Abstract
Previous research has shown that parent mental health is associated with asthma morbidity in children. However, the biological pathways explaining these relationships are not known. The present study tested whether parent psychological characteristics could predict longitudinal changes in inflammatory markers in children with asthma and a comparison group of healthy children.
For this, 33 healthy children (17m/16f; 13.5yrs) and 50 children with asthma (37m/13f; 13.3yrs) were assessed at two time points on average 208 days apart. Parent depression (CESD) and perceived stress (PSS) were assessed at baseline, child depression (CDI) and anxiety (RCMAS) at follow-up. Asthma-relevant inflammatory markers eosinophil cationic protein (ECP) and stimulated IL-4 production were measured at baseline and at follow-up.
Hierarchical regression analyses controlling for asthma severity and medication use revealed that higher levels of parental perceived stress at baseline were associated with greater increases over time in children’s IL-4 production (β=.29, p=.019) as well as ECP release (β =.27, p=.004). Additionally, higher levels of parental depression at baseline were associated with increases in ECP over time (β=.19, p=.046). There was no evidence that these associations were mediated by child depression or anxiety.
These results demonstrate that parental stress and depression at baseline predict increases in children’s inflammatory profiles over a six month period. This pattern appeared in both children with asthma and healthy children, and were not due to effects on child psychological states. These changes in inflammatory makers may represent one biological mechanism underlying the association between parental distress and child asthma morbidity.
INTRODUCTION
Asthma is a chronic condition that affects a large number of children. In the year 2000, more than five percent of all children in the United States below 18 years were reported to have had an asthma attack and, in 1999, asthma was estimated to be “responsible for 2 million emergency department visits, 478,000 hospitalizations with asthma as a primary diagnosis, and 4,426 deaths” in the US (NHLBI, 2002).
Research has documented that psychosocial factors play an important role in childhood asthma. Two of the most prominent psychosocial factors that have been linked to childhood asthma are parental stress and depression. For example, parental life stress has been linked to decreases in functional status and increases in hospitalizations (Weil et al., 1999), as well as increases in health care service utilization and symptoms (Shalowitz et al., 2001) in children with asthma. Furthermore, in a prospective study, stressful life events predicted an increased likelihood of asthma attacks in the weeks following an event (Sandberg et al., 2004; Sandberg et al., 2000). Similar findings have been reported for parental depression. Maternal depression has been linked to health care service utilization and symptoms (Shalowitz et al., 2001), hospitalizations (Brown et al., 2006; Weil et al., 1999), and emergency department visits (Bartlett et al., 2001) in children with asthma. Several studies further suggest that maternal depressive symptoms prospectively predicts asthma morbidity (Bartlett et al., 2004; Klinnert et al., 2001).
In summary, these studies consistently demonstrate that parental stress as well as parental depression play an important role in childhood asthma morbidity. Nevertheless, the question that remains is how the psychosocial experiences of one person (parent) get ‘under the skin’ of another person (child) to impact physical health. The present study takes one step toward answering this question by asking whether parent psychological states are linked with asthma-relevant inflammatory processes in children. If so, this would begin to provide researchers with plausible mechanistic models for the clinical research linking parent psychosocial traits to childhood asthma morbidity.
The primary goal of this study was to test the ability of parental depression and stress to predict children’s inflammatory profiles. Both parental depression and stress were included because they are the psychosocial variables most consistently linked to outcome measures in childhood asthma. However, perceived stress and depression are different constructs. Perceived stress relates to the extend a person appraises a situation as stressful, thus involving a cognitive appraisal process, while depression refers to changes in mood, which can be temporary or longer lasting and interfere with daily life (i.e., mood disorder). Therefore we investigated perceived stress and depression separately given the possibility that they might each operate via different pathways. In order for these associations to be meaningful, it is important to document whether parental psychological states can predict change over time in inflammatory markers relevant to asthma; hence, in the present study we measured inflammatory markers in children twice over a 6 month period. We were further interested whether these relationships would be specific to children with asthma, and therefore included a healthy comparison group of children. Lastly, we sought to address the possibility that one reason why parental stress and depression would be associated with child immune markers is because parents’ mood influences children’s mood, which in turns influences child’s immunological processes. We therefore tested whether child depression and anxiety represent one pathway by which parent psychological states get translated into child biological profiles.
STUDY DESIGN AND METHODS
Subjects
A total of 83 children and adolescents1 along with one of their parents were recruited from the Vancouver, BC community through advertisements in newspapers, magazines, and physicians’ offices. Children had to be between 9 and 18 years of age and English speaking. 50 children were physician-diagnosed with asthma according to National Heart, Lung, and Blood Institute (NHLBI) guidelines (NHLBI, 1997; NHLBI, 2002) and 33 children were medically healthy. Both groups of children had to be free of upper-respiratory illness, such as common cold, bronchitis, or pharyngitis, in the four weeks prior to each of the two visits at our laboratory, otherwise they were rescheduled. Upper-respiratory illness was an exclusion criterion because as an infection caused by viral or bacterial exposure could have influenced the immune markers of interest in this study. Having a psychiatric illness and having any other chronic medical illnesses besides asthma were exclusion criteria as well.
