Abstract
Evolutionary theory postulates that there should be a robust relationship between fecundity and longevity. Prior work has generally supported this concept, but has not shed much light on the mechanisms at play. In preceding work, we have developed and verified a mathematical model of Drosophila melanogaster female fecundity based on the analysis of empirical studies independently done by several different laboratories. Then we applied this technique to Mediterranean fruit fly (medfly) populations. In this article we analyze associations between individual longevity and the parameters of individual fecundity pattern in Drosophila and medfly. We cluster both Drosophila and medfly individuals by life span and discuss the differences. It allows us to demonstrate that only one fecundity-related parameter is associated with longevity in Drosophila, whereas two such parameters can be found in medflies. This difference demonstrates different ways of aging in various Diptera species. Finally, we discuss the possible implications of this finding.
Evolutionary theory postulates the existence of a robust relationship between fecundity and longevity. Drosophila has proven to be an ideal laboratory model for investigating the existence and mechanisms of this relationship. Prior work with this organism, involving both selection experiments as well as dietary manipulations, has generally supported this concept (1–8). All these studies yielded much the same picture, in that the population-averaged age-related fecundity pattern was described as starting rather quickly within the first 1–2 days after eclosion, achieving an apparent peak maximum value by 2–4 days, and then slowly decreasing to a negligible level (9–11).
Despite this general agreement on the descriptive aspects of this relationship, the prior work did not shed much light on the mechanisms underlying these widespread alterations in fecundity. In large part, this was because a robust analysis needed individual female, as opposed to population-based fecundity data. Although such individual female experiments have been done for some time (1,4,12–15), their intensive study began only recently (14,16–19).
Pretzlaff and Arking (20) were the first to note an exponential decrease in a mean-population fecundity in Drosophila. A theoretical prediction of optimal fecundity pattern in Drosophila was presented by Stearns and colleagues (21), but unfortunately that study did not report how the prediction was yielded. Muller and colleagues (22) successfully simulated the declining part of the age-related fecundity in individual Mediterranean fruit fly (medfly) females by an exponential function randomly beginning at an age of egg-laying peak, but unfortunately that study did not analyze the initial and mid-life part of the mean-population or individual fecundity pattern. Kindlmann and colleagues (23, p. 837) developed a model in which “inclusion of the senescence function … resulted in a triangular shaped fecundity function.” Nonetheless, these authors do not use any quantitative criteria to estimate the “triangularity” of the pattern. Dixon and Agarwala (24) compared such a form with a mean-population fecundity pattern in ladybird beetles, but they also did not go beyond the triangular appearance of the pattern. Finally, Rauser and colleagues (25) presented evolutionary reasons why mean fecundity values plateau at very advanced ages. None of these studies used a rigorous quantitative analysis.
Two other studies did attempt to quantitatively describe the overall fecundity pattern, but each yielded only rough approximations. Shanley and Kirkwood (26) used a cosine presentation of an overall fecundity pattern in a mouse population, and Cichon (27) calculated schematic curves of reproductive rates in Drosophila, presumably formed by optimal resource partitioning. There was no comparison with experimental data in either case.
These prior studies may generally be summarized as yielding a qualitative but not a quantitative understanding of the relationship between fecundity and longevity in this organism. As a consequence, no rigorous mechanistic understanding was possible.
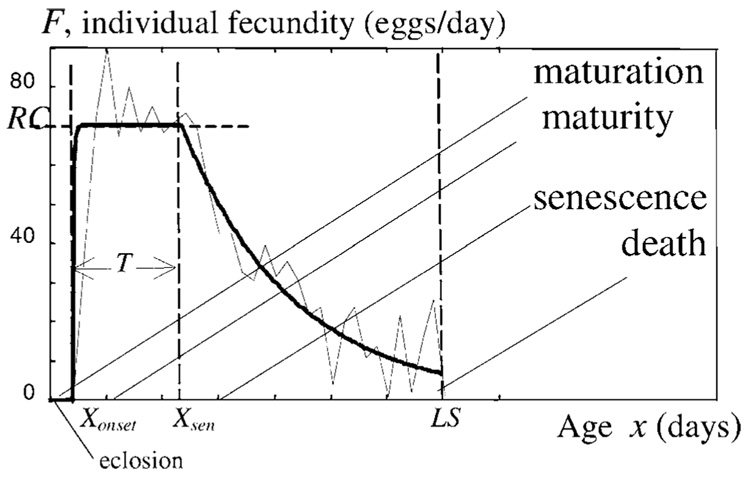
As described in our preceding articles, we have developed and verified a mathematical model of Drosophila melanogaster female fecundity which includes the three phases of maturation, maturity, and reproductive senescence. Initially, Novoseltsev and colleagues (28) analyzed the theoretical intrinsic mechanisms that relate egg production to longevity in individual fly females. This model assumed that the observed fecundity for any individual female represented the interactions and trade-offs between an age-related reproductive program and an age-related decline in the homeostatic capacity of the reproductive system (presumably due to increasing oxidative vulnerability). We showed that the individual rate of egg-laying followed a genetically prescribed constant maximal value. This means that, until the onset of senescence, the individual fecundity pattern is flat and has no maximum. After that time, it declines exponentially. To describe such a pattern in adult females, we assumed the existence of three age-related stages: maturation, maturity, and senescence. These phases are described by four parameters, namely Xonset, RC, T, and αsen (Figure 1).
Figure 1.
Individual fecundity in a Drosophila female (fly 193). The daily scores are shown by a thin line and the regular pattern by a thick line. The maturation stage is denoted by x < Xonset, maturity by Xonset ≤ x < Xsen, and senescence by Xsen ≤ x. The onset of senescence Xsen = Xonset + T. The senescence stage is described by F = RC·exp[−αsen·(x − Xsen)] and lasts S = (LS−T−Xonset) days. In the figure, Xonset=2, T=9.7, S=23.3, LS=35 (days), αsen· = 0.1014 (day−1), and RC = 70.6 (eggs per day). See the main text for further details.
Here, Xonset characterizes the onset of reproduction (days), RC is the reproductive capacity (eggs per day, the height of the fecundity plateau at which the reproductive machinery of an individual fly works at full strength), and T is the length of the maturity period (days). αsen is the rate of senescence. Maturity presumably ends at the onset of senescence Xsen = Xonset + T, after which the senescence lasts S days, S=(LS−T−Xonset). Here LS means life span.
The model was quantitated using a preexisting set of individual female longevity and fecundity data. This was subsequently verified as being applicable to other independently published Drosophila data sets (29). The model was then tested on independent data sets of female Medflies and was found to generally apply, although questions about the nature of the reproductive senescent phase in the medfly were left unanswered (e.g., the expected trade-off between fecundity and longevity was not observed in the medfly populations).
Thus our model generally mirrors the genetic and physiological features of the Diptera species, although the analysis to date suggests that the reproductive senescent phase of the medfly might involve alterations in different parameters in the medfly as compared to Drosophila (30).
