelial cells and pericytes. By contrast, CD34−/CD45+, Sca1−/Gr1+ and VEGFR1+ cells predominantly localize to sites outside the Ewing’s tumor vasculature. CD34−/CD45+ and Sca1−/Gr1+ cells differentiate into monocytes/macrophages.
Results
Migration and differentiation of human stem/progenitor subpopulations in vivo
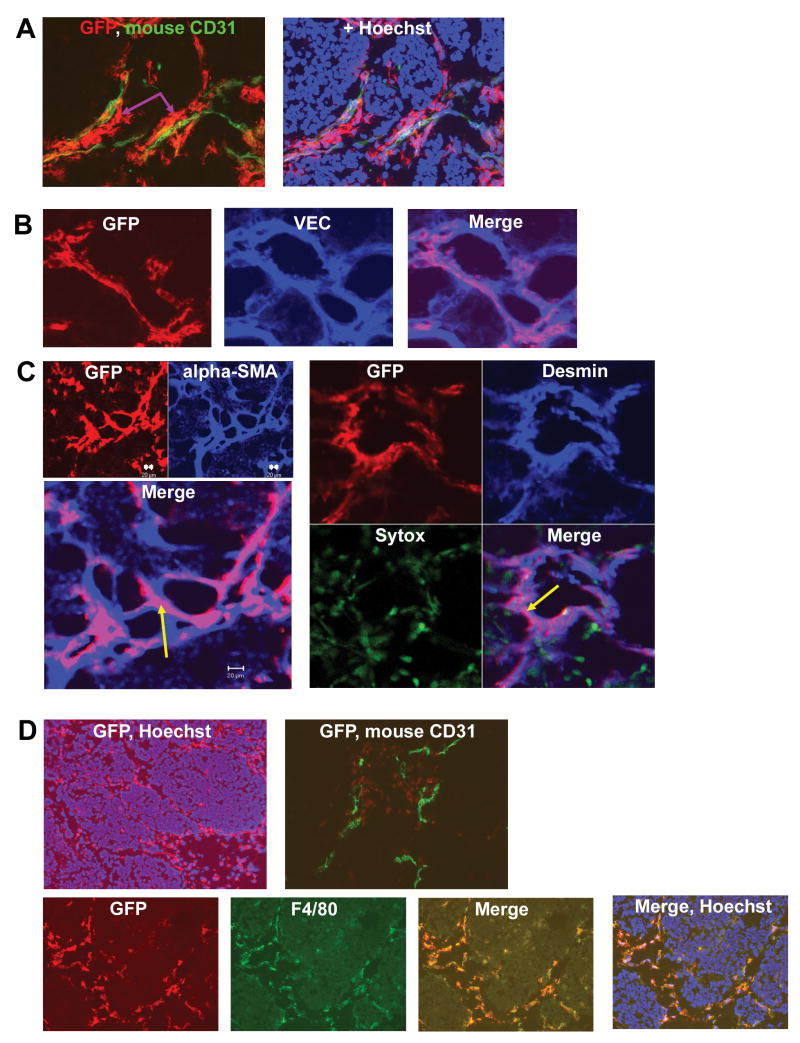
Fluorescence-activated cell sorting (BD FACSAria) was utilized to isolate CD34+/CD45+, CD34−/CD45+ and CD34+/CD38− cells (Fig. 1). To assess the in vitro endothelial differentiation capacity of human CD34+/CD45+ and CD34−/CD45+ cells, these subpopulations were cultured in endothelial differentiation medium, examined by light microscopy and immunostained for the endothelial marker human CD31. Cultured CD34+/CD45+ cells expressed CD31, with the formation of CD31+ colonies (data not shown). By contrast, no such colony formation was observed for CD34− cells. We next analyzed the capacity of CD34+/CD45+, CD34+/CD38−, and CD34−/CD45+ cells to migrate into TC71 tumors and participate in tumor neovasculature formation. The three human progenitor populations were gfp-labeled with Ad5/F35-gfp, and then separately i.v.-injected into nude mice bearing TC71 Ewing’s tumors. One week later, mice were sacrificed, tumors excised and sectioned. Migrated progenitor cells were detected within tumors by immunostaining with anti-gfp, followed by a secondary antibody conjugated to the Alexa594 fluorophore. Gfp+ cells were therefore red. To determine the location of these human stem/progenitor cells with respect to tumor microvessels, tumor sections were co-stained for gfp and mouse CD31 (Fig. 2A). The gfp+ migrated human CD34+/CD38− cells (red) were seen both in close association with as well as distant from mouse CD31+ microvessels (green). CD34+-derived cells formed continuous, elongated structures that ran adjacent to and surrounded the mouse CD31+ cells (Fig. 2A, purple arrows). Yellow areas, obtained from merging the gfp and CD31 images, indicated migrated cells that were either on top of or integrated into the tumor vessels. To determine if CD34+ cells were differentiating into endothelial cells, sections were co-stained for gfp and human vascular endothelial cadherin (hVEC) and analyzed by confocal microscopy. We have previously shown that there is no cross-reactivity between hVEC and mouse endothelial cells, therefore hVEC+ cells represent migrated human stem cells that differentiated into endothelial cells (14). Co-localization of gfp (red) and hVEC (blue), yielding purple fluorescence in the merged image, indicated that some migrated CD34+/CD38− cells (Fig. 2B) as well as CD34+/CD45+ cells (data not shown) had differentiated into human