Summary
Biodemographic studies of insects have significantly enhanced our understanding of the biology of aging. Eusocial insects have evolved to form different groups of colony members that are specialized for particular tasks and highly dependent on each other. These different groups (castes and sexes) also differ strongly in their life expectancy but relatively little is known about their mortality dynamics. In this study we present data on the age-specific flight activity and mortality of male honey bees from two different genetic lines that are exclusively dedicated to reproduction. We show that males initiating flight at a young age experience more flight events during their lifetime. No (negative) relation between the age at flight initiation and lifespan exists, as might be predicted on the basis of the antagonistic pleiotropy theory of aging. Furthermore, we fit our data to different aging models and conclude that overall a slight deceleration of the age-dependent mortality increase at advanced ages occurs. However, mortality risk increases according to the Gompertz–Makeham model when only days with flight activity (active days) are taken into account. Our interpretation of the latter is that two mortality components act on honey bee males during flight: increasing, age-dependent deaths (possibly from wear-and-tear), and age-independent deaths (possibly due to predation). The overall mortality curve is caused by the interaction of the distribution of age at foraging initiation and the mortality function during the active (flight) lifespan.
Keywords: aging, biodemography, drones, life history, mortality dynamics, reproduction, social evolution
Introduction
The emerging field of biodemography is only just beginning to explore the biological diversity of a taxonomically diverse set of model organisms. Demographic investigations into various organisms, such as the Mediterranean fruit fly (Carey et al., 1992) or weaver ants (Chapuisat & Keller, 2002), have triggered significant discoveries in the field of human demography (Oeppen & Vaupel, 2002) and provided important tests of general theories of aging (Keller & Genoud, 1997). Honey bees (Apis mellifera L.) represent a valuable, yet largely unexplored model organism for the biology of aging research (Rueppell et al., 2004) because they represent an advanced social system (Sherman et al., 1995). Many aspects of their biology, including their genome, are under intensive study, and spectacular intraspecific variation in longevity exists (Page & Peng, 2001). Males (drones), (female) workers and queens develop from the same genome in only 24, 21 and 16 days, respectively (Winston, 1987), but their adult lifespan potential differs dramatically. Whereas queens live for several years, workers live for 40–140 days depending on season, and drones are believed to reach maximally 60 days (Page & Peng, 2001), although records of up to 90 days exist (Fukuda & Ohtani, 1977).
Differences in life expectancy among drones, workers and queens, at least in part, may be attributable to different extrinsic mortality risks associated with the different lifestyles of each of these groups. Whereas the reproducing queen is largely shielded from external mortality causes inside the colony, workers and drones leave the colony regularly after a certain age to fulfil their colony functions, foraging for resources and finding a virgin queen with which to mate, respectively. Although drones have been studied extensively when looking at the mating biology of the honey bee (Currie, 1987; Gries & Koeniger, 1996) and are used in physiological studies (Berger et al., 1997; Panzenboeck & Crailsheim, 1997), their life history in general and their mortality dynamics in particular have not been studied in sufficient detail.
It is known that the haploid drone larvae develop slower than worker or queen larvae but with more weight gained (Winston, 1987). After emergence, drone maturation is believed to progress in several phases: independent feeding begins after 4–5 days, the first orientation flights are usually taken after a week and the reproductive organs take up to 12 days to mature (Ruttner, 1966). Mature drones spend the majority of their time in the hive resting, feeding and cleaning themselves (Ohtani, 1974), and flights take place when conditions are favorable, towards the afternoon (Ruttner, 1966). Orientation flights last only 1–6 min, whereas mating flights normally take 20–30 min (Witherell, 1971). Drones make on average 2–4 flights per day and fly up to 7 km from their hive (Currie, 1987). Lifespans seem to be highly variable, and means of 12–14, 21–24 and 54 days (Currie, 1987) have been reported.
One early life-table study exists that describes the survival of drones in different seasons, but no information beyond cohort survival is available, and data analyses were restricted to the generation of standard life-table statistics (Fukuda & Ohtani, 1977). In this study, we measured flight activity and survival of drones from two genetically different sources to investigate the relationship between the central life-history parameters ‘age at first flight’ (AFF), ‘flight activity’ and ‘lifespan’. We explored the drones’ mortality dynamics using general models of aging [‘Gompertz’: exponential increase in mortality risk, ‘Logistic’: mortality risk increases in a sigmoid fashion, and their combinations with a constant mortality risk (‘Makeham’) (Pletcher, 1999)].