Experimental Protocol
Upon arrival at our research center, the study procedure was explained in detail and written consent was obtained from the accompanying parent, and assent from the child. A local anesthetic cream (EMLA) was applied to the child’s arm in preparation for the blood draw. The accompanying parent was asked to fill out the Perceived Stress Scale (PSS) and the Center for Epidemiological Studies Depression Scale (CES-D). Meanwhile, blood for stimulated interleukin-4 (IL-4) and eosinophil cationic protein (ECP) determination was obtained from the child by antecubital venipuncture.
Six months later, children were asked to come to the laboratory a second time (visits 2). Children filled out the Child Depression Inventory (CDI) and the Revised Children’s Manifest Anxiety Scale (RCMAS) and another blood sample was obtained.
Parent Questionnaires
Perceived Stress
The Perceived Stress Scale (PSS; 10-item version) is a standardized self-reported questionnaire of globally perceived stress (Cohen et al., 1983; Cohen and Williamson, 1988). The questions ask about how stressful, overwhelming, and uncontrollable a person has found his/her life during the last month (e.g., “In the last month, how often have you felt that you were unable to control the important things in your life?”). Items are answered on a 0=never to 4=very scale and are summed, with higher scores indicating greater perceived stress. The PSS has been demonstrated to have adequate reliability (Cronbach’s alpha=.88) and validity (Cohen and Williamson, 1988).
Depressive Symptoms
Depressive symptoms were assessed using the Center for Epidemiological Studies Depression (CES-D) Scale (Radloff, 1977). The CES-D has been widely used in clinical trials in both general and psychiatric populations and has excellent psychometric characteristics (e.g., Cronbach’s alpha=.85). The 20 symptom-related items of the CES-D ask about how often one experienced depressive cognitions, affect, and behaviors during the past one week (e.g., feeling depressed and lonely, having poor appetite and sleep). Scores range from 0=none of the time or rarely (less than 1 day) to 3=most or all of the time (5–7 days). Scores are summed, with higher scores reflecting greater depressive symptoms. Scores of 16 reflect clinically significant levels of depressive symptoms.
Children Questionnaires
Depressive Symptoms
The Child Depression Inventory (CDI) is a self-report measure with high reliability (Cronbach’s alpha=.71–.89) and validity developed to assess depressive symptoms in 7–17-year-old children and adolescents (Kovacs, 1981; Romano and Nelson, 1988). It consists of 27 items which cover depression-related feelings, thoughts, and behaviors during the past two weeks, such as sadness, suicidal ideation, sleep, and appetite disturbances. Each item is answered on a scale ranging from 0=once in a while to 2=all the time. Items are summed, with higher scores indicating greater depressive symptoms. A total score of 20 or higher is indicative of depressive symptoms in the clinical range (Kovacs, 1981).
Anxiety
To assess anxiety, the Revised Children’s Manifest Anxiety Scale (RCMAS) was used (Reynolds and Richmond, 1997a; Reynolds and Richmond, 1997b). The RCMAS is a widely-used 37-item self-report measure with excellent psychometric properties (e.g., Kuber-Richardson(KR)20=.85) that assesses the level and nature of anxiety symptoms in 6–19-year-old children and adolescents. For example, the subject has to decide whether in general, the sentences “I am afraid of a lot of things”, “Often I feel sick in the stomach”, or “I worry when I go to bed at night” are true or not true. The RCMAS consists of three anxiety subscales plus a lie subscale. A total score can be calculated by summing the three anxiety scales, with higher scores indicating higher anxiety.
Inflammatory Markers
Blood was taken from the child at both visits for assessment of stimulated interleukin-4 (IL-4) production and eosinophil cationic protein concentrations (ECP).
IL-4 is an important cytokine in asthma, as its release by T helper II (Th-2) cells induces immunoglobulin-E (IgE) production by B cells. IgE in turn binds to mast cells and causes them to degranulate. This process initiates an acute inflammatory cascade, including the release of histamines and leukotrines, which leads to the typical asthma symptoms of airway constriction and edema (Bacharier and Geha, 2000; Chung and Barnes, 1999). Importantly, in the present study, we decided to investigate stimulated IL-4 production for two reasons. Firstly, in vivo IL-4 levels are extremely low and difficult to measure reliably, although usually higher levels of serum IL-4 have been found in adults with (acute) asthma (Hashimoto et al., 1993; Lee et al., 2001; Matsumoto et al., 1994). Secondly, we were specifically interested in the sensitivity of the immune system, i.e., the magnitude of the immune response to a stimulus. Hence we chose to stimulate immune cells in vitro with a mitogen and chose an unspecific mitogen so that responses between children with asthma and healthy children could be compared. Other researchers have found that while average cytokine production in response to unspecific mitogens may not differ between groups (Nurse et al., 1997), these stimulation protocols are often useful because factors have been found to predict variability within groups in cytokine response (Chen et al., 2003).
While stimulated IL-4 production assesses the capacity of peripheral leukocytes to induce this inflammatory cascade, this inflammatory process eventually also involves the recruitment of eosinophils to the airways. Activated eosinophils release among other mediators ECP. ECP has been shown to have cytotoxic effects against respiratory epithelial cells and to stimulate mucous production and histamine release, thereby promoting airway inflammation and obstruction. ECP is therefore considered a marker of on-going inflammation (Koh et al., 2007).