Thus, we now extend our prior analysis by focusing on the manner in which aging and senescence develop in Drosophila and medflies. We use a different analytical method to detect the parameters associated with longevity for each species. Having identified the species-specific parameters, we then proceed to analyze the manner by which reproductive senescence develops in each species. We conclude that reproductive senescence in these two species involves different parameters, and this finding may explain the different patterns of reproductive senescence observed in these two laboratory model organisms.
METHODS
For our study, we used the individual Drosophila melanogaster female fecundity and longevity data sets which were originally used to generate the population data reported in other articles (1,4,20). We also used the individual medfly female fecundity and longevity data sets originally analyzed and reported in other publications (16,30).
The Drosophila data used involved three control (i.e., normal-lived) lines in an artificial selection procedure for shortened and prolonged life span (1,4,31). Thus the united population data set consists of Ra, Rb, and Rc flies (149, 172, and 172 flies, relatively). Individual numbers from 1 to 493 were given to these flies.
A newly eclosed male and female fly were placed in vials which were changed daily, and fecundity and survival were tallied daily. Age at death was documented and longevity calculated. In the event of the death of a male in any of the assay vials, the dead fly was removed and replaced by another male of the same age from a cohort of reserve flies (1,4,20). However, the disturbed patterns were taken into account in our study.
Previously, egg-laying scores in medfly were approximated by regular three-stage patterns via the least-mean-square procedure (30). To test if it is possible to use this approach in Drosophila, we applied this fitting procedure to the individual fecundity scores of Drosophila females. This procedure allows us to divide the fecundity score into three parts, the first referred to as maturation, the second maturity, and the third senescence.
The methods used for the detection and analysis of critical points in the onset of senescence are described in conjunction with the results, as this approach is more informative to the reader.
RESULTS
Onset of Senescence Really Exists
The onset of senescence point arises when the individual resources devoted to reproduction (reproduction-related supply) cross downward over the line of constant resources, which is needed to fulfill the genetically predicted fecundity program (reproduction-related demand). Thus, the maturity stage proceeds T days and ends at the onset of senescence Xsen, which can be calculated as Xsen = Xonset + T.
After this point, the fecundity rate starts to decrease, presumably at the exponentially decelerated rate. αsen is the rate of senescence, which has a dimension of (1/day) and means that, at senescence, daily egg laying diminishes by exp[−αsen] each following day as related to the current day. Mathematically, αsen is the exponent of the exponential function that describes an age-related decrease in fecundity at older ages. Previously, we and others (28,31) used another parameter, τtail=(αsen)−1, instead of αsen. τtail is the time constant of the reproductive “tail” in the individual fecundity pattern. The senescence stage lasts for S days and ends when death occurs, at age LS.
Nonetheless, the random character of egg scores calls the existence of discrete times of onset of senescence into question. Indeed, in some patterns the location of this point is obvious, but in others, it is not. To test if there really exists a critical point that manifests the onset of senescence, we apply the following procedure.
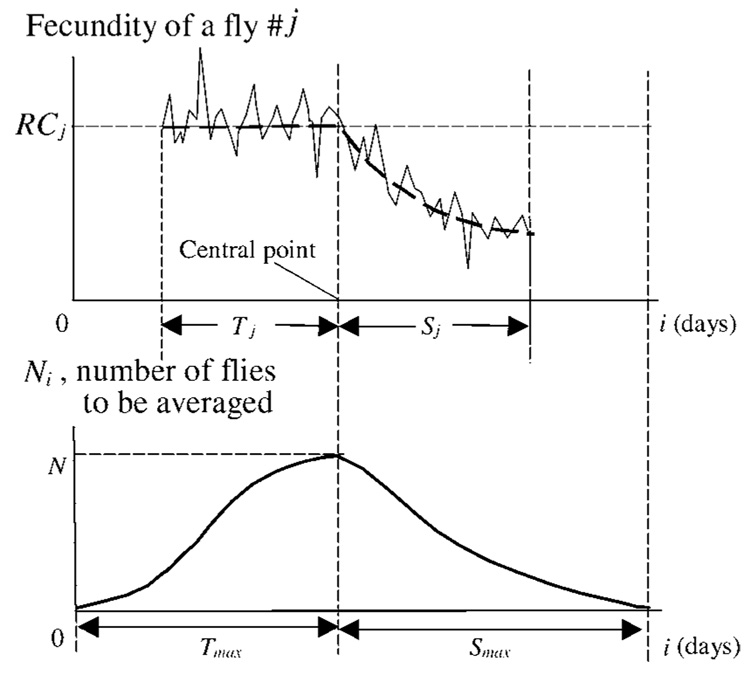
The least-mean-square procedure allows dividing maturity stage from senescence stage in a fly female by fitting the individual scores with a three-stage regular pattern. To the time axis we allocate all individual egg scores so as to put together individual change points predicted by least-mean-squares to be the onsets of senescence. An example of how this is done is shown in Figure 2. The egg scores for the jth fly are presented with the presumable onset of senescence placed in a central point. This point is positioned exactly between two time intervals. To the left of the point, the maximal length of the plateau (Tmax) is placed, and to the right, the maximal length of senescence tail in the studied population, Smax.
Figure 2.
The schematic presentation shows how the existence of the onset of senescence can be detected. Top: Egg scores and a regular fecundity pattern are presented for jth fly. The onset of senescence, predicted by least mean squares, is moved to the central point. The pattern starts at the centered age i = Tmax − Tj, where Tj is the length of the maturity stage, and Tmax is the maximal Tj in the analyzed population. The height of the individual fecundity plateau, RCj, and the lengths of the maturity and senescence stages, Tj and Sj, are shown. The data from this fly are subjected to a procedure of averaging the difference between egg scores and the fecundity plateau level at days from i=Tmax−Tj to i=Tmax + Sj. Bottom: Number of flies to be averaged at each age i is shown. Starting at day 0 from Ni=1, this number achieves on the central day i=Tmax the total quantity of flies in the population, N. Then Ni starts to decrease, and again Ni = 1 at day i = Tmax + Smax.
For each such score, we calculate the difference between its random points and the constant, equal to the respective RC. Then we average these differences across the corresponding number of flies. Being repeated many times, the averaging procedure eliminates the random errors but exposes the systematic ones. Thus, an acute angle will arise in the central point if senescence really originated in it.
The graphs below schematically show the changes in the number of flies that are to be averaged at each i, where the centered age is i = 0, 1, …, (Tmax + Smax). The number of flies is referred to as Ni. The graphs Ni(i) start from Ni=1 at point i = 0 (i.e., from the fly with the maximal length of maturity stage) and at the point i = Tmax arrives to the maximum value, which is equal to the population quantity, N. Then it moves downward to Ni = 1 at point i = Tmax + Smax (the fly with the maximal S length).