Results
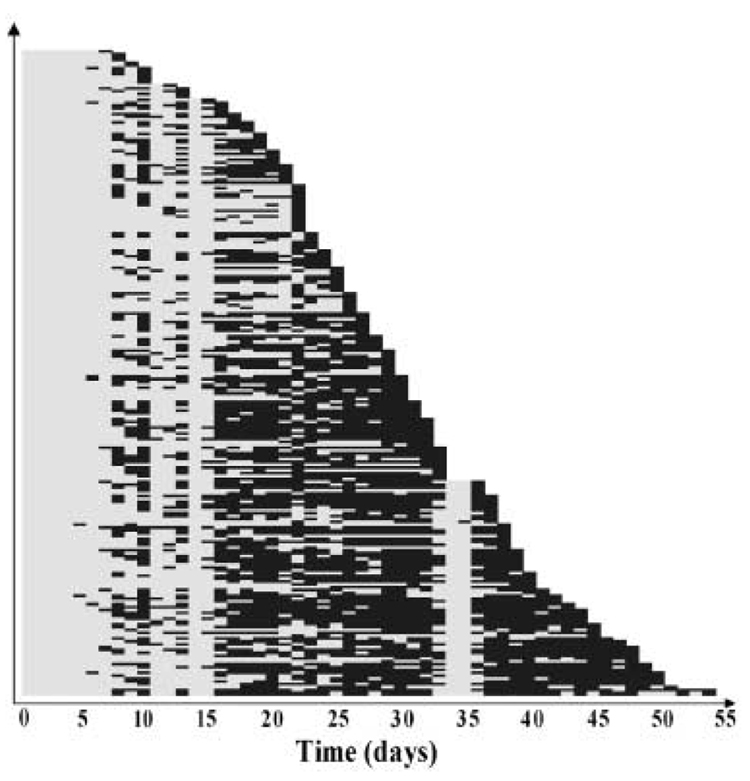
For all investigated parameters no differences existed between the ‘High’ and the ‘Low’ strain drones (Table 1) and consequently their data were pooled for subsequent analyses. Mean AFF was 11.1 days (± SD 4.8, range 5–29 days) and mean lifespan was 29.9 days (± 10.7, range 7–54 days). Once a drone initiated flying, the probability of flight activity was high (overall over 64%, Fig. 1), unless weather conditions were unfavorable, as apparent on days 34 and 35. In contrast to all other days, these two days were overcast, had significant rain precipitation (7.4 and 5.4 mm) and their average temperature was about 5 °C cooler.
Table 1.
Comparison of life history variables between the two drone sources
| Variable | Degrees of freedom | F-score | Significance |
|---|---|---|---|
| Lifespan | 1, 224 | 0.001 | 0.975 |
| Fly ratio | 1, 224 | 0.104 | 0.747 |
| Fly span | 1, 224 | 0.033 | 0.857 |
| AFF | 1, 224 | 0.128 | 0.721 |
| Fly days | 1, 224 | 0.001 | 0.976 |
Fig. 1.
Overview of the lifespan and flight activity of 226 investigated drones. Each ‘row’ represents a single drone with days (on the x-axis) coded in grey for no flight activity and in black for flight activity. Drones are sorted according to their lifespan and then their age of flight initiation.
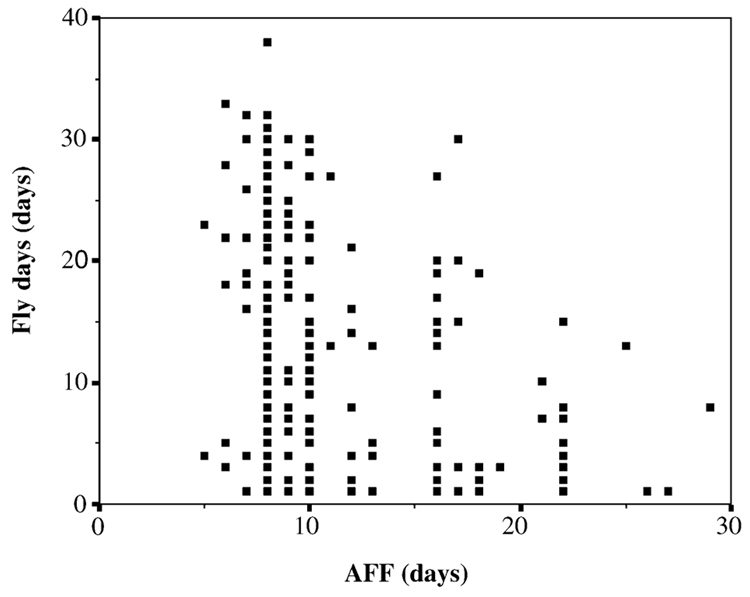
The regression of lifespan on AFF and fly ratio revealed no significant influence of either factor (r2 = 0.001, F2,223 = 0.15, P = 0.861; βAFF = −0.013, P = 0.847; βfly ratio = −0.033, P = 0.627). In contrast, the regression of fly span on AFF and fly ratio was highly significant (r2 = 0.176, F2,223 = 23.9, P < 0.001), which was due to a negative effect of AFF on fly span (βAFF = −0.415, P < 0.001) but not of fly ratio (βfly ratio = −0.033, P = 0.595). Evaluated alone, the effect of AFF on fly span remained virtually the same (r2 = 0.175, F1,224 = 47.6, P < 0.001). Practically the same relationship was true for AFF and fly days (Fig. 2), because fly days and fly span were highly correlated (r = 0.892, n = 226, P < 0.001). No significant relation existed between AFF and fly ratio (r2 = 0.013, F1,224 = 3.0, P = 0.085).
