Abstract
Given that erythropoietin (EPO) is no longer believed to have exclusive biological activity in the hematopoietic system, EPO is now considered to have applicability in a variety of nervous system disorders that can overlap with vascular disease, metabolic impairments, and immune system function. As a result, EPO may offer efficacy for a broad number of disorders that involve Alzheimer’s disease, cardiac insufficiency, stroke, trauma, and diabetic complications. During a number of clinical conditions, EPO is robust and can prevent metabolic compromise, neuronal and vascular degeneration, and inflammatory cell activation. Yet, use of EPO is not without its considerations especially in light of frequent concerns that may compromise clinical care. Recent work has elucidated a number of novel cellular pathways governed by EPO that can open new avenues to avert deleterious effects of this agent and offer previously unrecognized perspectives for therapeutic strategies. Obtaining greater insight into the role of EPO in the nervous system and elucidating its unique cellular pathways may provide greater cellular viability not only in the nervous system but also throughout the body.
Keywords: Alzheimer’s disease, Akt, angiogenesis, apoptosis, cancer, cardiac, caspases, diabetes, endothelial, erythropoietin, forkhead, GSK-3β, inflammation, microglia, mitochondria, neurodegeneration, NF-κB, renal, STATs, Wnt
1. Introduction
1.1 Historical Perspective of Hormones
“These chemical messengers, however, or hormones, as we might call them, have to be carried from the organ where they are produced to the organ which they affect by means of the blood stream and the continually recurring physiological needs of the organism must determine their repeated production and circulation throughout the body” (Starling, 1905). As part of his second Croonian lecture to the Royal College of Surgeons in 1905 entitled “The chemical control of the functions of the body,” Ernest Starling unexpectedly to the audience introduces the term “hormones” that was initially derived from the Greek term “excite” or “arouse.” He used this term to describe chemicals that can be set into action in the blood stream to elicit activity in different organs of the body (Maiese, 2007). How Starling selected the term “hormone” has many historical versions and the reasons that prompted him to present the term during this particular lecture may never be known, but the most accurate accounts appear to describe his conversations with William Hardy and the Greek poet scholar W. T. Vesey. These meetings sometimes focused upon the Greek verb “ormao” for “arouse” or “excite” (Henderson, 2005; Tata, 2005).
Although prior to this point the use of the term “hormone” in the scientific literature was considered to be minimal at best, early work during the mid-nineteenth century, such as by Claude Bernard, depicted processes responsible for internal secretion of chemicals as described with the release of glucose from glycogen in the liver (Bernard, 1855). During this period, other pioneers such as Arnold Adolphe Berthold spoke of the interaction and communication between the different organs in the body. As these concepts became more accepted, physicians later in the nineteenth century reported the use of extracts of animal thyroid, pancreas, and even adrenal glands to treat patients suspected of suffering from the loss of circulating chemicals.
By the early twentieth century, Starling and William Bayliss demonstrated that the duodenum, when stimulated with acid through local application, could lead to pancreatic secretion (Bayliss and Starling, 1901). They furthered these results by illustrating that duodenal extracts injected into the blood stream in animals also resulted in pancreatic secretion (Bayliss and Starling, 1902). From these studies, Starling and Bayliss suggested that the agent released from the duodenum should be termed “secretin.” The Nobel Laureate Pavlov was initially impressed with these results that had suggested the presence of several mechanisms in the control of the digestive system, but later stood firm to promote his personal concepts that pancreatic secretion and the organs of the gut were controlled principally by innervation of the nervous system during his acceptance of the Nobel Peace Prize for his work in 1904 (Pavlov, 1904).
In spite of the political undercurrents, subsequent investigations in endocrinology and the study of hormones have fostered the development of numerous fields that involve vascular biology, neuroscience, physiology, genetics, metabolomics, development, cancer, and molecular medicine. Clinically, the advances from these fields that rely upon the understanding of the chemistry of hormones have resulted in remarkable strides for treatment protocols that involve the care and management of diabetes, the replenishment of hormone deficiencies, the success of fertility treatments that utilize in vitro fertilization, and the treatment of disorders associated with anemia.
1.2 The Discovery of Erythropoietin (EPO)
The initial studies by pioneers such as Starling, Bernard, Berthold, and Bayliss have led us to remarkable advances in clinical medicine and exposed us to the novel and protean effects that agents functioning as hormones can impart upon the body. Our progressive knowledge of the cellular and molecular processes that involve these agents have alerted us to the intimate relationship that exists between the intricate cellular systems and organs of the body that may be “aroused” or “excited” by a single agent. These discoveries bring us to the novel discussion of the hormone, growth factor, and cytokine termed erythropoietin (EPO).
First presented as “hemopoietine,” EPO became known as a factor that could stimulate new red blood cell development through the pioneering work of Carnot and Deflandre in 1906 (Carnot and DeFlandre, 1906; Fisher, 2003). These investigators demonstrated that plasma removed from rabbits following a bleeding stimulus that was later injected into control untreated rabbits would lead to the development of immature red blood cells. A number of other investigations followed these studies, which showed similar findings demonstrating that plasma from bled animals would yield a significant reticulocytosis (Erslev, 1974; Gibelli, 1911; Sandor, 1932). More elegant experiments subsequently demonstrated that a rise in hemoglobin levels with reticulocytosis occurred in parabiotic rats when only one partner was exposed to hypoxia, illustrating that depressed oxygen tensions could stimulate EPO production (Reissmann, 1950). Later, human EPO protein was purified, which paved the way for the cloning of the EPO gene and the development of recombinant EPO for clinical use (Jacobs et al., 1985; Lin et al., 1985).
2. Structural and Molecular Determinants of EPO Activity
EPO is a 30.4 kDa glycoprotein with approximately half of its molecular weight derived from carbohydrates that can vary among species (Maiese et al., 2005c). EPO contains four glycosylated chains including three N-linked and one O-linked acidic oligosaccharide side chains. N-linked glycosylation sites occur at the positions 24, 38, and 83 of aspartyl residues, while the O-linked glycosylation site is at Serine126. Three N-glycan chains of human EPO consist of the tetra-antennary structure with or without N-acetyllactosamine repeating units (Tsuda et al., 1988). The O-linked sugar chain is composed of Gal-GalNAc and sialic acids (Sasaki et al., 1987). The production and secretion of the mature EPO also relies upon the integrity of the N- and O-linked chains. The EPO gene is located on chromosome 7, exists as a single copy in a 5.4 kb region of the genomic DNA, and encodes a polypeptide chain containing 193 amino acids (Jacobs et al., 1985). During the production and secretion of EPO, a 166 amino acid peptide is initially generated following the cleavage of a 27 amino acid hydrophobic secretory leader at the amino-terminal (Imai et al., 1990). In addition, a carboxy-terminal arginine in position 166 is removed both in the mature human and recombinant human EPO (rhEPO) resulting in a circulatory mature protein of 165 amino acids (Chong et al., 2002a).
The glycosylated chains are important for the biological activity of EPO and can protect EPO from oxygen radical degradation. EPO is stabilized by the carbohydrate chains (Toyoda et al., 2000) and the oligosaccharides in EPO may protect the protein from oxygen radical activity (Uchida et al., 1997). The N-glycosylated chains are believed to contribute to the thermal stability of EPO (Tsuda et al., 1988). In addition, the N- and O-linked chains may be necessary for the production and secretion of the mature EPO (Krantz, 1991). Replacement of asparagines 38 and 83 by glutamate or serine 126 by glycine can decrease the production and secretion of EPO (Dube et al., 1988). The presence of the carbohydrates also are important in the control of the metabolism of EPO, since EPO molecules with high sialic acid content can be easily cleared by the body through specific binding in the liver (Tsuda et al., 1990).
In addition, the biological activity of EPO also relies upon two disulfide bonds formed between cysteines at positions 7 and 160 and at positions 29 and 33 (Li et al., 2004a). The requirement of these disulfide bridges has been demonstrated by the evidence that reduction of these bonds results in the loss of the biologic activity of EPO. Alkylation of the sulfhydryl groups results in irreversible loss of the biological activity of EPO. Re-oxidization of EPO after reduction by guanidine restores eighty-five percent of the biological activity of EPO (Wang et al., 1985). Cysteine 33 replacement with proline also reduces the biological function of EPO.
3. Expression and Signal Transduction for EPO and its Receptor
3.1 Cellular Expression of EPO
EPO can be detected in the breath of healthy individuals (Schumann et al., 2006), suggesting its ubiquitous presence in the body (Maiese et al., 2007a; Maiese et al., 2007c). In addition, it has been suggested that EPO may provide developmental cognitive support in humans with the recent observations that elevated EPO concentrations during infant maturation have been correlated with increased Mental Development Index scores (Bierer et al., 2006). The primary organs of EPO production and secretion are the kidney, liver, brain, and uterus. EPO production and secretion occurs foremost in the kidney (Fliser and Haller, 2007). The kidney peritubular interstitial cells are responsible for the production and secretion of EPO (Fisher, 2003). With the use of cDNA probes derived from the EPO gene, peritubular endothelial cells (ECs), tubular epithelial cells, and nephron segments in the kidney also have been demonstrated to be vital cells for the production and secretion of EPO (Lacombe et al., 1991; Mujais et al., 1999). During periods of acute renal failure, EPO may provide assistance for the protection of nephrons (Sharples et al., 2005; Sharples and Yaqoob, 2006).
Secondary sites of EPO production and secretion occur in the liver and the uterus (Chong et al., 2002a). Hepatocytes, hepatoma cells, and Kupffer cells of the liver can produce EPO (Fisher, 2003) and, in turn, EPO may provide a protective environment for these cells (Schmeding et al., 2007). In regards to the uterine production of EPO, it is believed that the occurrence of neonatal anemia that can take place in the early weeks after birth may partly result from the loss of EPO production and secretion by placenta (Davis et al., 2003).
EPO is approved by the Food and Drug Administration for the treatment of anemia, but a body of recent work has revealed that EPO is not only required for erythropoiesis, but also functions in other organs and tissues, such as the brain, heart, and vascular system (Chong et al., 2002b; 2003b; Chong and Maiese, 2007a; Mikati et al., 2007; Moon et al., 2006; Um et al., 2007). It is the discovery of EPO and its receptor in the nervous and vascular systems that has resulted in a heightened level of interest and enthusiasm for the potential clinical applications of EPO, such as in Alzheimer’s disease, cardiac insufficiency (Assaraf et al., 2007; Palazzuoli et al., 2006), and cardiac transplantation (Gleissner et al., 2006; Mocini et al., 2007). In the nervous system, the major sites of EPO production and secretion are in the hippocampus, internal capsule, cortex, midbrain, cerebral ECs, and astrocytes(Digicaylioglu et al., 2004; Genc et al., 2004; Maiese et al., 2004; 2005c). Further work has revealed several other organs as secretory tissues for EPO that include peripheral ECs (Anagnostou et al., 1994), myoblasts (Ogilvie et al., 2000), insulin-producing cells (Fenjves et al., 2003), and cardiac tissue (Fliser and Haller, 2007; Maiese et al., 2005c).
3.2 Signal Transduction of EPO and the EPO receptor
After the EPO gene was cloned (Jacobs et al., 1985; Lin et al., 1985), work was initiated to identify a receptor for EPO. The EPO receptor (EPOR) was found to be expressed in both normal and transformed erythroid cells (D’Andrea and Zon, 1990). The EPOR is part of the type 1 super-family of cytokine receptors and is activated via homodimerization (Bazan, 1990; Watowich et al., 1994). This receptor family shares a common domain structure consisting of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain. The extracellular domain is necessary for the initial binding of EPO and the intracellular domain is responsible for the transduction of intracellular signaling (Mulcahy, 2001).
EPO regulates bone marrow erythroid cell proliferation, differentiation, and survival through its binding to an erythroid progenitor cell surface EPOR. The EPOR also is expressed in numerous non-erythroid blood lines, which include neurons, microglia, astrocytes, and in cerebral ECs (Anagnostou et al., 1994; Fliser and Haller, 2007; Genc et al., 2004; Maiese et al., 2004; 2005c) as well as on myelin sheaths of radicular nerves in human peripheral nerves (Hassan et al., 2004), suggesting both a developmental and potential protective role for EPO in the central and peripheral nervous systems. The EPOR also is expressed in primary cerebral ECs (Chong et al., 2003a; c) as well as in human umbilical veins, bovine adrenal capillaries, and rat brain capillaries (Anagnostou et al., 1994; Yamaji et al., 1996).
During the development of an organism, production of EPO and the expression of its receptor are altered. Elevated expression of the EPOR occurs in early embryonic neuronal tissues at levels similar to that observed in the adult spleen and bone marrow (Liu et al., 1994). Yet, the level of endogenous EPOR expression is significantly reduced following the maturation of the brain (Liu et al., 1997). During gestation, EPO production is increased, but later becomes suppressed following birth to be regulated by the tissue oxygen supply (Chong et al., 2002c). A deficiency in tissue oxygen results in the production of EPO and an increase in the expression of the EPOR not only in peripheral organs (Fliser and Haller, 2007; Li et al., 2004a; Maiese et al., 2004; 2005c) but also in the brain (Li et al., 2007a), which may be responsible for hypoxic tolerance in some species (Ravid et al., 2007). EPO secretion in the brain appears to be more sustained than in peripheral organs such as the kidney (Chikuma et al., 2000), suggesting that EPO production may originate in the brain and possibly cross the blood-brain barrier to reach the blood and peripheral organs (Li et al., 2004a). Work performed in vivo with subjects exposed to hypoxia also demonstrates an increase in expression of EPO and EPOR mRNA following reduced oxygenation (Marti et al., 1996). Furthermore, both primary neurons (Chikuma et al., 2000; Liu et al., 2006) and neuronal cell lines (Stolze et al., 2002) have been found to retain the capacity to express EPO in an oxygen-dependent manner.
Although EPO is recognized as a critical modulator of erythropoiesis, a low concentration of red blood cells alone does not directly stimulate EPO production, but requires the presence of a diminished oxygen tension. Once a hypoxic stimulus is received, EPO is subsequently released into the peripheral blood circulation, and upon arrival in the bone marrow, EPO binds to its receptor that is highly expressed on the surface of erythroid progenitor cells and leads to erythropoiesis (Broudy et al., 1991). This results in an elevation in the number of mature erythrocytes and the improvement of oxygen supply. EPO also functions to stimulate colony-forming erythroid cells to induce these cells to proliferate, mature into erythrocytes, and possibly assist with reticulocyte release to the blood (Sathyanarayana et al., 2007).
