Abstract
Malaria and helminth infections are two of the most prevalent parasitic diseases globally. While concomitant infection is common, mechanisms contributing to altered disease outcomes during co-infection remain poorly defined. We have previously reported exacerbation of normally non-lethal Plasmodium yoelii malaria in BALB/c mice chronically infected with the intestinal trematode Echinostoma caproni. The goal of the present studies was to determine the effect of helminth infection on IFN-γ and other key cytokines during malaria co-infection in the P. yoelii-E. caproni and P. yoelii-Heligmosomoides polygyrus model systems. Polyclonally-stimulated spleen cells from both E. caproni- and H. polygyrus-infected mice produced significantly lower amounts of IFN-γ during P. yoelii co-infection than malaria-only infected mice. Furthermore, the magnitude of IFN-γ suppression was correlated with the relative amounts of IL-4 induced by these helminths (E. caproni = low; H. polygyrus = high), but not IL-10. Concurrent malaria infection also suppressed helminth-associated IL-4 responses, indicating that immunologic counter-regulation occurs during co-infection with malaria and intestinal helminths.
Keywords: malaria, helminths, co-infection, Plasmodium yoelii, Echinostoma caproni, Heligmosomoides polygyrus, IFN-γ, IL-4, cytokines, immune regulation, trematode, nematode
INTRODUCTION
Malaria and helminthiases are the two most prevalent parasitic diseases globally, with approximately 500 million people suffering from malaria (Snow et al., 2005) and an estimated 2 billion infected with helminths (de Silva et al., 2003). Since the epidemiologic distributions of these diseases overlap in many tropical areas, concomitant infection is a frequent occurrence (Mwangi et al., 2006). Studies from human populations reveal that helminth infection can have a negative effect on host response to malaria, including increased susceptibility to Plasmodium infection and increased severity of disease (Nacher et al., 2002; Sokhna et al., 2004; Spiegel et al., 2003; Tshikuka et al., 1996). Conversely, protective effects, such as decreased risk of cerebral malaria and lower incidence of malaria, have also been reported for those infected with helminths (Briand et al., 2005; Lyke et al., 2005; Murray et al., 1977; Nacher et al., 2000).
Rodent models of co-infection offer a tractable means of defining the underlying mechanisms, and results from various models reflect the spectrum of interactions observed in humans (Hartgers and Yazdanbakhsh, 2006). We have previously reported that BALB/c mice infected with an intestinal trematode, Echinostoma caproni, were susceptible to increased duration and intensity of malaria parasitemia, resulting in approximately 50% fatality, when infected with otherwise non-lethal P. yoelii (Noland et al., 2005). Similar exacerbation of murine malaria during helminth co-infection has been reported for E. caproni (referred to as E. revolutum in the paper) (Christensen et al., 1988), as well as for other helminths such as the blood trematode Schistosoma mansoni (Legesse et al., 2004; Lwin et al., 1982; Helmby et al., 1998; Yoshida et al., 2000) and the intestinal nematode Heligmosomoides polygyrus (Su et al., 2005).
Host immune response to blood stage malaria infection consists of an orchestrated cytokine and antibody response. IFN-γ, the hallmark Th1 cytokine, plays a central role in the anti-malaria response by limiting parasite growth through the classical activation of macrophages (Stevenson and Tam 1993; Li et al., 2001), and promoting cytophilic antibody production (Snapper and Paul, 1987), which, in humans, has been associated with protective anti-malarial responses (Bouharoun-Tayoun and Druilhe, 1992). The necessity for IFN-γ is shown by studies of genetic knock-out mice, which are susceptible to hyper-parasitemia and death following challenge with P. chabaudi (Su and Stevenson, 2000). Co-infection of mice with H. polygyrus also impairs IFN-γ production during P. yoelii co-infection, rendering mice susceptible to hyper-parasitemia and death (Su et al., 2005).
Here, we evaluated whether chronic E. caproni infection in BALB/c mice similarly leads to reduced IFN-γ response during concurrent P. yoelii infection. We also investigated whether IFN-γ suppression in this model was associated with production of the hallmark Th2 cytokine IL-4 or the regulatory cytokine IL-10, both of which have demonstrated activity against Th1 effector function (Groux et al., 1996; Seder et al., 1992; Skapenko et al., 2004). While the humoral immune response to E. caproni infection in various rodent hosts has been well described (Toledo et al., 2006), the cellular cytokine response in BALB/c mice is largely unknown. Therefore, to control for the relative production of IL-4, we compared the immune response during E. caproni- malaria co-infection in BALB/c mice with that of mice co-infected with H. polygyrus, an intestinal nematode of mice that induces a vigorous IL-4 response (Urban et al., 1991).
MATERIALS AND METHODS
Animals and parasites
For all experiments, age-matched male BALB/c mice were purchased from the National Cancer Institute (Bethesda, Maryland) and maintained in a pathogen-free micro-isolation facility in accordance with the National Institutes of Health guidelines for the humane use of laboratory animals. Mice were infected orally with either 10–15 E. caproni metacercarial cysts or 200 third stage H. polygyrus larvae (L3) as previously described (Noland et al., 2005; Urban et al., 1991). At three weeks post helminth infection, groups of helminth-infected and worm-free age-matched controls were infected with 105 P. yoelii 17X non-lethal (NL) strain parasitized erythrocytes by intraperitoneal (i.p.) injection. Infections were monitored by Giemsa-stained blood smears prepared from tail bleeds, and parasitemia calculated from examining approximately 1,000 red blood cells (RBCs).