endothelial cells. Because the transduction efficiency of Ad5/F35-gfp in human subpopulations was < 50%, migrated CD34+-derived, hVEC+ cells that did not express gfp were also found within the Ewing’s tumor vasculature (Fig. 2B Merge, blue cells). To determine whether stem cells also contributed to pericyte formation, tumor sections were co-stained for gfp and the pericyte differentiation markers α-smooth muscle actin (SMA) and desmin, and then analyzed by confocal microscopy (Fig. 2C). CD34+/CD38− cells expressing alpha-SMA or desmin were detected in the tumor vascular network, indicating that a fraction of this migrated progenitor subpopulation had differentiated into pericytes/vascular smooth muscle cells (arrows, Fig. 2C). The migration of CD34+/CD38− and CD34+/CD45+ cells to TC71 tumors and their contribution to neovasculature formation confirms that vasculogenesis plays a role in the growth of Ewing’s sarcoma.
Figure 1.
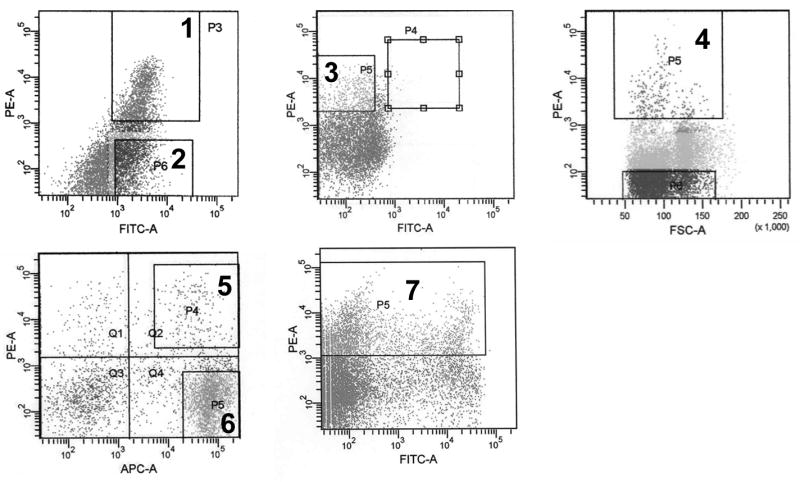
Isolation of human and murine stem/progenitor subpopulations. Fluorescence-activated cell sorting (FACS) of human umbilical cord blood- or BM-derived ex-vivo expanded CD34+ cells was utilized to isolate CD34+/CD45+ (1), CD34−/CD45+ (2) and CD34+/CD38− cells (3). Similarly, VEGFR2+ (4), Sca1+/Gr1+ (5), Sca1−/Gr1+ (6) and VEGFR1+ (7) cells were isolated from whole gfp-transgenic mouse BM.
Figure 2.
Migrated CD34+ cells differentiate into both endothelial cells and pericytes in the expanding tumor vessel network, while CD34− cells differentiate into macrophages. (A) Sorted CD34+/CD38− cells were gfp-labeled and i.v.-injected into nude mice with TC71 tumors. IHC (20×) identified migrated gfp+ CD34+/CD38− cells (red) near and around mouse CD31+ vessels (green). Hoechst 33342 identifies cell nuclei. (B) Confocal microscopy; migrated CD34+/CD38−cells differentiated into endothelial cells expressing hVEC (40×). (C) Confocal microscopy; CD34+/CD38− cells (arrows) expressing pericyte markers α-SMA (left; GFP & α-SMA: 20×; Merge: 40×) and desmin (right; 20×) were present within the tumor vascular smooth muscle cell network. The merged images demonstrate that α-SMA+ and desmin+ pericyte networks were mosaics comprised of locally-derived (blue) as well as CD34+/CD38− progenitor-derived (magenta) cells. Sytox was used to stain nuclei green. (D) Sorted CD34−/CD45+ cells were gfp-labeled and i.v.-injected into nude mice with TC71 tumors. IHC of tumor sections revealed migrated CD34− cells (red) throughout the tumor area (top left, 10×). CD34− progenitors (red) were single cells in the vicinity of green CD31+ tumor vessels (top right, 20×). CD34− progenitors differentiated into cells expressing macrophage antigen F4/80 (bottom, 20×).