Let r(i,j) denote a number of eggs laid by fly j at day i. The difference Di between the scores and the respective plateau levels, averaged in series at all ages, yields
| [1] |
Here, Di is the averaged difference at the centered age i, and rc(i, j)=r(Xonset,j−Tmax+Tj+i, j) is the centered egg score for the fly j at day i.
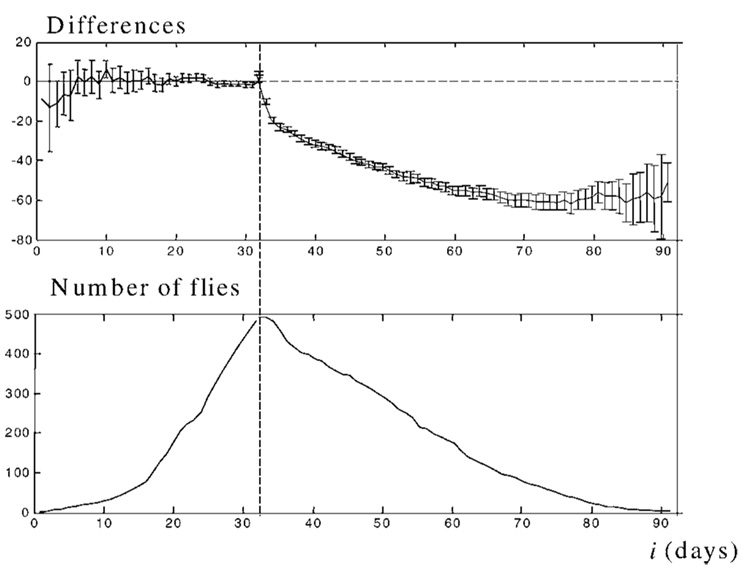
The pattern Di for the Drosophila population and the Ni pattern are presented in Figure 3. Having been averaged, the deviations of the individual plateau levels Di calculated from 493 individual scores yields a two-part pattern. Left of the central point the differences are averaged to give 0, whereas to the right they produce the curve, which is close to the exponential curve.
Figure 3.
The pattern demonstrates the existence of the onset of senescence in Drosophila flies. Top: Averaged differences in the Drosophila population respective to the individual plateau level. The vertical dotted line denotes the central point for the individual onsets of senescence detected by least mean squares. The individual onsets are aligned to day 32. In the population, Tmax = 34 days, but to make the result clearer, the data from the first 2 days are eliminated from each individual score before averaging. Thus, for each individual score, the averaging procedure starts at i = Tmax − Tj + 2 = 32 − Tj. Bars represent 95% confidence intervals. Bottom: Number of flies, across which the averaging is performed.
The acute angle seen at the central point of the graph clearly demonstrates the existence of senescence onset in the Drosophila population. As this Drosophila strain is the only one studied individually to date, we assume the existence of the onset of senescence to be true for all Drosophila species. Analogous results in medfly (data not shown) demonstrate that this property is common in Diptera organisms.
Fecundity Rate at Maturity in Drosophila and Medfly Is Constant
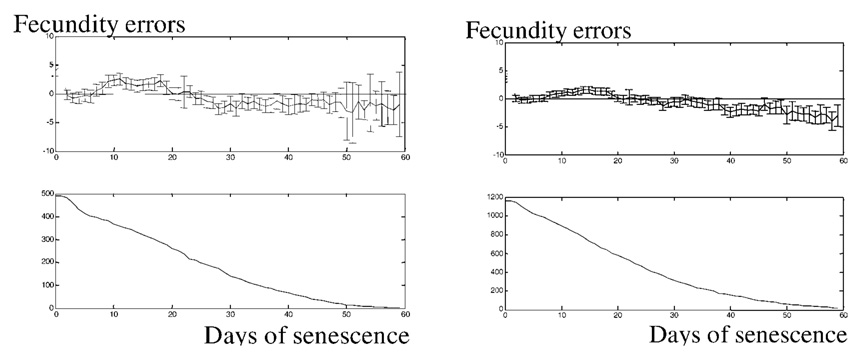
Egg scores in Figure 1 represent the “noisy” components, over which the regular individual patterns are superimposed. But how flat is the noisy reproductive plateau at maturity in individuals, on average? Figure 3 is too rough to see the details of the plateau structure. To answer that question, we estimate individual errors between the random scores in each fly and its plateau level, and then average them over the population. This procedure allows us to evaluate how close these scores are, on average, to their regular patterns.
For each day of maturity we average only the errors of the mature individuals. Thus, the average gives
| [2] |
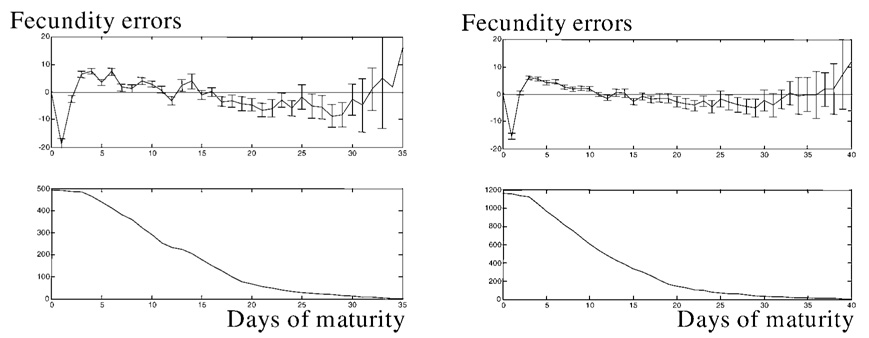
where EMi is an error at the ith day of maturity, rM (i, j) = r(Xonset,j + i, j), and (rij − RCj) is an individual error of fly j on that day. Then, j = 1, …, Ni, where Ni is the number of flies that are mature at day i.
Errors EMi for 493 Drosophila and 1170 Medflies are presented in Figure 4. It can be seen that there are no maxima in the patterns on these graphs. This means that maximal values in the scores are evenly scattered over the maturity period. Thus individual “noisy” fecundity allows for a flat approximation at maturity. The patterns show the number of mature flies, and this number smoothly diminishes from the initial value because the flies leave the “steady-state” mature group after having achieved the onset of senescence. Thus, we present experimental confirmation of our theoretical finding that the flat maturity plateau exists in the life course of individual fruit flies of the species Drosophila and medfly.
Figure 4.
Systematic errors at maturity in the Drosophila population and in 1170 medflies studied in our previous work (29). The pattern for Drosophila is shown at top left, and that for medflies at top right. All onsets of reproduction are moved to the origin. Top: Averaged error scores for the first, second, third, etc., day of individual maturity as compared to the corresponding plateau level. Bottom: Number of mature flies. Bars represent 95% confidence intervals of the systematic errors.
Fecundity Rate at Senescence Exponentially Decelerates
Novoseltsev and colleagues (28,30) also proposed that the individual reproductive tail follows an exponentially decreasing dependence. But, how close does the averaged fecundity at senescence follow the exponentially decreased curve?