Spleen cell cultures
Spleens were harvested aseptically from worm-free, P. yoelii-infected, E. caproni-infected, E. caproni-P.yoelii co-infected, H. polygyrus-infected, and H. polygyrus-P. yoelii co-infected mice at days 3, 5, and 10 post malaria infection (n=3 per time point). Spleens were placed into wells containing cDMEM (Mediatech, Herndon, VA) supplemented with 10% FBS (Sigma, St. Louis, MO), 25 mM HEPES (Gibco, Carlsbad, CA), and 100 U/ml penicillin-100 ug/ml streptomycin (Gibco) (complete medium). Spleens were crushed between the frosted ends of sterile glass slides, filtered through nylon mesh, and RBCs lysed with ACK lysis buffer (Invitrogen, Carlsbad, CA). Cells were then washed with complete medium, and viability determined by trypan blue (Gibco) exclusion. Cells were re-suspended in complete medium supplemented with 50 uM 2-mercaptoethanol (Sigma) and plated at a concentration of 5×106 cells/ml in triplicate aliquots of 750 ul into 48-well tissue culture plates pre-coated overnight with either anti-CD3 (BD Biosciences, San Jose, CA; 1 ug in 100 ul) or PBS alone. Cells were cultured for 48 h at 37°C in a humidified CO2 incubator and, following centrifugation, supernatants were collected and stored at −80°C.
Cytokine ELISAs
Levels of IFN-γ, IL-4, and IL-10 were measured from splenocyte culture supernatants by sandwich ELISA using paired capture and detection antibodies and concentration calculated against a standard curve of recombinant cytokines (BD Biosciences). Preliminary assays were carried out to ensure that responses fell within the linear range of the standard curve. Capture and detect antibodies, respectively, for each cytokine were as follows: R4-6A2 and XMG1.2 for IFN-γ; 11B11 and BVD6-24G2 for IL-4; JES5-2A5 and SXC-1 for IL-10 (all BD Biosciences). 96-well Immulon 2 plates (Thermo Electron) were coated overnight at 4°C with 100 ul capture antibody (2 ug / ml) diluted in PBS. Wells were washed with PBS-0.05% Tween 20, then blocked for 1 h at 37°C with blocking buffer: PBS / 5% non-fat dry milk for IL-4, IL-10; or PBS / 1% BSA (Sigma) for IFN-γ. Following washing with PBS-0.05% Tween 20, 100 ul of recombinant cytokines standards, duplicate samples diluted 1:10 in blocking buffer, or negative samples of blocking buffer alone were added to plates, and incubated for 1–2 h at 37°C. Plates were washed again, then incubated for 1 h at 37°C with 100 ul of biotinylated detection antibody (1 ug / ml) diluted in blocking buffer, followed by an additional wash and incubation with 100 ul streptavidin-peroxidase (2 ug / ml; Sigma). After extensive washing, plates were developed with 100 ul ABTS (2, 2′-azino-di[3-ethylbenzthiazoline-6-sulfonate]; Kirkegaard & Perry Laboratories, Gaithersburg, MD), and absorbance read at 405 nm. Net cytokine levels were calculated by subtracting the mean amount of cytokine produced spontaneously in unstimulated PBS cultures from that produced in anti-CD3 stimulated cultures. Samples from each time point were analyzed simultaneously to minimize variability.
IFN-γ neutralization
Mice were given i.p. injections (1 mg/mouse in endotoxin-free PBS) of anti-IFN-γ (XMG.6, a kind gift from Dr. Tom Richie at the US Navy Medical Research Center), rat IgG as control, or PBS alone at days 4, 6, and 8 post P. yoelii infection. For measurement of IFN-γ in these mice during malaria infection, blood was drawn by tail bleeds at days 5 and 10 post P. yoelii infection and allowed to clot over-night at 4°C. Serum was then collected and stored at −80°C, and levels of IFN-γ analyzed by ELISA exactly as described above for splenocyte cultures.
Statistical analysis
Differences in cytokine levels between groups were compared using the nonparametric Wilcoxon rank-sum (Mann-Whitney) test. Association between IL-4 production and IFN-γ production in helminth infected mice was calculated using Spearman’s rank correlation. All analysis was performed using Stata 9.2 (StataCorp, College Station, TX), and a P value < 0.05 was considered significant. Results from cytokine characterization are reflective of two independent experiments.
RESULTS
IFN-γ kinetics during P. yoelii infection
To first determine the kinetics of IFN-γ production in BALB/c mice infected only with non-lethal P. yoelii, we characterized cytokine production from spleen cells over the course of malaria infection in response to polyclonal stimulation with anti-CD3. Production of IFN-γ peaked early in infection, around day 5, and then returned to baseline levels by day 10 (Fig. 1). This precipitous decline preceded peak parasitemia by several days, and is consistent with the shift from Th1 to Th2 anti-malaria responses observed in other rodent malaria models (Meding et al., 1990; Stevenson and Tam, 1993).
Fig. 1.