Stimulated Interleukin-4 (IL-4) Production
Blood was drawn into Cell Preparation Tubes (CPTs; Becton Dickinson, Franklin Lakes, NJ) containing sodium heparin. After density-gradient centrifugation and three subsequent wash steps, isolated peripheral blood mononuclear cells (PBMCs) were resuspended in complete culture medium (RPMI 1640 with HEPES, L-Glutamine, 10% FCS, 1% penicillin-streptomycin; all reagents: Sigma-Aldrich Canada Ltd., Oakville, ON) in a concentration of 3 × 106 cells/ml. Cells were immediately incubated with phorbol 12- myristate 13-acetate (PMA; 25ng/ml f.c.) and ionomycin (1µg/ml f.c.) for 48 hours at 37°C, 5% CO2 in 6-well plates (Sarstedt, Newton, NC). After 48 hours, plates were centrifuged and the supernatants were frozen at −80°C until assayed.
IL-4 levels were determined by a commercially available high-sensitivity enzyme-linked immunosorbent assay (ELISAs, R&D System, Minneapolis, MN). Intra- and inter-assay variance was below 10%. Due to technical problems, measures of stimulated IL-4 production for both visit 1 and visit 2 were only available for a subgroup of 76 children consisting of 44 children with asthma and 32 healthy children.
Eosinophil Cationic Protein (ECP)
For measures of ECP concentrations, blood was drawn into serum tubes (SSTs; Becton Dickinson, Franklin Lakes, NJ) and incubated for 90 minutes at room temperature, during which activated eosinophils release ECP. The final measure of ECP concentrations thus combines ECP found already in the peripheral blood plus ECP released during incubation. After incubation, the blood was centrifuged and serum was frozen at −20°C until assayed. ECP concentrations were measured using the ImmunoCAP system (ImmunoCAP 100€ Phadia AB, Uppsala, Sweden). Briefly, anti-ECP antibodies coupled to ImmunoCAPs bind ECP in the sample and standards. Then enzyme-labeled anti-ECP antibodies are added, and the complex is incubated with a developing agent. Fluorescence is measured and ECP concentrations are calculated using the standard curve (reagents: Somagen Diagnostics, Edmonton, AB).
Control Variables
Medical variables potentially influencing inflammatory measures were included as control variables. This included asthma severity and asthma medication use.
Asthma severity was classified as mild intermittent asthma, mild persistent asthma, moderate asthma, and severe asthma, according to the procedure described by Bacharier et al. (2004), i.e., based on the NAEPP/EPR2 Guidelines and using the higher of symptom frequency and medication use.
Asthma medication was assessed by asking participants the number of times during the past two weeks they had used asthma medications. Asthma medications were divided into inhaled corticosteroids and β-agonists, and both the number of days a participant used inhaled corticosteroids and the number of days a participant used β-agonists were included as covariates.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Science Version 11.0.4 (SPSS Institute, Chicago, IL). IL-4 and ECP measures at each time point were z-transformed to account for any potential non-systematic variation due to laboratory procedures. Analyses followed a two-step approach:
Preliminary Analyses
(a) Group differences: Student’s T-tests and chi-square tests were computed to compare differences between children with asthma and healthy children with regard to demographic variables, psychological data (including parent psychological data) and inflammatory markers. (b) Pearson correlations were calculated to test associations between parent and child psychological data. (c) Multiple regressions were computed to test whether demographic variables (age, gender, age × gender interaction) were associated with any psychological or immune variables.
Hierarchical Regression Analyses
To test the primary study hypotheses about relationships between parent psychological variables and changes over time in child immunological parameters, hierarchical regression analyses were calculated. These analyses tested whether the relationship between parent psychological variables and change in child immune parameters differed by group status using recommended procedures for testing interaction effects in multiple regression analyses (Aiken and West, 1991). Visit 2 IL-4 production and ECP concentrations formed the dependent variable and all analyses controlled for visit 1 levels of IL-4 and ECP, respectively. In addition, all regression analyses controlled for asthma severity and medication (i.e., number of days over a period of two weeks participant took inhaled corticosteroids and beta-agonists). Regression analyses thus included visit 1 immune variables in step 1, severity and medication variables in step 2, followed by the main effect of group (asthma vs. healthy; step 3) and the main effect of parent psychological state (e.g., stress; step 4), and finally the interaction between group and parent perceived stress in step 5. Identical procedures were followed for testing the role of parent depression. Separate regressions were conducted predicting ECP changes over time and predicting changes in IL-4 production over time.
Subsequently, the hypothesis was tested that child psychological state would mediate some of the relationships between parent psychological state and child immune parameters. According to Baron and Kenny (1986), three requirements have to be met to test for mediation: a significant association of predictor and outcome variable, predictor and assumed mediator, and assumed mediator and outcome variable. Thus, first correlations were computed between parental questionnaire scores, children’s questionnaire scores, and children’s immune measure. In case of the three corresponding associations being significant, the above described regressions were repeated, this time adding child depressive symptoms and anxiety levels to the regression models before adding the parent variables, i.e., at step 4. Changes in the predictive value of parent psychological state from significant to non-significant were considered consistent with the hypothesis that child psychological state mediated the effects of parental psychological state on child immune markers.
For all analyses, p-values of p<.05 were considered significant.
RESULTS
Preliminary Analyses
Group Differences
Demographic Variables
See Table 1 for a summary of study variables. Children with asthma and healthy children did not differ significantly in age (t (81) = −0.47, p = .64). With regard to gender, the group of children with asthma consisted of significantly more boys compared to the healthy group (χ2 = 4.42, p=.035). The proportion of mothers versus fathers participating in the study did not differ by group (χ2 = 0.46, p = .50). Child gender and age was not associated with parent psychological data or child immune measures (age: all p ≥ .13; gender: all p ≥ .103), and controlling for age and gender did not change the pattern of results below.