To answer the question, one needs to calculate the individual errors, compare them at each age with the respective value of the decreasing exponential curve, and then average the errors. The results will be conditional, based on the premise that the fly is at the senescence stage of life on the day of averaging. Thus one has
| [3] |
where ESi is an error at the ith day of senescence, rS (i,j) = r(Xsen,j + i, j), Ni is the number of senescing flies at a day i, and αsen is the rate of senescence in the jth fly. The other notations are the same as in Equation 2.
The errors ESi are presented in Figure 5, with the x-axis showing the day of senescence. All onsets of senescence are placed at the origin. No specific features are seen in the patterns for both species. This finding definitely confirms our previous assumption about the exponential deceleration of individual fecundity at senescence.
Figure 5.
Systematic errors at senescence (day 0 is the onset of senescence) in the Drosophila (top left) and medfly populations (top right). All onsets of senescence are moved to the origin. Bottom: Number of flies at their senescence stage. All notations are the same as in Figure 3.
The patterns in the second row of diagrams show the number of flies at each age of senescence. At the onset of senescence, all flies are alive because no flies formally die during the maturity stage. Even the flies that die at their reproductive plateau, strictly speaking, have very short tails, and all are shown in the figure as senescing ones. The number of senescing flies diminishes due to natural mortality, but the diminution slows because of new flies achieving the onset of senescence and joining the senescing group.
Individual Longevity and Fecundity
The relationship between individual fecundity and senescence is intriguing. Indeed, it would be interesting to prospectively know which flies will live long. What are the individual parameters that differ in flies and thus make it possible to distinguish the long-living ones from the others in a population?
Unfortunately, individual data on Drosophila and medfly fecundity are stochastic and heterogeneous, thus preventing a direct exploration of the relationships between fecundity and longevity. The correlation coefficients between the parameters in both populations (Drosophila and 1000-fly Carey’s medfly) are presented in Table 1.
Table 1.
Correlation Coefficients Between the Parameters
| Parameters | RC | T | αsen* | LS |
|---|---|---|---|---|
| RC | ||||
| D | 1 | −0.095 | 0.210 | 0.060 |
| M | 1 | −0.106 | 0.285 | 0.060 |
| T | ||||
| D | — | 1 | −0.340 | 0.140 |
| M | — | 1 | −0.267 | 0.474 |
| αsen | ||||
| D | — | — | 1 | 0.632 |
| M | — | — | 1 | 0.423 |
Note: Coefficients calculated for the reversed values (αsen)−1.
RC = reproductive capacity (eggs per day); T = length of maturity period; αsen = rate of senescence; LS = life span; D = Drosophila; M = medfly.
We can see that the correlation is not robust. Only three figures are noteworthy in the table, one for the Drosophila and two others for medfly. The correlation coefficient between life span, LS, and rate of senescence, αsen, in both cases, is rather high: r = 0.632 in Drosophila and r = 0.423 in medfly [the correlation coefficients were calculated for the reversed parameters, τtail = (αsen)−1]. Additionally, r = 0.474 between the life span and length of maturity period in medfly.
To overcome difficulties related to the extreme stochasticity in the data, we divide both total populations into 10-decile clusters each. Each cluster contains one-tenth of the total scores (50 in Drosophila and 94 in medfly, with the exception of the first cluster in each) sorted in ascending order of LS. Then we calculated the correlation coefficients between the cluster parameters.
Drosophila and medfly data are presented in Table 2. One can see that a correlation arises, resulting in higher values of all coefficients. The most striking are 0.96 and 0.95 values of the correlation coefficients between αsen and life span in Drosophila and medfly; another is the 0.98 value of the medfly correlation between T and LS. The other large value (correlation coefficient 0.91 between T and αsen in medfly) is not essential for our analysis.
Table 2.
Correlation Coefficients Between the Cluster Parameters
| Parameters | RC | T | αsen* | LS |
|---|---|---|---|---|
| RC | ||||
| D | 1 | 0.38 | 0.54 | 0.49 |
| M | 1 | −0.34 | −0.28 | −0.39 |
| T | ||||
| D | — | 1 | 0.62 | 0.61 |
| M | — | 1 | 0.91 | 0.98 |
| αsen | ||||
| D | — | — | 1 | 0.96 |
| M | — | — | 1 | 0.95 |
Note: Coefficients calculated for the reversed values (αsen)−1.
RC = reproductive capacity (eggs per day); T = length of maturity period; αsen = rate of senescence; LS = life span; D = Drosophila; M = medfly.
To find out which parameters are associated with longevity, we take the most long-lived Drosophila, which are in the 10th cluster of flies. It is surprising that these long-lived flies do not differ from the rest of the population in their reproductive capacity, RC (60.3 ± 5.5 vs 60.47 ± 4.3 eggs/day), or in the length of their maturity stage, T (14.3 ± 3.6 vs 13 ± 4.2 days).
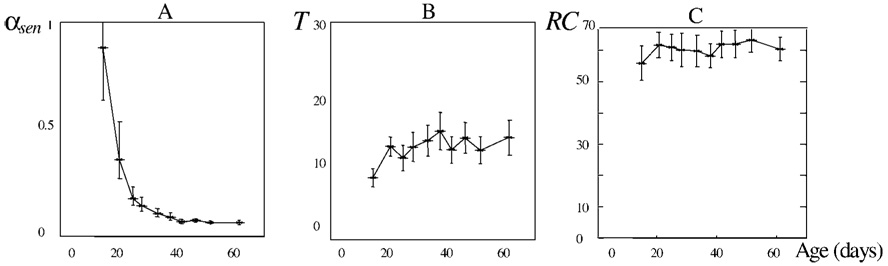
The overall dependencies between the mean decile values of the individual parameters and the mean decile life spans are shown in Figure 6. We can see that, indeed, neither RC nor T is related to prolongation of life span in Drosophila. Only the exponent αsen is clearly related to prolonged senescence. The smaller this exponent is, the slower is the decrease in egg-laying after the onset of senescence, and the longer is life span. The maturity period and the reproductive capacity tend to be constant in all clusters. This means that longevity in Drosophila is associated only with the rate of senescence.
Figure 6.
A decrease of the exponent αsen in long-lived Drosophila is the only parameter associated with senescence. Rate of senescence, αsen (A), the length of the maturity stage, T (B), and the reproductive capacity, RC (C) are shown. The population is divided into 10 clusters by longevity increase so that 10% of the total quantity of flies enter each decile cluster. In each cluster, the mean value of the corresponding parameter is calculated and plotted against the average life span in the cluster. Thus, 10 points in the patterns present the age-related changes in the parameters. Points are connected with a solid line. Confidence intervals of 95% are shown.