Fig. 2.
The older a male at the initiation of flight (AFF), the fewer number of days it is found flying during its life (fly days). The corresponding regression is highly significant (r2 = 0.128, F1,224 = 33.0, P < 0.001).
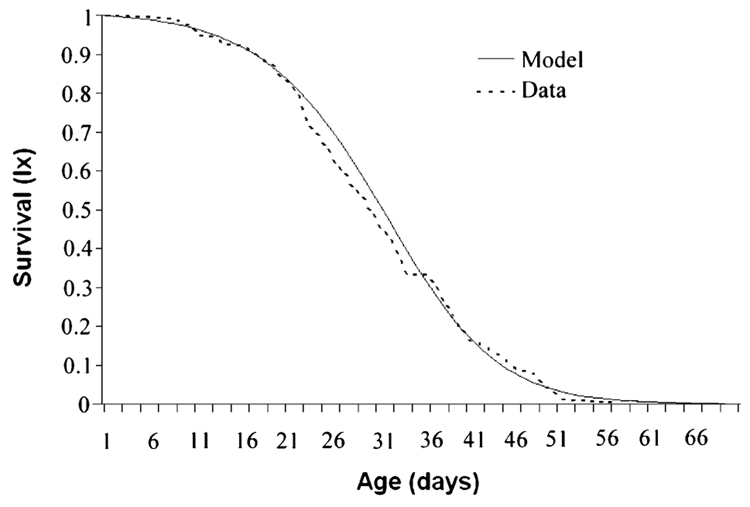
Overall drone mortality rates were best described with a logistic model [likelihood ratio comparison using the Gompertz model χ2 = 7.3, d.f. = 1, P = 0.007; Logistic–Makeham did not improve likelihood (Pletcher, 1999; Tu et al., 2002)]. Parameters estimation yielded λ = 0.00167, γ = 0.14108 and s = 0.76940. A sensitivity analysis varying the start parameters λ (0.0001–0.01), γ (0.01–0.3) and s (0.1–0.9) revealed that these parameters are robust. This result is also corroborated by the close concordance between data and model (Fig. 3).
Fig. 3.
Cumulative survival and logistic model (for honey bee male lifespan). ‘High’ and ‘Low’ pollen hoarding lines are pooled because their mortality dynamics are similar (see Fig. 4).
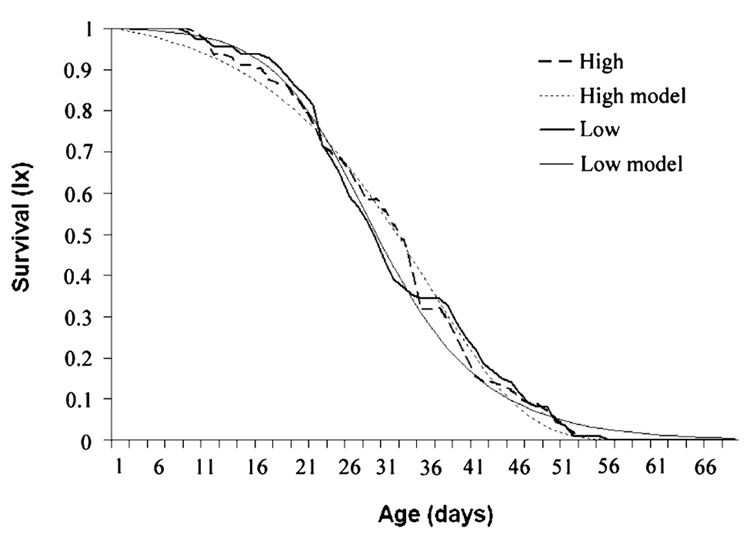
In spite of the similar lifespan means of ‘High’ and ‘Low’ strain males, separate model fitting suggests that the age-specific mortality differs between them. The mortality rates of ‘High’ strain drones are best described by a Gompertz model (λ = 0.00379, γ = 0.08933) because the logistic model did not fit the data significantly better (χ2 = 3.1, d.f. = 1, P = 0.078), and the Makeham extensions of both models did not result in any improvement. The mortality rates of ‘Low’ strain drones are best described by the logistic model (λ = 0.00063, γ = 0.20486 and s = 1.75968) because it provides a significantly improved fit over the Gompertz model (χ2 = 6.2, d.f. = 1, P = 0.012) and its Makeham extension did not result in any improved fit. However, visual inspection of the survival curves (Fig. 4) suggests that the two cohorts have similar mortality dynamics.