Hypoxia-dependent expression of EPO and EPOR are controlled by hypoxia-inducible factor 1 (HIF-1) in both vascular and neuronal systems. HIF-1 is essential for the production and secretion of EPO in response to hypoxia (Ikeda, 2005). At the transcriptional level, the hypoxia-dependent gene transcription of EPO and EPOR directly results from the activation of the HIF-1 pathway under hypoxic conditions. Gene transcription of EPO is mediated by the transcription enhancer located in the 3′-flanking region of the EPO gene that specifically binds to HIF-1 (Wang and Semenza, 1995).
HIF-1 is a basic helix-loop-helix heterodimeric transcription factor containing two subunits, HIF-1α and HIF-1β. HIF-1β is a constitutively expressed 91-94 kDa subunit that was characterized previously as aryl hydrocarbon receptor nuclear translocator (ARNT) (Hoffman et al., 1991). HIF-1α is a 120 kDa oxygen-labile subunit that is degraded through the ubiquitin-proteasome pathway under normoxic conditions (Huang et al., 1998). During hypoxia or conditions such as iron chelation that can mimic hypoxia, degradation of HIF-1α is impaired by blocking its association with von Hippel-Lindau protein that targets HIF-1α for proteasome destruction (Maxwell et al., 1999). HIF-1α subsequently translocates to the nucleus and heterodimerizes with HIF-1β to form a stable HIF-1 complex. The HIF complex then binds to the conserved sequence (5′RCGTG3′) near the 5′ end of the hypoxia-responsive enhancer of the EPO gene to up-regulate EPO gene transcription (Bunn et al., 1998). Increased DNA binding activity of HIF-1 occurs in rat cortical neurons during oxidative stress, suggesting that HIF-1 may function as oxygen sensor regulating adaptive gene transcription and resulting in the production and secretion of the EPO protein during hypoxia in the nervous system (Maiese et al., 2004; 2005c). It is important to note that each of the HIF family members HIF-1α, HIF-1β, and HIF-3α plays important roles in regulating the expression of EPO and the EPOR to foster protection against hypoxic cell injury (Heidbreder et al., 2003).
Hypoxia is not the only factor responsible for the expression of EPO and the EPOR. The production and secretion of EPO in female reproductive organs is estrogen-dependent. During the cyclic development of the uterine endometrium, 17β-estradiol can lead to a rapid and transient increase in EPO mRNA in the uterus (Yasuda et al., 1998), oviducts, and ovaries (Masuda et al., 2000). Hypoxic induced EPO mRNA expression in uterine tissue occurs only in the presence of 17β-estradiol. EPO mRNA expression by hypoxia in the uterus is less pronounced than the EPO expression that occurs in the kidney and the brain (Chikuma et al., 2000). Interestingly, a variety of cellular disturbances may lead to either increased or decreased EPO expression through the control of HIF, such as hypoglycemia, cadmium exposure, raised intracellular calcium, or intense neuronal depolarizations generated by mitochondrial reactive oxygen species (Chong et al., 2002c; Genc et al., 2004; Obara et al., 2003). Anemic stress, insulin release, and several cytokines, including insulin-like growth factor, tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) (Nagai et al., 2001), also can lead to increased expression of EPO and the EPOR (Maiese et al., 2004; 2005c).
4. The Impact of EPO Upon Cellular Metabolism, Survival, and Proliferation
4.1 EPO and Cellular Metabolism
In both clinical and basic experimental studies, EPO has been intimately associated with the modulation of cellular metabolism. When one considers cellular dysfunction in relation to cellular metabolism, diabetes mellitus (DM) comes to mind since it represents a significant health concern in the clinical population (Maiese et al., 2007a). DM occurs in at least 16 million individuals in the United States and more than 165 million individuals worldwide (Quinn, 2001). Furthermore, by the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions (Wild et al., 2004). Type 2 DM represents at least 80 percent of all diabetics and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index (Laakso, 2001). Type 1 insulin-dependent diabetes mellitus accounts for only 5–10 percent of all diabetics (Maiese et al., 2007c), but is increasing in adolescent minority groups (Dabelea et al., 2007). Yet, the incidence of undiagnosed diabetes, impaired glucose tolerance, and fluctuations in serum glucose in the young raises further concerns (Jacobson et al., 2007). Individuals with impaired glucose tolerance have greater than two times the risk for the development of diabetic complications than individuals with normal glucose tolerance (Harris and Eastman, 2000). Healthcare costs for diabetic complications are a significant driver for government resource consumption with costs of $214.8 million for outpatient expenditures and $1.45 billion for inpatient expenditures (Maciejewski and Maynard, 2004). If one examines cognitive impairments resulting from diabetes in the general population that can mimic Alzheimer’s disease (Chong et al., 2005f), annual costs equal $100 billion (Maiese and Chong, 2004; McCormick et al., 2001; Mendiondo et al., 2001).
Both type 1 and type 2 DM represent important health concerns whether they begin early or later in life (Maiese et al., 2007a), since each can result in long-term complications throughout the body (Daneman, 2006). In regards to the vascular and nervous systems, patients with DM can develop severe neurological and vascular disease (Donahoe et al., 2007) that can lead to an increased risk for cognitive decline especially from vascular disease (Chong et al., 2005e; Li et al., 2006a; Schnaider Beeri et al., 2004). Disease of the nervous system can become the most debilitating complication for DM and affect sensitive cognitive regions of the brain, such as the hippocampus, which modulates memory function, resulting in significant functional impairment and dementia (Awad et al., 2004; Gerozissis, 2003). DM also has been found to increase the risk for vascular dementia in elderly subjects (Schnaider Beeri et al., 2004; Xu et al., 2004). DM also may affect the course of Alzheimer’s disease. Although some studies have found that diabetic patients may have less neuritic plaques and neurofibrillary tangles than non-diabetic patients (Beeri et al., 2005), contrasting work suggests a modest adjusted relative risk of Alzheimer’s disease in patients with diabetes as compared with those without diabetes to be 1.3 (Luchsinger et al., 2001).
Closely tied to the development of insulin resistance and the complications of DM in the nervous and vascular systems is the presence of cellular oxidative stress and the release of reactive oxygen species (Maiese et al., 2007c). In patients with DM, elevated levels of ceruloplasmin are suggestive of increased reactive oxygen species (Memisogullari and Bakan, 2004), and acute glucose fluctuations may promote oxidative stress (Monnier et al., 2006). Hyperglycemia can lead to increased production of reactive oxygen species in endothelial cells, liver and pancreatic β-cells (Ceriello et al., 1996; Ihara et al., 1999; Ling et al., 2003; Yano et al., 2004). Prolonged duration of hyperglycemia is not necessary to lead to oxidative stress injury, since even short periods of hyperglycemia generate reactive oxygen species, such as in vascular cells (Yano et al., 2004). Recent clinical correlates support these experimental studies to show that acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms during type 2 DM, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes (Monnier et al., 2006).
The preservation of cellular energy reserves is dependent upon the maintenance of mitochondrial integrity during DM (Newsholme et al., 2007). For example, free fatty acids, which can lead to reactive oxygen species, have been shown to also contribute to mitochondrial DNA damage and impaired pancreatic β-cell function (Rachek et al., 2006). In patients with type 2 DM, skeletal muscle mitochondria have been described to be smaller than those in control subjects (Kelley et al., 2002). Furthermore, a decrease in the levels of mitochondrial proteins and mitochondrial DNA in adipocytes has been correlated with the development of type 2 DM (Choo et al., 2006). Insulin resistance in the elderly also has been associated with elevation in fat accumulation and reduction in mitochondrial oxidative and phosphorylation activity (Petersen et al., 2003). In addition, an association exists with insulin resistance and the impairment of intramyocellular fatty acid metabolism in young insulin-resistance offspring of parents with type 2 DM (Petersen et al., 2004).
Given that administration of antioxidants during elevated glucose concentrations can block free radical production and prevent the production of advanced glycation endproducts (AGEs) known to produce reactive oxygen species during DM (Giardino et al., 1996), EPO may offer an attractive alternative therapy to maintain proper cellular metabolism and mitochondrial membrane potential during DM (Table 1). In clinical studies with DM, plasma EPO is often low in diabetic patients with anemia (Mojiminiyi et al., 2006) or without anemia (Symeonidis et al., 2006). Furthermore, the failure of these individuals to produce EPO in response to a declining hemoglobin level suggests an impaired EPO response in diabetic patients (Thomas et al., 2005). Yet, increased EPO secretion during diabetic pregnancies may represent the body’s attempt at endogenous protection against the complications of DM (Teramo et al., 2004). Similar to the potential protective role of insulin (Duarte et al., 2006), EPO administration has been shown both in diabetics as well as non-diabetics with severe, resistant congestive heart failure to decrease fatigue, increase left ventricular ejection fraction, and significantly decrease the number of hospitalization days (Silverberg et al., 2006). In vitro studies with vascular cells exposed to elevated glucose also have elucidated a strong cytoprotective effect of EPO. Administration of EPO can significantly improve EC survival in a 1.0 ng/ml range (Chong et al., 2007c). EPO administration in patients also can significantly increase plasma levels of EPO well above this range of 1.0 ng/ml that has been associated with potential EPO cellular protection in patients with cardiac or renal disease (Mason-Garcia et al., 1990; Namiuchi et al., 2005), suggesting that the effects of EPO observed during in vitro studies may parallel the cellular processes altered by EPO in patients with DM (Bierer et al., 2006). Furthermore, EPO can block apoptotic DNA degradation in ECs during elevated glucose similar to other models of oxidative stress in cardiac and vascular cell models (Avasarala and Konduru, 2005; Chong et al., 2002b; 2003a; Chong and Maiese, 2007a; Moon et al., 2006).
Table 1.
Therapeutic Potential and Adverse Aspects of Erythropoietin (EPO)
| Therapeutic Potential/ Clinical Trials | Outcomes | Selected References |
|---|---|---|
|
| ||
| Anemia | Left ventricular ejection improved, stroke volume increased | Goldberg et al., 1992, Silverberg et al., 2001 |
| Cancer Treatment Synergy | Improve efficacy of chemotherapy | Ning et al., 2005; Sigounas et al., 2004 |
| Congestive Heart Failure | Cardiac output improved, medical resource utilization decreased | Silverberg et al., 2003; 2006 |
| Chronic Cardiac Insufficiency | Excise tolerance increased, left ventricular function improved, renal function improved decreased | Mancini et al., 2003; Palazzuoli et al., 2006; 2007 |
| Diabetes | Improved cardiac function, protection of vascular cells | Silverberg et al., 2003; Chong et al., 2007c |
| Pulmonary Distress | Improved pulmonary function | Wu et al., 2006 |
| Renal Transplantation | Prevention of allograft rejection | Reinders et al., 2006 |
| Trauma | Mortality decreased | Corwin et al., 2007 |
|
| ||
| Section II Adverse effects/Conditions | ||
| Breast Cancer | Apoptosis of cancer cells inhibited | Hardee et al., 2006 |
| Cancer with Chemotherapy | Myocardial infarction, pyrexia, vomiting, paresthesias, upper respiratory infection increased | Henry et al., 2004 |
| Congestive Heart Failure, Hypertension | Associated with disease severity, mean arterial pressure increased | van der Meer et al., 2004b; Kanbay et al., 2007 |
| Head and Neck Cancer | Survival decreased | Henke et al., 2003; Henke et al., 2006 |
| Metastatic Disease | Further disease progression | Leyland-Jones et al., 2005; Lai and Grandis, 2006 |
| Radiotherapy | Decreased efficacy | Ceelen et al., 2007 |
| Trauma | Incidence of thrombosis increased | Corwin et al., 2007 |
| Vascular Thrombosis | Potential vascular stenosis, thrombosis | Corwin et al., 2007; Reddy et al., 2007 |
Cytoprotection by EPO also is related to the maintenance of mitochondrial membrane potential (ΔΨm). Loss of ΔΨm through the opening of the mitochondrial permeability transition pore represents a significant determinant for cell injury and the subsequent induction of apoptosis (Leuner et al., 2007; Maiese and Chong, 2004). EPO has the capacity to prevent the depolarization of the mitochondrial membrane that also affects the release of cytochrome c (Chong et al., 2002b; Chong et al., 2003e; Miki et al., 2006).
4.2 EPO and Neurodegeneration
As a robust cytoprotectant, EPO can enhance the survival of cells during several types of injury models in the nervous system (Lykissas et al., 2007; Maiese et al., 2004; 2005c). In cells that involve the brain or the retina, EPO can prevent injury from hypoxic ischemia (Chong et al., 2002b; 2003b; Liu et al., 2006; Meloni et al., 2006; Wei et al., 2006; Yu et al., 2005), excitotoxicity (Montero et al., 2007; Yamasaki et al., 2005), infection (Kaiser et al., 2006), free radical exposure (Chong et al., 2003a; Chong et al., 2003e; Yamasaki et al., 2005), staurosporine (Pregi et al., 2006), and dopaminergic cell injury (Demers et al., 2005; McLeod et al., 2006; Signore et al., 2006). In addition, administration of EPO also represents a viable option for the prevention of retinal cell injury during glutamate toxicity (Zhong et al., 2007) and glaucoma (Tsai et al., 2007). Systemic application of EPO also can improve functional outcome and reduce cell loss during spinal cord injury (King et al., 2007; Okutan et al., 2007), traumatic cerebral edema (Verdonck et al., 2007), cortical trauma (Cherian et al., 2007), and epileptic activity (Mikati et al., 2007; Nadam et al., 2007).
Interestingly, EPO may provide hope for individuals that suffer from cognitive disability, such as memory loss or psychiatric illness (Chong et al., 2005c; f; Ehrenreich et al., 2007; Pacary et al., 2006). In animal studies, EPO has been shown to reduce cognitive loss during mechanical injury to the hippocampus (Mala et al., 2005). As a result, Alzheimer’s disease has become a prime consideration for the applications of EPO. Alzheimer’s disease leads to a progressive deterioration of cognitive function with memory loss and injury to hippocampal neurons. The generation of extracellular plaques of amyloid-β peptide aggregates composed of a 39–42 amino acid peptide (Aβ) is considered to be one of the pathological mechanisms that may promote the development of Alzheimer’s disease (Chong et al., 2005f). Accumulation of Aβ can lead to apoptotic injury with chromatin condensation, DNA fragmentation, and cellular membrane PS exposure (Chong et al., 2005c; f). Aβ also can release reactive oxygen species and lead to toxicity in neurons. In addition, Aβ can not only precipitate a significant inflammatory response with microglial activation and the secretion of TNF-α (Bornemann et al., 2001), but also Aβ can elicit the neuronal expression of inducible nitric oxide synthase, peroxinitrite production, and neuronal apoptosis during an acute inflammatory response (Chong et al., 2005e; Combs et al., 2001). Furthermore, Aβ may lead to the induction of caspase mediated pathways (Nakagawa et al., 2000; Troy et al., 2001) that work in concert with oxidative stress (Tamagno et al., 2003). As a result, therapeutic strategies that address the toxicity of Aβ as a result of oxidative stress may foster novel developments for the treatment of Alzheimer’s disease. EPO appears to be both necessary and sufficient to protect neurons from Aβ toxicity. For example, application of a blocking antibody of EPO, which can bind to EPO and block its biological activities in cells (Koshimura et al., 1999), can otherwise negate the protective effects of EPO to increase neuronal hippocampal cell survival and prevent apoptotic injury during Aβ exposure (Chong et al., 2005d).