TABLE 1.
Demographics, questionnaire data, and inflammatory data of study participants (mean, range, and SD: standard deviation).
| Asthma | Healthy | p | |
|---|---|---|---|
| gender (child) | 37 male, 13 female | 17 male, 16 female | .04* |
| age (child) | 13.3 (8–18, SD=2.7) | 13.5 (9–17, SD=2.0) | .64 |
| asthma severitya | 8 mild intermittent | ||
| 22 mild persistent | |||
| 13 moderate | |||
| 7 severe | |||
| inhaled corticosteroidsb | 4.1 (0–14, SD=5.8) | ||
| β-agonistsb | 4.6 (0–14, SD=5.9) | ||
| CDI (child) | 6.1 (0–16, SD=4.5) | 7.5 (0–27, SD=6.1) | .26 |
| RCMAS (child) | 6.8 (0–19, SD=5.3) | 6.9 (0–20, SD=4.6) | .95 |
| PSS (parent) | 16.2 (5–33, SD=7.0) | 5.7 (9–28, SD=4.1) | .73 |
| CES-D (parent) | 10.7 (0–38, SD=9.0) | 10.8 (0–28, SD=7.0) | .92 |
| ECP visit 1 (µg/l) | 13.5 (0.3–47.0, SD=12.9) | 8.2 (1.1–29.6, SD=6.2) | .03* |
| ECP visit 2 (µg/l) | 10.7 (0.7–52.9, SD=10.4) | 5.7 (0.8–23.6, SD=6.0) | .02* |
| IL-4 visit 1 (pg/ml) | 13.1 (0.5–43.3, SD=11.0) | 21.2 (1.5–56.0, SD=13.9) | <.01* |
| IL-4 visit 2 (pg/ml) | 7.9 (0.3–41.5, SD=10.2) | 7.1 (0.2–50.1, SD=11.6) | .74* |
p<.05
Based on medications and symptoms according to NHLBI guidelines (NHLBI, 1997; NHLBI, 2002)
number of days over a period of two weeks participants took inhaled corticosteroids and β-agonists (8 participants were on no medication, 30 were on inhaled corticosteroids, and 42 were on β-agonists).
Psychological Data
Children with asthma and healthy children did not differ on depression or anxiety (CDI: t (81) = −1.15, p = .26; RCMAS: t (81) = −0.06, p = .95). Furthermore, parents of children with asthma did not differ from parents of healthy children on depression or stress (CES-D: t (81) = −0.10, p = .92; PSS: t (81) = 0.34, p = .73). In both parents and children, the respective two questionnaire scores correlated highly (CES-D and PSS: r = .75, p < .001; CDI and RCMAS: r = .79, p < .001). However, parents depressive symptoms and perceived stress scores did not correlate with children’s depressive symptoms and anxiety scores (CES-D and CDI: r = −.08, p = .49; CES-D and RCMAS: r = .05, p = .63; PSS and CDI: r = −.03, p = .76; PSS and RCMAS: r = .07, p = .52).
Inflammatory Data
ECP and IL-4 values were detectable in both groups with similar ranges and variances across children with asthma and healthy children (see Table 1). However, as expected, children with asthma showed significantly higher ECP levels than healthy children at both baseline and visit 2 (visit 1: t (81) = 2.18, p = .03; visit 2: t (81) = 2.48, p = .015). Furthermore, although IL-4 production at visit 1 was higher in healthy children, IL-4 production at visit 2 did not differ in children with asthma compared to healthy children (visit 1: t (74) = −2.87, p = .005; visit 2: t (74) = 0.33, p = .74). Both ECP levels as well as IL-4 productions decreased slightly but significantly over time (ECP: F (1,82) = 106.7, p >.001; IL-4: F (1,75) = 155.6, p > .001).
Hierarchical Regressions: Changes in Children’s Immune Parameter
Parents’ Perceived Stress
We found a main effect of parental perceived stress, such that higher parental perceived stress at baseline was associated with increases over time in children’s ECP concentrations, explaining 7.2% of the variance in ECP changes over time (p = .004, see Fig. 1A and Table 2). The interaction effect of group × PSS was marginal, indicating that parental perceived stress predicted increases in ECP more strongly in children with asthma, while healthy children showed a weaker relationship between parental stress and changes in ECP (p = .061).
Fig. 1.