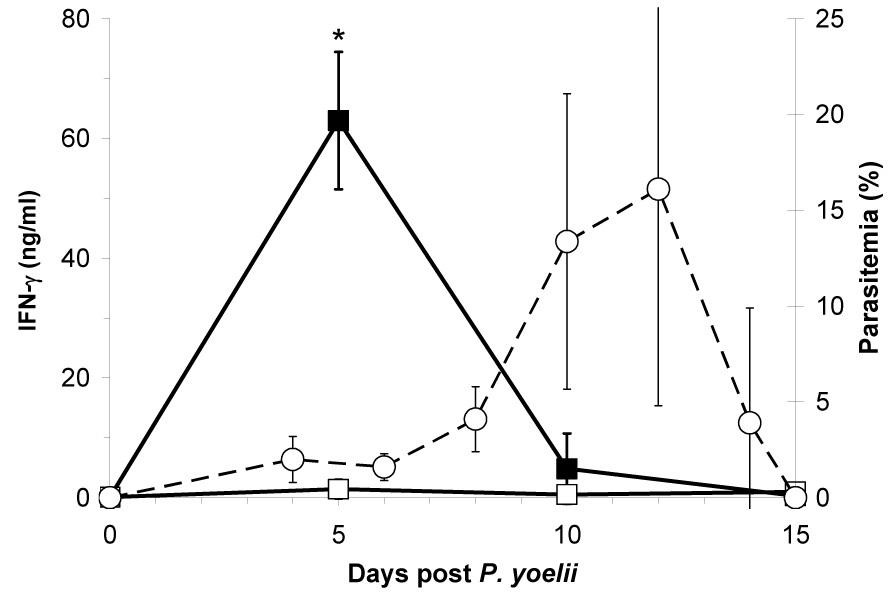
Mean (±SD) IFN-γ production (solid line) from anti-CD3 stimulated spleen cells from Plasmodium yoelii-infected (■) and uninfected naive (□) BALB/c mice at days 0, 5, 10, and 15 post malaria infection (n=3 per time point). Dotted-line indicates mean (±SD) malaria parasitemia in P. yoelii infected mice (○) (n≥11 per time point). Asterisk (*) indicates significantly different (P<0.05) from uninfected mice.
Helminth-induced IFN-γ suppression
Next we determined whether IFN-γ production during this period (days 3–10 post P. yoelii infection), was impaired in mice chronically infected with either E. caproni or H. polygyrus. Beginning at day 3 post malaria infection, levels of IFN-γ were significantly elevated in all malaria-infected mice, whether alone or in combination with chronic helminth infection, compared to naïve control mice or helminth-only infected mice (Fig. 2). By day 5, co-incident with rising parasitemia (Table 1), large amounts of IFN-γ were produced by spleen cells from all malaria infected mice (Fig. 2). However, when compared to malaria-only infected mice, levels of IFN-γ were significantly reduced in both E. caproni-P. yoelii and H. polygyrus-P. yoelii co-infected mice (Fig. 2). By day 10 post malaria infection, IFN-γ production returned to baseline levels in all groups.
Fig. 2.
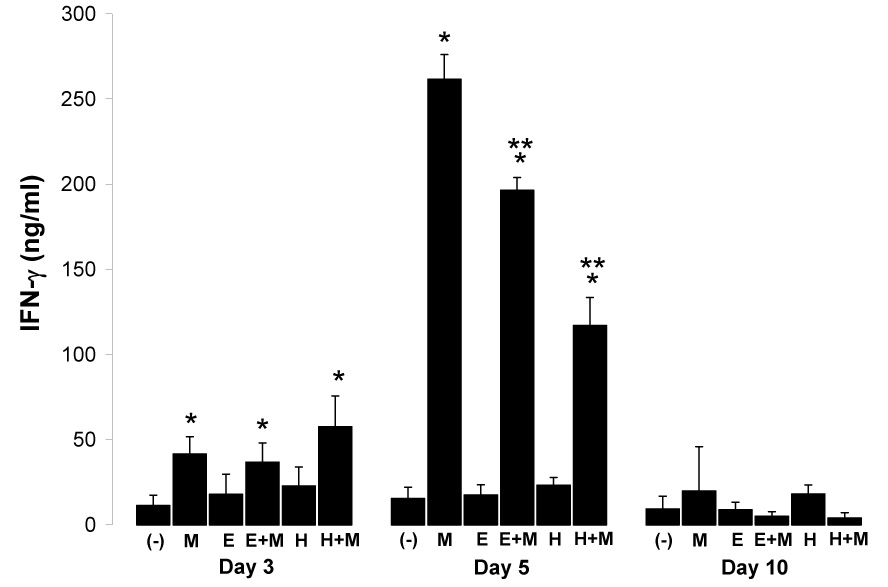
Mean (+SD) net IFN-γ production from anti-CD3-stimulated spleen cells from uninfected (−), P. yoelii-infected (M), E. caproni-infected (E), E. caproni-P.yoelii co-infected (E+M), H. polygyrus-infected (H), and H. polygyrus-P. yoelii co-infected (H+M) BALB/c mice at days 3, 5, and 10 post malaria infection (n = 3 per group per time point). Malaria infection was initiated three weeks post helminth infections. Single and double asterisks (*, **) indicate significantly different (P<0.05) from uninfected control (−) or malaria-only infected mice (M), respectively.
Table 1.