Let us compare the Drosophila data with the analogous data for medflies, as presented in Figure 7. A surprising difference between the Drosophila and medfly patterns is the rapid change of the length of the maturity phase in medflies with life span (Figure 7B). This means that the length of the maturity stage tends to be longer in the long-living group of medflies than in the short-living animals. Thus, there are two parameters associated with longevity of medflies, namely, the exponent, αsen, and the length of the maturity stage, T.
Figure 7.
A decrease of the exponent αsen (A) and an increase of the length of the maturity stage T (B) in long-lived medflies. αsen and T are the two parameters associated with senescence. Reproductive capacity, RC (C), is more or less constant (see text for details). The other notations are as in Figure 6.
The beginning of the RC curve (Figure 7C) is moved downward by the numerous short-living flies having a smaller RC. At the other end of the curve, in the 10th decile, the same effect arises due to the relatively high quantity of flies in the cluster that have a long postreproductive period and a smaller reproductive capacity.
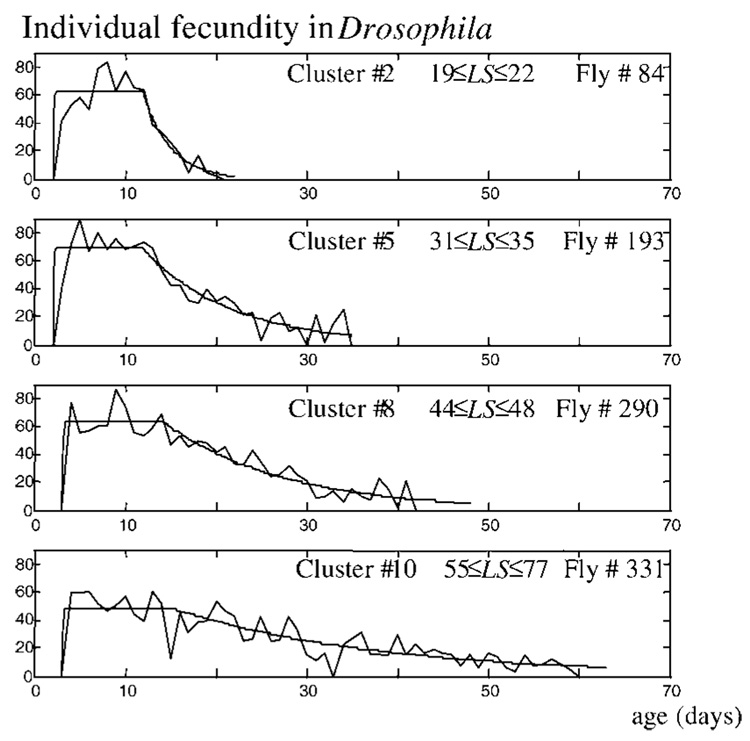
Representative examples taken from different clusters of Drosophila and medflies confirm this finding. Four such patterns from the Drosophila population are presented in Figure 8. Flies from clusters 2, 5, 8, and 10 have a similar plateau period but drastically differ in the lengths of the senescence stage. Analogous examples from medflies (data not shown) demonstrate that the length of both the maturity stage and the senescence stage increases from one cluster to the following one.
Figure 8.
Representative fecundity patterns from different clusters show that the senescence period in Drosophila females is created totally by the increase of the reproductive “tail.” Flies from clusters 2, 5, 8, and 10 have similar plateau periods but drastically differ in the lengths of the senescence stage.
DISCUSSION
The fruit fly Drosophila melanogaster is one of the most widely used laboratory model organisms in the study of aging (32). Its life history is divided into distinct morphological phases so that growth and development can be easily distinguished from the mature phase, in which only the aging process is uncovered. In this article, we continue our detailed analysis of individual fecundity and longevity in Drosophila. We characterized the parameters regulating the individual life histories in Drosophila and compared the results with previously obtained medfly data, using more than 1500 flies (1170 medfly and 493 Drosophila). We showed that two points divide the three periods of individual life history in both medfly and Drosophila: The onset of reproduction opens the maturity stage, and the onset of senescence opens the senescence stage. We presented elsewhere (28) the genetic and physiological reasons underlying the existence of this three-stage pattern. That analysis, plus the data that supports its application in both Drosophila (33) and medfly (30), allows us to conclude that the same model can explain the onset of reproductive (and somatic) senescence in these two species and perhaps in all Diptera species.
To describe the three-stage pattern, we used a set of parameters. These are the onset of reproduction, Xonset, the height of a fecundity plateau at maturity, RC, the length of the maturity period, T, and the rate of senescence, αsen. The last parameter characterizes the rate of the exponential decrease of fecundity in a senescing individual. The egg-laying rate diminishes each day as related to the current one by exp[−αsen]. In this study, the rate of the senescence parameter was used instead of τtail, namely αsen = (τtail)−1 used previously.
Due to the high degree of heterogeneity, a special technique was applied to test if the onset of senescence really exists, namely, whether the rate of egg laying at the maturity stage is constant, and if fecundity at senescence exponentially decelerates.
That analysis confirmed that individual fecundity plateaus, on average, are flat despite the existence of the high “noise” level observed in individual scores of fecundity. Because egg-laying shows a rhythmic activity (34), at least part of this noise may be caused by the stroboscopic effect of daily periodic cycles. An obvious maximum was not observed at maturity, on average. This means that sporadic maxima in the individual patterns are evenly scattered over the maturity ages.
The changes in the mathematical parameters described herein have predictive value and are interesting, but one would like to know the nature of the genetic and physiological events underlying these mathematically described transitions. At the onset of senescence, Xsen, the slow diminution in fecundity undergoes a drastic decrease, and this can likely be related to an alteration of the signals regulating the development of the ripening eggs in the ovaries. It is known that fecundity and life span in Drosophila are under the joint control of the insulin-like signaling pathway (35,36) and the nutritional status of the organism (37). These two inputs modulate the animal’s relative hormone levels (e.g., juvenile hormone [JH] and 20-hydroxyecdysone [20E]). Well fed flies with a good nutritional status have higher levels of JH and lower levels of 20E, whereas starved flies have the opposite situation (38). These hormones regulate the expression of various genes, including the various isoforms of the Broad-Complex (BR-C) gene (38). The high JH/low 20E levels characteristic of well fed flies activate the Z1 isoform of the BR-C gene during stages 8–9 of egg development; this isoform represses apoptosis and allows the eggs to develop normally. The reverse situation, characteristic of flies with a nutritional shortage, activates BR-C isoforms Z1, Z2, and Z3 during that same checkpoint stage; this expression pattern subsequently induces apoptosis in that egg chamber. Thus it seems that the hormones known to affect fly fecundity (39) operate by changing the number of immature eggs allowed to develop in each egg chamber (ovariole) of the ovary. Individual egg chambers autonomously sense the nutritional status of the animal, some entering apoptosis and others not. This sensing then permits a graded fecundity response from each individual female. Integrating our observations with the existing genetic and physiological data allows the generalization that the individual fecundity plateau occurs when nutrition is good, the insulin-like signaling pathway is activated, JH level is high, ecdysone level is lower (but present), BR-C Z1 isoform is expressed, and apoptosis is repressed. Under these conditions, characteristic of the mature female adult, almost all eggs that enter the ovarioles are allowed to develop. However, when functional decline of nutritional mechanisms and degeneration of the soma as a whole arise due to individual senescence, then the nutritional status of the organism could change to yield internal conditions somewhat similar to those observed in the experimentally starved flies in the Terashima and Bownes study (38). Then JH level drops, ecdysone level increases, BR-C isoform Z1 is activated, and apoptosis is activated. Because a constantly increasing proportion of developing eggs are signaled to undergo apoptosis, significantly fewer eggs are laid, and the reproductive senescence stage begins.