In direct relation to the potential protective cognitive effects of EPO, enhanced survival by EPO also extends to afford protection of the neurovascular unit during cerebral vascular disease (Demers et al., 2005; Dzietko et al., 2004; Maiese et al., 2004; Wei et al., 2006). In addition, EPO can protect sensitive hippocampal neurons from both focal and global ischemic brain injury (Keogh et al., 2007; Wei et al., 2006; Yu et al., 2005; Zhang et al., 2006). Systemic administration of EPO also represents a viable option for several other disorders. EPO administration for retinal cell injury can protect retinal ganglion cells from apoptosis (Grimm et al., 2002), EPO can improve functional outcome and reduce lipid peroxidation during spinal cord injury (Kaptanoglu et al., 2004), and EPO can maintain autoregulation of cerebral blood flow, reverse basilar artery vasoconstriction, and enhance neuronal survival and functional recovery following subarachnoid hemorrhage (Olsen, 2003).
4.3 EPO and Inflammatory Cell Activation
Of equal importance to the functional preservation of cells is the role of EPO during cellular inflammation. In particular, one can consider the role of microglia in the brain that can lead to the phagocytic removal of both neurons and vascular cells (Chong et al., 2005a; Chong et al., 2004a; Kang et al., 2003b). During inflammation, microglial cells require the activation of intracellular cytoprotective pathways (Chong et al., 2007b; Li et al., 2006b) to proliferate and remove injured cells (Li et al., 2005; Mallat et al., 2005). Subsequently, microglia can form a barrier for the removal of foreign microorganisms from the central nervous system and promote tissue repair during neuronal and vascular cell injury (Chong et al., 2007b; Dringen, 2005). Yet, microglia also may lead to cellular damage through the generation of reactive oxygen species (Maiese and Chong, 2004; Sankarapandi et al., 1998) and through the production of cytokines (Benzing et al., 1999; Mehlhorn et al., 2000). Furthermore, microglial activation has been correlated with several neurodegenerative disorders, such as Alzheimer’s disease with the co-localization of microglia and amyloid plaque development (Sheng et al., 1997).
Given the impact that inflammatory cells, such as microglia, may have upon the progression or resolution of degenerative insults throughout the body, it becomes essential to consider agents that can control inflammatory pathways. To this end, cytoprotective agents that are known to modulate inflammatory cell function may offer attractive therapeutic considerations. EPO appears to fill such a need in regards to its role during periods of cellular inflammation. EPO can reduce cytokine gene expression in endothelial cells exposed to tumor necrosis factor (Avasarala and Konduru, 2005), prevent ulcer progression in cases of scleroderma (Ferri et al., 2007), and block primary microglial activation and proliferation during oxidative stress (Chong et al., 2003b; Chong et al., 2005d) (Figure 1). Furthermore, EPO can block microglial cell activation and proliferation to prevent phagocytosis of injured cells through pathways that involve cellular membrane PS exposure, protein kinase B (Chong et al., 2004a), and the regulation of caspases (Chong et al., 2003a; b; Wu et al., 2007a). EPO can directly inhibit several pro-inflammatory cytokines, such as IL-6, TNF-α, and monocyte chemoattractant protein 1 (Li et al., 2004a; Maiese et al., 2005c), as well as reduce leukocyte inflammation (Contaldo et al., 2007). In addition, EPO may foster the preservation of microglial cells for neuronal and vascular restructuring by preventing apoptotic injury in microglia (Li et al., 2006b; Vairano et al., 2002). In regard to the capacity of EPO to maintain microglial cellular integrity, EPO retains its capacity to prevent early apoptotic injury with membrane PS externalization as well as later stages of apoptotic injury involving DNA fragmentation in microglia (Li et al., 2006b) similar to other cell systems of neurovascular origin (Chong et al., 2002b; 2003b; Chong et al., 2005d; Parsa et al., 2003; Sharples et al., 2004).
Figure 1. Erythropoietin (EPO) prevents the activation and proliferation of microglia during oxygen-glucose deprivation (OGD).
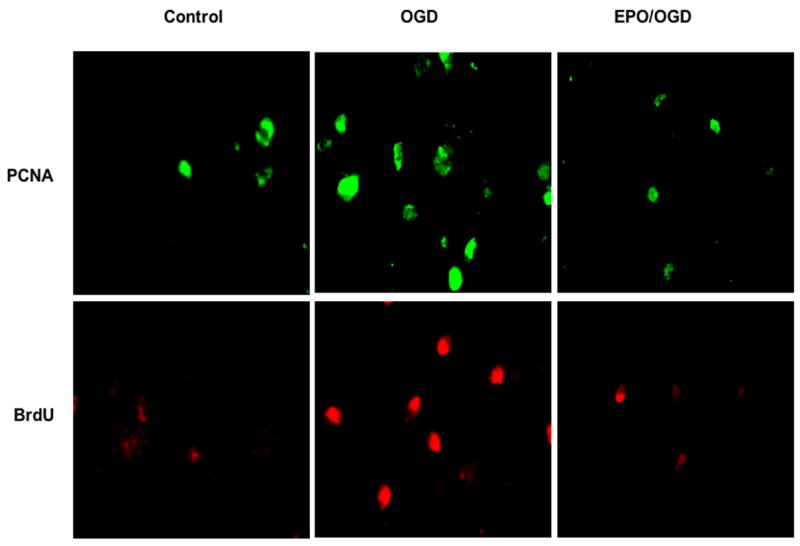
EPO (10ng/ml) was applied to microglial cultures (EOC-2) 1 hour prior to a 6 hour period of OGD. Proliferating cell nuclear antigen (PCNA) that can assess activation of microglia and bromodeoxyuridine (BrdU) that can follow microglia proliferation were performed with anti-mouse antibody against PCNA (1:100) or BrdU (1:100) and visualized through fluorescence conjugated anti-mouse IgG (1:50) for PCNA and Texas Red conjugated anti-mouse IgG for BrdU. BrdU (10 μM) and fluorodexyuridine (1 μM) were applied 1 hour prior to the time of fixation. Untreated control microglia have minimal PCNA and BrdU expression. Expression of PCNA and BrdU in microglia significantly increases during OGD exposure. In contrast, PCNA expression and BrdU expression is significantly less in microglia treated with EPO (10 ng/ml), illustrating the ability of EPO to prevent the activation and proliferation of inflammatory microglia during oxidative stress.
4.4 EPO and Cardiovascular-Renal Protection
Clinical studies have suggested an important role for EPO in the cardiovascular system (Maiese et al., 2004; 2005c) and in the renal system (Sharples and Yaqoob, 2006) that ultimately can affect the function and integrity of the nervous system. For example, in patients with anemia EPO administration can increase left ventricular ejection fraction and stroke volume (Goldberg et al., 1992). This work has been followed by randomized control studies with EPO administration in patients with congestive heart failure or diabetes combined with congestive heart failure that demonstrate improved cardiac output and a decrease in medical resource utilization (Silverberg et al., 2003). More recent studies have shown that patients with acute myocardial infarction have increased plasma EPO levels within seven days of the cardiac insult, suggesting a possible protective response from the body (Ferrario et al., 2007). In addition, EPO administration in patients with anemia and congestive heart failure can improve exercise tolerance, renal function, and left ventricular systolic function (Palazzuoli et al., 2006; Palazzuoli et al., 2007).
Randomized control studies with EPO administration in patients with congestive heart failure or diabetes combined with congestive heart failure also demonstrate an improved cardiac output and a decrease in medical resource utilization (Maiese et al., 2005c; Silverberg et al., 2006). Tightly integrated with cardiac performance, pulmonary function also is believed to be enhanced during EPO administration, especially in the setting of ischemic reperfusion injury of the lung (Wu et al., 2006). Serum levels of EPO also may function as a biomarker for cardiovascular injury (Fu and Van Eyk, 2006). Work from experimental studies illustrates that EPO plays a critical role in the vascular and renal systems with the maintenance of erythrocyte (Foller et al., 2007) and podocyte (Eto et al., 2007) integrity, regulates the survival of ECs (Chong et al., 2002b; 2003a), and may act as a powerful endogenous protectant during cardiac injury (Asaumi et al., 2007).
It is important to note that as a large molecule, EPO may maintain the establishment of EC communication and function, which could become crucial in a number of scenarios, such as repair of the blood-brain barrier during injury (Martinez-Estrada et al., 2003). In addition, by assuring EC integrity, EPO prevents ischemic cardiac demise by reducing myocardial injury and cardiomyocyte apoptosis (Burger et al., 2006), lessening myocardial ischemia (Bullard et al., 2005), modulating cardiac remodeling (Miki et al., 2006; Toma et al., 2007), reducing ventricular dysfunction (Parsa et al., 2004; Parsa et al., 2003), and improving cardiac function (Gao et al., 2007; Westenbrink et al., 2007). Overall, EPO can protect against myocardial cell apoptosis and decrease infarct size, resulting in improved left ventricular contractility. In isolated rat heart preparations following ischemia/reperfusion experiments, beneficial effects of treatment with EPO have been shown to significantly improve post-ischemic recovery of left ventricular pressure (Moon et al., 2003; van der Meer et al., 2004a). EPO treatment also can prevent myocardial cell apoptosis and decrease infarct size, resulting in enhanced cardiac function and recovery (Parsa et al., 2004). At the onset of coronary artery occlusion, EPO administered also can significantly inhibit apoptosis in the central region of myocardial ischemia (Tramontano et al., 2003). Even in acute scenarios following coronary artery ligation, EPO leads to a decrease in apoptotic cells by fifty percent in the myocardium and significantly improves cardiac function (Moon et al., 2003; Parsa et al., 2003).
Some of the results from experimental studies with EPO have correlated well with a number of positive clinical observations for EPO in cardiac patients. Clinical studies in patients with anemia or on chronic hemodialysis have indicated that administration of EPO can increase left ventricular ejection fraction, stroke volume, and cardiac output, suggesting improved cardiac function secondary to the correction of anemia (Maiese et al., 2004; 2005c; Silverberg et al., 2006). Other clinical randomized control studies in patients with mild anemia and severe or resistant congestive heart failure have demonstrated that EPO in combination with intravenous iron can lead to increased left ventricular ejection fraction and a reduction in hospitalization days by almost eighty percent (Silverberg et al., 2001). In addition to the correction of anemia, EPO can promote microvascular growth in the heart, suggesting that functional cardiac recovery with EPO may ensue also from the generation of new blood vessels (Westenbrink et al., 2007).
4.5 EPO and Angiogenesis
In the vascular system, EPO not only offers direct preservation of EC integrity (Chong et al., 2002a; b; 2003a), but also promotes new capillary formation from pre-existing vessels into an avascular area, a process known as angiogenesis (Chong et al., 2002a). Angiogenesis is present during embryogenesis, during menstruation, and during pathological processes that involve wound healing, chronic inflammation, and tumor growth (Risau, 1997). EPO has both a mitogenic and chemotactic effect that can lead to matrix metalloproteinase-2 production, cell proliferation, and vessel formation in EC lines (Maiese et al., 2004; 2005c). In cultured human and bovine ECs, EPO stimulates EC proliferation and fosters the migration of ECs (Anagnostou et al., 1990). In neonatal mesenteric microvascular ECs, EPO also leads to vasculogenesis (Ashley et al., 2002). In clinical studies, EPO serum levels are significantly associated with the number and function of circulating endothelial progenitor cells and EPO can stimulate postnatal neovascularization by increasing endothelial progenitor cell mobilization from the bone marrow (Heeschen et al., 2003). Angiogenesis also has been observed in endothelial samples derived from human adult myocardial tissue following treatment with EPO (Jaquet et al., 2002). In addition, the uterine endometrium and the ovaries are dependent upon EPO for the induction of angiogenesis to compensate for lost vessels during the estrus cycle. EPO has been shown to be necessary to foster blood vessel formation in the endometrium in ovariectomized mice and to be required for the formation of a capillary network for the development of follicles and the corpora lutea (Yasuda et al., 1998).
Angiogenesis by EPO offers an additional level of cytoprotection in various cell systems. For example, in models of cerebral ischemia, EPO promotes factors for angiogenesis such as Tie-2 and Angiopoietin-2 that may assist with the restoration of cerebral blood flow to pre-ischemic levels (Li et al., 2007a). EPO controlled angiogenesis also may play a significant role during renal inflammation and prevention of allograft rejection (Reinders et al., 2006). In addition, EPO may promote the viability of transplanted marrow stromal cells and enhance capillary density during experimental cardiac ischemia (Zhang et al., 2007a). On the converse side, it also is vital to consider paradigms that require inhibition of angiogenesis. Although EPO induced angiogenesis may impart beneficial effects to ischemic cells of the nervous and cardiovascular systems for nutrient and oxygen supply, other scenarios that involve ocular neovascularization may seek to block or limit angiogenesis by EPO to prevent disease progression (Zhang and Ma, 2007).
5. EPO and the Modulation of Critical Cellular Pathways
5.1 EPO and Cellular Oxidative Stress
EPO regulates several signal transduction pathways during cellular oxidative stress that can involve protein kinase B, signal transducer and activator of transcription pathways, forkhead transcription factors, caspases, and nuclear factor κB. Strongly associated to these pathways of EPO that control cell longevity are the injury mechanisms associated with apoptosis. Oxidative stress occurs as a result of the development of reactive oxygen species that consist of oxygen free radicals and other chemical entities.