Mean (±SD) malaria parasitemia at indicated days post Plasmodium yoelii infection.
| Group | Day 3 (n=9) | Day 5 (n=6) | Day 10 (n=3) |
|---|---|---|---|
| M | 0.50 (±0.32) | 2.78 (±1.40) | 10.10 (±8.96) |
| E+M | 0.34 (±0.35) | 2.87 (±0.78) | 14.70 (±2.36) |
| H+M | 0.30 (±0.26) | 3.00 (±0.98) | 15.47 (±1.97) |
Differential IL-4 response to helminth infection and correlation with IFN-γ suppression
The hallmark Th2 cytokine, IL-4, is a potent inhibitor of IFN–γ (Seder et al., 1992; Skapenko et al., 2004). Therefore, we evaluated whether suppression of IFN-γ during malaria co-infection was related to IL-4 production in helminth infected mice. Because it has previously been reported that E. caproni infection induces only a moderate IL-4 response (Brunet et al., 2000), the magnitude of IL-4 production in E. caproni-infected mice was compared with that of H. polygyrus, a prototypic IL-4 inducing parasite (Urban et al., 1991). We found that between 4 and 5 weeks post helminth infections (the period corresponding to the initiation of malaria infection in co-infected mice), levels of IL-4 produced by anti-CD3 stimulated spleen cells from E. caproni-infected mice were significantly greater (P=0.03) than levels produced by naïve uninfected control BALB/c mice (Fig. 3). However, the IL-4 response in E. caproni infected mice was significantly lower than the robust IL-4 response from H. polygyrus infected mice (P=0.0002). Based on this categorization (H. polygyrus = high; E. caproni = low), there was a significant inverse correlation between IL-4 and IFN-γ in these groups of mice at day 5 of concurrent P. yoelii infection (Spearman’s ρ= −0.88; P=0.02)—i.e. H. polygyrus co-infected mice displayed a higher degree of IFN-γ suppression than E. caproni co-infected mice.
Fig. 3.
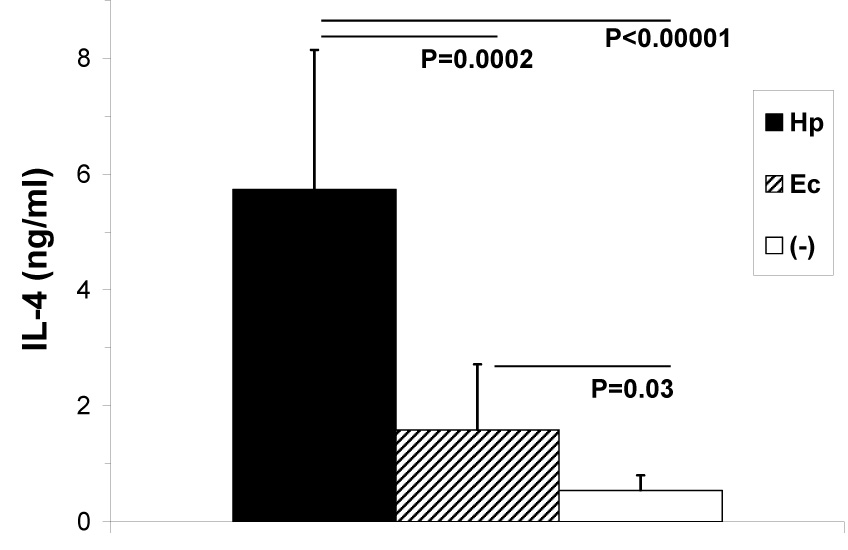
Mean (+SD) net IL-4 production by spleen cells from H. polygyrus (Hp), E. caproni (Ec), or naïve control (−) BALB/c mice measured by ELISA (n=12 per group). Spleens were harvested from groups of mice at four time points between 4 and 5 weeks post helminth infections, and cultured in the presence of anti-CD3.
IL-10 characterization during malaria and helminth infections
The regulatory cytokine IL-10 also exerts potent anti-inflammatory properties (Groux et al., 1996), and has been shown to play a critical role in malaria disease progression (Li et al., 1999). We therefore evaluated whether this cytokine was associated with helminth-induced IFN-γ suppression. Mice infected only with H. polygyrus produced significantly more IL-10 (8.28 ± 3.98_ng/ml, mean ± SD; P = 0.0002) over the course of the experiment than either mice infected with E. caproni alone (3.25 ± 2.80 ng/ml) or uninfected control mice (3.10 ± 1.17 ng/ml, n=12 per group). This likely accounts for the elevated IL-10 observed at day 3 post malaria infection in H. polygyrus-malaria co-infected mice versus those infected only with malaria (P<0.05; Fig. 4). Infection with P. yoelii in worm-free and helminth co-infected mice induced a significant IL-10 response by day 5 post malaria infections (Fig. 4). However, there was no significant difference in levels between these groups, suggesting that IL-10 production from spleen cells of helminth-infected mice had little effect on the suppression of IFN-γ observed in helminth-malaria co-infected mice.
Fig. 4.