The exponentially decelerated decline of fecundity is characterized by the exponent, or the rate of senescence, αsen. Mathematically, it means that fecundity in senescing flies is described by the exponential function RC · exp[−αsen · (x − Xsen)], where x is age (x > Xsen), RC is steady-state fecundity at maturity, and Xsen is the onset of senescence. The rate of senescence, αsen, is the exponent in the exponential function.
No specific dynamic properties were observed in the senescence-related parts of the fecundity patterns. This statement is even stronger than the thesis about the constancy of the egg-laying rate at the plateau stage. It calls into question the claim by Rauser and colleagues (25) that a late-life fecundity plateau exists. In any case, this question must be studied more thoroughly, especially when keeping in mind that Rauser and colleagues have studied population and not individual fecundity, and have presented serious evolutionary reasons for why mean fecundity values must plateau at very advanced ages.
Individual Senescence in Drosophila and Medfly
The specificity of the individual fecundity patterns in the two species poses a number of questions. What are the genetic properties related to senescence in Drosophila and medfly? Are aging and senescence similar in the two species?
In search of an answer to those questions, let us draw schematically the fecundity patterns in each species (Figure 9). Figure 9A shows six cluster-related patterns in Drosophila, and Figure 9B shows six such patterns for medflies. The schemes differ in that in Drosophila only the senescence phase changes with a cluster number whereas the maturity stage is constant. In medflies, both the length of the maturity phase and the exponent, αsen, change. The height of the reproductive plateau at maturity is shown as constant.
Figure 9.
Schematic presentation of individual senescence in Drosophila (A) and medfly (B). There is a crucial difference in senescence between the species. In Drosophila, a constant length of maturity exists so that the only parameter associated with longevity is the exponent of the fecundity decrease. Schematic patterns (A) present the original patterns shown in Figure 8. In medfly, both the maturity period and the exponent are prolonged with the increase of life span.
In Drosophila, the time of onset of senescence is fixed in all the patterns. In medflies, a different pattern appears, such that the point that indicates the onset of senescence moves along the age axis to the right when LS increases. The length of the maturity stage extends in the more longer-living clusters of the medfly.
Thus it is clear that longevity is associated with different parameters; namely, in Drosophila with αsen only, and in medflies with T and αsen. These parameters may present a solid basis for prediction of life spans in individual flies, especially in Drosophila.
Senescence in Drosophila and medfly follows different scenarios. At the moment, the difference between the two species cannot be empirically explained. However, if we assume that the same genetic and physiological mechanisms known to be operative in Drosophila are also operative in medfly, albeit with species-specific variation in particular regulatory steps, then we may consider a possible molecular basis for this difference in their kinetics of reproductive senescence. Both mechanisms probably begin to work at the onset of senescence. The Drosophila process probably involves the mechanism determining the ability of each egg in the ovary to choose between development and apoptosis (38). The medfly process might well be a diminution of the number of eggs entering the ovary from germarium. A different role of these mechanisms can produce the observed effects in the two species. This topic will be the subject of a future investigation.
Thus it is possible that, in Drosophila, genetic mechanisms might regulate the length of the maturity stage, sustaining it at some constant period. In humans, the length of the period between menarche and menopause is under strict genetic control (40). Should our supposition about Drosophila be confirmed, then such a fact would add Drosophila to the two species known for such a trait, namely humans and pilot whales (41). If so, then perhaps we might in the future use Drosophila not only as a model for human diseases (42) but also for reproductive processes.
Mair and colleagues (43) have demonstrated that the lifespan extension observed in Drosophila females subjected to dietary restriction does not seem to be caused by a reduction in vitellogenesis or ovarian activity. Thus the processes regulating reproductive senescence in Drosophila seem to be separable from those influencing longevity and somatic senescence. Given that finding, it is reasonable to assume that reproductive senescence is regulated in part by those processes centered on the expression of the BR-C and related genes, whereas the longevity and somatic senescence processes may be influenced by the organism’s stress-resistance processes centered on the expression of the dFOXO transcription factor and its downstream targets. If so, then the reproductive and somatic senescent processes may be indirectly related within the individual organism by the power available for reproduction, Smax [see Figure 3 of (28)], which is itself related to overall oxygen consumption rate and oxidative vulnerability (29).
Individual and Population Aging and Senescence
Aging, senescence, and reproduction are inevitable features of living beings (43,44). Following the definition by Rose (44), aging is a persistent decline in age-specific components of an organism due to internal physiological deterioration, which is accompanied by an increase in the risk of death. Decline of reproduction (45–48) and physical activity (19,49–51) and age-related changes in accumulation of reserves (52,53) usually characterizes aging in individuals. Generally, such functional decline is used to present senescence.
In this study we definitely discriminate mortality rate and individual senescing. The Gompertz equation,
| [4] |
describes the mortality rate µ(x) as a function of age x, and a and b represent the age-independent mortality rate and the age-dependent acceleration of mortality rate, respectively.
It is common belief that the mortality-related Gompertz parameter b may be considered to be a measure of the demographic “rate of aging.” The slope of the logarithm of the mortality curve is often associated with changes in individual aging. Nonetheless, such interpretation may be incorrect: The changes in the slope of this curve do not necessarily correspond to the individual changes in aging. For example, the improvement in survival in developed countries in the first half of the past century is characterized by a rectangularization of the survival curve. This trend corresponds to an increase in the slope of the respective logarithm of the mortality rate. Naturally, this does not mean that the rate of individual aging increases as well (54).
To describe individual aging, we use the homeostatic approach, in which aging is treated as an age-related decrease of the homeostatic capacity (55,56). Such a decrease continues until the death of the individual. The homeostatic capacity S(x), a basic notion of the approach, describes the ability of the systemic mechanisms in the organism to convert into Adenosine triphosphate (ATP) substances delivered from external sources. Aging is governed by a quasiexponential function
| [5] |
which describes the age-related decrease of the capacity in various organs and systems of the organism. In agreement with the oxidative damage theory, as it is treated in (55–57), the rate of aging in each organ is proportional to the respective oxygen consumption rate, W(x), modified by the corresponding defensive and reparative ability. At age x,
| [6] |
where the β(x) function describes the age-related oxidative vulnerability of the organismal systems. Thus, individual aging is reflected by a decrease of the homeostatic capacity, S(x), in each organismal system at a decelerated rate. Novoseltsev and colleagues (28) hypothesized that, in the reproductive system at advanced ages, this function converges to a pure exponential one,
| [7] |
.