Oxygen consumption in organisms, or at least the rate of oxygen consumption in organisms, has intrigued a host of investigators and may have had some of its original origins with the work of Pearl. Pearl proposed that increased exposure to oxygen through an increased metabolic rate could lead to a shortened life span (Pearl, 1928). Subsequent work by multiple investigators has furthered this hypothesis by demonstrating that increased metabolic rates could be detrimental to animals in an elevated oxygen environment (Muller et al., 2007). When one moves to more current work, oxygen free radicals and mitochondrial DNA mutations have become associated with oxidative stress injury, aging mechanisms, and accumulated toxicity for an organism (Yui and Matsuura, 2006).
In clinical terms, oxygen free radicals can be generated in elevated quantities during the reduction of oxygen and subsequently lead to cell injury and apoptosis. Reactive oxygen species can involve superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite (Chong et al., 2005e). Most species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase, and small molecule substances such as vitamins C and E. Other closely linked pathways to oxidative stress may be tempered by different vitamins, such as vitamin D3 (Regulska et al., 2007) and the amide form of niacin or vitamin B3, nicotinamide (Chlopicki et al., 2007; Chong et al., 2002d; Feng et al., 2006; Hara et al., 2007; Ieraci and Herrera, 2006; Lin et al., 2000; Maiese and Chong, 2003).
Oxidative stress represents a significant mechanism for the destruction of cells that can involve apoptotic cell injury and neuronal or vascular degeneration (Chong et al., 2006a; De Felice et al., 2007; Lin and Maiese, 2001). In fact, it has recently been shown that genes involved in the apoptotic process are replicated early during processes that involve cell replication and transcription, suggesting a much broader role for these genes than originally anticipated (Cohen et al., 2007). Apoptotic induced oxidative stress in conjunction with processes of mitochondrial dysfunction can contribute to a variety of disease states such as diabetes, ischemia, general cognitive loss, Alzheimer’s disease, and trauma (Chong et al., 2005e; f; Harris et al., 2007; Leuner et al., 2007; Okouchi et al., 2007). Oxidative stress can lead to apoptosis in a variety of cell types that involve neurons, ECs, cardiomyocytes, and smooth muscle cells through multiple cellular pathways (Chong et al., 2004a; Chong et al., 2007b; Harris et al., 2007; Kang et al., 2003b; Karunakaran et al., 2007; Verdaguer et al., 2007).
Membrane phosphatidylserine (PS) externalization is an early event during cell apoptosis (Maiese et al., 2000; Mari et al., 2004) and can become a signal for the phagocytosis of cells (Chong et al., 2005a; Li et al., 2006b; Lin and Maiese, 2001). As an example, externalization of membrane PS residues occurs in neurons during anoxia (Maiese, 2001; Maiese and Boccone, 1995; Vincent and Maiese, 1999a), nitric oxide exposure (Chong et al., 2003f; Maiese et al., 1997), and during the administration of agents that induce the production of reactive oxygen species, such as 6-hydroxydopamine (Salinas et al., 2003). Membrane PS externalization on platelets also has been associated with clot formation in the vascular cell system (Leytin et al., 2006).
The translocation of membrane PS residues from the inner cellular membrane to the outer surface is a necessary component under most conditions for the removal of apoptotic cells (Maiese et al., 2003; Maiese and Vincent, 2000a; b). The loss of membrane phospholipid asymmetry leads to the externalization of membrane PS residues and assists microglia to target cells for phagocytosis (Chong et al., 2003d; Kang et al., 2003a; b; Maiese and Chong, 2003; Mallat et al., 2005). This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress (Li et al., 2006a; c), since blockade of PSR function in microglia prevents the activation of microglia (Chong et al., 2003b; Kang et al., 2003a).
In contrast to the early externalization of membrane PS residues, the cleavage of genomic DNA into fragments (Maiese et al., 1999; Maiese and Vincent, 2000a; b) is considered to be a later event during apoptotic injury (Dombroski et al., 2000; Jessel et al., 2002; Kang et al., 2003b; Maiese and Vincent, 2000b). Endonucleases lead to DNA degradation and have been differentiated based on their ionic sensitivities to zinc (Torriglia et al., 1997), magnesium (Sun and Cohen, 1994), and calcium (Maiese et al., 1999), an important regulator that can independently impair cell survival. In the nervous system, three separate endonuclease activities are present. These include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease (Chong et al., 2005f; Vincent and Maiese, 1999b; Vincent et al., 1999a).
Oxidative stress also can impair mitochondrial function. Mitochondrial membrane transition pore permeability is increased (Chong et al., 2003a; Di Lisa et al., 2001; Kang et al., 2003b; Lin et al., 2000) and leads to a significant loss of mitochondrial NAD+ stores and subsequent apoptotic cell injury (Chong et al., 2005g; Maiese and Chong, 2003). In addition, mitochondria are a significant source of superoxide radicals that are associated with oxidative stress (Chong et al., 2005e; Maiese and Chong, 2004). Blockade of the electron transfer chain at the flavin mononucleotide group of complex I or at the ubiquinone site of complex III results in the active generation of free radicals, which can impair mitochondrial electron transport and enhance free radical production (Chong and Maiese, 2007b; Li et al., 2006a). Furthermore, mutations in the mitochondrial genome have been associated with the potential development of a host of disorders, such as hypertension, hypercholesterolemia, and hypomagnesemia (Li et al., 2004b; Wilson et al., 2004). Reactive oxygen species also may lead to the induction of acidosis-induced cellular toxicity and subsequent mitochondrial failure (Chong et al., 2005f). Disorders, such as hypoxia (Roberts and Chih, 1997), diabetes (Cardella, 2005; Kratzsch et al., 2006), and excessive free radical production (Ito et al., 1997; Vincent et al., 1999a; b) can result in the disturbance of intracellular pH. In the consideration of oxidative stress-induced pathways, EPO offers a unique opportunity to prevent the exposure of membrane PS residues, inhibit the committed stages of genomic DNA destruction, and block cell injury.
5.2 EPO and Jak2, STATS, ERKs, Caspases
Cellular signal transduction with EPO requires the activation of the EPOR which specifically binds to and activates Janus-tyrosine kinase 2 (Jak2) through phosphorylation. Jak2 is a member of a family of Janus-type protein-tyrosine kinases including Jak1, Jak2, Jak3, and Tyk2, which are characterized by a kinase domain in the carboxyl portion, a kinase-like domain, and a large amino-terminal domain (Wilks et al., 1991). The amino-terminal domain of Jak2 is responsible for the binding of Jak2 with the β-subunit of the EPOR at a region proximal to the membrane that contains the Box 1 sequence (Zhao et al., 1995). EPO can prevent apoptotic injury through its reliance on Jak2 phosphorylation (Kawakami et al., 2001; Sharples et al., 2004), since loss of Jak2 activity reduces protection by EPO (Digicaylioglu et al., 2004; Lipton, 2007) (Figure 2).
Figure 2. Erythropoietin (EPO) prevents apoptotic injury through a series of interconnected cellular pathways.
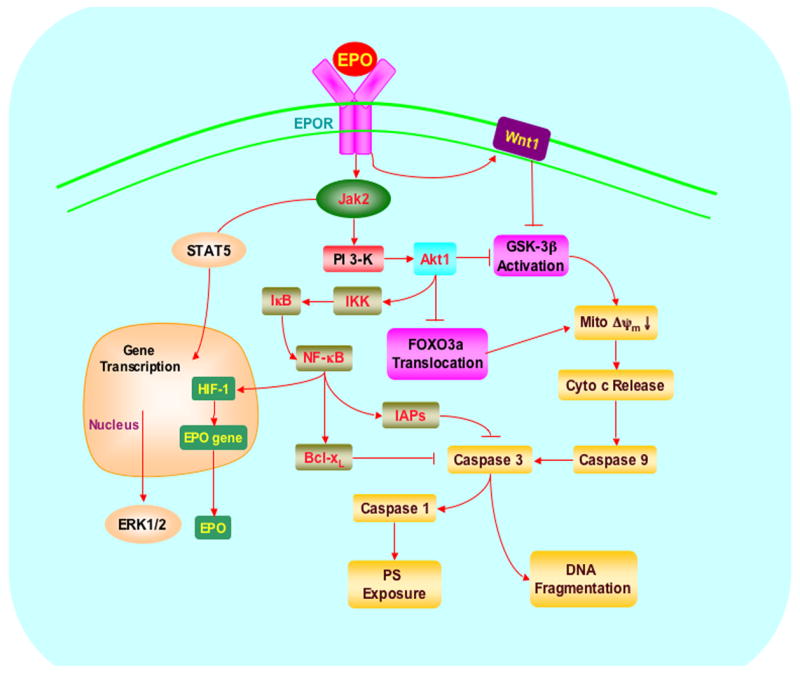
With HIF-1 activation, EPO and the EPO receptor (EPOR) can increase cell survival, promote progenitor cell development, and control inflammatory cell activation through pathways that involve the Janus-tyrosine kinase 2 (Jak2) protein, protein kinase B (Akt), and signal transducer and activator of transcription (STAT) proteins. Interconnected pathways involve Wnt1, IκB kinase (IKK), IκB, inhibitors of apoptotic protein (IAPs), extracellular signal-related kinases (ERKs), the forkhead family member FOXO3a, glycogen synthase kinase-3β(GSK-3β), nuclear factor-κB (NF-κB), mitochondrial membrane potential (Δψm), cytochrome c, (Cyto-c), and caspases. Ultimately these pathways converge upon early apoptotic injury with phosphatidylserine (PS) exposure and later apoptotic DNA degradation.
The signal transducer and activator of transcription (STAT) proteins are direct substrates of Janus kinases. Seven mammalian STAT genes encoding proteins exist and are considered to be latent DNA binding factors that can be activated by tyrosine phosphorylation (Reich, 2007). Activation of Janus kinases results in tyrosine phosphorylation and dimerization of STATs. Once activated, STATs translocate to the nucleus and bind to specific DNA sequences in the promoter regions of responsive genes to lead to gene transcription. Associated with these transcription pathways are the mitogen-activated protein kinases, which include the extracellular signal-related kinases (ERKs), the c-Jun-amino terminal kinases, and p38 MAP kinase, which can oversee erythroid proliferation and differentiation (Nagata et al., 1998). Yet, in regard to cytoprotection, EPO has been shown to not only activate STAT 3 (Asaumi et al., 2007; Chong and Maiese, 2007a; Parsa et al., 2003), STAT 5 (Chong and Maiese, 2007a; Menon et al., 2006b; Moon et al., 2006; Um and Lodish, 2006; Wei et al., 2006), and ERK 1/2 (Bullard et al., 2005; Menon et al., 2006a), but also to employ these pathways for cell development and cell protection (Figure 2). For example, EPO significantly activates STAT3, STAT5, and ERK 1/2 in primary cerebral vascular cells, suggesting that EPO may require these cellular pathways to confer EC cytoprotection during oxidative stress (Chong and Maiese, 2007a) (Table 2). In addition, activation of STAT5 also can modulate EPO proliferation as well as protection against cellular apoptosis (Damen et al., 1995). In erythroleukemic cell lines, EPO-dependent cell survival is accompanied by sustained STAT5 DNA-binding activity. Stable expression of the truncated STAT5a has been shown to enhance STAT5-DNA binding activity and reduce the induction of apoptosis (Bittorf et al., 2000). In contrast, induction of apoptosis can be observed in cells that lack STAT5 (STAT5a−/−/5b−/) function (Socolovsky et al., 2001). For example, STAT5a−/−5b−/−fetal liver erythroid progenitors show higher levels of apoptosis and are less responsive to the presence of EPO (Socolovsky et al., 1999).
Table 2.
Novel Cellular Pathways Modulated by Erythropoietin (EPO)
Downstream from Janus kinases, STATS, and the ERKs are the apoptotic pathways of the caspase family. Caspases are a family of cysteine proteases that are synthesized as inactive zymogens, which are proteolytically cleaved into subunits at the onset of apoptosis (Li et al., 2006a; Maiese et al., 2005a; Okouchi et al., 2007). Caspases are composed of three domains including an N-terminal prodomain, a large subunit, and a small subunit (Earnshaw et al., 1999). As a result of their activation sequence, caspases are classified as either initiator caspases (also known as apical caspases) or effector caspases (Shi, 2004). An initiator caspase cleaves and subsequently activates an effector caspase. The apoptotic-associated caspases include initiator caspases, such as caspase 2, 8, 9, and 10, that activate downstream effector caspases, resulting in an amplification of cascade activity. The initiator caspases consist of long N-terminal prodomains that contain caspase recruitment domains (CARDs) in caspase 2 and caspase 9 or death effector domains (DEDs) in caspase 8 and caspase 10 (Hofmann et al., 1997). The effector caspases consist of caspase 3, 6, and 7, which function to directly cleave crucial cellular protein substrates to result in cell destruction.
The caspases 1 and 3 have each been linked to the independent apoptotic pathways of genomic DNA cleavage and cellular membrane PS exposure (Chong et al., 2003a; Chong et al., 2003e; Takahashi et al., 1999). These caspases, in addition to caspase 8 and 9, are also tied to the direct activation and proliferation of microglia (Chong et al., 2003b; Kang et al., 2003a; b). Caspase 1 is believed to be principally responsible for the externalization of membrane PS residues in several cell systems that can subsequently activate microglial phagocytosis (Maiese and Vincent, 2000b; Vanags et al., 1996). Furthermore, caspase 9 is activated through a process that involves the cytochrome c -apoptotic protease-activating factor-1 (Apaf-1) complex (Chong et al., 2004b; Li et al., 1997). In addition, caspase 8 serves as an upstream initiator of executioner caspases, such as caspase 3, and also leads to the mitochondrial release of cytochrome c (Engels et al., 2000; Stegh et al., 2002). Following caspase 8 and caspase 9 activation, caspase 3 directly leads to genomic DNA degradation.
Modulation of caspase activity by EPO may offer several avenues for protection against cell injury (Table 2). The ability of EPO to prevent specific caspase 1- and caspase 3-like activities appears to play a significant role in its cellular protection (Chong et al., 2002b; 2003b; Chong et al., 2003e; Digicaylioglu et al., 2004; Li et al., 2007a; Okutan et al., 2007; Wu et al., 2007a). In regards to caspase 1, EPO prevents PS externalization primarily through the inhibition of caspase 1-like activity and, to a lesser degree, through other caspases such as 3, 8, and 9 (Chong et al., 2002b; 2003a; b; Chong et al., 2003e) (Figure 2). EPO also can block genomic DNA degradation through the inhibition of cytochrome c and the subsequent inhibition of caspase 3-like activity (Chong et al., 2003b). Regulation of caspase 3-like activity by EPO has recently been linked to a unique regulatory mechanism that blocks the proteolytic degradation of phosphorylated forkhead transcription factors by caspase 3. Given that specific pro-apoptotic transcription factors, such as FoxO3a which is a member of the mammalian FoxO proteins assigned to the O class of the forkhead transcription superfamily, have been shown to be a substrate for caspase 3-like proteases at the consensus sequence DELD304A (Charvet et al., 2003), current work demonstrates that blockade of caspase 3-like activity prevents the destruction of the inactive phosphorylated FoxO3a during oxidative stress to increase cell survival (Chong and Maiese, 2007a). In relation to caspase 8 and caspase 9, EPO can also target these pathways (Chong et al., 2003a; b; Chong et al., 2003e; Sharples et al., 2004; Signore et al., 2006). EPO prevents cellular apoptosis through parallel pathways that prevent the induction of Apaf-1 and caspase 9 as well as by preserving mitochondrial membrane potential in conjunction with enhanced Bcl-xL expression (Chong et al., 2003a).