CD34−/CD45+ cells also migrated to the TC71 tumor area. These migrated cells were observed at the tumor periphery and also deeper into the tumor tissue (Fig. 2D, top left). But unlike the CD34+ subpopulations, CD34−/CD45+ cells were found mostly as single cells (Fig. 2D, top right), as opposed to large clusters/arrangements (Fig 2A). CD34−- derived cells did not express either mouse CD31 (Fig. 2D, top right) or hVEC (data not shown). Rather, the CD34−/CD45+ migrated cells expressed F4/80, demonstrating that they had differentiated into macrophages (Fig. 2D, bottom).
VEGFR2+ and Sca1+Gr1+ cells differentiate into both endothelial cells and pericytes in Ewing’s sarcoma tumors
We next investigated the migration and participation of murine BM-derived progenitor cell types in the formation of the TC71 tumor vasculature. Sca1+/Gr1+, Sca1−/Gr1+, VEGFR2+ and VEGFR1+ subpopulations were isolated from fresh gfp+ BM using FACS (Fig. 1) and then i.v.-injected separately into tumor-bearing mice. Mice were sacrificed one week later, tumors excised and analyzed by immunohistochemistry. The migrated murine BM progenitor subpopulations were detected using anti-gfp. All four subpopulations migrated to the tumor. However, their distributions within the Ewing’s tumors varied. As shown in Fig. 3A, migrated VEGFR2+ cells (red) were detected throughout the tumor area, predominantly in the vicinity of tumor vessels (green). Yellow fluorescence in the merged image (Fig. 3A, arrows) demonstrated that some of the migrated VEGFR2+ cells expressed CD31, indicating they had differentiated into mouse endothelial cells. Gfp+ cells expressing desmin (data not shown) and α-SMA (Fig. 3B) were also present within the vasculature, indicating that some of the VEGFR2+ progenitor cells had differentiated into pericytes/vascular smooth muscle cells. Similar to the VEGFR2+ cells, Sca1+/Gr1+ progenitor cells (Fig. 3C) migrated into the tumor and were associated with CD31+ microvessels. Using confocal microscopy, we demonstrated that Sca1+/Gr1+ cells differentiated into CD31+ endothelial cells (purple cells) and were directly incorporated into vessels, thereby forming a mosaic pattern of BM progenitor-derived (purple) and locally-derived (blue) endothelial cells adjacent to each other within the vascular endothelial lining (Fig. 3C, left). Sca1+/Gr1+-derived cells expressing α-SMA (Fig. 3C, right; arrow) and desmin (data not shown) were also seen, indicating pericyte differentiation.
Figure 3.
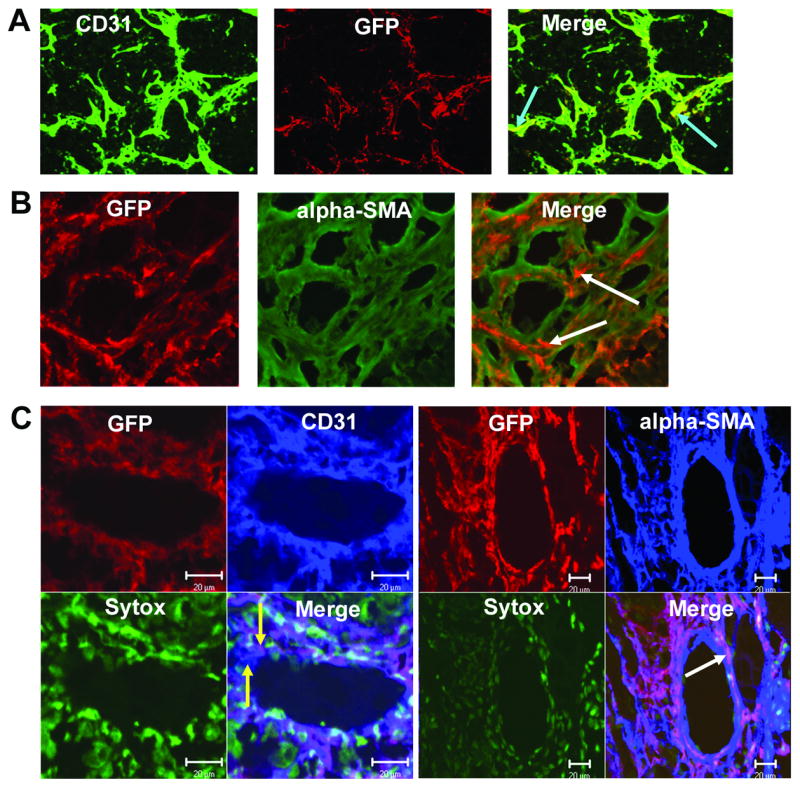
VEGFR2+ and Sca1+/Gr1+ cells differentiate into both endothelial cells and pericytes within the TC71 vasculature. Gfp+ VEGFR2+ and Sca1+/Gr1+ cells were i.v.-injected into nude mice with TC71 tumors. (A) VEGFR2+ cells differentiated into CD31+ endothelial cells (arrows). (B) Confocal microscopy (20×); VEGFR2+ cells also differentiated into α-SMA+ pericytes (arrows), adjacent to locally-derived cells (green) within the vascular smooth muscle cell network. (C) Confocal microscopy; Left (40×): Sca1+/Gr1+-derived cells differentiated into CD31+ endothelial cells that were incorporated into the vascular endothelial lining; yellow arrows indicate adjacent locally-derived (blue) and Sca1+-derived (purple) endothelial cells within the mosaic vessel. Right (20×): Sca1+/Gr1+ cells also differentiated into α-SMA+ cells that comprised the pericyte network (arrow). Sytox was used to stain nuclei green.