Fig. 4.
Survival curves of males from ‘High’ and ‘Low’ pollen hoarding lines separately. We fitted to the ‘High’ group a Gompertz model and to the ‘Low’ group a logistic model, as suggested by statistical analysis (for estimated parameters see text).
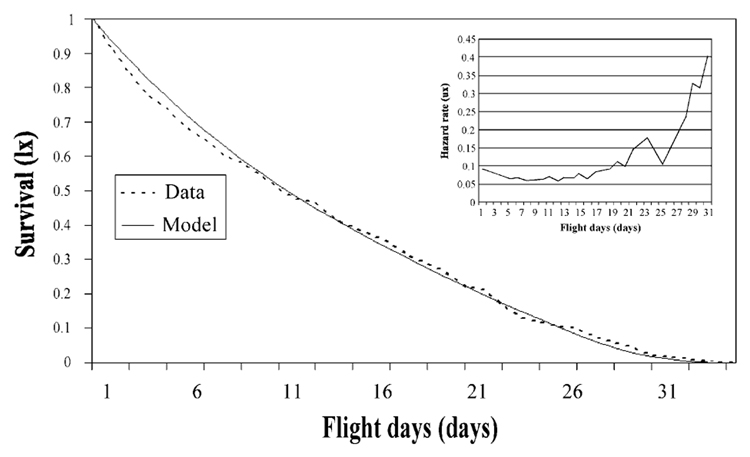
The duration of reproductively active drone life (fly days) was best represented as a Gompertz–Makeham function (likelihood ratio comparison using the Gompertz model χ2 = 7.3, d.f. = 1, P = 0.007) and robust optimal parameter estimates were λ = 0.00059, γ = 0.21221 and c = 0.06175. However, the age-specific hazard shows a more complex pattern: initial flight days are associated with a slightly higher mortality than subsequent flight days, and drones incurred the lowest mortality between the seventh and eleventh day of flying (Fig. 5). Subsequently, mortality risk increases exponentially, with a notable short-term reversal of this trend around the 25th flight day.
Fig. 5.
Survival of honey bee males relative to the amount of flight activity (flight days). Mortality contains a significant age-independent component, reflected in the Gompertz–Makeham model that was fitted to the data. Inset: age-specific hazard rate (smoothed using 3-day means).
Discussion
Honey bee drones are a particularly interesting class of organism for studying aging because they are integrated into the societal unit of the honey bee hive, where they have an exclusively reproductive function (Winston, 1987). Therefore, they may be compared with germ cells within multicellular organisms, although differences certainly exist (Ratnieks & Reeve, 1992). The functionally sterile workers can be compared to somatic tissue, receiving less resource allocation and suffering higher mortality (Kirkwood, 1987; Amdam et al., 2004). However, this seems not to be the case, as our observed male lifespans fall well within reported mean worker lifespans (Page & Peng 2001), and in fact are similar to some worker lifespans measured under similar circumstances from the same stocks of bees (our unpublished data).
This study is the first to investigate the mortality of honey bee drones in relation to their flight activity and to compare their mortality patterns with general models of aging (Bell, 1984). Some intriguing relationship patterns emerge among principal fitness determinants in honey bee drones that are crucial for understanding the drones’ life history evolution. Drones are believed to mature as adults in the colony without contributing to colony productivity, before initiating flight. In contrast, they use resources through feeding, resting and being groomed (Winston, 1987). Therefore, negligible aging (Finch, 1990) was expected during the preflight period. However, our data show no relation between the onset of flight and longevity. Drones that remain longer in the hive have a higher mortality after initiating flight. This suggests that some aging (defined as an increase in mortality risk) occurs prior to the onset of flight (reproduction). The conclusion that flight activity itself is not the single most important mortality cause is corroborated by the insignificance of the factor ‘fly ratio’ in our analyses. The result of a finite lifespan for drones cannot be explained by drone execution by workers at the end of the season because our experiments were performed in the middle of the local reproductive season (Page, 1981). Therefore, other age-limiting factors must operate in drones that are, in contrast to workers (Neukirch, 1982), largely activity-independent. Caution is warranted concerning the causality of the age of first flight and lifespan, because the positive correlation between fast maturation and long reproductive life (flight days) could also be caused independently by a high-quality genome (Partridge & Harey, 1988) or an environmental ‘silver-spoon’ effect (Grafen, 1988).