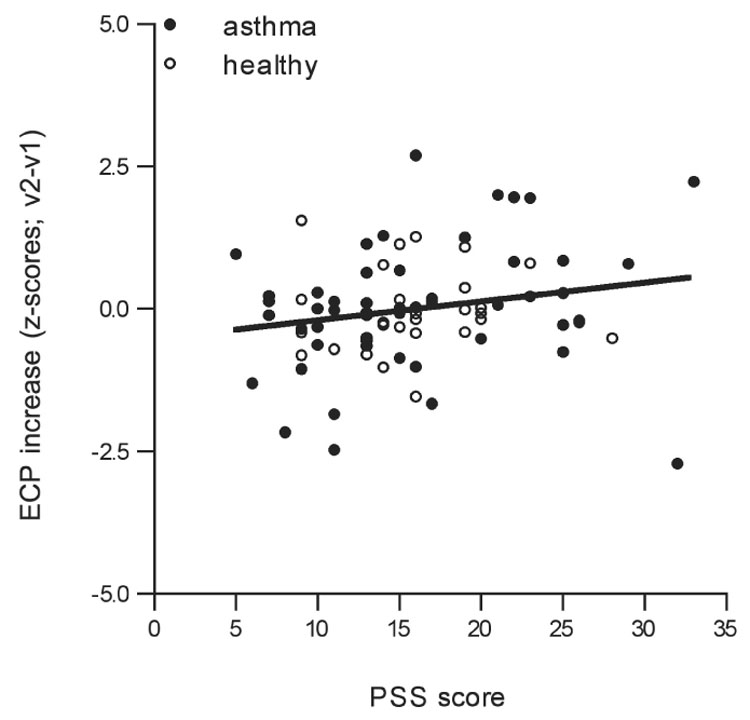

Fig. 1A. Scatterplot of parent perceived stress (PSS) predicting changes over time in ECP levels in children with asthma (filled circles) and healthy children (clear circles; PSS: p = .004, group: p = .42). Regression line is shown for the total group, i.e., including both children with asthma and healthy children.
Fig. 1B. Scatterplot of parent perceived stress (PSS) predicting changes over time in IL-4 production in children with asthma (filled circles) and healthy children (clear circles; PSS: p = .019, group: p = .58). Regression line is shown for the total group, i.e., including both children with asthma and healthy children.
TABLE 2.
Hierarchical regression analyses predicting changes in ECP concentrations and changes in IL-4 productions from parental perceived stress (PSS).
| β | t | p | ΔR2 | ΔF | df1,df2 | ΔFp | ||
|---|---|---|---|---|---|---|---|---|
| 1 | ECP visit 1 | .52 | 5.50 | <.001* | .272 | 30.29 | 1,81 | <.001* |
| asthma severity | −.08 | −0.81 | .42 | |||||
| 2 | icsa | .23 | 1.31 | .20 | .031 | 1.14 | 3,78 | .34 |
| β-agonists | −.11 | −0.65 | .52 | |||||
| 3 | group | −.18 | −0.82 | .42 | .006 | 0.67 | 1,77 | .42 |
| 4 | PSS | .27 | 2.98 | .004* | .072 | 8.86 | 1.76 | .004* |
| 5 | PSS-by-group | −.19 | −1.90 | .061+ | .028 | 3.61 | 1,75 | .061+ |
| 1 | IL-4 visit 1 | .01 | 0.09 | .93 | .000 | 0.01 | 1,74 | .93 |
| asthma severity | −.05 | −0.42 | .68 | |||||
| 2 | ics* | −.12 | −0.57 | .57 | .010 | 0.25 | 3,71 | .86 |
| β-agonists | .02 | 0.09 | .93 | |||||
| 3 | group | −.16 | −0.56 | .58 | .004 | 0.31 | 1,70 | .58 |
| 4 | PSS | .29 | 2.40 | .019* | .076 | 5.77 | 1,69 | .019* |
| 5 | PSS-by-group | .01 | 0.09 | .93 | .000 | 0.01 | 1,68 | .93 |
p<.05
p<.10
inhaled corticosteroids
We further found a main effect of parental perceived stress with regard to changes in IL-4 production, such that higher parental perceived stress was associated with increases over time in children’s stimulated IL-4 production (p = .019; see Fig. 1B and Table 2), explaining 7.6% of variance in changes in IL-4 production. No interaction of group × PSS was observed (p = .93).
Parents’ Depressive Mood
Similar to perceived stress, greater parental depressive symptoms at baseline predicted increases in children’s ECP levels over time (p = .046, see Table 3), explaining 3.6% of variance in changes in ECP levels. This association was true for both healthy children and children with asthma (group × CES-D: p = .12). Contrary, parental depressive symptoms did not predict changes over time in children’s stimulated IL-4 production (main effect CES-D: p = .19, group × CES-D: p = .96, see Table 3).
TABLE 3.
Hierarchical regression analyses predicting changes in ECP concentrations and changes in IL-4 productions from parental depressive symptoms (CES-D).
| β | t | p | ΔR2 | ΔF | df1,df2 | ΔFp | ||
|---|---|---|---|---|---|---|---|---|
| 1 | ECP visit 1 | .52 | 5.50 | <.001* | .272 | 30.29 | 1,81 | <.001* |
| asthma severity | −.08 | −0.81 | .42 | |||||
| 2 | icsa | .23 | 1.31 | .20 | .031 | 1.14 | 3,78 | .34 |
| β-agonists | −.11 | −0.65 | .52 | |||||
| 3 | group | −.18 | −0.82 | .42 | .006 | 0.67 | 1,77 | .42 |
| 4 | CES-D | .19 | 2.03 | .046* | .036 | 4.13 | 1,76 | .046* |
| 5 | CES-D-by-group | −.17 | −1.56 | .12 | .021 | 2.43 | 1,75 | .12 |
| 1 | IL-4 visit 1 | .01 | 0.09 | .93 | .000 | 0.01 | 1,74 | .93 |
| asthma severity | −.05 | −0.42 | .68 | |||||
| 2 | icsa | −.12 | −0.57 | .57 | .010 | 0.25 | 3,71 | .86 |
| β-agonists | .02 | 0.09 | .93 | |||||
| 3 | group | −.16 | −0.56 | .58 | .004 | 0.31 | 1,70 | .58 |
| 4 | CES-D | .16 | 1.31 | .19 | .024 | 1.72 | 1,69 | .19 |
| 5 | CES-D-by-group | .01 | 0.05 | .96 | .000 | 0.00 | 1,68 | .96 |
p<.05
p<.10
inhaled corticosteroids
Children’s Psychological State as Mediator
As mentioned above, neither parents depressive symptoms nor perceived stress scores correlated with children’s depressive symptoms and anxiety scores. This was also true when calculated for children with asthma and healthy children separately (asthma – CES-D and CDI: r = −.11, p = .45; CES-D and RCMAS: r = .02, p = .87; PSS and CDI: r = −.07, p = .65; PSS and RCMAS: r = .10, p = .49; healthy – CES-D and CDI: r = −.04, p = .82; CES-D and RCMAS: r = .12, p = .51; PSS and CDI: r = .03, p = .85; PSS and RCMAS: r = −.01, p = .96) and when additionally controlling for asthma severity and medication in the former group (CES-D and CDI: r45 = −.12, p = .43; CES-D and RCMAS: r45 = .03, p = .82; PSS and CDI: r45 = −.09, p = .56; PSS and RCMAS: r45 = .09, p = .56).