Sca1−Gr1+ and VEGFR1+ progenitor subpopulations do not differentiate into vascular endothelial cells
While Sca1−/Gr1+ progenitor cells also migrated to the TC71 tumors, very few were found to reside in the tumor vessel vicinity. Similar to what was seen with CD34−cells, none of the Sca1−/Gr1+-derived cells expressed CD31 (data not shown), indicating that this BM subpopulation did not differentiate into endothelial cells (Table 2). Similar to the human CD34− subpopulation, Sca1−/Gr1+-derived cells expressed CD14, a monocyte/macrophage marker (Table 2). Migrated VEGFR1+-derived cells were found in close proximity to CD31+ tumor vessels as well as away from the vessels (data not shown). However, these VEGFR1+-derived cells did not express CD31, again indicating that this murine BM subpopulation did not differentiate into endothelial cells within Ewing’s sarcoma tumors (Table 2).
Table 2.
Differentiation of selected stem/progenitor subpopulations within the Ewing’s sarcoma tumor microenvironment
| Stem/progenitor subpopulation | Differentiation into Endothelial cells | Differentiation into Pericytes | Differentiation into inflammatory cell types (do not contribute to neovessel formation) |
|---|---|---|---|
| CD34+38− | + | + | |
| CD34+45+ | + | + | |
| CD34−45+ | − | + (macrophages) | |
| Sca1+Gr1+ | + | + | |
| Sca1−Gr1+ | − | + (macrophages) | |
| VEGFR1+ (Flt1+) | − | ||
| VEGFR2+ (Flk1+) | + | + |
DISCUSSION
The data presented demonstrate that both human and mouse BM-derived stem/progenitor subpopulations fall into two categories: those that differentiate into endothelial cells and pericytes and participate in Ewing’s sarcoma tumor neovasculature formation, and those that differentiate into myeloid cells and do not contribute to tumor vessel development. We demonstrated that human CD34+/CD38−, CD34+/CD45+ and CD34−/CD45+ stem/progenitor cells migrated into TC71 tumors. However, only the CD34+ cells differentiated into endothelial cells and pericytes. Evidence of endothelial differentiation in vivo was established using human VEC staining, a marker of human endothelial cells. We have previously shown that human VEC does not cross-react with mouse endothelial cells, allowing us to separate out the endothelial cells derived from human CD34+ cells. These human CD34+-derived endothelial cells may be forming functional anastomoses to tumor vessels comprised of murine endothelium. We further demonstrated that many migrated CD34+ cells were associated with mouse CD31+ vessels and expressed the pericyte differentiation markers α-SMA and desmin. By contrast, migrated CD34−cells did not differentiate into endothelial cells and mostly resided distant from the tumor vessels. Because we showed that CD34+ but not CD34− stem/progenitor subpopulations contributed to the tumor vessel architecture, our findings refute the contention that migration of progenitor cells to human tumors is non-specific.
