Abstract
Neuroblastomas are the most common extra-cranial tumors of childhood and well known for their heterogenous clinical behavior associated with certain genetic aberrations. Radiation therapy is an important modality for the treatment of high-risk neuroblastomas. In this study, we investigated whether ionizing irradiation modulate the migration and invasiveness of human neuroblastoma cells and expression of proangiogenic molecules known to be involved in tumor progression and metastasis. Irradiation of neuroblastoma cells resulted in increased migration and invasion as measured by spheroid migration and matrigel invasion assay respectively. Zymographic analysis revealed an increase in enzyme activity of MMP-9 and uPA in conditioned medium of irradiated neuroblastoma cells compared with nonirradiated cells. An increase in VEGF levels was also found in lysates of irradiated neuroblastoma cells. The upregulation of uPA, MMP-9 and VEGF transcripts was also confirmed by RT-PCR analysis. Next, we examined the irradiated tumor cell-mediated modulation of endothelial cell behavior. Conditioned media from irradiated neuroblastoma cells enhanced capillary-like structure formation of microvascular endothelial cells. In a coculture system, irradiation of neuroblastoma cells enhanced endothelial cell invasiveness through Matrigel matrix. Endothelial cells treated with irradiated tumor cell conditioned medium were also analyzed for expression of uPA, MMP-9 and VEGF and compared to cells treated with nonirradiated tumor cell conditioned medium. These findings suggest that the irradiation effects of tumor cells could influence endothelial angiogenesis present in nonirradiated fields.
Keywords: Irradiation, invasion, angiogenesis, neuroblastoma, endothelial cells
Introduction
Neuroblastoma, the most common pediatric solid tumor, is derived from neural crest cells and is remarkable for its clinical heterogeneity (1,2). The prognosis of neuroblastoma is multifactorial and depends on an assortment of clinical and biological factors. Radiotherapy remains an important form of local and regional cancer therapy (3,4). Typical cellular changes resulting from radiation depend on the dose level and time elapsed after exposure. Cancer cells acquire resistance to radiation because they are genetically unstable. Nonetheless, many tumors are poorly controlled by radiation therapy due to radiation resistance. The invasive, metastatic and hypervascular nature of stage IV neuroblastoma is refractory to all conventional therapeutic modalities and is associated with a dismal prognosis (5). Studies have reported apparently improved local control rates with radiation therapy or increasing radiation doses to primary sites of disease (6). Unfortunately, the cure rate of children with high-risk neuroblastoma remains at <20%, providing a compelling reason to better understand the molecular mechanisms that can be targeted to treat this disease.
Radiation therapy, a mainstay of tumor treatment, can stimulate multiple signal transduction pathways simultaneously and, in turn, these pathways may alter the expression of proangiogenic molecules in surviving cells. Since angiogenesis is essential for tumor progression, we examined the effects of irradiation on the expression of proangiogenic factors uPA, MMP-9 and VEGF in human neuroblastoma cells and in vitro angiogenic process.
Materials and Methods
Cell Culture
The human neuroblastoma SK-N-AS cell line was obtained from American Type Culture Collection (Manassas, VA) and were cultured in DMEM supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 ag/ml) and maintained at 37°C in a 95% air/5% CO2 humidified incubator. SK-N-AS cells were grown in complete DMEM to subconfluent monolayers. Thereafter, culture medium was replaced (after washing cells twice with sterile PBS) by serum-free DMEM before irradiating them at 5, 10 and 20 Gy. Conditioned medium was collected 24 hours after irradiation, centrifuged, and used for in vitro angiogenesis and Matrigel invasion assays. Human microvascular endothelial cells (HMECs) were maintained as described earlier (7).
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA thus obtained was further purified by digesting with DNase for 20 min at 37°C and then reverse-transcribed using the cDNA cycle kit (Invitrogen) with random primers (8). To amplify the cDNA, the reverse-transcribed cDNA was subjected to 30 cycles of PCR in 25 al of PCR Master Mix (Promega, Madison, WI) containing 100 pmol of sense and antisense primers. The efficiency of cDNA synthesis was estimated by PCR with GAPDH-specific primers. The following sense (S) and antisense (AS) primers were used in the RT-PCR reactions: uPA (S, 5’TGCGTCCTGGTCGTGAGCGA 3’; AS 5’CAAGCGTGTCAGCGCTGTAG); VEGF (S, 5’ ATGAACTTTCTGCTGTCTTGGGT 3’; AS, 5’-TCACCGCCTCGGCTTGTCAC-3’); MMP-9 (S, 5’ TGGACGATGCCTGCAACGTG 3’; AS, 5’ GTCGTGCGTGTCCAAAGGCA 3’); GAPDH (S, 5’-CGGAGTCAACGG ATTTGGTCGTAT-3’; AS, 5’-AGCCTTCTCCATGGTGGTGA AGAC-3’). Samples were subjected to electrophoresis on a 1.5% agarose gel and photographed as ethidium bromide fluorescent bands.