Drones experience selection pressure for fast adult maturation (Page, 1981). They have to leave the hive as early as possible in order to maximize the number of days they can be on the wing to find a queen willing to mate. This selection should lead to a relatively narrow time window of flight initiation. Our data support this because most drones (65%) initiated flight within 3 days, compared with workers of the same honey bee lines that initiated flight over a much larger time window (Pankiw & Page, 2001). Presumably, the time spent flying is directly related to the probability of mating, although sexual selection may occur (Koeniger et al., 1979). Our experimental set-up did not provide information on the duration of mating flights on a given day. Therefore, it is currently not possible to decide whether the absence of the expected correlation between early maturation and short life (Partridge, 2001) is due to an increased reproductive effort of late-maturing males per day, and environmental correlation (Grafen, 1988) or reflects the lack of antagonistic pleiotropy (Williams, 1957). Males die shortly after copulation (Winston, 1987) but this cannot explain the short reproductive lifespan of the late-maturing drones because overall male mating chances are low due to the extremely male-biased numerical sex ratio (c. 20 000 : 1) in honey bees (Page & Metcalf, 1984).
Our experimental set-up did not allow us to monitor preflight mortality, which would have been of interest because the result of a similar aging rate inside and outside the hive is in contrast with earlier studies (Witherell, 1972). Drone survival was related to ‘fly days’ by a Gompertz–Makeham function, which indicates that mortality is increasing exponentially with flight activity in addition to a flight activity-independent mortality component. We interpret the increasing mortality risk as wear-and-tear, possibly coupled with some physiological depletion (Neukirch, 1982). The age-independent component may be due to mortality from predators. The elevated mortality risk during the first flight days can be explained by experience-related mortality factors, such as misorientation.
The high probability of flying after initiation of flight activity found might be peculiar to the favorable mating flight conditions in Davis, California. However, the high correlation between ‘fly span’ and ‘fly days’ strengthens the presumed fitness advantage of fast-maturing males. We failed to find an effect of flight intensity (measured as the proportion of days with flight activity once flying was initiated) on longevity. No trade-off between intensity and duration of reproductive effort seems to exist in drones under the examined circumstances. However, our data only qualify this statement under the assumption that drones do not differ systematically in their duration of mating flights. Number of flight days and number of flights are generally correlated (Witherell, 1972), but some of the variation may also be due to behavioral differences within days, a subject that requires further investigation.
The idea of a limited lifespan and an exponentially increasing mortality risk during flight are difficult to accommodate within a logistic pattern of overall drone survival (Pletcher, 1999). We have to qualify ‘limited overall lifespan’ in that we do not intend to claim that no drone can reach an age beyond 54 days. Any such statement would be problematic (Carey, 2001) because (maximal) lifespan measurement obviously depends on sample size and environment. Our statement that the drone overall lifespan seems limited is intended to stress that lifespan is not prolonged proportionally to the postponement of flight activity, as is the case in honey bee workers, which show a more constant foraging lifespan (Guzman-Novoa et al., 1994; Omholt & Amdam, 2004). Presumably, the slowing of the mortality increase at advanced ages is due to a combination of the function for the age at first flight and the (almost quadratic) survival function of day with flight activity. Another contributing factor might be the marked drop in mortality around the 25th flight day, for which we have little explanation.
The mortality models fitted to the data are robust and differences in their likelihood are significant; however, the conclusions drawn, such as that in the previous paragraph, should be viewed with caution because of our relatively small sample size. The power of the maximum likelihood estimation (MLE) approach (Pletcher, 1999) to distinguish different patterns in survival curves is demonstrated in this study. Whereas standard statistical estimators did not differentiate between ‘High’ and ‘Low’ males, different mortality models were fitted to the data. However, we do not want to overinterpret these differences, because the data sets are actually a lot closer to each other than are the resulting models.
In conclusion, social evolution has led to honey bee drones that specialize exclusively in reproduction but their mortality dynamics are not very distinct from that of other, non-social insects (Carey et al., 1992). Ultimately, selection in both groups acts similarly to maximize reproduction. Honey bee drones are supported by the remainder of the hive with shelter and food, but this apparently does not generate rigorously different mortality dynamics. Drones provide one of the rare opportunities to measure longevity and reproductive effort under natural conditions. Interestingly, the only other such study that we are aware of (Bonduriansky & Brassil, 2002) also failed to find a negative correlation between early maturation and longevity.
Experimental procedures
Experiment
Honey bee strains underwent bidirectional selection for pollen hoarding capacity over 20 generations (Page & Fondrk, 1995) before ten sister queens were raised from each strain. Three queens of each strain were introduced into colonies of larger than 15 000 individuals to ensure drone rearing. After 1 month, queens were simultaneously caged on empty drone combs in their respective colonies to induce maximal egg-laying. When drones were beginning to emerge from these combs, all bees were brushed off, and the combs were transferred to an incubator for controlled emergence (34 °C, 60% relative humidity).