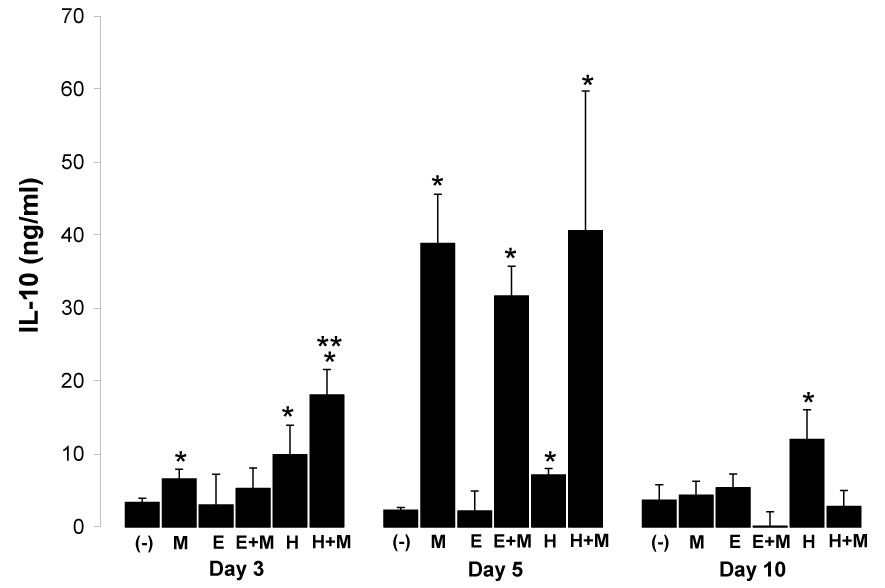
Mean (+SD) net IL-10 production from anti-CD3 stimulated spleen cells from worm-free (−), P. yoelii-infected (M), E. caproni-infected (E), E. caproni-P.yoelii co-infected (E+M), H. polygyrus-infected (H), and H. polygyrus-P. yoelii co-infected (H+M) BALB/c mice at days 3, 5, and 10 post malaria infection (n=3 per group). Malaria infection was initiated three weeks post helminth infections. Single and double asterisks (*, **) indicate significantly different (P<0.05) from uninfected control (−) or malaria-only infected mice (M), respectively.
Malaria-induced IL-4 suppression
These studies also permitted a detailed examination of the effect of concurrent malaria infection on the host immune response to helminth infection. Co-infection of H. polygyrus-infected mice with P. yoelii progressively reduced the high levels of IL-4 normally produced in H. polygyrus infection to near base-line amounts by day 10 of malaria infection (Fig. 5). Likewise, even the relatively low levels of IL-4 produced in response to infection only with E. caproni were significantly reduced by day 10 post malaria infection in E. caproni-P. yoelii co-infected mice.
Fig. 5.
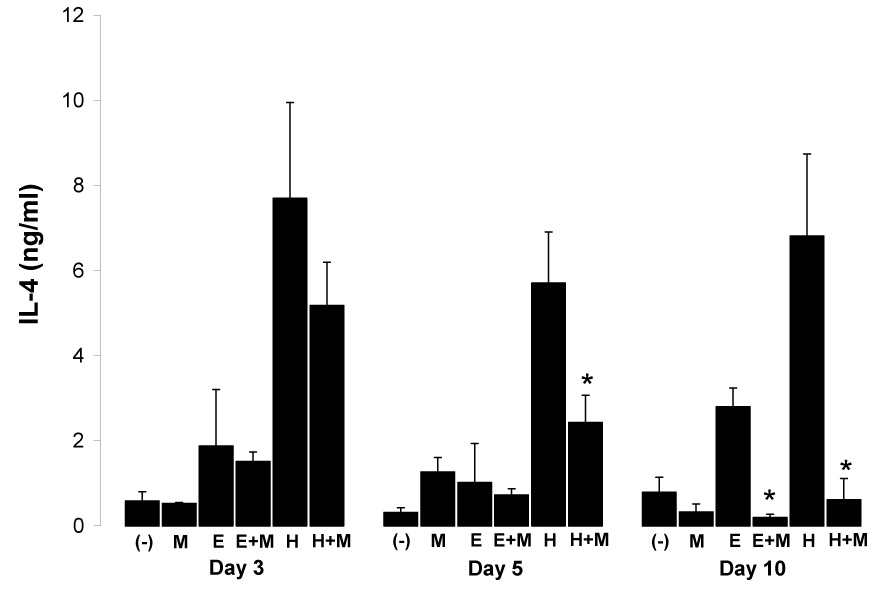
Mean (+SD) net IL-4 production from anti-CD3 stimulated spleen cells from worm-free (−), P. yoelii-infected (M), E. caproni-infected (E), E. caproni-P.yoelii co-infected (E+M), H. polygyrus-infected (H), and H. polygyrus-P. yoelii co-infected (H+M) BALB/c mice at days 3 (a), 5 (b), and 10 (c) post malaria infection (n=3 per group). Asterisks (*) indicates helminth co-infected group significantly different (P<0.05) from helminth-only infected group.
Effect of IFN-γ neutralization during acute malaria infection
In order to determine whether neutralization of serum IFN–γ in the P. yoelii system would recapitulate the exacerbation previously observed during helminth – P. yoelii co-infection (Noland et al., 2005), we administered neutralizing and control antibodies at three time points during early malaria infection (days 4, 6, and 8), and analyzed serum levels of IFN-γ at days 5 and 10 post P. yoelii infection. Administration of IFN-γ-neutralizing antibody significantly reduced the amount of serum IFN-γ compared to mice receiving control antibodies (Fig. 6a). Despite this reduction, malaria parasitemia was only slightly enhanced in this group (Fig. 6b) and fatalities did not occur.
Fig. 6.