A significant association of predictor and assumed mediator is one central requirement that has to be met to test for mediation (Baron and Kenny, 1986). Since none of the parent psychological variables correlated with the child psychological variables, no further mediational testing was conducted.
DISCUSSION
The results of the present study demonstrate that parental stress and parental depression at baseline predict increases in children’s inflammatory profiles over a six month period. This pattern appeared in both children with asthma and healthy children, and could not be explained by child psychological states.
The present data are the first we are aware of that longitudinally link parent psychological states to changes over time in inflammatory markers in children. Specifically, parental perceived stress as well as parental depressive mood predicted increases in children’s ECP levels over the following 6 months and parental perceived stress predicted increases in stimulated IL-4 production in children over the following 6 months. Parents’ stress thereby accounted for 7.2–7.6% of the variance in children’s inflammatory measures. These changes in inflammatory profiles over time could have detrimental implications, particularly for children with asthma. Both IL-4 and ECP are closely connected to the inflammatory processes leading to asthma symptoms such as airway constriction/obstruction and edema (Bacharier and Geha, 2000; Chung and Barnes, 1999; Koh et al., 2007). Thus, they may represent one biological mechanism underlying the association between parental distress and childhood asthma morbidity found in previous research (Beautrais et al., 1982; Boyce et al., 1977; Sandberg et al., 2004; Sandberg et al., 2000; Shalowitz et al., 2001; Weil et al., 1999).
So far, only a few studies have examined the effects of parental stress and depression on children’s biology. These studies are generally consistent with the present study. For example, among young children predisposed to atopy, high levels of parental stress over time were associated with higher immunoglobulin E levels and increased proliferative responses to selected antigens (Wright et al., 2004). However, because immune outcomes were only measured once in this study, it is not clear how parental stress affects change in these markers over time. In healthy children ages 5–10, natural killer cell function was found to be enhanced when parents reported more chronic stress (Wyman et al., 2007). A couple of studies have investigated effects of parent mental health on biological profiles, specifically cortisol, in children. Young et al. (2006) reported that children in families where one parent had current major depression showed higher cortisol basally and elevated cortisol in responses to different doses of dexamethasone. In contrast, Yehuda et al. (2002) showed that if both parents had posttraumatic stress disorder, the adult offspring had lower 24-hour cortisol excretion.
In the present study, we extended previous research by including a group of children with a chronic medical illness and found that relationships between parent psychological states and child inflammatory markers were similar in children with asthma and healthy children. The only hint of a difference between groups was a marginal group × stress interaction for ECP, suggesting that the relationship between stress and ECP changes over time was stronger among children with asthma. This is in line with previous findings (Casey et al., 2004; Zuckerman and Beardslee, 1987). The similarity in patterns of associations between parental stress and depression and children’s immune measures across children with asthma and healthy children suggests some intriguing possibilities about how psychosocial factors might contribute to disease. The current study findings suggest that psychosocial factors may be able to alter immune processes, but that this link is not sufficient to cause disease, given that these relationships were present in children with no chronic illnesses. Rather, psychosocial factors may need to be accompanied by other exposures (e.g., physical environment triggers) or by underlying vulnerabilities (e.g., genetic predisposition) for the immune changes to influence disease course (Chen and Miller, 2007).
In addition, the connection between parents’ psychosocial states and children’s inflammatory profiles appeared to be generally stronger for parental perceived stress compared to parental depression, given that parental depression predicted changes in ECP levels over time, but not changes in IL-4 production. This finding suggests that parental stress may have more robust effects across various child inflammatory markers perhaps because children are more attune to the stresses their parents experience than they are to the emotional states of their parents.
The present data did not find support for the hypothesis that one reason for these associations is because of the effects of parent psychological distress on child distress, which in turn influences children’s inflammatory profiles. Although child distress may be one of the most obvious pathways, given the links between child anxiety/depression and asthma morbidity (Fritz et al., 1987; Goodwin et al., 2003; Kaptein, 1982; Miller, 1987; Ortega et al., 2002; Strunk et al., 1985), our findings as well as others suggest that there may be other pathways explaining links between parental distress and child inflammatory profiles (Wade et al., 1997). For example, Wright et al. (1998), suggest that parent perceived stress may have effects on children’s perceptions of control and self-efficacy, both of which are predictive of health outcomes. Another possibility is that distress influences families’ problem-solving and asthma-management skills (Bartlett et al., 2004; Fehrenbach and Peterson, 1989; Kaugars et al., 2004). Future research should probe these types of variables to better understand pathways connecting parental psychosocial states and children’s biological profiles, and in turn, health outcomes.