This means that the rate of individual aging of the reproductive system becomes approximately constant, equal to αsen. The aging process continues all the time, but only the onset of reproductive senescence made it explicit. A diminution of reproduction after the onset of senescence can be observed in individual fecundity patterns.
Thus, in fruit flies we relate senescence with reproductive changes. Senescence arises when aging results in an inability of the mechanisms supporting the reproductive system to fulfill the endowed reproductive program, sustaining the constant rate of egg-laying. Being closely related to mechanistic causes of senescence (28), the αsen parameter can be treated as a general characteristic of senescence in fruit flies.
A large difference between the Gompertz-based measure of aging, b, and the parameter αsen is that the first one describes the demographic rate of population aging, whereas the second is directly related to senescing in an individual. The first one characterizes the processes at all ages, whereas αsen describes only the last period of life history, its senescence stage.
In general, the parallel study of the two species suggests that the three-stage approach is applicable individually to a wide range of animals. The parameters describing individual fecundity change during laboratory selection. Existing genetic and physiological data identify the molecular mechanisms most likely to be responsible for the modeled changes in fecundity and longevity of these two Dipteran species. It is possible to use the technique to analyze individual fecundity in other Drosophila studies as well as in differing species such as ladybird beetles (24).
ACKNOWLEDGMENTS
This work was partly supported by National Institutes of Health National Institute on Aging grant AG08761-01.
We thank James W. Vaupel for the opportunity to complete this work at the Max-Planck Institute for Demographic Research. We also thank A. I. Mikhalski, A. A. Butov, and A. A. Romaniukha for discussion on the paper and for valuable comments.
Address correspondence to A. I. Yashin, Duke University Center for Demographic Studies, 2117 Campus Drive, Box 90408, Durham, NC 27708. E-mail: yashin@cds.duke.edu
REFERENCES
- 1.Luckinbill LS, Arking R, Clare MJ, Cicocco WC, Buck SA. Selection for delayed senescence in Drosophila melanogaster. Evolution. 1984;38:996–1003. doi: 10.1111/j.1558-5646.1984.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 2.Rose MR. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 3.Partridge L, Fowler K, Trevitt S, Sharp W. An examination of the effects of males on the survival and egg-production rates of females Drosophila melanogaster. J Insect Physiol. 1986;32:925–929. [Google Scholar]
- 4.Arking R. Successful selection for increased longevity in Drosophila: analysis of the survival data and presentation of a hypothesis on the genetic regulation on longevity. Exp Gerontol. 1987;22:199–220. doi: 10.1016/0531-5565(87)90040-4. [DOI] [PubMed] [Google Scholar]
- 5.Partridge L, Fowler K. Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution. 1992;46:76–91. doi: 10.1111/j.1558-5646.1992.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 6.Butov AA, Carey JR, Volkov MA, Sehl ME, Yashin AI. Reproduction and survival in Mediterranean fruit flies: a “protein and energy” free radical model of aging. Biogerontology. 2003;4:387–395. doi: 10.1023/B:BGEN.0000006559.59110.10. [DOI] [PubMed] [Google Scholar]
- 7.Romaniukha AA, Carey JR, Karkach AS, Yashin AI. The impacts of diet switching on resource allocation to reproduction and longevity in Mediterranean fruitflies. Proc Roy Soc Lond Biol Sci. 2004;271:1319–1324. doi: 10.1098/rspb.2004.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad NG, Joshi A. What have two decades of laboratory life-history evolution studies on Drosophila melanogaster taught us? J Genet. 2003;82:45–76. doi: 10.1007/BF02715881. [DOI] [PubMed] [Google Scholar]
- 9.Robertson FW, Sang JH. The ecological determinants of population growth in a Drosophila culture. I. Fecundity of adult flies. Proc Roy Soc Lond Biol Sci. 1944;132:258–277. [Google Scholar]
- 10.Maynard Smith J. The effects of temperature and eggs laying on the longevity of Drosophila subobscura. J Exp Biol. 1959;35:832–841. [Google Scholar]
- 11.Lints FA, Lints CV. Respiration in Drosophila. II. Respiration in relation to age by wild, inbred and hybrid Drosophila melanogaster imagos. Exp Gerontol. 1968;3:341–349. doi: 10.1016/0531-5565(68)90047-8. [DOI] [PubMed] [Google Scholar]
- 12.Aigaki T, Ohba S. Individual analysis of age-associated changes in reproductive activity and lifespan of Drosophila virilis. Exp Gerontol. 1984;19:13–23. doi: 10.1016/0531-5565(84)90027-5. [DOI] [PubMed] [Google Scholar]
- 13.Le Bourg E, Lints FA, Delinge J, Lints CV. Reproductive fitness and longevity in Drosophila melanogaster. Exp Gerontol. 1988;23:491–500. doi: 10.1016/0531-5565(88)90061-7. [DOI] [PubMed] [Google Scholar]
- 14.Whittier TS, Shelley TE. Productivity of singly vs. multiply mated female Mediterranean fruit flies (Diptera, Tephritidae) J Kansas Entomol Soc. 1993;66:200–209. [Google Scholar]
- 15.Marden JH, Rogina B, Montooth KL, Helfand SL. Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. Proc Natl Acad Sci. 2003;100:3369–3373. doi: 10.1073/pnas.0634985100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey JR, Liedo P, Muller HG, Wang JL, Chiou JM. Relationship of age patterns of fecundity to mortality, longevity, and lifetime reproduction in a large cohort of Mediterranean fruit fly females. J Gerontol Biol Sci. 1998;53A:B245–B251. doi: 10.1093/gerona/53a.4.b245. [DOI] [PubMed] [Google Scholar]
- 17.Blay S, Yuval B. Oviposition and fertility in the Mediterranean fruit fly (Diptera: Tephritidae): effect of male and female body size and the availability of sperm. Ann Entomol Soc Am. 1999;92:278–284. [Google Scholar]
- 18.Shelley TE. Fecundity of female oriental fruit flies (Diptera: Tephriditae): effects of methyl eugenol-fed and multiple mates. Ann Entomol Soc Am. 2000;93:559–564. [Google Scholar]
- 19.Papadopoulos NT, Carey JR, Katsoyannis BI, Koulossis NA, Muller HG, Liu XL. Supine behavior predicts the time to death in male Mediterranean fruit flies (Ceratita capitatis) Proc Roy Soc Lond Biol Sci. 2002;269:1633–1637. doi: 10.1098/rspb.2002.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretzlaff R, Arking R. Patterns of amino acid incorporation in long-lived genetic strains of Drosophila melanogaster. Exp Gerontol. 1989;24:67–81. doi: 10.1016/0531-5565(89)90036-3. [DOI] [PubMed] [Google Scholar]