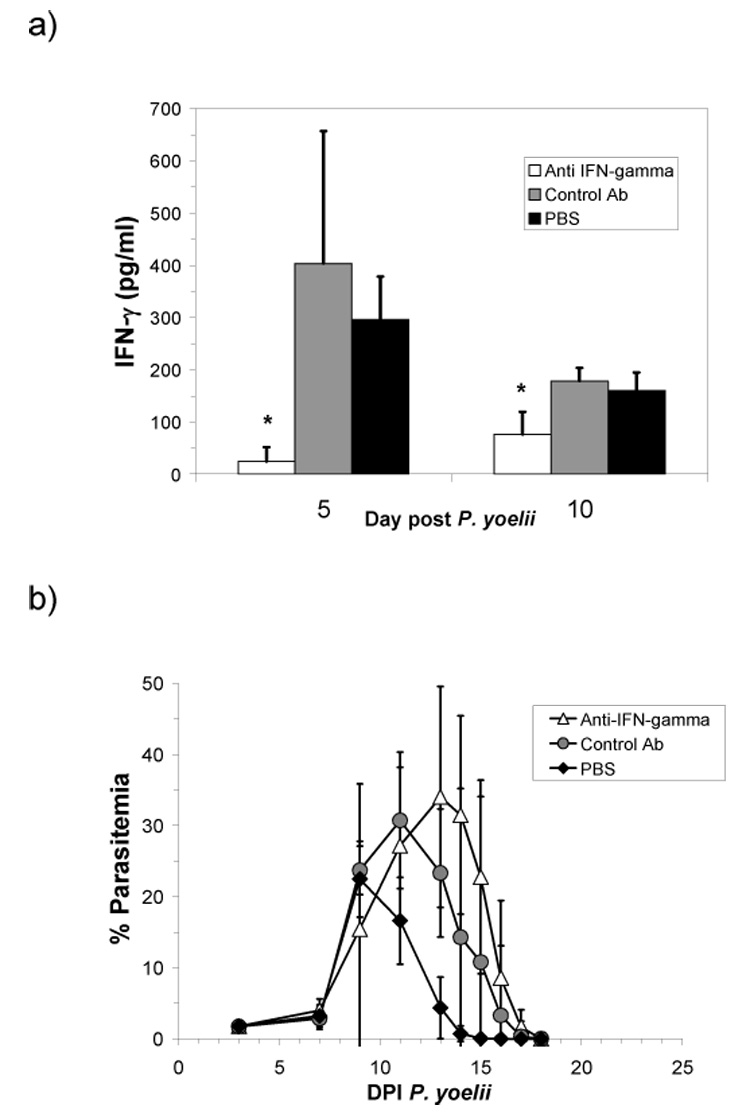
Mean (+SD) serum IFN-γ levels as measured by ELISA at days 5 and 10 post P. yoelii infection (a) and mean (±SD) parasitemia (b) in mice treated with neutralizing IFN-γ antibody, a control rat IgG antibody, or PBS alone on days 4, 6, and 8 post P. yoelii infection (n = 5 per group). Asterisk (*) indicates significantly less (P < 0.05) than either control Ab, or PBS groups.
DISCUSSION
Previously, we reported that BALB/c mice concurrently infected with the intestinal trematode E. caproni and the normally non-lethal rodent malaria P. yoelii were susceptible to enhanced malaria parasitemia and death during co-infection (Noland et al., 2005). IFN–γ plays a central role in controlling blood stage malaria infection (Li et al., 2001), and mice unable to produce this cytokine are likewise susceptible to hyper-parasitemia and death (Su and Stevenson, 2000). The goal of the present studies was to determine the effect of intestinal trematode and nematode infections on IFN-γ and other key cytokines during malaria co-infection in the P. yoelii-E. caproni and P. yoelii-H. polygyrus model systems. Previous studies have shown that a variety of inbred mouse strains mount a strong IFN–γ response to P. chabaudi malaria infection, with peak activity occurring around day 5 post infection (Meding et al., 1990; Stevenson et al., 1990)—a result confirmed in this study with P. yoelii. We therefore focused our attention on modulation of cytokine response during early malaria infection.
The present study demonstrated that chronic infection with either of two intestinal parasites, E. caproni or H. polygyrus, impaired peak IFN–γ production from polyclonally-stimulated spleen cells of BALB/c mice during concurrent P. yoelii infection. Helminth co-infected mice also displayed increased malaria parasitemia at day 10 post malaria infection. Although this result was not statistically significant due to small samples sizes in the present study, previous results in the E. caproni-P. yoelii model indicate that significant differences in malaria parasitemia were not observed until after day 14 of co-infection (Noland et al., 2005). A similar deficit in malaria-specific IFN-γ has been observed in P. chabaudi-H. polygyrus co-infected C57BL/6 mice, which were also susceptible to hyper-parasitemia and death (Su et al., 2005). Taken together, these studies indicate that intestinal helminth infections in mice adversely affect host immune response to concurrent malaria infection, and furthermore suggest that the exacerbation of late-stage disease is associated with impairment of IFN-γ response early in infection.
In contrast to the findings of Su et al. (2005), we found no effect of concurrent helminth infection on humoral IFN-γ response (data not shown), suggesting that control of malaria may depend more critically on cellular INF-γ. In line with this hypothesis, we observed minimal exacerbation of disease when serum IFN-γ was neutralized through injection of antibodies throughout P. yoelii infection. Such results are consistent with similar experiments in the P. chabaudi model (Meding et al., 1990;Stevenson et al., 1990).