5.3 EPO and Akt, Forkhead Transcription Factors
The ability of EPO to enhance cell survival during injury also directly relies upon the phosphatidylinositol 3-kinase (PI 3-K) pathway through protein kinase B (Akt). Phosphorylation of Akt in conjunction with EPO administration leads to its activation and protects against genomic DNA degradation and membrane PS exposure (Chong et al., 2003a; b; Chong et al., 2003e). Up-regulation of Akt activity during multiple injury paradigms, such as vascular and cardiomyocyte ischemia (Miki et al., 2006; Parsa et al., 2003), free radical exposure (Chong et al., 2003b; Matsuzaki et al., 1999), matrix detachment (Rytomaa et al., 2000), neuronal axotomy (Namikawa et al., 2000), N-methyl-D-aspartate toxicity (Dzietko et al., 2004), hypoxia (Chong et al., 2002b; Zhang et al., 2007b),β-amyloid toxicity (Chong et al., 2005d; Martin et al., 2001), DNA damage (Chong et al., 2002b; 2004a; Henry et al., 2001; Kang et al., 2003a), metabotropic receptor signaling (Chong et al., 2005a; Chong et al., 2006b; Maiese et al., 2005a), cell metabolic pathways (Chong et al., 2005g; Maiese and Chong, 2003), and oxidative stress (Chong et al., 2004a; Kang et al., 2003a; b), increases cell survival. Akt also can directly control microglial activation through the prevention of Bcl-xL degradation (Chong et al., 2004a) and the inhibition of caspase 1-, 3-, and 9-like activities (Chong et al., 2005a; Kang et al., 2003a; b).
In addition, modulation of Akt activity can critically affect cell survival during hyperglycemia and the outcome of diabetic complications. Furthermore, endoplasmic reticulum stress inducers can lead to dephosphorylation and inactivation of Akt with subsequent cell death (Hyoda et al., 2006). On the converse side, overexpression of Akt, such as in endothelial cells, can protect cells from injury during elevated glucose concentrations (Varma et al., 2005). Therefore, Akt may be an essential component for EPO protection especially during disease processes such as diabetes, since inhibition of Akt activity blocks cellular protection and anti-inflammatory mechanisms by EPO (Chong et al., 2003a; b; Chong et al., 2003e). EPO has been shown to employ the PI 3-K/Akt pathway in a variety of experimental models of injury (Bahlmann et al., 2004; Chong et al., 2002b; 2003b; Chong et al., 2003e; Chong and Maiese, 2007a; Li et al., 2006b; Miki et al., 2006; Parsa et al., 2003; Sharples et al., 2004; Um et al., 2007; Um and Lodish, 2006; Wu et al., 2007b) (Figure 2). These can involve transcription factor regulation (Chong and Maiese, 2007a), maintenance of ΔΨm, prevention of cytochrome c release (Chong et al., 2003a; b; Chong et al., 2003e), and blockade of caspase activity (Chong et al., 2002b; 2003a; b) (Table 2).
Interestingly, a number of novel pathways that may mediate the ability of EPO to prevent cellular apoptosis are linked to Akt. For example, Akt is a central regulatory element for the mammalian forkhead transcription factor family that oversees processes that can involve cell metabolism, hormone modulation, and apoptosis (Cuesta et al., 2007; Maiese et al., 2007a; Maiese et al., 2007b). The mammalian forkhead transcription factor family functions as transcription factors by preferentially binding to the core consensus DNA sequence 5′-TTGTTTAG-3′, the forkhead response element (Chong et al., 2005e; Chong et al., 2004c; Wijchers et al., 2006). The first member of this family was the Drosophila melanogaster gene Fork head. Since this time, greater than 100 forkhead genes and 19 human subgroups are known to exist that extend from FOXA to FOXS (Maiese et al., 2007b; Wijchers et al., 2006). The forkhead box (FOX) family of genes is characterized by a conserved forkhead domain commonly noted as a “forkhead box” or a “winged helix” as a result of the butterfly-like appearance on X-ray crystallography (Clark et al., 1993) and nuclear magnetic resonance (Jin et al., 1998). All Fox proteins contain the 100-amino acid winged helix domain, but it should be noted that not all winged helix domains are Fox proteins (Larson et al., 2007).
Of the mammalian forkhead transcription factors assigned to the O class, FoxO3a has emerged as a versatile target for a number of disorders. Akt can phosphorylate FoxO3a and inhibit its activity to sequester FoxO3a in the cytoplasm by association with 14-3-3 proteins (Brunet et al., 2002; Chong and Maiese, 2007a; Dong et al., 2007; Kino et al., 2005; Munoz-Fontela et al., 2007). In the absence of inhibitory Akt1 phosphorylation, FoxO3a can translocate to the nucleus, and controls a variety of functions that involve cell cycle progression, cell longevity, and apoptosis (Lehtinen et al., 2006; Li et al., 2006a; Maiese et al., 2007a). As a result, control of FoxO3a is considered to be a viable therapeutic target for agents such as metabotropic glutamate receptors (Chong et al., 2006b), neurotrophins (Zheng et al., 2002), and NAD+ precursors (Chong et al., 2004c; Li et al., 2006a; b) to increase cell survival (Figure 2). In addition, FoxO3a interfaces with several pathways that regulate cellular lifespan (Lehtinen et al., 2006) and function to control neoplastic growth (Li et al., 2007b). In a similar manner, EPO controls the phosphorylation and degradation of FoxO3a to retain it in the cytoplasm through binding to 14-3-3 protein and foster vascular cell protection during oxidative stress (Chong and Maiese, 2007a) (Table 2).
5.4 EPO and Wnt, GSK-3β, NF-κB
Wnt proteins, derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes, have been shown to play a role in both cell development and cell demise with recent recognition that the Wnt pathway also is dependent upon Akt signaling (Chong et al., 2007a; Chong et al., 2007c; Li et al., 2006c; Speese and Budnik, 2007). Wnt proteins are secreted cysteine-rich glycosylated proteins that play a role in a variety of cellular functions that involve embryonic cell proliferation, differentiation, survival, and death (Li et al., 2006c; Patapoutian and Reichardt, 2000; Wodarz and Nusse, 1998). In general, all Wnt signaling pathways are initiated by interaction of Wnt proteins with Frizzled receptors and the binding of the Wnt protein to the Frizzled transmembrane receptor in the presence of the co-receptor LRP-5/6 (Mao et al., 2001). Once Wnt protein binds to the Frizzled transmembrane receptor and the co-receptor LRP-5/6, this is followed by recruitment of dishevelled, a cytoplasmic multifunctional phosphoprotein (Li et al., 2005; Patapoutian and Reichardt, 2000; Salinas, 1999).
Of interest, Wnt signaling can prevent cell injury through a variety of mechanisms. Wnt prevents apoptosis through β-catenin/Tcf transcription mediated pathways (Chen et al., 2001) and also can protect cells against c-myc induced apoptosis through cyclooxygenase-2 and Wnt induced secreted protein (You et al., 2002). Wnt signaling also can inhibit apoptosis during oxidative stress (Chong and Maiese, 2004) and β-amyloid toxicity that may require modulation of glycogen synthase kinase-3 β (GSK-3β) and β-catenin (Chong et al., 2007a).
Current experimental work suggests that abnormalities in the Wnt signaling pathways, such as with transcription factor 7-like 2 gene, may impart increased risk for type 2 diabetes in some populations (Grant et al., 2006; Lehman et al., 2007; Scott et al., 2006) as well as have increased association with obesity (Guo et al., 2006). Yet, intact Wnt family members may offer glucose tolerance and increased insulin sensitivity (Wright et al., 2007) as well as protect glomerular mesangial cells from elevated glucose induced apoptosis (Lin et al., 2006). These observations suggest a potential protective cellular mechanism for EPO through Wnt signaling to improve clinical cardiac function in diabetic patients (Silverberg et al., 2006) and decrease complications in women with diabetic pregnancies (Teramo et al., 2004) (Figure 2). New in vitro studies demonstrate that the Wnt1 protein is necessary and sufficient to impart cellular protection during elevated glucose exposure (Chong et al., 2007c) (Table 2). Administration of exogenous Wnt1 protein can significantly prevent apoptotic EC injury during elevated glucose exposure. Interestingly, EPO maintains the expression of Wnt1 during elevated glucose exposure and prevents loss of Wnt1 expression that would occur in the absence of EPO during elevated glucose. More importantly, blockade of Wnt1 with a Wnt1Ab can neutralize the protective capacity of EPO, illustrating that Wnt1 is a critical component in the cytoprotection of EPO during elevated glucose exposure (Chong et al., 2007c).
In the Wnt pathway, dishevelled is phosphorylated by casein kinase Iε to form a complex with Frat1 and inhibit GSK-3β activity. Inhibition of GSK-3β activity can increase cell survival during oxidative stress and, as a result, GSK-3β is considered to be a therapeutic target for some neurodegenerative disorders (Balaraman et al., 2006; Chong et al., 2005e; Nurmi et al., 2006; Qin et al., 2006). GSK-3β also may influence inflammatory cell survival (Chong et al., 2007b) and activation (Tanuma et al., 2006). In regard to metabolic disease, inactivation of GSK-3β by small molecule inhibitors or RNA interference prevents toxicity from high concentrations of glucose and increases rat beta cell replication, suggesting a possible target of GSK-3β for pancreatic beta cell regeneration (Mussmann et al., 2007). Clinical applications for GSK-3β are attractive (Rowe et al., 2007), especially in concert with EPO. For example, both the potential benefits of EPO to improve cardiovascular function in diabetic patients (Silverberg et al., 2006; Silverberg et al., 2001) and the positive effects of exercise to improve glycemic control during DM (Maiorana et al., 2002) appear to rely upon the inhibition of GSK-3β activity. EPO blocks GSK-3β activity (Chong et al., 2007c; Li et al., 2006b; Wu et al., 2007a) and combined with exercise may offer synergistic benefits, since physical exercise also has been shown to phosphorylate and inhibit GSK-3β activity (Howlett et al., 2006).
Expression and cytoprotection of EPO also is dependent, in part, upon Akt and the activation of nuclear factor-κB (NF-κB). NF-κB proteins are composed of several homo- and heterodimer proteins that can bind to common DNA elements. It is the phosphorylation of IκB proteins by the IκB kinase (IKK) and their subsequent degradation that lead to the release of NF-κB for its translocation to the nucleus to initiate gene transcription (Hayden and Ghosh, 2004). Dependent upon Akt controlled pathways, the transactivation domain of the p65 subunit of NF-κB is activated by IKK and the IKKα catalytic subunit to lead to the induction of protective anti-apoptotic pathways (Chong et al., 2005b). Increased expression of NF-κB during injury models can occur in inflammatory microglial cells (Chong et al., 2005d; 2007b; Guo and Bhat, 2006) and in neurons (Sanz et al., 2002). NF-κB does represent a critical pathway that is responsible for the activation of inhibitors of apoptotic proteins (IAPs), the maintenance of Bcl-xL expression, (Chen et al., 2000; Chong et al., 2005f), and protection against cell injury during oxidative stress (Chong et al., 2005d). NF-κB also is strongly associated with the cytoprotection of trophic factors that includes EPO (Chong et al., 2005d; Nakata et al., 2004; Sae-Ung et al., 2005). NF-κB also plays a key role in the expression of EPO during HIF-1 induction. Akt can significantly increase NF-κB and HIF-1 activation resulting in the enhancement of EPO expression. Although NF-κB has not consistently been found to be beneficial in all cell systems (Esposito et al., 2006; Jacobsen et al., 2006) and may sometimes not be cytoprotective (Nurmi et al., 2006; Xu et al., 2005), EPO subsequently uses NF-κB to prevent apoptosis through the enhanced expression and translocation of NF-κB to the nucleus to elicit anti-apoptotic gene activation (Bittorf et al., 2001; Chong et al., 2005d; Li et al., 2006b; Spandou et al., 2006).
6. Future Perspectives and Considerations for EPO
Since EPO has been identified as a candidate treatment for a number of disease entities that involve disorders of cardiac, nervous, and vascular systems, it may not be surprising to learn that marketing expenditures for EPO by manufacturers continues to rise at a fast pace that has been reported to equal 31% of the total marketing budget geared to consumer advertising (Donohue et al., 2007). Furthermore, United States annual sale revenues for EPO have recently been reported to approach 9 billion dollars (Donohue et al., 2007). At present, there are at least 100 trials with the National Institutes of Health website (clinicaltrials.gov) that are either recruiting or in preparation to examine the clinical effects of EPO in patients with a variety of disorders that include anemia, cancer, cardiac ischemia, or spinal cord trauma. Although some cardiac injury experimental models do not consistently demonstrate a benefit with EPO (Olea et al., 2006), initial studies in patients with anemia or on chronic hemodialysis have suggested a direct cardiac benefit from EPO administration (Goldberg et al., 1992; Silverberg et al., 2001) (Table 1). Subsequent work has demonstrated that EPO administration can improve exercise tolerance either during cardiac or renal insufficiency in patients with anemia and congestive heart failure (Mancini et al., 2003; Palazzuoli et al., 2006) and that this may be tied to improved pulmonary function (Wu et al., 2006). Of significant interest is a recent randomized, concealed, multicenter trail of 1460 patients who received 40,000 U of epoetin alfa up to a 3 week maximum following intensive care unit admission and demonstrated a reduced mortality in patients with trauma (Corwin et al., 2007).