Zymographic assays
Human neuroblastoma cells SK-N-AS were plated at equal numbers and irradiated with different radiation doses under serum free conditions. After 24 h, supernatants were collected and then resolved under nonreducing conditions on 10% SDS-PAGE gels embedded with fibrinogen/plasminogen (9) or gelatin (10). Gels were rinsed three times in 2.5% Triton X-100 for 30 min at room temp and then incubated in 100mM glycine buffer pH 8.0 (for uPA fibrin zymography) or 50 mM Tris-HCl, 10mM CaCl2 buffer pH 7.6 (for gelatin zymography) overnight at 37°C. Gels were stained with Amido Black and areas of lysis were visualized as transparent bands. Bands of lysis representing gelatinase activity were then visualized against a dark background.
SDS-PAGE and Western blot analysis
Cells were extracted in a buffer solution containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1mM sodium fluoride, 1mM PMSF, 10 ag/ml aprotinin on ice for 20 min. Samples were subjected to SDS-PAGE and separated proteins were transferred onto membrane followed by blocking of membrane with 5% nonfat milk powder (w/v) in Tris- buffered saline (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature or overnight at 4°C. Membranes were probed for the VEGF protein using specific primary antibodies followed by peroxidase-conjugated appropriate secondary antibody, and visualized by an enhanced chemiluminescence detection system. Membranes were stripped and reprobed with β-actin antibody as a protein loading control.
Cell migration from spheroids
Migration from spheroids was assayed as described previously (9). Single multicellular spheroids were placed in the center of each well of a 96-well microplate and were cultured for 24 h, after which the spheroids were fixed and stained with Hema-3 and cellular migration from the spheroids was assessed under light microscopy.
Matrigel invasion assay
Cells were plated on Matrigel-coated cell-culture inserts in Transwell chambers (Corning Inc, Corning, NY) containing 6.5-mm filters (pore size 8 am) as described earlier (8). Cells were added to the Matrigel-coated chamber, and after a 24 h incubation period, cells on the Matrigel-coated side of the filter were removed with a cotton swab and the migrating cells remaining on the bottom part of the filters were fixed and stained with Hema-3. Cells were counted and the percentage of cells that had migrated through the matrigel was determined. A modified coculture model of Matrigel invasion assay was performed to assess the effects on endothelial cells after selective radiation of tumor cells. Human neuroblastoma cells SK-N-AS were first seeded in 24-well plates. After irradiation of the neuroblastoma cells, Matrigel-coated 8-μm pore size transwell inserts with HMECs were added in the upper compartment and allowed to migrate toward the lower neuroblastoma compartment. After 24 h of incubation, HMECs that had invaded into the underside of the membrane were fixed, stained and photographed.
Capillary-like structure formation
Human microvascular endothelial cells were seeded onto 48-well plates and grown in the presence of serum-free conditioned medium collected from human neuroblastoma SK-N-AS cells for 16 h. Cells were stained with Hema-3 and photographed (9). The capillary length was determined by computer-assisted image analysis with the Image-pro Discovery program.
Statistical Analyses
Student’s t-test was carried out for comparison of paired mean experimental values.