Helminth species do not seem uniform, however, in their effect on host response to concurrent malaria infection. S. mansoni-infected C57BL/6 mice, which were susceptible to enhanced P. chabaudi malaria, displayed only sporadic reduction in INF-γ, whereas levels of TNF-α, another Th1-associated cytokine implicated in control of rodent malaria infection (Jacobs et al., 1996), were significantly reduced (Helmby et al., 1998). Other investigators have found that mice infected with either S. mansoni or the filarial nematode Litomosoides sigmodontis produced increased levels of IFN-γ during P. chabaudi infection (Graham et al., 2005; Yoshida et al., 2000). Such results may be explained by the fact that schistosomes and filarial worms exhibit both Th1 and Th2 properties (Maizels and Yazdanbakhsh, 2003), and effects of these tissue-borne parasites may be more heterogenous than intestinal parasite infection.
In contrast to the Th1-associated protective immune response to malaria, helminth infection generally results in strong Th2 polarization, characterized by the production of IL-4, IL-5, IL-9, and IL-13, IgE antibodies, as well as subsequent activation of mast cells, eosinophils, and basophils (MacDonald et al., 2002). Indeed, H. polygyrus elicited high levels of IL-4 in these experiments, consistent with previous studies (Urban et al., 1991). E. caproni infection, however, resulted in relatively minor IL-4 production. This result is in agreement with a previous report of moderate IL-4 response to E. caproni infection in C57BL/6 mice (Brunet et al., 2000). The low level IL-4 response during E. caproni infection is remarkable as nearly all other helminths, including gastrointestinal nematodes (Finkleman et al., 1997), filarial nematodes (Lawrence et al., 1994), and S. mansoni (Pearce et al., 2004), induce a robust Th2 response during some or all stages of infection. It is likely that the exclusively enteric and non-invasive nature of the E. caproni life cycle in the mammalian host (Fried and Huffman, 1996) plays a major role in this finding, though a definitive mechanism has yet to be established. The E. caproni model thus offers a unique system in which the confounding effects of systemic tissue phase disease and gut invasion are eliminated from characterization of host immune response to intestinal parasites, and continued examination of this aspect should prove valuable for the immunology of echinostomes and helminths in general.
In this study we observed a strong correlation between the relative production of IL-4 by helminth infected mice and the magnitude of IFN-γ suppression during co-infection with P. yoelii. IL-4 directly suppresses Th1 activity and IFN–γ production (Seder et al., 1992; Skapenko et al., 2004), and previous work has also shown that this occurs in a dose dependent manner (Peleman et al., 1989). Th2 cells produce IL-4 under the control of the transcription factor GATA-3 (Murphy and Reiner, 2002). GATA-3 has already been implicated in Th1 down modulation in human helminth infections (Babu et al., 2006), and it would be interesting to evaluate its expression during malaria co-infection in the E. caproni and H. polygyrus models.
IL-10 also exerts potent anti-inflammatory properties (Groux et al., 1996) and plays a central role in regulating immune pathology to both malaria (Li et al., 1999) and helminth infections (Hoffmann et al., 2000). Indeed, in these studies, the down-regulation of IFN-γ in all malaria infected groups by day 10 post malaria infection was preceded by a vigorous IL-10 response in these groups at day 5. H. polygyrus infection alone also induced a significant IL-10 response, in agreement to previous findings (Su et al., 2005). However, as spleen cells from mice infected with E. caproni failed to produce levels of IL-10 greater than naïve uninfected mice, IL-10 production by spleen cells did not appear to be a causative factor in helminth-associated IFN–γ suppression. This does not exclude the possibilities, however, that IL-10 from other cellular sources such as dendritic cells or macrophages, or other regulatory factors play a role in these interactions. Indeed, recent studies have shown that H. polygyrus induces CD4(+)CD25(+) regulatory T cells (Finney et al., 2007) and CD8+ regulatory T cells (Setiawan et al., 2007) that modify responses to non-parasite antigens and immune pathology at sites distal to worm infection. Exploration of the interaction of these cells with Plasmodium infection should prove fruitful.
Here we were also able to examine the reciprocal effects of malaria infection on cytokine response to existing helminth infection. We found that the vigorous Th1 anti-malaria response down-regulated the established IL-4 response to both E. caproni and H. polygyrus infection. This also corroborates similar observations in the H. polygyrus-P. chabaudi system (Su et al., 2005). However, such modulation does not seem to alter viability or fecundity of E. caproni, as malaria had no effect on E. caproni fecal egg production during co-infection (Noland et al., 2005), and initiation of E. caproni infection simultaneous to, or during early malaria infection had no effect on worm establishment (data not shown). In contrast, concurrent P. yoelii infection has previously been found to enhance establishment of S. mansoni infection (Christensen et al., 1988)
In conclusion, these studies demonstrate that concurrent intestinal helminth and malaria infections can reciprocally regulate host immune response to either pathogen. Furthermore, findings presented here support the hypotheses that helminth-induced exacerbation of malaria is associated with a deficit in IFN-γ production, and that suppression of IFN-γ is related to IL-4 production during chronic helminth infection. Results from the co-infection models employed here provide insight into the complex interactions that have emerged from human co-infection studies, and continue to offer convenient systems to explore the mechanistic determinants.