Limitations to the present study include the use of questionnaires, rather than interviews, to assess parental stress and depression. Furthermore, we were also limited in the variables that we could test as mediators of the relationship between parent psychological state and child inflammatory profiles. Not all study variables were assessed at all time points, and hence for example, we could not test associations with changes in child psychological variables over time. While this might not be an issue with regard to the child anxiety measure, which shows high stability over long periods of time (e.g., nine months; Reynolds, 1981), it could be an issue with the child depression measure, which has a reference period of two weeks. Future studies are needed to more comprehensively test the role of child mood as a mediator between parent psychological state and child inflammatory profiles. It should further be noted that parental depression and perceived stress explained between 3.6 and 7.6 percent of the variance in immune measures. These modest effect sizes suggest that there are a variety of other factors that likely contribute to changes in children’s inflammatory profiles over time. Future studies are needed to determine the relative importance of psychosocial factors when considered in conjunction with other contributors to inflammation. Lastly, future studies should assess not only whether changes in biological markers are linked to changes in clinical asthma outcomes, but also whether the described relationships can be found in healthy children and children with chronic illnesses for other immune markers beyond those directly related to asthma.
In sum, the present study addressed the intriguing question of how parents’ psychological states come to impact the health of their child. Our findings are the first we are aware of to show that high parental stress and depression prospectively predict increases in inflammatory markers in children over a six month period. Interestingly, these relationships emerged in both children with asthma and healthy children, and could not be explained by children’s own levels of anxiety and depression.
The present findings have several implications. They suggest that children are sensitive to parents’ psychological distress and may internalize it biologically. Among children with asthma, this biological profile of elevations in inflammatory markers over time may impact morbidity outcomes such as symptoms. Furthermore, although healthy children might not be symptomatic, they do show the same biological responses as children with asthma and thus are important to study in terms of identifying children who might be at risk for future health problems. Finally, future research is needed to understand the family and child factors that explain how parent psychosocial states come to be linked to child health. The present study represents a first important step in this direction, and suggests that interventions targeting parents’ psychosocial characteristics may be beneficial, at least biologically, to their children.
ACKNOWLEDGEMENT
Funding for this study was provided by NIH Grant HL073975, the William T. Grant Foundation, and the Michael Smith Foundation for Health Research (MSFHR).
Footnotes
In the following, children and adolescents will be referred to as children. If not stated otherwise, children will therefore always refer to the whole group of subjects, not just the younger participant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jutta M. Wolf, University of British Columbia, Department of Psychology
Gregory E. Miller, University of British Columbia, Department of Psychology
Edith Chen, University of British Columbia, Department of Psychology
REFERENCES
- Aiken L, West S. Multiple Regression: Testing and Interpreting Interaction Effects. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105:S547–S558. doi: 10.1016/s0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med. 2001;155:347–353. doi: 10.1001/archpedi.155.3.347. [DOI] [PubMed] [Google Scholar]
- Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113:229–237. doi: 10.1542/peds.113.2.229. [DOI] [PubMed] [Google Scholar]
- Beautrais AL, Fergusson DM, Shannon FT. Life events and childhood morbidity: a prospective study. Pediatrics. 1982;70:935–940. [PubMed] [Google Scholar]
- Boyce WT, Jensen EW, Cassel JC, Collier AM, Smith AH, Ramey CT. Influence of life events and family routines on childhood respiratory tract illness. Pediatrics. 1977;60:609–615. [PubMed] [Google Scholar]
- Brown ES, Gan V, Jeffress J, Mullen-Gingrich K, Khan DA, Wood BL, Miller BD, Gruchalla R, Rush AJ. Psychiatric symptomatology and disorders in caregivers of children with asthma. Pediatrics. 2006;118:e1715–e1720. doi: 10.1542/peds.2006-1119. [DOI] [PubMed] [Google Scholar]
- Casey P, Goolsby S, Berkowitz C, Frank D, Cook J, Cutts D, Black MM, Zaldivar N, Levenson S, Heeren T, Meyers A. Maternal depression, changing public assistance, food security, and child health status. Pediatrics. 2004;113:298–304. doi: 10.1542/peds.113.2.298. [DOI] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–857. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the U.S. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Fehrenbach AM, Peterson L. Parental problem-solving skills, stress, and dietary compliance in phenylketonuria. J Consult Clin Psychol. 1989;57:237–241. doi: 10.1037//0022-006x.57.2.237. [DOI] [PubMed] [Google Scholar]
- Fritz GK, Rubinstein S, Lewiston NJ. Psychological factors in fatal childhood asthma. Am J Orthopsychiatry. 1987;57:253–257. doi: 10.1111/j.1939-0025.1987.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry. 2003;60:1125–1130. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Amemiya E, Tomita Y, Kobatashi T, Arai K, Yamaguchi M, Horie T. Elevation of soluble IL-2 receptor and IL-4, and nonelevation of IFN-gamma in sera from patients with allergic asthma. Ann Allergy. 1993;71:455–458. [PubMed] [Google Scholar]
- Kaptein AA. Psychological correlates of length of hospitalization and rehospitalization in patients with acute, severe asthma. Soc Sci Med. 1982;16:725–729. doi: 10.1016/0277-9536(82)90463-4. [DOI] [PubMed] [Google Scholar]
- Kaugars AS, Klinnert MD, Bender BG. Family influences on pediatric asthma. J Pediatr Psychol. 2004;29:475–491. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108:E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: Is it useful in asthma? A systematic review. Respir Med. 2007;101:696–705. doi: 10.1016/j.rmed.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–315. [PubMed] [Google Scholar]
- Lee YC, Lee KH, Lee HB, Rhee YK. Serum levels of interleukins (IL)-4, IL-5, IL-13, and interferon-gamma in acute asthma. J Asthma. 2001;38:665–671. doi: 10.1081/jas-100107544. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Taki F, Miura M, Matsuzaki M, Takagi K. Serum levels of soluble IL-2R, IL-4, and soluble Fc epsilon RII in adult bronchial asthma. Chest. 1994;105:681–686. doi: 10.1378/chest.105.3.681. [DOI] [PubMed] [Google Scholar]
- Miller BD. Depression and asthma: a potentially lethal mixture. J Allergy Clin Immunol. 1987;80:481–486. doi: 10.1016/0091-6749(87)90080-7. [DOI] [PubMed] [Google Scholar]
- NHLBI. Guidelines for the Diagnosis and Management of Asthma. National Asthma Education and Prevention Program Vol. publication no. 97-4051. Bethesda MD: US Department of Health and Human Services; National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) 1997 Expert Panel 2, 1997.