CD34+-derived human endothelial cells were observed wrapped around the tumor vessels and appeared distinct from the tumor vessel mouse endothelium. For this reason, we were concerned that species mismatch may interfere with the ability of recruited human progenitor cells to interact with the local mouse endothelial cells in the formation of tumor vessels. We therefore also evaluated whether mouse BM-derived stem/progenitor subpopulations showed a similar pattern of migration and differentiation. Sca1 is a marker of hematopoietic stem cells found in the mouse BM, while Gr1 is a myeloid differentiation antigen (Table 1). We selected to evaluate Sca1+/Gr1+ and Sca1−/Gr1+ cells because these subpopulations represented the mouse counterparts of human CD34+/CD45+ and CD34−/CD45+ cells, respectively. Sca1+/Gr1+ and Sca1−/Gr1+ cells, as well as VEGFR2+ and VEGFR1+ cells, all migrated into Ewing’s tumors. Similar to the human CD34+ subpopulation, VEGFR2+- and Sca1+/Gr1+-derived cells were within the expanding tumor vascular network. It is unclear at this point if these cells proliferated once within the tumor tissue or whether the large clusters of bone marrow-derived cells resulted solely from migration. A portion of the VEGFR2+- and Sca1+/Gr1+-derived cells co-localized with CD31 and incorporated into tumor vessels, indicating that they had differentiated into endothelial cells (Fig. 3 A, C). A substantial fraction, however, resided in very close proximity to tumor vessels but did not express CD31. Rather, these recruited Sca1+/Gr1+- and VEGFR2+-derived cells expressed the pericyte markers a -SMA and desmin (Fig. 3 B, C). These data indicate that, similar to human CD34+ cells, these mouse BM subsets also contribute to the tumor vessel pericyte/vascular smooth muscle cell network. More importantly, our confocal studies (Fig. 3 B, C) indicate for the first time that the endothelial and smooth muscle cell components of the tumor vasculature are mosaics comprised of both BM- and locally-derived cells. Indeed, the vessel wall is comprised of BM-derived and local endothelial cells side by side. This finding suggests that BM cells play an important, perhaps even critical, role in tumor vessel formation and that the BM serves as a source for the needed progenitor cells as the tumor vasculature expands during tumor growth. Interestingly, the incorporation of BM-derived cells in tumor vessels is not a uniform process, and these cells do not distribute homogeneously throughout the tumor tissue. Rather, recruited BM-derived cells form “hotspots” of incorporation at distinct sites within the tumor neovasculature. In our analysis of the Ewing’s tumor vasculature, certain microscopic tumor fields were found to contain numerous BM-derived endothelial cells and pericytes, with nearby microscopic fields often demonstrating a relative absence of BM-derived cells. Previous work in our laboratory has shown that, on average, 10% of Ewing’s tumor vessels contain at least one BM-derived cell (1).
Table 1.
Significance of selected stem/progenitor subpopulations
| Stem/progenitor subpopulation | Significance |
|---|---|
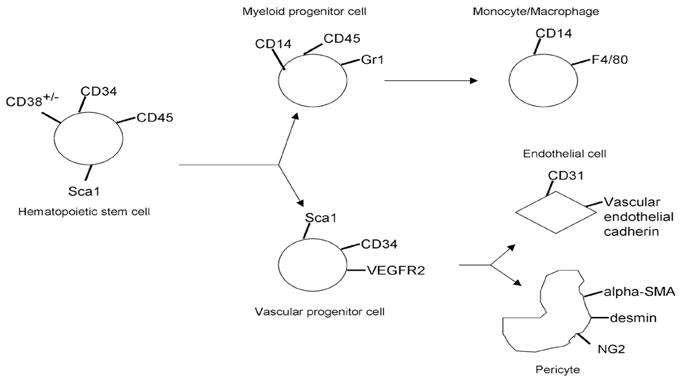
| CD34+38− | Primitive CD34+ cell; important in late engraftment & enriched in cord blood |
| CD34+45+ | Human hematopoietic stem cell |
| CD34−45+ | Human myeloid progenitor cell |
| Sca1+Gr1+ | Hematopoietic stem cell of mouse BM (also displaying evidence of myeloid differentiation) |
| Sca1−Gr1+ | Expresses Gr -1 (Ly6G) myeloid differentiation differentiation antigen, found on bone marrow monocytes and granulocytes as well as peripheral neutrophils |
| VEGFR2+ (Flk1+) (vascular endothelial growth factor receptor 2) | consensus marker for EPCs (also important in multiple angiogenic processes) |
| VEGFR1+ (Flt1 (Flt1+) (vascular endothelial growth factor receptor 1) | Expressed by a subpopulation of hematopoietic stem cells; receptor for VEGF and Placental Growth Factor |
| |
Our findings are consistent with other recent reports showing that BM-derived cells differentiate into tumor pericytes in melanoma, pancreatic carcinoma and neuroblastoma (10, 16–18). Pericytes envelop the endothelial lining of blood vessels, supporting and regulating blood vessel formation (16). Pericytes in tumor blood vessels provide an escape mechanism to anti-angiogenic therapy by promoting endothelial cell survival/stability (19). Here we have identified three BM subpopulations, namely CD34+, VEGFR2+ and Sca1+/Gr1+ cells, which act as both pericyte precursors as well as endothelial cell precursors in Ewing’s sarcoma. Interfering with the homing of these particular progenitor cell types to Ewing’s tumors might provide a dual insult to the tumor vasculature by interfering with the supply of endothelial cells and pericytes that are needed to form functional tumor vessels. By disrupting vasculature expansion, this strategy could potentially allow better inhibition of tumor growth.