ACKNOWLEDGEMENTS
GSN was supported by a predoctoral fellowship from the Johns Hopkins Malaria Research Institute. Research in the Kumar laboratory is supported by grants from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. Journal of Immunology. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infection and Immunity. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand V, Watier L, Le Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in Senegalese children? American Journal of Tropical Medicine and Hygiene. 2005;72:702–707. [PubMed] [Google Scholar]
- Brunet LR, Joseph S, Dunne DW, Fried B. Immune responses during the acute stages of infection with the intestinal trematode Echinostoma caproni. Parasitology. 2000;120(Pt 6):565–571. doi: 10.1017/s0031182099006009. [DOI] [PubMed] [Google Scholar]
- Christensen NO, Furu P, Kurtzhals J, Odaibo A. Heterologous synergistic interactions in concurrent experimental infection in the mouse with Schistosoma mansoni, Echinostoma revolutum, Plasmodium yoelii, Babesia microti, and Trypanosoma brucei. Parasitology Research. 1988;74:544–551. doi: 10.1007/BF00531632. [DOI] [PubMed] [Google Scholar]
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends in Parasitology. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annual Review of Immunology. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. European Journal of Immunology. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried B, Huffman JE. The biology of the intestinal trematode Echinostoma caproni. Advances in Parasitology. 1996;38:311–368. doi: 10.1016/s0065-308x(08)60037-8. [DOI] [PubMed] [Google Scholar]
- Graham AL, Lamb TJ, Read AF, Allen JE. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. Journal of Infectious Diseases. 2005;191:410–421. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. Journal of Experimental Medicine. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunology. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- Helmby H, Kullberg M, Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infection and Immunity. 1998;66:5167–5174. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. Journal of Immunology. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Radzioch D, Stevenson MM. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infection and Immunity. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RA, Allen JE, Osborne J, Maizels RM. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. Journal of Immunology. 1994;153:1216–1224. [PubMed] [Google Scholar]
- Legesse M, Erko B, Balcha F. Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghei co-infected mice. Acta Tropica. 2004;91:161–166. doi: 10.1016/j.actatropica.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infection and Immunity. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Seixas E, Langhorne J. Rodent malarias: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Medical Microbiology and Immunology. 2001;189:115–126. doi: 10.1007/s430-001-8017-8. [DOI] [PubMed] [Google Scholar]
- Lwin M, Last C, Targett GA, Doenhoff MJ. Infection of mice concurrently with Schistosoma mansoni and rodent malarias: contrasting effects of patent S. mansoni infections on Plasmodium chabaudi, P. yoelii and P. berghei. Annals of Tropical Medicine and Parasitology. 1982;76:265–273. doi: 10.1080/00034983.1982.11687541. [DOI] [PubMed] [Google Scholar]
- Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, Guindo A, Traore K, Daou M, Diarra I, Sztein MB, Plowe CV, Doumbo OK. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. American Journal of Tropical Medicine and Hygiene. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- MacDonald AS, Araujo MI, Pearce EJ. Immunology of parasitic helminth infections. Infection and Immunity. 2002;70:427–433. doi: 10.1128/iai.70.2.427-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Reviews Immunology. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Meding SJ, Cheng SC, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infection and Immunity. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature Reviews Immunology. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Murray AB, Murray MB, Murray CJ. Parotid enlargement, forehead edema, and suppression of malaria as nutritional consequences of ascariasis. American Journal of Clinical Nutrition. 1977;30:2117–2121. doi: 10.1093/ajcn/30.12.2117. [DOI] [PubMed] [Google Scholar]
- Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Annals of Tropical Medicine and Parasitology. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite immunology. 2000;22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R, Looareesuwan S. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. Journal of Parasitology. 2002;88:55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Noland GS, Graczyk TK, Fried B, Fitzgerald EJ, Kumar N. Exacerbation of Plasmodium yoelii malaria in Echinostoma caproni infected mice and abatement through anthelmintic treatment. Journal of Parasitology. 2005;91:944–948. doi: 10.1645/GE-456R.1. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, M Kane C, Sun J, J Taylor J, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunological Reviews. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- Peleman R, Wu J, Fargeas C, Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. Journal of Experimental Medicine. 1989;170:1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. Journal of Experimental Medicine. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infection and Immunity. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A, Niedobitek GU, Kalden JR, Lipsky PE, Schulze-Koops H. Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. Journal of Immunology. 2004;172:6427–6434. doi: 10.4049/jimmunol.172.10.6427. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malaria Journal. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:198–199. doi: 10.1016/s0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- Stevenson MM, Tam MF. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clinical and Experimental Immunology. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MM, Tam MF, Belosevic M, van der Meide PH, Podoba JE. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infection and Immunity. 1990;58:3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infection and Immunity. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infection and Immunity. 2000;68:4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo R, Esteban JG, Fried B. Immunology and pathology of intestinal trematodes in their definitive hosts. Advances in Parasitology. 2006;63:285–365. doi: 10.1016/S0065-308X(06)63004-2. [DOI] [PubMed] [Google Scholar]
- Tshikuka JG, Scott ME, Gray-Donald K, Kalumba ON. Multiple infection with Plasmodium and helminths in communities of low and relatively high socio-economic status. Annals of Tropical Medicine and Parasitology. 1996;90:277–293. doi: 10.1080/00034983.1996.11813053. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Maruyama H, Kumagai T, Amano T, Kobayashi F, Zhang M, Himeno K, Ohta N. Schistosoma mansoni infection cancels the susceptibility to Plasmodium chabaudi through induction of type 1 immune responses in A/J mice. International Immunology. 2000;12:1117–1125. doi: 10.1093/intimm/12.8.1117. [DOI] [PubMed] [Google Scholar]