Over a 2-day period, 200 emerging drones of each strain were individually tagged and all were introduced into an unrelated, large hive within 24 h of emergence. This hive was divided with a queen excluder, impenetrable for drones, into an upper ‘core’ nest and a bottom area. The ‘core’ consisted of two standard-sized hive bodies and was equipped with a one-way exit. Beneath the queen excluder, we had one medium-sized hive body on a bottom-board with a regular hive entrance.
Drones were initially introduced to the ‘core’ hive, which they could only leave for flight through the one-way exits. To re-enter the hive, returning drones had to use the regular hive entrance leading to the bottom area of the hive. All marked drones were collected from the frames of this bottom part of the hive, recorded and re-introduced into the upper ‘core’ hive.
Analyses
We extracted the following five variables from the experiment: (1) AFF, (2) lifespan, (3) number of days flown (fly days), (4) time from initiation of flight to death (fly span) and (5) relative flight activity, measured as fly days/fly span (fly ratio). Normality of data was assessed with Kolmogorov-Smirnov tests, which indicated significant deviations for AFF and fly days. To improve the fit to normality, AFF and fly days were transformed (logarithmic and square-root, respectively) before parametrical analyses.
Group differences between ‘High’ and ‘Low’ males were evaluated by one-way anovas. We investigated the effect of AFF and fly ratio on lifespan and fly span by multiple regressions, and the effect of AFF on fly span, fly days and fly ratio by single regression. Additionally, we used Pearson’s product-moment correlations. To test for effects of the data violating parametric assumptions, significance was also tested by bootstrapping (Manly, 1997) but no significant deviations were found.
Survival analysis was performed on the lifespan and fly days data according to standard demographic procedures. Using the program Winmodest (Pletcher, 1999), we investigated the fit of our data to Gompertz, Gompertz–Makeham, logistic and logistic–Makeham models, and estimated the parameters of the best fitting model. Furthermore, we calculated for lifespan, AFF and fly span the life-table functions lx (cohort survival), qx (age-specific mortality) and ex (age-specific life expectancy) (Carey, 2001) directly from the data (see Appendix).
Acknowledgments
We would like to thank James R. Carey, as well as the other members of the Program Project ‘Biodemographic Determinants of Lifespan’ for their advice and encouragement. Financial support came from the National Institute on Aging (PO1 AG22500).
Appendix
Life table statistics for lifespan, lifespan before flight initiation (AFF) and days with flight activity (fly days)
| Lifespan |
AFF |
Fly-days |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| x (days) | l(x) | q(x) | e(x) | l(x) | q(x) | e(x) | l(x) | q(x) | e(x) |
| 1 | 1.00 | 0.00 | 29.4 | 1.00 | 0.00 | 9.6 | 1.00 | 0.16 | 11.1 |
| 2 | 1.00 | 0.00 | 28.4 | 1.00 | 0.00 | 8.6 | 0.84 | 0.07 | 12.1 |
| 3 | 1.00 | 0.00 | 27.4 | 1.00 | 0.00 | 7.6 | 0.78 | 0.06 | 12.0 |
| 4 | 1.00 | 0.00 | 26.4 | 1.00 | 0.01 | 6.6 | 0.73 | 0.07 | 11.7 |
| 5 | 1.00 | 0.00 | 25.4 | 0.99 | 0.03 | 5.6 | 0.69 | 0.05 | 11.5 |
| 6 | 1.00 | 0.00 | 24.4 | 0.96 | 0.05 | 4.8 | 0.65 | 0.07 | 11.1 |
| 7 | 1.00 | 0.00 | 23.4 | 0.92 | 0.37 | 4.0 | 0.61 | 0.04 | 10.8 |
| 8 | 1.00 | 0.01 | 22.5 | 0.58 | 0.18 | 5.0 | 0.58 | 0.06 | 10.2 |
| 9 | 0.98 | 0.01 | 21.8 | 0.47 | 0.42 | 5.0 | 0.55 | 0.08 | 9.8 |
| 10 | 0.97 | 0.03 | 21.0 | 0.27 | 0.03 | 7.3 | 0.50 | 0.05 | 9.7 |
| 11 | 0.95 | 0.00 | 20.6 | 0.27 | 0.13 | 6.5 | 0.48 | 0.03 | 9.2 |
| 12 | 0.95 | 0.00 | 19.6 | 0.23 | 0.08 | 6.4 | 0.46 | 0.10 | 8.4 |
| 13 | 0.94 | 0.02 | 18.6 | 0.21 | 0.00 | 5.9 | 0.42 | 0.05 | 8.4 |
| 14 | 0.92 | 0.00 | 18.0 | 0.21 | 0.00 | 4.9 | 0.39 | 0.06 | 7.8 |
| 15 | 0.92 | 0.00 | 17.0 | 0.21 | 0.33 | 3.9 | 0.37 | 0.06 | 7.2 |
| 16 | 0.92 | 0.02 | 16.1 | 0.14 | 0.16 | 4.7 | 0.35 | 0.11 | 6.7 |