In contrast to CD34+ and Sca1+/Gr1+ cells, we found that CD34− and Sca1−/Gr1+ progenitor cells had markedly reduced endothelial differentiation capacity in vitro and in vivo. Human CD34− and mouse BM-derived Sca1−/Gr1+ cells did not contribute to the TC71 tumor neovasculature. Tumor-infiltrating CD34− cells and Sca1−/Gr1+ cells differentiated into macrophages/monocytes within the TC71 microenvironment. As was the case for the BM-derived endothelial cells and pericytes described above, BM-derived macrophages did not appear to distribute uniformly throughout the tumor tissue. While distinct areas within the Ewing’s tumor demonstrated an abundance of BM-derived macrophages, other areas demonstrated many host-derived (non-graft-derived) macrophages. While these specific BM subpopulations appear to play no role in the formation of tumor vessels, they nevertheless may have a different role that contributes to tumor cell growth and survival. Macrophage/monocyte progenitors may promote tumor growth via the production of angiogenic factors. For example, human stem/progenitor cells expressing monocyte/macrophage markers have previously been shown to secrete VEGF, hepatocyte growth factor, Granulocyte colony-stimulating factor and Granulocyte-macrophage colony-stimulating factor (4). Furthermore, Bingle et al., utilized breast cancer spheroids to demonstrate that macrophages can initiate tumor angiogenesis in vivo (20). Not only have tumor-infiltrating macrophages been linked to tumor progression (21), but specifically eliminating these cells suppressed tumor growth and metastasis (22, 23). Targeting the specific BM stem/progenitor cell types which migrate to tumors and promote tumor growth by differentiating into macrophages may represent another potential therapeutic approach.
The objective of these studies was to determine whether specific BM subpopulations contributed to the formation of the tumor neovasculature. We have demonstrated that human CD34+ hematopoietic stem cells and the mouse counterpart Sca1+/Gr1+ cells are the key progenitor cells that contribute to tumor vessel formation and that these cells migrate to the tumor and differentiate into either endothelial cells or pericytes. It is impossible to uniquely label each individual BM subsets within a single animal and distinguish their individual contributions to the tumor vasculature. We therefore characterized each BM subpopulation of interest separately in a tumor-bearing animal. It is important to note that the surface markers expressed by the BM-derived subpopulations analyzed are certainly not mutually exclusive. Specifically, we demonstrated that 52.5% of VEGFR2+ cells were also Sca1+, while 58.2% of Sca1+ cells co-expressed VEGFR2. In addition, 8.5% of Sca1+ cells also expressed VEGFR1, while 38.5% of VEGFR1+ cells co-expressed Sca1. 6.0% of VEGFR2+ cells were also VEGFR1+, while 29.4% of VEGFR1+ cells co-expressed VEGFR2.
Determining the relative importance of each subpopulation is beyond the scope of this work and cannot be ascertained by the methodology described. As an initial step towards comparing various progenitor subpopulations, an equal number of sorted cells of each type could be i.v.-injected into tumor-bearing mice and the relative numbers of migrated cells assessed. However, the detection of a larger number of migrated cells of a particular type within the tumor may not necessarily equate to greater importance. It is likely that the migrated cells divide, and the present detection system cannot identify daughter cells from parental migrated cells. For this reason, determining the percentage of i.v.-injected BM cells of a particular type, such as Sca1+Gr1+ cells, that contribute to the vessel network was not possible in this work, as the number of these cells within the final tumor is potentially very different from that introduced initially. Different BM subpopulations are also likely to proliferate at different rates within tumors, making it difficult to meaningfully interpret any quantitative comparison between various BM subsets. Also, a few BM cells of a specific type may exert great influence on tumor growth via production of cytokines affecting both resident tumor cells and vascular endothelial cells. At the same time, the tumor microenvironment may in part dictate the functional roles assumed by infiltrating BM stem/progenitor cells. A better understanding of the specific types of BM cells that migrate to tumors and how these progenitor cells contribute to the expansion of the tumor vascular network within the tumor microenvironment may aid in the identification of new therapeutic targets. Additionally, understanding this process may help in ascertaining how some tumors circumvent the effect of anti-angiogenic therapy.