Results
Ionizing radiation enhances production of proangiogenic molecules in neuroblastoma cells
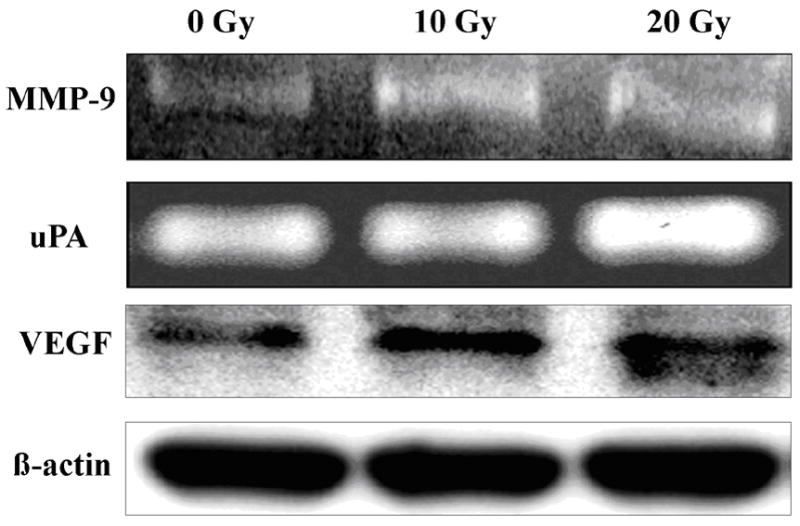
We performed zymography to examine the enzyme activities of uPA, MMP-2, MMP-9 on conditioned medium and immunoblot for protein levels of VEGF on lysates of irradiated and non-irradiated SK-N-AS neuroblastoma cells. Ionizing radiation enhanced enzyme activities of uPA and MMP-9 released in a dose dependent manner and the maximal production of uPA and MMP-9 was observed at doses of 20 and 10Gy in SK-N-AS cells respectively (Fig 1). The molecular mass of MMP-9 was 92 kDa corresponding to pro form. The active forms were not detected. MMP-2 was found as a 72kDa band corresponding to the proform, and this band was little affected by ionizing radiation. Western blot analysis showed enhanced VEGF protein levels after exposure to radiation doses of 5 to 10Gy (Fig. 1).
Figure 1.
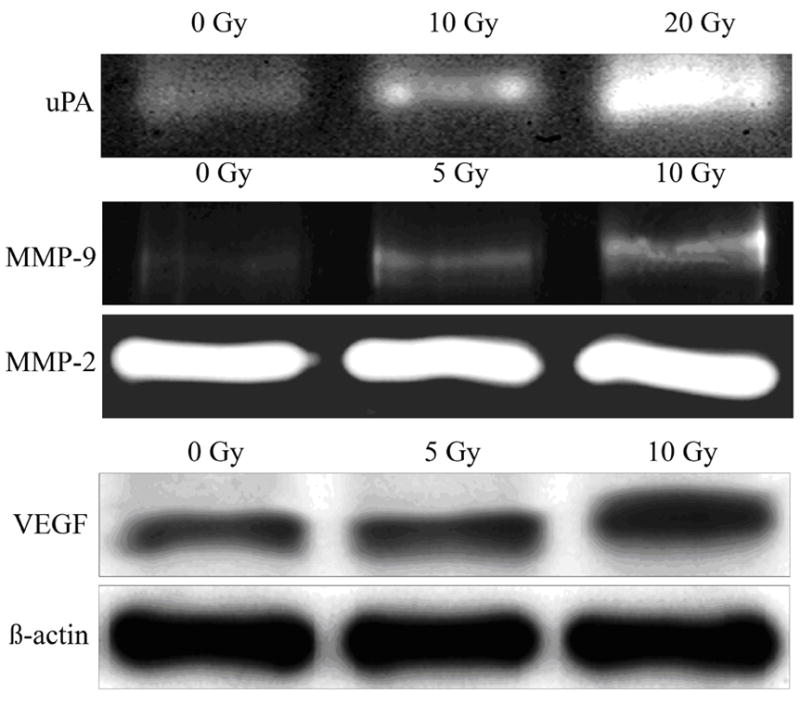
Effect of radiation on enzyme activity of uPA and MMP-9 and VEGF protein level in human neuroblastoma cells. Human neuroblastoma cells SK-N-AS were plated at equal numbers and irradiated with different radiation doses (10 Gy and 20 Gy). Serum-free conditioned medium was collected after 24 hr and electrophoresed under nonreducing conditions on 10% SDS-PAGE gels embedded with fibrinogen/plasminogen or gelatin. Gels were rinsed in 2.5% Triton X-100 for 30 min. at room temp and then incubated in 100mM glycine buffer pH 8.0 (for uPA zymography) or 50 mM Tris-HCl, 10mM CaCl2 buffer pH 7.6 (gelatin zymography) overnight at 37 0C. Gels were stained with Amido Black and areas of lysis were visualized as transparent bands. Irradiated and nonirradiated cells were extracted with lysis buffer containing protease inhibitors and electrophoresed on SDS-PAGE for analysis of VEGF and β-actin levels by immunoblotting.