| 17 | 0.90 | 0.01 | 15.4 | 0.12 | 0.15 | 4.4 | 0.31 | 0.07 | 6.4 |
| 18 | 0.89 | 0.02 | 14.6 | 0.10 | 0.04 | 4.1 | 0.29 | 0.08 | 5.9 |
| 19 | 0.87 | 0.03 | 13.9 | 0.10 | 0.00 | 3.3 | 0.27 | 0.17 | 5.4 |
| 20 | 0.85 | 0.03 | 13.3 | 0.10 | 0.09 | 2.3 | 0.22 | 0.04 | 5.3 |
| 21 | 0.82 | 0.04 | 12.7 | 0.09 | 0.80 | 1.5 | 0.21 | 0.21 | 4.5 |
| 22 | 0.79 | 0.09 | 12.1 | 0.02 | 0.00 | 4.3 | 0.17 | 0.21 | 4.6 |
| 23 | 0.72 | 0.04 | 12.3 | 0.02 | 0.00 | 3.3 | 0.13 | 0.10 | 4.7 |
| 24 | 0.69 | 0.04 | 11.8 | 0.02 | 0.25 | 2.3 | 0.12 | 0.11 | 4.1 |
| 25 | 0.66 | 0.06 | 11.3 | 0.01 | 0.33 | 1.8 | 0.11 | 0.08 | 3.6 |
| 26 | 0.62 | 0.05 | 10.9 | 0.01 | 0.50 | 1.5 | 0.10 | 0.23 | 2.9 |
| 27 | 0.59 | 0.06 | 10.5 | 0.00 | 0.00 | 1.5 | 0.08 | 0.24 | 2.6 |
| 28 | 0.56 | 0.04 | 10.1 | 0.00 | 1.00 | 0.5 | 0.06 | 0.23 | 2.2 |
| 29 | 0.54 | 0.07 | 9.5 | 0.00 | n/a | n/a | 0.04 | 0.50 | 1.7 |
| 30 | 0.50 | 0.08 | 9.2 | 0.00 | n/a | n/a | 0.02 | 0.20 | 1.9 |
| 31 | 0.46 | 0.06 | 9.0 | 0.00 | n/a | n/a | 0.02 | 0.50 | 1.3 |
| 32 | 0.43 | 0.10 | 8.5 | 0.00 | n/a | n/a | 0.01 | 0.50 | 1.0 |
| 33 | 0.38 | 0.14 | 8.5 | 0.00 | n/a | n/a | 0.00 | 1.00 | 0.5 |
| 34 | 0.33 | 0.00 | 8.7 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 35 | 0.33 | 0.00 | 7.7 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 36 | 0.33 | 0.07 | 6.7 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 37 | 0.31 | 0.14 | 6.2 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 38 | 0.27 | 0.15 | 6.1 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 39 | 0.23 | 0.16 | 6.1 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 40 | 0.19 | 0.14 | 6.2 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 41 | 0.16 | 0.05 | 6.1 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 42 | 0.15 | 0.09 | 5.4 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 43 | 0.14 | 0.06 | 4.8 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 44 | 0.13 | 0.17 | 4.1 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 45 | 0.11 | 0.20 | 3.9 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 46 | 0.09 | 0.05 | 3.7 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 47 | 0.08 | 0.11 | 2.9 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 48 | 0.08 | 0.35 | 2.1 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 49 | 0.05 | 0.27 | 2.0 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 50 | 0.04 | 0.63 | 1.6 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 51 | 0.01 | 0.33 | 2.5 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 52 | 0.01 | 0.00 | 2.5 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 53 | 0.01 | 0.00 | 1.5 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 54 | 0.01 | 1.00 | 0.5 | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
| 55 | 0.00 | n/a | n/a | 0.00 | n/a | n/a | 0.00 | n/a | n/a |
References
- Amdam GV, Simoes ZLP, Hagen A, Norberg K, Schroder K, Mikkelsen O, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vittelogenin regulates immune function and longevity in honey bees. Exp. Gerontol. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bell G. Evolutionary and nonevolutionary theories of senescence. Am. Natur. 1984;124:600–603. [Google Scholar]
- Berger B, Crailsheim K, Leonhard B. Proline, leucine and phenylalanine metabolism in adult honeybee drones (Apis mellifica carnica Pollm) Insect Biochem. Mol. Biol. 1997;27:587–593. [Google Scholar]
- Bonduriansky R, Brassil CE. Rapid and costly ageing in wild male flies. Nature. 2002;420:377. doi: 10.1038/420377a. [DOI] [PubMed] [Google Scholar]
- Carey JR. Insect biodemography. Annu. Rev. Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proc. R. Soc. London B. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie RW. The biology and behaviour of drones. Bee World. 1987;68:129–143. [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. Chicago: The University of Chicago Press; 1990. [Google Scholar]
- Fukuda H, Ohtani T. Survival and lifespan of drone honeybees. Res. Popul. Ecol. 1977;19:51–68. [Google Scholar]