Unfortunately, agents such as EPO may not be tolerated by all individuals, especially those with co-morbid conditions such as congestive heart failure, hypertension, and neoplasms. Some studies suggest that elevated plasma levels of EPO independent of hemoglobin concentration can be associated with increased severity of disease in individuals with congestive heart failure (van der Meer et al., 2004b) and that EPO may contribute to vascular stenosis with intima hyperplasia (Reddy et al., 2007) (Table 1). Adverse effects during treatment with EPO are not uncommon, such as an increased incidence of thrombotic vascular effects (Corwin et al., 2007) or the use of EPO in cancer patients receiving chemotherapy that has been associated with nonfatal myocardial infarction, pyrexia, vomiting, shortness of breath, paresthesias, and upper respiratory tract infection (Henry et al., 2004). In addition, the use of EPO in patients with hypertension must proceed with caution, since both acute and long-term administration of EPO can significantly elevate mean arterial pressure (Kanbay et al., 2007).
The potential progression of cancer has been another significant concern raised with EPO administration (Kokhaei et al., 2007; Maiese et al., 2005b). Not only have both EPO and its receptor been demonstrated in tumor specimens, but under some conditions EPO expression has been suggested to block tumor cell apoptosis through Akt (Hardee et al., 2006), enhance tumor progression, increase metastatic disease, (Lai and Grandis, 2006), and negate the effects of radiotherapy by assisting with tumor angiogenesis (Ceelen et al., 2007) (Table 1). In studies of patients with head and neck cancer, EPO decreased disease progression-free survival and overall survival (Henke et al., 2003). Similar results were reported in trials with metastatic breast cancer (Leyland-Jones et al., 2005) and the expression of the EPOR in tumors appeared to suggest a worse prognosis (Henke et al., 2006). It should be noted though that the potential risk of EPO administration to either initiate tumor growth or lead to tumor progression is not entirely understood. In regards to the possible tumor promoting ability of EPO (Rades et al., 2007), a number of competing factors must be considered that include the possible benefits of EPO administration in patients with cancer that involve the synergistic effects of EPO with chemotherapeutic modalities (Ning et al., 2005; Sigounas et al., 2004), potential protection against chemotherapy tissue injury (Joyeux-Faure, 2007), and the treatment of cancer-related anemia. The deployment of further large scale prospective trials that can more clearly examine the attributes and contraindications for EPO, especially in patients with neoplastic disease, is required.
However, in addition to the concerns outlined in patients with cancer, other important considerations for EPO exist. Irrespective of the problems associated with EPO abuse and gene doping (Baoutina et al., 2007; Diamanti-Kandarakis et al., 2005; Segura et al., 2007), EPO has been correlated with the alteration of red cell membrane properties leading to a cognitive decrement in rodent animal models (Li et al., 2004a; Maiese et al., 2004; 2005c). In addition, development of potentially detrimental side-effects during EPO therapy, such as for cerebral ischemia with increased metabolic rate and blood viscosity (Frietsch et al., 2007), could severely limit or halt the use of EPO for neurovascular diseases. As a result, alternate strategies have been suggested. New investigations are studying the role of targeted bioavailability for EPO such as in bone marrow stromal cells genetically engineered to secrete EPO (Eliopoulos et al., 2006) and controlled release of EPO from encapsulated cells (Orive et al., 2005; Ponce et al., 2006). The passage of EPO entry into the central nervous system continues to attract significant interest (Doolittle et al., 2007) as well as does the use of novel intranasal routes for EPO administration (Yu et al., 2005). Other avenues of study are directed to the development of derivations of EPO to reduce erythropoietic activity and the potential associated vascular complications (Montero et al., 2007). Yet, these lines of investigation are not without limitations, since chemical derivatives of EPO can become absent of clinical efficacy (Maiese et al., 2004; 2005c) as well as possibly lose the ability to promote sustainable cytoprotective effects, such as neurogenesis (Gonzalez et al., 2007) and angiogenesis (Li et al., 2007a; Reinders et al., 2006; Slevin et al., 2006; Zhang and Ma, 2007).
Use of EPO is now considered a promising strategy not only for erythropoiesis but also for cellular maintenance, survival, and the modulation of inflammatory pathways. New work conducted through basic research as well as through clinical trials should continue to broaden the therapeutic applications for EPO. However, precise focus upon the intricate cellular pathways governed by EPO is required to elucidate the benefits and risks of this agent. With this approach in place, EPO or the pathways that determine its biological effects should be warranted for the treatment of patients with a variety of disorders that can involve neurodegeneration, cardiac insufficiency, diabetes, and cancer.
Acknowledgments
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
Abbreviations
- Aβ
β-amyloid
- AGES
advanced glycation endproducts
- Akt
protein kinase B
- Apaf-1
apoptotic protease-activating factor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- CARD
caspase recruitment domain
- DED
death effector domain
- DM
diabetes mellitus
- EC
endothelial cell
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- ERK
extracellular signal-related kinase
- FOX
Forkhead box
- GSK-3β
glycogen synthase kinaseβ
- IAP
inhibitor of apoptosis protein
- IKK
IκB kinase
- IL-1β
interleukin-β
- IL-6
interleukin-6
- Jak
Janus-tyrosine kinase
- ΔΨm
mitochondrial membrane potential
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PI 3-K
phosphatidylinositol 3-kinase
- PS
phosphatidylserine
- PSR
phosphatidylserine receptor
- STAT
signal transducer and activator of transcription
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
- Wg
Drosophila Wingless
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaumi Y, Kagaya Y, Takeda M, Yamaguchi N, Tada H, Ito K, Ohta J, Shiroto T, Shirato K, Minegishi N, Shimokawa H. Protective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload-induced left ventricular dysfunction in mice. Circulation. 2007;115:2022–2032. doi: 10.1161/CIRCULATIONAHA.106.659037. [DOI] [PubMed] [Google Scholar]
- Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res. 2002;51:472–478. doi: 10.1203/00006450-200204000-00012. [DOI] [PubMed] [Google Scholar]
- Assaraf MI, Diaz Z, Liberman A, Miller WH, Jr, Arvanitakis Z, Li Y, Bennett DA, Schipper HM. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol. 2007;66:389–398. doi: 10.1097/nen.0b013e3180517b28. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25:183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, Kirsch T, de Groot K, Laudeley R, Niemczyk E, Guler F, Menne J, Haller H, Fliser D. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baoutina A, Alexander IE, Rasko JE, Emslie KR. Potential use of gene transfer in athletic performance enhancement. Mol Ther. 2007;15:1751–1766. doi: 10.1038/sj.mt.6300278. [DOI] [PubMed] [Google Scholar]
- Bayliss W, Starling E. The movements and innervation of the small intestine. J Physiol. 1901;26:125–138. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W, Starling E. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Bernard C. Remarques sur le sécrétion du sucre dans la foie, faites à l’occasion de la communication de M Lehman. Comptes rendus Academies de Sciences. 1855;40:589–592. [Google Scholar]
- Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Bittorf T, Sasse T, Wright M, Jaster R, Otte L, Schneider-Mergener J, Brock J. cDNA cloning and functional analysis of a truncated STAT5a protein from autonomously growing FDCP-1 cells. Cell Signal. 2000;12:721–730. doi: 10.1016/s0898-6568(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am J Pathol. 2001;158:63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77:2583–2590. [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–828. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Gu J, Huang LE, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–59. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Cardella F. Insulin therapy during diabetic ketoacidosis in children. Acta Biomed 76 Suppl. 2005;3:49–54. [PubMed] [Google Scholar]
- Carnot P, DeFlandre C. Sur l’activite hemopoietique de serum au cours de la regeneration du sang. C R Acad Sci (Paris) 1906;143:384–386. [Google Scholar]
- Ceelen W, Boterberg T, Smeets P, Van Damme N, Demetter P, Zwaenepoel O, Cesteleyn L, Houtmeyers P, Peeters M, Pattyn P. Recombinant human erythropoietin alpha modulates the effects of radiotherapy on colorectal cancer microvessels. Br J Cancer. 2007;96:692–700. doi: 10.1038/sj.bjc.6603568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45:471–477. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, Deckert M. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Chikuma M, Masuda S, Kobayashi T, Nagao M, Sasaki R. Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am J Physiol Endocrinol Metab. 2000;279:E1242–1248. doi: 10.1152/ajpendo.2000.279.6.E1242. [DOI] [PubMed] [Google Scholar]
- Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J, Gebicki J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152:230–239. doi: 10.1038/sj.bjp.0707383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005a;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002a;11:863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002b;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002c;22:503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003a;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003b;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin: cytoprotection in vascular and neuronal cells. Curr Drug Targets Cardiovasc Haematol Disord. 2003c;3:141–154. doi: 10.2174/1568006033481483. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003d;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004a;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004b;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005b;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Employing new cellular therapeutic targets for Alzheimer’s disease: a change for the better? Curr Neurovasc Res. 2005c;2:55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005d;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005e;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005f;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006a;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006b;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007a;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007b;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003e;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003f;23:561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005g;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002d;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004c;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007a;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007b;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007c;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Cordeiro-Stone M, Kaufman DG. Early replication and the apoptotic pathway. J Cell Physiol. 2007;213:434–439. doi: 10.1002/jcp.21156. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contaldo C, Meier C, Elsherbiny A, Harder Y, Trentz O, Menger MD, Wanner GA. Human recombinant erythropoietin protects the striated muscle microcirculation of the dorsal skinfold from postischemic injury in mice. Am J Physiol Heart Circ Physiol. 2007;293:H274–283. doi: 10.1152/ajpheart.01031.2006. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- Cuesta I, Zaret KS, Santisteban P. The forkhead factor FoxE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure. Mol Cell Biol. 2007;27:7302–7314. doi: 10.1128/MCB.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea AD, Zon LI. Erythropoietin receptor. Subunit structure and activation. J Clin Invest. 1990;86:681–687. doi: 10.1172/JCI114763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. Jama. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Damen JE, Wakao H, Miyajima A, Krosl J, Humphries RK, Cutler RL, Krystal G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. Embo J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- Davis LE, Widness JA, Brace RA. Renal and placental secretion of erythropoietin during anemia or hypoxia in the ovine fetus. Am J Obstet Gynecol. 2003;189:1764–1770. doi: 10.1016/s0002-9378(03)00874-3. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58:297–301. doi: 10.1203/01.PDR.0000169971.64558.5A. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Konstantinopoulos PA, Papailiou J, Kandarakis SA, Andreopoulos A, Sykiotis GP. Erythropoietin abuse and erythropoietin gene doping: detection strategies in the genomic era. Sports Med. 2005;35:831–840. doi: 10.2165/00007256-200535100-00001. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci U S A. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody- dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- Dong S, Kang S, Gu TL, Kardar S, Fu H, Lonial S, Khoury HJ, Khuri F, Chen J. 14-3-3 integrates prosurvival signals mediated by the AKT and MAPK pathways in ZNF198-FGFR1-transformed hematopoietic cells. Blood. 2007;110:360–369. doi: 10.1182/blood-2006-12-065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med. 2007;357:673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, Peereboom DM, Christoforidis GA, Hall WA, Palmieri D, Brock PR, Campbell KC, Dickey DT, Muldoon LL, O’Neill BP, Peterson DR, Pollock B, Soussain C, Smith Q, Tyson RM, Neuwelt EA. Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the Eleventh Annual Blood-Brain Barrier Consortium meeting. J Neurooncol. 2007;81:81–91. doi: 10.1007/s11060-006-9209-y. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Duarte AI, Proenca T, Oliveira CR, Santos MS, Rego AC. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes. 2006;55:2863–2870. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- Dube S, Fisher JW, Powell JS. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. J Biol Chem. 1988;263:17516–17521. [PubMed] [Google Scholar]
- Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S, Jahn H, Degner D, Ritzen M, Mohr A, Wagner M, Schneider U, Bohn M, Huber M, Czernik A, Pollmacher T, Maier W, Siren AL, Klosterkotter J, Falkai P, Ruther E, Aldenhoff JB, Krampe H. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Gagnon RF, Francois M, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Janicke RU, Porter AG, Belka C, Gregor M, Schulze-Osthoff K, Wesselborg S. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19:4563–4573. doi: 10.1038/sj.onc.1203824. [DOI] [PubMed] [Google Scholar]
- Erslev AJ. In vitro production of erythropoietin by kidneys perfused with a serum-free solution. Blood. 1974;44:77–85. [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett. 2006;399:91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Eto N, Wada T, Inagi R, Takano H, Shimizu A, Kato H, Kurihara H, Kawachi H, Shankland SJ, Fujita T, Nangaku M. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int. 2007;72:455–463. doi: 10.1038/sj.ki.5002311. [DOI] [PubMed] [Google Scholar]
- Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69:117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenjves ES, Ochoa MS, Cabrera O, Mendez AJ, Kenyon NS, Inverardi L, Ricordi C. Human, nonhuman primate, and rat pancreatic islets express erythropoietin receptors. Transplantation. 2003;75:1356–1360. doi: 10.1097/01.TP.0000062862.88375.BD. [DOI] [PubMed] [Google Scholar]
- Ferrario M, Massa M, Rosti V, Campanelli R, Ferlini M, Marinoni B, De Ferrari GM, Meli V, De Amici M, Repetto A, Verri A, Bramucci E, Tavazzi L. Early haemoglobin-independent increase of plasma erythropoietin levels in patients with acute myocardial infarction. Eur Heart J. 2007;28:1805–1813. doi: 10.1093/eurheartj/ehm065. [DOI] [PubMed] [Google Scholar]
- Ferri C, Giuggioli D, Sebastiani M, Colaci M. Treatment of severe scleroderma skin ulcers with recombinant human erythropoietin. Clin Exp Dermatol. 2007;32:287–290. doi: 10.1111/j.1365-2230.2007.02363.x. [DOI] [PubMed] [Google Scholar]
- Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- Fliser D, Haller H. Erythropoietin and treatment of non-anemic conditions--cardiovascular protection. Semin Hematol. 2007;44:212–217. doi: 10.1053/j.seminhematol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Foller M, Kasinathan RS, Koka S, Huber SM, Schuler B, Vogel J, Gassmann M, Lang F. Enhanced susceptibility to suicidal death of erythrocytes from transgenic mice overexpressing erythropoietin. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1127–1134. doi: 10.1152/ajpregu.00110.2007. [DOI] [PubMed] [Google Scholar]
- Frietsch T, Maurer MH, Vogel J, Gassmann M, Kuschinsky W, Waschke KF. Reduced cerebral blood flow but elevated cerebral glucose metabolic rate in erythropoietin overexpressing transgenic mice with excessive erythrocytosis. J Cereb Blood Flow Metab. 2007;27:469–476. doi: 10.1038/sj.jcbfm.9600360. [DOI] [PubMed] [Google Scholar]
- Fu Q, Van Eyk JE. Proteomics and heart disease: identifying biomarkers of clinical utility. Expert Rev Proteomics. 2006;3:237–249. doi: 10.1586/14789450.3.2.237. [DOI] [PubMed] [Google Scholar]
- Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ. Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol. 2007;293:H60–68. doi: 10.1152/ajpheart.00227.2007. [DOI] [PubMed] [Google Scholar]
- Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- Gerozissis K. Brain insulin: regulation, mechanisms of action and functions. Cell Mol Neurobiol. 2003;23:1–25. doi: 10.1023/A:1022598900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibelli C. Uber den wert des serums anamisch gemachten tiere bei der regeneration des blutes. Arch Exp Pathol Pharmacol. 1911;65:284–302. [Google Scholar]
- Gleissner CA, Klingenberg R, Staritz P, Koch A, Ehlermann P, Wiggenhauser A, Dengler TJ. Role of erythropoietin in anemia after heart transplantation. Int J Cardiol. 2006;112:341–347. doi: 10.1016/j.ijcard.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Goldberg N, Lundin AP, Delano B, Friedman EA, Stein RA. Changes in left ventricular size, wall thickness, and function in anemic patients treated with recombinant human erythropoietin. Am Heart J. 1992;124:424–427. doi: 10.1016/0002-8703(92)90608-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Guo G, Bhat NR. Hypoxia/Reoxygenation Differentially Modulates NF-kappaB Activation and iNOS Expression in Astrocytes and Microglia. Antioxid Redox Signal. 2006;8:911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Rabbani ZN, Arcasoy MO, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Blackwell KL. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther. 2006;5:356–361. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16:230–236. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K, Gross B, Simri W, Rubinchik I, Cohen H, Jacobi J, Shasha SM, Kristal B. The presence of erythropoietin receptors in the human peripheral nervous system. Clin Nephrol. 2004;61:127–129. doi: 10.5414/cnp61127. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- Heidbreder M, Frohlich F, Johren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3alpha mRNA expression. Faseb J. 2003;17:1541–1543. doi: 10.1096/fj.02-0963fje. [DOI] [PubMed] [Google Scholar]
- Henderson J. Ernest Starling and ‘Hormones’: an historical commentary. J Endocrinol. 2005;184:5–10. doi: 10.1677/joe.1.06000. [DOI] [PubMed] [Google Scholar]
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24:4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- Henry DH, Bowers P, Romano MT, Provenzano R. Epoetin alfa. Clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004;164:262–276. doi: 10.1001/archinte.164.3.262. [DOI] [PubMed] [Google Scholar]
- Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98:834–841. doi: 10.1182/blood.v98.3.834. [DOI] [PubMed] [Google Scholar]
- Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P, Tschopp J. The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci. 1997;22:155–156. doi: 10.1016/s0968-0004(97)01043-8. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55:1046–1052. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2- dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyoda K, Hosoi T, Horie N, Okuma Y, Ozawa K, Nomura Y. PI3K-Akt inactivation induced CHOP expression in endoplasmic reticulum-stressed cells. Biochem Biophys Res Commun. 2006;340:286–290. doi: 10.1016/j.bbrc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med. 2006;3:e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- Ikeda E. Cellular response to tissue hypoxia and its involvement in disease progression. Pathol Int. 2005;55:603–610. doi: 10.1111/j.1440-1827.2005.01877.x. [DOI] [PubMed] [Google Scholar]
- Imai N, Kawamura A, Higuchi M, Oh-eda M, Orita T, Kawaguchi T, Ochi N. Physicochemical and biological comparison of recombinant human erythropoietin with human urinary erythropoietin. J Biochem (Tokyo) 1990;107:352–359. doi: 10.1093/oxfordjournals.jbchem.a123050. [DOI] [PubMed] [Google Scholar]
- Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95:2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Ananieva O, Brown ML, Chang Y. Growth, differentiation, and malignant transformation of pre-B cells mediated by inducible activation of v-Abl oncogene. J Immunol. 2006;176:6831–6838. doi: 10.4049/jimmunol.176.11.6831. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Marsden I, Chen X, Liao X. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998;37:6179–6187. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- Joyeux-Faure M. Cellular protection by erythropoietin: new therapeutic implications? J Pharmacol Exp Ther. 2007;323:759–762. doi: 10.1124/jpet.107.127357. [DOI] [PubMed] [Google Scholar]
- Kaiser K, Texier A, Ferrandiz J, Buguet A, Meiller A, Latour C, Peyron F, Cespuglio R, Picot S. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- Kanbay M, Akcay A, Delibasi T, Uz B, Kaya A, Koca C, Turgut F, Bavbek N, Uz E, Duranay M, Yigitoglu R. Comparison of effects of darbepoetin alfa and epoetin alfa on serum endothelin level and blood pressure. Adv Ther. 2007;24:346–352. doi: 10.1007/BF02849903. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. Faseb J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Keogh CL, Yu SP, Wei L. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. J Pharmacol Exp Ther. 2007;322:521–528. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26:90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes. 2005;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kokhaei P, Abdalla AO, Hansson L, Mikaelsson E, Kubbies M, Haselbeck A, Jernberg-Wiklund H, Mellstedt H, Osterborg A. Expression of erythropoietin receptor and in vitro functional effects of epoetins in B-cell malignancies. Clin Cancer Res. 2007;13:3536–3544. doi: 10.1158/1078-0432.CCR-06-2828. [DOI] [PubMed] [Google Scholar]
- Koshimura K, Murakami Y, Sohmiya M, Tanaka J, Kato Y. Effects of erythropoietin on neuronal activity. J Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Kratzsch J, Knerr I, Galler A, Kapellen T, Raile K, Korner A, Thiery J, Dotsch J, Kiess W. Metabolic decompensation in children with type 1 diabetes mellitus associated with increased serum levels of the soluble leptin receptor. Eur J Endocrinol. 2006;155:609–614. doi: 10.1530/eje.1.02261. [DOI] [PubMed] [Google Scholar]
- Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. 2001;249:225–235. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- Lacombe C, Da Silva JL, Bruneval P, Casadevall N, Camilleri JP, Bariety J, Tambourin P, Varet B. Erythropoietin: sites of synthesis and regulation of secretion. Am J Kidney Dis. 1991;18:14–19. [PubMed] [Google Scholar]
- Lai SY, Grandis JR. Understanding the presence and function of erythropoietin receptors on cancer cells. J Clin Oncol. 2006;24:4675–4676. doi: 10.1200/JCO.2006.08.1190. [DOI] [PubMed] [Google Scholar]
- Larson ET, Eilers B, Menon S, Reiter D, Ortmann A, Young MJ, Lawrence CM. A winged-helix protein from sulfolobus turreted icosahedral virus points toward stabilizing disulfide bonds in the intracellular proteins of a hyperthermophilic virus. Virology. 2007 doi: 10.1016/j.virol.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Lehman DM, Hunt KJ, Leach RJ, Hamlington J, Arya R, Abboud HE, Duggirala R, Blangero J, Goring HH, Stern MP. Haplotypes of Transcription Factor 7-Like 2 (TCF7L2) Gene and Its Upstream Region Are Associated With Type 2 Diabetes and Age of Onset in Mexican Americans. Diabetes. 2007;56:389–393. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu X, Vercammen E. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006;4:2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004a;13:265–289. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004b;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006a;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006c;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007a;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007b;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z, et al. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Ling PR, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metabolism. 2003;52:868–874. doi: 10.1016/s0026-0495(03)00057-x. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Liu C, Shen K, Liu Z, Noguchi CT. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–32400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Chin K, Noguchi CT. Tissue specific expression of human erythropoietin receptor in transgenic mice. Dev Biol. 1994;166:159–169. doi: 10.1006/dbio.1994.1304. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Lykissas MG, Korompilias AV, Vekris MD, Mitsionis GI, Sakellariou E, Beris AE. The role of erythropoietin in central and peripheral nerve injury. Clin Neurol Neurosurg. 2007;109:639–644. doi: 10.1016/j.clineuro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Maciejewski ML, Maynard C. Diabetes-related utilization and costs for inpatient and outpatient services in the Veterans Administration. Diabetes Care. 2004;27 Suppl 2:B69–73. doi: 10.2337/diacare.27.suppl_2.b69. [DOI] [PubMed] [Google Scholar]
- Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16:633–644. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- Maiese K. Exciting news from the messenger. Curr Neurovasc Res. 2007;4:152. doi: 10.2174/156720207781387196. [DOI] [PubMed] [Google Scholar]
- Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15:440–449. doi: 10.1038/jcbfm.1995.55. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Kang J. Transformation into treatment: Novel therapeutics that begin within the cell. In: Maiese K, editor. Neuronal and Vascular Plasticity: Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. Kluwer Academic Publishers; Norwell, MA: 2003. pp. 1–26. [Google Scholar]
- Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005a;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanisitic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007a;14:1689–1699. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007b;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin and cancer. JAMA. 2005b;293:1858–1859. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005c;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007c;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem. 1997;68:710–714. doi: 10.1046/j.1471-4159.1997.68020710.x. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62:257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000a;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000b;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–123. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- Mala H, Alsina CG, Madsen KS, Sibbesen EC, Stick H, Mogensen J. Erythropoietin improves place learning in an 8-arm radial maze in fimbria-fornix transected rats. Neural Plast. 2005;12:329–340. doi: 10.1155/NP.2005.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Martin D, Salinas M, Lopez-Valdaliso R, Serrano E, Recuero M, Cuadrado A. Effect of the Alzheimer amyloid fragment Abeta(25–35) on Akt/PKB kinase and survival of PC12 cells. J Neurochem. 2001;78:1000–1008. doi: 10.1046/j.1471-4159.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Rodriguez-Millan E, Gonzalez-De Vicente E, Reina M, Vilaro S, Fabre M. Erythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeability. Eur J Neurosci. 2003;18:2538–2544. doi: 10.1046/j.1460-9568.2003.02987.x. [DOI] [PubMed] [Google Scholar]
- Mason-Garcia M, Beckman BS, Brookins JW, Powell JS, Lanham W, Blaisdell S, Keay L, Li SC, Fisher JW. Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int. 1990;38:969–975. doi: 10.1038/ki.1990.299. [DOI] [PubMed] [Google Scholar]
- Masuda S, Kobayashi T, Chikuma M, Nagao M, Sasaki R. The oviduct produces erythropoietin in an estrogen- and oxygen- dependent manner. Am J Physiol Endocrinol Metab. 2000;278:E1038–1044. doi: 10.1152/ajpendo.2000.278.6.E1038. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73:2037–2046. [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McCormick WC, Hardy J, Kukull WA, Bowen JD, Teri L, Zitzer S, Larson EB. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49:1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- McLeod M, Hong M, Mukhida K, Sadi D, Ulalia R, Mendez I. Erythropoietin and GDNF enhance ventral mesencephalic fiber outgrowth and capillary proliferation following neural transplantation in a rodent model of Parkinson’s disease. Eur J Neurosci. 2006;24:361–370. doi: 10.1111/j.1460-9568.2006.04919.x. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584–593. doi: 10.1002/jnr.20755. [DOI] [PubMed] [Google Scholar]
- Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Mendiondo MS, Kryscio RJ, Schmitt FA. Models of progression in AD: predicting disability and costs. Neurology. 2001;57:943–944. doi: 10.1212/wnl.57.6.943. [DOI] [PubMed] [Google Scholar]
- Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006a;107:2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006b;116:683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Hokayem JA, Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at p10. Epilepsia. 2007;48:175–181. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Mocini D, Leone T, Tubaro M, Santini M, Penco M. Structure, production and function of erythropoietin: implications for therapeutical use in cardiovascular disease. Curr Med Chem. 2007;14:2278–2287. doi: 10.2174/092986707781696627. [DOI] [PubMed] [Google Scholar]
- Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with Type 2 diabetes mellitus. Diabet Med. 2006;23:839–844. doi: 10.1111/j.1464-5491.2006.01893.x. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Montero M, Poulsen FR, Noraberg J, Kirkeby A, van Beek J, Leist M, Zimmer J. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp Neurol. 2007;204:106–117. doi: 10.1016/j.expneurol.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Mujais SK, Beru N, Pullman TN, Goldwasser E. Erythropoietin is produced by tubular cells of the rat kidney. Cell Biochem Biophys. 1999;30:153–166. doi: 10.1007/BF02737888. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. The erythropoietin receptor. Semin Oncol. 2001;28:19–23. doi: 10.1016/s0093-7754(01)90208-8. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Munoz-Fontela C, Marcos-Villar L, Gallego P, Arroyo J, Da Costa M, Pomeranz KM, Lam EW, Rivas C. Latent protein LANA2 from Kaposi’s sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J Virol. 2007;81:1511–1516. doi: 10.1128/JVI.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of glycogen synthase kinase (GSK) 3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007 doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- Nadam J, Navarro F, Sanchez P, Moulin C, Georges B, Laglaine A, Pequignot JM, Morales A, Ryvlin P, Bezin L. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, Era T, Kanakura Y. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J Biol Chem. 2004;279:55578–55586. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiuchi S, Kagaya Y, Ohta J, Shiba N, Sugi M, Oikawa M, Kunii H, Yamao H, Komatsu N, Yui M, Tada H, Sakuma M, Watanabe J, Ichihara T, Shirato K. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S, Hartley C, Molineux G, Knox SJ. Darbepoietin alfa potentiates the efficacy of radiation therapy in mice with corrected or uncorrected anemia. Cancer Res. 2005;65:284–290. [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Obara N, Imagawa S, Nakano Y, Suzuki N, Yamamoto M, Nagasawa T. Suppression of erythropoietin gene expression by cadmium depends on inhibition of HIF-1, not stimulation of GATA-2. Arch Toxicol. 2003;77:267–273. doi: 10.1007/s00204-003-0444-0. [DOI] [PubMed] [Google Scholar]
- Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- Okutan O, Solaroglu I, Beskonakli E, Taskin Y. Recombinant human erythropoietin decreases myeloperoxidase and caspase-3 activity and improves early functional results after spinal cord injury in rats. J Clin Neurosci. 2007;14:364–368. doi: 10.1016/j.jocn.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Olea FD, Vera Janavel G, De Lorenzi A, Cuniberti L, Yannarelli G, Cabeza Meckert P, Cearras M, Laguens R, Crottogini A. High-dose erythropoietin has no long-term protective effects in sheep with reperfused myocardial infarction. J Cardiovasc Pharmacol. 2006;47:736–741. doi: 10.1097/01.fjc.0000211766.59636.0d. [DOI] [PubMed] [Google Scholar]
- Olsen NV. Central nervous system frontiers for the use of erythropoietin. Clin Infect Dis. 2003;37 Suppl 4:S323–330. doi: 10.1086/376912. [DOI] [PubMed] [Google Scholar]
- Orive G, De Castro M, Ponce S, Hernandez RM, Gascon AR, Bosch M, Alberch J, Pedraz JL. Long-term expression of erythropoietin from myoblasts immobilized in biocompatible and neovascularized microcapsules. Mol Ther. 2005;12:283–289. doi: 10.1016/j.ymthe.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pacary E, Petit E, Bernaudin M. Erythropoietin, a cytoprotective and regenerative cytokine, and the hypoxic brain. Neurodegener Dis. 2006;3:87–93. doi: 10.1159/000092098. [DOI] [PubMed] [Google Scholar]
- Palazzuoli A, Silverberg D, Iovine F, Capobianco S, Giannotti G, Calabro A, Campagna SM, Nuti R. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152:1096, e1099–1015. doi: 10.1016/j.ahj.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Palazzuoli A, Silverberg DS, Iovine F, Calabro A, Campagna MS, Gallotta M, Nuti R. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007;154:645, e649–615. doi: 10.1016/j.ahj.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Kim J, Riel RU, Pascal LS, Thompson RB, Petrofski JA, Matsumoto A, Stamler JS, Koch WJ. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem. 2004;279:20655–20662. doi: 10.1074/jbc.M314099200. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I. Nobel Lecture (Physiology or Medicine) Amsterdam: Elsevier; 1904. The Physiology of Digestion; pp. 141–155. [Google Scholar]
- Pearl R. The rate of living. University of London Press; London: 1928. [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce S, Orive G, Hernandez RM, Gascon AR, Canals JM, Munoz MT, Pedraz JL. In vivo evaluation of EPO-secreting cells immobilized in different alginate-PLL microcapsules. J Control Release. 2006;116:28–34. doi: 10.1016/j.jconrel.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pregi N, Vittori D, Perez G, Leiros CP, Nesse A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim Biophys Acta. 2006;1763:238–246. doi: 10.1016/j.bbamcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Qin W, Peng Y, Ksiezak-Reding H, Ho L, Stetka B, Lovati E, Pasinetti GM. Inhibition of cyclooxygenase as potential novel therapeutic strategy in N141I presenilin-2 familial Alzheimer’s disease. Mol Psychiatry. 2006;11:172–181. doi: 10.1038/sj.mp.4001773. [DOI] [PubMed] [Google Scholar]
- Quinn L. Type 2 diabetes: epidemiology, pathophysiology, and diagnosis. Nurs Clin North Am. 2001;36:175–192. v. [PubMed] [Google Scholar]
- Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006;55:1022–1028. doi: 10.2337/diabetes.55.04.06.db05-0865. [DOI] [PubMed] [Google Scholar]
- Rades D, Golke H, Schild SE, Kilic E. The Impact of Tumor Expression of Erythropoietin Receptors and Erythropoietin on Clinical Outcome of Esophageal Cancer Patients Treated with Chemoradiation. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Ravid O, Shams I, Ben Califa N, Nevo E, Avivi A, Neumann D. An extracellular region of the erythropoietin receptor of the subterranean blind mole rat Spalax enhances receptor maturation. Proc Natl Acad Sci U S A. 2007;104:14360–14365. doi: 10.1073/pnas.0706777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MK, Vasir JK, Hegde GV, Joshi SS, Labhasetwar V. Erythropoietin induces excessive neointima formation: a study in a rat carotid artery model of vascular injury. J Cardiovasc Pharmacol Ther. 2007;12:237–247. doi: 10.1177/1074248406297326. [DOI] [PubMed] [Google Scholar]
- Regulska M, Leskiewicz M, Budziszewska B, Kutner A, Jantas D, Basta-Kaim A, Kubera M, Jaworska-Feil L, Lason W. Inhibitory effects of 1,25-dihydroxyvitamin D(3) and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol Rep. 2007;59:393–401. [PubMed] [Google Scholar]
- Reich NC. STAT dynamics. Cytokine Growth Factor Rev. 2007;18:511–518. doi: 10.1016/j.cytogfr.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- Reissmann K. Studies on the mechanism of erythropoietin stimulation in parabiotic rats during hypoxia. Blood. 1950;5:347–380. [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Roberts E, Jr, Chih CP. The influence of age of pH regulation in hippocampal slices before, during, and after anoxia. J Cereb Blood Flow Metab. 1997;17:560–566. doi: 10.1097/00004647-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytomaa M, Lehmann K, Downward J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- Sae-Ung N, Matsushima T, Choi I, Abe Y, Winichagoon P, Fucharoen S, Nawata H, Muta K. Role of NF-kappa B in regulation of apoptosis of erythroid progenitor cells. Eur J Haematol. 2005;74:315–323. doi: 10.1111/j.1600-0609.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–109. [PubMed] [Google Scholar]
- Sandor G. Uber die blutbidende wirkung des serums von tieren, die in verdunnter luft gehalten wuren. Z Gesante Exp Med. 1932;82:633–646. [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Sanz O, Acarin L, Gonzalez B, Castellano B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J Neurosci Res. 2002;67:772–780. doi: 10.1002/jnr.10140. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sasaki Y, Kanno K, Hidaka H. Disorganization by calcium antagonists of actin microfilament in aortic smooth muscle cells. Am J Physiol. 1987;253:C71–78. doi: 10.1152/ajpcell.1987.253.1.C71. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana P, Menon MP, Bogacheva O, Bogachev O, Niss K, Kapelle WS, Houde E, Fang J, Wojchowski DM. Erythropoietin modulation of podocalyxin and a proposed erythroblast niche. Blood. 2007;110:509–518. doi: 10.1182/blood-2006-11-056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeding M, Neumann UP, Boas-Knoop S, Spinelli A, Neuhaus P. Erythropoietin reduces ischemia-reperfusion injury in the rat liver. Eur Surg Res. 2007;39:189–197. doi: 10.1159/000101009. [DOI] [PubMed] [Google Scholar]
- Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- Schumann C, Triantafilou K, Krueger S, Hombach V, Triantafilou M, Becher G, Lepper PM. Detection of erythropoietin in exhaled breath condensate of nonhypoxic subjects using a multiplex bead array. Mediators Inflamm. 2006;2006:18061. doi: 10.1155/MI/2006/18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, Narisu N, Duren WL, Chines PS, Stringham HM, Erdos MR, Valle TT, Tuomilehto J, Bergman RN, Mohlke KL, Collins FS, Boehnke M. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006;55:2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- Segura J, Pascual JA, Gutierrez-Gallego R. Procedures for monitoring recombinant erythropoietin and analogues in doping control. Anal Bioanal Chem. 2007;388:1521–1529. doi: 10.1007/s00216-007-1316-x. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Thiemermann C, Yaqoob MM. Mechanisms of disease: Cell death in acute renal failure and emerging evidence for a protective role of erythropoietin. Nat Clin Pract Nephrol. 2005;1:87–97. doi: 10.1038/ncpneph0042. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Yaqoob MM. Erythropoietin in experimental acute renal failure. Nephron Exp Nephrol. 2006;104:e83–88. doi: 10.1159/000094546. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol (Berl) 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Signore AP, Weng Z, Hastings T, Van Laar AD, Liang Q, Lee YJ, Chen J. Erythropoietin protects against 6-hydroxydopamine-induced dopaminergic cell death. J Neurochem. 2006;96:428–443. doi: 10.1111/j.1471-4159.2005.03587.x. [DOI] [PubMed] [Google Scholar]
- Sigounas G, Sallah S, Sigounas VY. Erythropoietin modulates the anticancer activity of chemotherapeutic drugs in a murine lung cancer model. Cancer Lett. 2004;214:171–179. doi: 10.1016/j.canlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, Tchebiner JZ, Sheps D, Keren G, Schwartz D, Baruch R, Yachnin T, Shaked M, Schwartz I, Steinbruch S, Iaina A. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18:141–146. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006;26:296–306. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, Tsakiris D. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21:330–336. doi: 10.1093/ndt/gfi177. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling E. Croonian Lecture: On the chemical correlation of the functions of the body II. Lancet. 1905;2:423–425. [Google Scholar]
- Stegh AH, Barnhart BC, Volkland J, Algeciras-Schimnich A, Ke N, Reed JC, Peter ME. Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein. J Biol Chem. 2002;277:4351–4360. doi: 10.1074/jbc.M108947200. [DOI] [PubMed] [Google Scholar]
- Stolze I, Berchner-Pfannschmidt U, Freitag P, Wotzlaw C, Rossler J, Frede S, Acker H, Fandrey J. Hypoxia-inducible erythropoietin gene expression in human neuroblastoma cells. Blood. 2002;100:2623–2628. doi: 10.1182/blood-2001-12-0169. [DOI] [PubMed] [Google Scholar]
- Sun XM, Cohen GM. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J Biol Chem. 1994;269:14857–14860. [PubMed] [Google Scholar]
- Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, Vagenakis A, Zoumbos N. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006;85:79–85. doi: 10.1007/s00277-005-1102-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res. 1999;249:291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp Neurol. 2003;180:144–155. doi: 10.1016/s0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- Tata JR. One hundred years of hormones. EMBO Rep. 2005;6:490–496. doi: 10.1038/sj.embor.7400444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramo K, Kari MA, Eronen M, Markkanen H, Hiilesmaa V. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia. 2004;47:1695–1703. doi: 10.1007/s00125-004-1515-3. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Cooper ME, Tsalamandris C, MacIsaac R, Jerums G. Anemia with impaired erythropoietin response in diabetic patients. Arch Intern Med. 2005;165:466–469. doi: 10.1001/archinte.165.4.466. [DOI] [PubMed] [Google Scholar]
- Toma C, Letts DP, Tanabe M, Gorcsan J, 3rd, Counihan PJ. Positive effect of darbepoetin on peri-infarction remodeling in a porcine model of myocardial ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:130–136. doi: 10.1016/j.yjmcc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Torriglia A, Chaudun E, Courtois Y, Counis MF. On the use of Zn2+ to discriminate endonucleases activated during apoptosis. Biochimie. 1997;79:435–438. doi: 10.1016/s0300-9084(97)86153-6. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Itai T, Arakawa T, Aoki KH, Yamaguchi H. Stabilization of human recombinant erythropoietin through interactions with the highly branched N-glycans. J Biochem (Tokyo) 2000;128:731–737. doi: 10.1093/oxfordjournals.jbchem.a022809. [DOI] [PubMed] [Google Scholar]
- Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma. 2007;16:567–571. doi: 10.1097/IJG.0b013e318156a556. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Goto M, Murakami A, Akai K, Ueda M, Kawanishi G, Takahashi N, Sasaki R, Chiba H, Ishihara H, et al. Comparative structural study of N-linked oligosaccharides of urinary and recombinant erythropoietins. Biochemistry. 1988;27:5646–5654. doi: 10.1021/bi00415a038. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R. The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem. 1990;188:405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- Uchida E, Morimoto K, Kawasaki N, Izaki Y, Abdu Said A, Hayakawa T. Effect of active oxygen radicals on protein and carbohydrate moieties of recombinant human erythropoietin. Free Radic Res. 1997;27:311–323. doi: 10.3109/10715769709065769. [DOI] [PubMed] [Google Scholar]
- Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Um M, Lodish HF. Antiapoptotic Effects of Erythropoietin in Differentiated Neuroblastoma SH-SY5Y Cells Require Activation of Both the STAT5 and AKT Signaling Pathways. J Biol Chem. 2006;281:5648–5656. doi: 10.1074/jbc.M510943200. [DOI] [PubMed] [Google Scholar]
- Vairano M, Dello Russo C, Pozzoli G, Battaglia A, Scambia G, Tringali G, Aloe-Spiriti MA, Preziosi P, Navarra P. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16:584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Lipsic E, Henning RH, de Boer RA, Suurmeijer AJ, van Veldhuisen DJ, van Gilst WH. Erythropoietin improves left ventricular function and coronary flow in an experimental model of ischemia-reperfusion injury. Eur J Heart Fail. 2004a;6:853–859. doi: 10.1016/j.ejheart.2004.03.012. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Voors AA, Lipsic E, Smilde TD, van Gilst WH, van Veldhuisen DJ. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. 2004b;44:63–67. doi: 10.1016/j.jacc.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ, Hobson RW, 2nd, Duran WN. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, Remy C, Segebarth C, Payen JF. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999a;47:661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999b;246:290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp Neurol. 1999a;155:79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J Neurobiol. 1999b;40:171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Wang FF, Kung CK, Goldwasser E. Some chemical properties of human erythropoietin. Endocrinology. 1985;116:2286–2292. doi: 10.1210/endo-116-6-2286. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, Koster J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zurcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11:2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, Macdougald OA. Wnt10b Inhibits Obesity in ob/ob and Agouti Mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Wu H, Ren B, Zhu J, Dong G, Xu B, Wang C, Zheng X, Jing H. Pretreatment with recombined human erythropoietin attenuates ischemia-reperfusion-induced lung injury in rats. Eur J Cardiothorac Surg. 2006;29:902–907. doi: 10.1016/j.ejcts.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007a;12:1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun S, Liu R. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur J Pharmacol. 2007b;564:47–56. doi: 10.1016/j.ejphar.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Xu B, Dong GH, Liu H, Wang YQ, Wu HW, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35:161–168. [PubMed] [Google Scholar]
- Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- Yamaji R, Okada T, Moriya M, Naito M, Tsuruo T, Miyatake K, Nakano Y. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem. 1996;239:494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K, Minamoto A, Inaba T. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050:15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9:111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Yui R, Matsuura ET. Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat Res. 2006;594:155–161. doi: 10.1016/j.mrfmmm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang F, Zhang Y, Gao X, Li C, Ma W, Cao K. Erythropoietin enhances the angiogenic potency of autologous bone marrow stromal cells in a rat model of myocardial infarction. Cardiology. 2007a;108:228–236. doi: 10.1159/000096803. [DOI] [PubMed] [Google Scholar]
- Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol. 2007b;292:H2573–2581. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wagner F, Frank SJ, Kraft AS. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony- stimulating factor receptor beta c chain. J Biol Chem. 1995;270:13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Yao H, Deng L, Cheng Y, Zhou X. Promotion of neurite outgrowth and protective effect of erythropoietin on the retinal neurons of rats. Graefes Arch Clin Exp Ophthalmol. 2007;245:1859–1867. doi: 10.1007/s00417-007-0671-9. [DOI] [PubMed] [Google Scholar]