- NHLBI. Guidelines for the Diagnosis and Management of Asthma. National Asthma Education and Prevention Program. Bethesda MD: US Department of Health and Human Services; National Institutes of (NIH), National Heart, Lung, and Blood Institute (NHLBI) 2002 Expert Panel Report - update on selected topics.
- Nurse B, Haus M, Puterman AS, Weinberg EG, Potter PC. Reduced interferon-gamma but normal IL-4 and IL-5 release by peripheral blood mononuclear cells from Xhosa children with atopic asthma. J Allergy Clin Immunol. 1997;100:662–668. doi: 10.1016/s0091-6749(97)70171-4. [DOI] [PubMed] [Google Scholar]
- Ortega AN, Huertas SE, Canino G, Ramirez R, Rubio-Stipec M. Childhood asthma, chronic illness, and psychiatric disorders. J Nerv Ment Dis. 2002;190:275–281. doi: 10.1097/00005053-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reynolds CR. Long-term stability of scores on the Revised-Children's Manifest Anxiety Scale. Percept Mot Skills. 1981;53:702. doi: 10.2466/pms.1981.53.3.702. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children's Manifest Anxiety Scale (RCMAS) Manual. 6th ed. Los Angeles: Western Psychological Services; 1997a. [Google Scholar]
- Reynolds CR, Richmond BO. What I Think and Feel: a revised measure of Children's Manifest Anxiety. J Abnorm Child Psychol. 1997b;25:15–20. doi: 10.1023/a:1025751206600. [DOI] [PubMed] [Google Scholar]
- Romano BA, Nelson RO. Discrimination and concurrent validity of measures of children's depression. J Clin Child Psychol. 1988;17:255–259. [Google Scholar]
- Sandberg S, Jarvenpaa S, Penttinen A, Paton JY, McCann DC. Asthma exacerbations in children immediately following stressful life events: a Cox's hierarchical regression. Thorax. 2004;59:1046–1051. doi: 10.1136/thx.2004.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambul Pediatr. 2001;1:185–193. doi: 10.1367/1539-4409(2001)001<0185:trolsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Mrazek DA, Fuhrmann GS, LaBrecque JF. Physiologic and psychological characteristics associated with deaths due to asthma in childhood. A case-controlled study. Jama. 1985;254:1193–1198. [PubMed] [Google Scholar]
- Wade S, Weil C, Holden G, Mitchell H, Evans R, 3rd, Kruszon-Moran D, Bauman L, Crain E, Eggleston P, Kattan M, Kercsmar C, Leickly F, Malveaux F, Wedner HJ. Psychosocial characteristics of inner-city children with asthma: a description of the NCICAS psychosocial protocol. National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:263–276. doi: 10.1002/(sici)1099-0496(199710)24:4<263::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104:1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Rodriguez M, Cohen S. Review of psychosocial stress and asthma: an integrated biopsychosocial approach. Thorax. 1998;53:1066–1074. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman PA, Moynihan J, Eberly S, Cox C, Cross W, Jin X, Caserta MT. Association of family stress with natural killer cell activity and the frequency of illnesses in children. Arch Pediatr Adolesc Med. 2007;161:228–234. doi: 10.1001/archpedi.161.3.228. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Bierer LM. Cortisol levels in adult offspring of Holocaust survivors: relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology. 2002;27:171–180. doi: 10.1016/s0306-4530(01)00043-9. [DOI] [PubMed] [Google Scholar]
- Young EA, Vazquez D, Jiang H, Pfeffer CR. Saliva cortisol and response to dexamethasone in children of depressed parents. Biol Psychiatry. 2006;60:831–836. doi: 10.1016/j.biopsych.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Zuckerman BS, Beardslee WR. Maternal depression: a concern for pediatricians. Pediatrics. 1987;79:110–117. [PubMed] [Google Scholar]