- Grafen A. On the uses of data on lifetime reproductive success. In: Clutton-Brock TH, editor. Reproductive success. Chicago, IL, USA: Univ. Chicago Press; 1988. pp. 454–471. [Google Scholar]
- Gries M, Koeniger N. Straight forward to the queen: Pursuing honey-bee drones (Apis mellifera L.) adjust their body axis to the direction of the queen. J. Comparative Physiol. A-Sensory Neural. Behav. Physiol. 1996;179:539–544. [Google Scholar]
- Guzman-Novoa E, Page RE, Jr, Gary NE. Behavioral and life-history components of division of labor in honey bees (Apis mellifera L.) Behav. Ecol. Sociobiol. 1994;34:409–417. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Kirkwood TB. Immortality of the germ-line versus disposability of the soma. Basic Life Sci. 1987;42:209–218. doi: 10.1007/978-1-4613-1939-9_15. [DOI] [PubMed] [Google Scholar]
- Koeniger G, Koeniger N, Fabritius M. Some detailed observations of mating in the honeybee. Bee World. 1979;60:53–57. [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. London: Chapman & Hall; 1997. [Google Scholar]
- Neukirch A. Dependence of lifespan of the honeybee (Apis mellifera) upon flight performance and energey consumption. J. Comparative Physiol. B. 1982;146:35–40. [Google Scholar]
- Oeppen J, Vaupel JW. Demography – Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Ohtani T. Behavior repertoire of adult drone honeybee within observation hives. J. Fac. Sci. Hokkaido University. 1974;19:706–721. [Google Scholar]
- Omholt SW, Amdam GV. Epigenetic regulation of aging in honeybee workers. Sci. Aging Knowl. Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- Page RE., Jr Protandrous reproduction in honey bees. Ann. Entomol. Soc. Am. 1981;10:359–362. [Google Scholar]
- Page RE, Jr, Fondrk MK. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behav. Ecol. Sociobiol. 1995;36:135–144. [Google Scholar]
- Page RE, Jr, Metcalf RA. A population investment sex ratio for the honey bee (Apis mellifera L.) Am. Natur. 1984;124:680–702. [Google Scholar]
- Page RE, Jr, Peng CYS. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE., Jr Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav. Ecol. Sociobiol. 2001;51:87–94. [Google Scholar]
- Panzenboeck U, Crailsheim K. Glycogen in honeybee queens, workers and drones (Apis mellifera carnica Pollm) J. Insect Physiol. 1997;43:155–165. doi: 10.1016/s0022-1910(96)00079-0. [DOI] [PubMed] [Google Scholar]
- Partridge L. Evolutionary theories of ageing applied to long-lived organisms. Exp. Gerontol. 2001;36:641–650. doi: 10.1016/s0531-5565(00)00232-1. [DOI] [PubMed] [Google Scholar]
- Partridge L, Harey PH. The ecological context of life-history evolution. Science. 1988;241:1449–1455. doi: 10.1126/science.241.4872.1449. [DOI] [PubMed] [Google Scholar]
- Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 1999;12:430–439. [Google Scholar]
- Ratnieks FLW, Reeve HK. Conflict in single-queen Hymenopteran societies: the structure of conflict and processes that reduce conflict in advanced eusocial species. J. Theoret. Biol. 1992;158:33–65. [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Jr, Carey JR. From genes to society: social insects as models for research on aging. Sci. Aging Knowl. Environ. 2004 doi: 10.1126/sageke.2004.5.pe5. http://sageke.sciencemag.org/cgi/content/full/2004/5/pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttner F. The life and flight activity of drones. Bee World. 1966;47:93–100. [Google Scholar]
- Sherman PW, Lacey EA, Reeve HK, Keller L. The eusociality continuum. Behav. Ecol. 1995;6:102–108. [Google Scholar]
- Tu M-P, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell. 2002;1:75–80. doi: 10.1046/j.1474-9728.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; 1987. [Google Scholar]
- Witherell PC. Duration of flight and of interflight time of drone honeybees, Apis mellifera. Ann. Entomol. Soc. Am. 1971;64:609–612. [Google Scholar]
- Witherell PC. Flight activity and nautral mortality of normal and mutant drone honeybees. J. Apicultural Res. 1972;11:65–75. [Google Scholar]