Materials and Methods
Ewing’s sarcoma cell lines
TC71 human Ewing’s sarcoma cells were cultured in Eagle’s modified essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM non-essential amino acids, I mM penicillin-streptomycin, and 2-fold vitamin solution (Gibco BRL, Grand Island, NY). These cells have the Ewing’s sarcoma t(11; 22) translocation as detected by reverse-transcriptase polymerase chain reaction.
Mice and murine bone marrow cells
All experiments were approved by the Institutional Animal Care and Use committee of the University of Texas M. D. Anderson Cancer Center. All animals were housed in a specific pathogen-free facility. Four- to six-week-old athymic (T-cell deficient) nu/nu nude mice were purchased from the National Cancer Institute (NCI). GFP transgenic mice (Jackson Laboratories strain 003115), were purchased from M.D. Anderson Cancer Center, Genetic Engineering Mouse Facility (GEMF). This transgenic strain expresses an Enhanced Green Fluorescent Protein driven by chicken beta-actin promoter and CMV intermediate early enhancer; intensity of GFP expression varies in a tissue-dependent manner. GFP transgenic mice were used as BM donors. Bone marrow (BM) cells were isolated from donors by flushing hind femurs with PBS. Freshly-isolated BM cells were resuspended in PBS and kept on ice until injected into the tail veins of recipient nude mice.
Isolation of human and murine stem/progenitor cell subpopulations
Human CD34+ cells were isolated from umbilical cord blood obtained from the St. Louis Cord Blood Bank (St. Louis, MO) as previously described (14). Alternatively, frozen, bone marrow-derived CD34+ cells were purchased from the National Disease Research Interchange (NDRI, Philadelphia, PA). CD34+ cells were cultured at 37°C in Serum-free Expansion Medium (Stemcell Technologies, Vancouver, British Columbia) supplemented with Flt-3 Ligand, Stem Cell Factor, IL-3 and IL-6 (StemSpan CC100 cytokine cocktail, Stemcell Technologies). Culture-expanded CD34+ cells were analyzed using the following antibodies: phycoerythrin (PE)-conjugated mouse anti-human CD34 (BD Biosciences, San Jose, CA), fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD45 (BD Biosciences), and FITC-conjugated mouse anti-human CD38 (BD Biosciences). Isotypic mouse IgG1κ antibodies conjugated to either PE or FITC were used as negative controls (BD Biosciences). Fluorescence-activated cell sorting (BD FACSAria) was utilized to isolate CD34+/CD45+, CD34−/CD45+ and CD34+/CD38− cells (Fig. 1).
Whole bone marrow obtained from GFP transgenic mice was used as the source of all murine progenitor cell types and analyzed using the following antibodies: PE-conjugated anti-mouse Flk1 (VEGF-R2, Ly-73) (BD Biosciences), PE-conjugated anti-mouse Sca-1 (Ly-6A/E) (BD Biosciences), allophycocyanin (APC)-conjugated anti-mouse Gr-1 (Ly-6G) (BD Biosciences) and PE-conjugated rat anti-mouse VEGFR1 (Flt1) (R & D Systems, Minneapolis, MN). Isotypic mouse IgG1κ antibodies conjugated to either PE, FITC (BD Biosciences) or APC (R & D Systems) were used as negative controls. Fluorescence-activated cell sorting (BD FACSAria) of freshly-isolated whole mouse BM was utilized to isolate Flk1+, Sca1+/Gr1+, Sca1−/Gr1+ and Flt1+ cells (Fig. 1). The significance of these human and mouse lineages is described in Table 1.
Transduction of human stem/progenitor cell subpopulations with Ad5/F35-gfp
CD34+/CD45+, CD34−/CD45+ and CD34+/CD38− cells were cultured for 24 hours with the adenovirus vector Ad5/F35-gfp. Ad5/F35 binds cells in a CAR-independent manner, allowing effective gene delivery to human stem/progenitor cells (15).
In vitro endothelial progenitor cell culture
To determine the ability of human CD34+/CD45+ and CD34−/CD45+ cells to differentiate into endothelial cells in vitro, these sorted cell populations were cultured in fibronectin-coated chamber slides (BD Biocoat Human Fibronectin Cellware, BD Discovery Labware, Bedford, MA) in endothelial differentiation medium (Endocult Liquid Medium Kit, Stemcell Technologies, Vancouver, British Columbia). Cells were cultured in either Endocult Basal Medium alone, medium + Endocult Supplements (Stemcell Technologies), or medium + supplements + recombinant human VEGF (200 ng/mL, Stemcell Technologies). After 5 days, cell proliferation and endothelial colony formation was assessed by direct observation via light microscopy.