- 21.Stearns SC, Ackermann M, Doebeli M. The experimental evolution of aging in fruitflies. Exp Gerontol. 1998;33:785–792. doi: 10.1016/s0531-5565(98)00021-7. [DOI] [PubMed] [Google Scholar]
- 22.Muller HG, Carey JR, Wu DQ, Liedo P, Vaupel JW. Reproductive potential predicts longevity of female Mediterranean fruitflies. Proc Roy Soc Lond B. 2001;268:445–450. doi: 10.1098/rspb.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindlmann P, Dixon AFG, Dostalkova I. Role of ageing and temperature in shaping reaction norms and fecundity functions in insects. J Evol Biol. 2001;5:835–840. [Google Scholar]
- 24.Dixon AFG, Agarwala BK. Triangular fecundity function and ageing in ladybird beetles. Ecol Entomol. 2002;27:433–440. [Google Scholar]
- 25.Rauser CL, Mueller LD, Rose MR. Aging, fertility, and immortality. Exp Gerontol. 2003;38:27–33. doi: 10.1016/s0531-5565(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 26.Shanley DP, Kirkwood TBL. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 27.Cichon M. Diversity of age-specific reproductive rates may result from aging and optimal resource allocation. J Evol Ecol. 2001;14:180–185. doi: 10.1046/j.1420-9101.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 28.Novoseltsev VN, Novoseltseva JA, Yashin AI. What does a fly’s individual fecundity pattern look like? The dynamics of resource allocation in reproduction and ageing. Mech Age Dev. 2003;124:605–617. doi: 10.1016/s0047-6374(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 29.Novoseltsev VN, Novoseltseva JA, Boyko SI, Yashin AI. What fecundity patterns indicate about aging and longevity: insights from Drosophila studies. J Gerontol Biol Sci. 2003;58A:484–494. doi: 10.1093/gerona/58.6.b484. [DOI] [PubMed] [Google Scholar]
- 30.Novoseltsev VN, Carey RJ, Novoseltseva JA, Papadopoulos NT, Blay S, Yashin AI. Systemic mechanisms of individual reproductive life history in female medflies. Mech Age Dev. 2004;125:77–87. doi: 10.1016/j.mad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Arking R, Burde V, Graves K, et al. Identical longevity phenotypes are characterized by different patterns of gene expression and oxidative damage. Exp Gerontol. 2000;35:353–373. doi: 10.1016/s0531-5565(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 32.Helfand SL, Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Ann Rev Genet. 2003;37:329–348. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 33.Novoseltsev VN, Arking R, Novoseltseva JA, Yashin AI. Evolutionary optimality applied to Drosophila experiments: hypothesis of constrained reproductive efficiency. Evolution. 2002;56:1136–1149. doi: 10.1111/j.0014-3820.2002.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 34.Sheeba V, Sharma VK, Chandrashecaran MK, Joshi A. The effect of different light regimes on adult lifespan in Drosophila melanogaster is partly mediated through reproductive output. J Biol Rhythms. 2000;15:380–392. doi: 10.1177/074873000129001477. [DOI] [PubMed] [Google Scholar]
- 35.Ginnakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with over-expressed dFOXO in adult fat body. Science. 2004;305:36. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 36.Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 37.Britton ES, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 38.Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167:1711–1719. doi: 10.1534/genetics.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- 40.Perls TT, Fretts RC. The evolution of menopause and human life span. Ann Hum Biol. 2001;28:237–245. doi: 10.1080/030144601300119052. [DOI] [PubMed] [Google Scholar]
- 41.Austad SN. Menopause, an evolutionary perspective. Exp Gerontol. 1994;29:255–263. doi: 10.1016/0531-5565(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 42.O’Kane C. Modelling human diseases in Drosophila and Caenorhabditis. Semin Cell Dev Biol. 2003;14:3–10. doi: 10.1016/s1084-9521(02)00162-3. [DOI] [PubMed] [Google Scholar]
- 43.Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Rose MR. Evolutionary Biology of Aging. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 45.Arking R. Biology of Aging: Observations and Principles. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- 46.Partridge L, Andrews B. The effect of reproductive activity on the longevity of male Drosophila melanogaster is not caused by an acceleration of ageing. J Insect Physiol. 1985;31:393–395. [Google Scholar]
- 47.Partridge L. Sexual activity and life span. In: Collatz KG, Sohal RS, editors. Insect Aging. Strategies and Mechanisms. Berlin: Springer; 1986. pp. 45–54. [Google Scholar]
- 48.Lints FA. The rate of living theory revisited. Gerontology. 1989;35:36–57. doi: 10.1159/000212998. [DOI] [PubMed] [Google Scholar]
- 49.Berrigan D, Partridge L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp Biochem Physiol. 1997;118A:1301–1307. doi: 10.1016/s0300-9629(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 50.Sohal RS, Buchan PB. Relationship between physical activity and life span in the adult housefly, Musca domestica. Exp Gerontol. 1981;16:157–162. doi: 10.1016/0531-5565(81)90040-1. [DOI] [PubMed] [Google Scholar]
- 51.Sohal RS. The rate of living theory: a contemporary interpretation. In: Collatz KG, Sohal RS, editors. Insect Aging. Strategies and Mechanisms. Berlin: Springer; 1986. pp. 23–43. [Google Scholar]
- 52.Driver C, Georgiu A, Georgiu G. The contribution by mitohondrially induced oxidative damage to aging in Drosophila melanogaster. Biogerontology. 2004;5:185–192. doi: 10.1023/B:BGEN.0000031156.75376.e3. [DOI] [PubMed] [Google Scholar]
- 53.Djawdan M, Chippindale AK, Rose MR, Bradley TJ. Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol Zool. 1998;71:584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- 54.Luckinbill LS, Foley P. The role of metabolism in aging. J Am Aging Assoc. 2000;23:85–93. doi: 10.1007/s11357-000-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yashin AI, Ukraintseva SV, Boiko SI, Arbeev KG. Individual aging and mortality rate: how are they related? Soc Biol. 2002;49:206–217. doi: 10.1080/19485565.2002.9989059. [DOI] [PubMed] [Google Scholar]
- 56.Novoseltsev VN, Carey JR, Liedo P, Novoseltseva JA, Yashin AI. Anticipation of oxidative damage decelerates aging in virgin female medflies: hypothesis tested by statistical modeling. Exp Gerontol. 2000;35:971–987. doi: 10.1016/s0531-5565(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 57.Novoseltsev VN, Novoseltseva JA, Yashin AI. A homeostatic model explains paradoxes observed in earlier aging experiments: fusion and extension of older theories of aging. Biogerontology. 2001;2:127–138. doi: 10.1023/a:1011511100472. [DOI] [PubMed] [Google Scholar]