Assessment of in vivo migration of stem/progenitor subpopulations to Ewing’s tumors
Nude mice were injected subcutaneously with TC71 Ewing’s sarcoma cells plus growth factor-depleted matrigel basement membrane matrix (BD Matrigel Basement Membrane Matrix, BD Biosciences) (2 × 106 cells/0.3 mL Matrigel). One week following tumor cell inoculation, one of the BM-derived mouse progenitor subpopulations or Ad5/F35-infected human subpopulations was separately injected intravenously (i.v.) into a Ewing’s tumor-bearing mouse. Mice were euthanized one week later, tumors resected, placed in Optimal Cutting Temperature (OCT) compound, snap-frozen in liquid nitrogen and stored at −80°C.
Immunohistochemistry
For in vitro analysis of endothelial progenitor cell differentiation, chamber slides (see above) were fixed in acetone, and incubated with mouse anti-human CD31 (DakoCytomation, Denmark). For in vivo detection of mouse platelet-endothelial cell adhesion molecule (mCD31), green fluorescent protein (gfp), human vascular endothelial cadherin (VEC), α-smooth muscle actin (SMA), desmin, PDGFR-β, CD14 or F4/80, frozen tissue sections were fixed in acetone, blocked using 4% fish gelatin in PBS, and incubated with the appropriate primary antibody. The following antibodies were used: rat anti-mouse CD31/PECAM (BD Biosciences), rabbit anti-gfp (Santa Cruz Biotechnologies, Santa Cruz, CA), mouse anti-human VEC (CHEMICON International Inc, Temecula, CA), mouse anti-SMA (Abcam, Cambridge, MA), rabbit anti-desmin (Abcam), rabbit anti-PDGFR-β (Santa Cruz Biotechnologies), mouse anti-CD14 (Abcam), rat anti-mouse F4/80 (Serotec, Raleigh, NC) When using mouse monoclonal antibodies, slides were preblocked overnight with F(ab) fragment (Jackson ImmunoResearch) prior to incubation with primary antibody. The following secondary antibodies were utilized: goat anti-rat Alexa Fluor 488, goat anti-rabbit Alexa Fluor 594, goat anti-rabbit Alexa Fluor 488, or goat anti-mouse Alexa Fluor 488 (Invitrogen, Carlsbad, California). All antibodies were diluted in PBS containing 4% fish gelatin. Hoescht 33342 dye (Molecular Probes) was used to stain cell nuclei. Fluorescent images were captured at 10× (eyepiece) and 10× or 20× (objective) with Optimas imaging software (San Diego, CA).
For confocal microscopy, the following secondary antibodies were utilized: goat anti-mouse Cy-3 (Cy-3 –conjugated AffiniPure F(ab’)2 Fragment Goat Anti-Mouse IgG + IgM, Jackson Immunoresearch Laboratories Inc, West Grove, PA), goat anti-mouse Cy-5 (Jackson Immunoresearch), goat anti-rabbit Cy-3 (Jackson Immunoresearch), goat anti-rabbit Cy-5 (Jackson Immunoresearch), goat anti-rat Cy-3 (Jackson Immunoresearch) and goat anti-rat Cy5 (Jackson Immunoresearch). Sytox Green (Invitrogen, Carlsbad, California) was used to stain cell nuclei. Images were collected using a Zeiss Laser Confocal Microscope (Carl Zeiss MicroImaging, Inc. Thornwood, New York).
References
- 1.Bolontrade MF, Zhou RR, Kleinerman ES. Vasculogenesis Plays a Role in the Growth of Ewing’s Sarcoma in Vivo. Clin Cancer Res. 2002;8:3622–7. [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 4.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 5.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 6.Elsheikh E, Uzunel M, He Z, et al. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–55. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 7.Salven P, Mustjoki S, Alitalo R, et al. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–72. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 8.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 9.Duda DG, Cohen KS, Kozin SV, et al. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–6. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song S, Ewald AJ, Stallcup W, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte dizzerentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–95. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 12.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Lee TH, Bolontrade MF, Worth LL, et al. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. Int J Cancer. 2006;119:83946. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 15.Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M, Davis A. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 2001;8:930–7. doi: 10.1038/sj.gt.3301488. [DOI] [PubMed] [Google Scholar]
- 16.Lamagna C, Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677–81. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 17.Rajantie I, Ilmonen M, Alminaite A, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jodele S, Chantrain CF, Blavier L, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9-dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 19.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 20.Bingle L, Lewis CE, Corke KP, et al. Macrophages promote angiogenesis in human breast tumor spheroids in vivo. Br J Cancer. 2006;94:101–7. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–7. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhanaeva SY, Korolenko TA, Nekrasov BG, et al. Stimulation of macrophages increases, while suppression of these cells inhibits metastatic dissemination of two transplantable mouse tumors in the liver and lungs. Bull Exp Biol Med. 2005;140:449–51. doi: 10.1007/s10517-005-0516-7. [DOI] [PubMed] [Google Scholar]