Transcription of angiogenic molecules is induced by irradiation of neuroblastoma cells
In order to see if the above-mentioned increases in proangiogenic factors secretion correlate with changes in the expression of the corresponding genes, we tested the effect of irradiation on their mRNAs levels by sensitive semi-quantitative RT-PCR. The effect of ionizing radiation on uPA, MMP-9 and VEGF mRNA expression in SK-N-AS cells is shown in Fig 2. After ionizing radiation, there was a dose-dependent increase in uPA, MMP-9 and VEGF mRNA levels at 24h. The maximal expression of uPA mRNA was observed at dose of 20 Gy; the levels of MMP-9 and VEGF mRNA peaked at 10 Gy after ionizing radiation and the results were normalized to the levels of the housekeeping gene GAPDH (Fig. 2)
Figure 2.
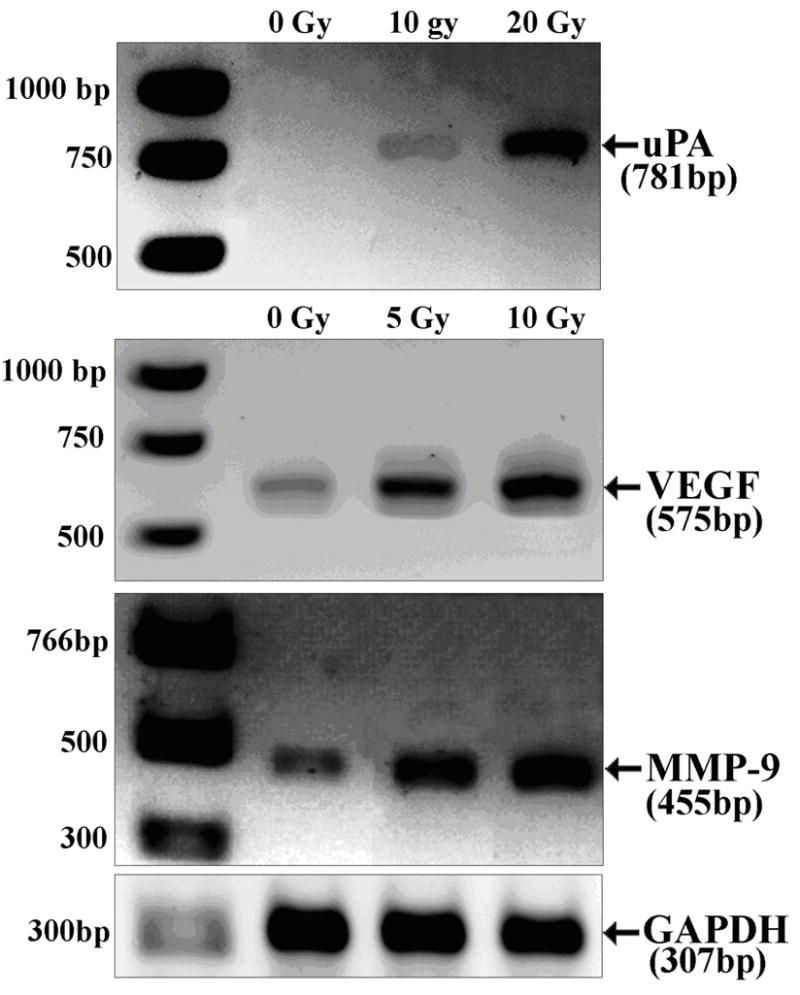
Radiation induced changes in the transcripts of uPA, MMP-9 and VEGF in human neuroblastoma cells. Total RNA was extracted from irradiated and nonirradiated human neuroblastoma cells SK-N-AS and then reverse-transcribed using the cDNA cycle kit (Invitrogen). The cDNA was amplified with sense and antisense primers specific to uPA, MMP-9, VEGF and GAPDH. The amplified cDNA fragments were subjected to electrophoresis on a 1.5% agarose gel and photographed as ethidium bromide fluorescent bands. Densitometric values of uPA, MMP-9 and VEGF were normalized to GAPDH measurements.
Radiation promoted tumor cell migration and invasion
To determine the effect of radiation on cell motility, we analyzed the migration of human neuroblastoma cells before and after irradiation using the spheroid migration assay. Single multicellular spheroids of irradiated and non-irradiated neuroblastoma cells were cultured for 48 h and cellular migration from the spheroids was assessed under light microscopy. Compared with untreated controls, tumor cells irradiated at dose of 10 Gy showed significantly higher numbers of migrated cells ( Fig. 3). In a further set of experiments, we investigated whether irradiation enhanced invasion of neuroblastoma cells through Matrigel, a reconstituted basement membrane. Control and irradiated tumor cells were examined for their invasive ability in Matrigel transwell chambers. Compared with nonirradiated controls, SK-N-AS cells irradiated at dose of 10 Gy showed higher numbers of invading cells (Fig. 4).
Figure 3.
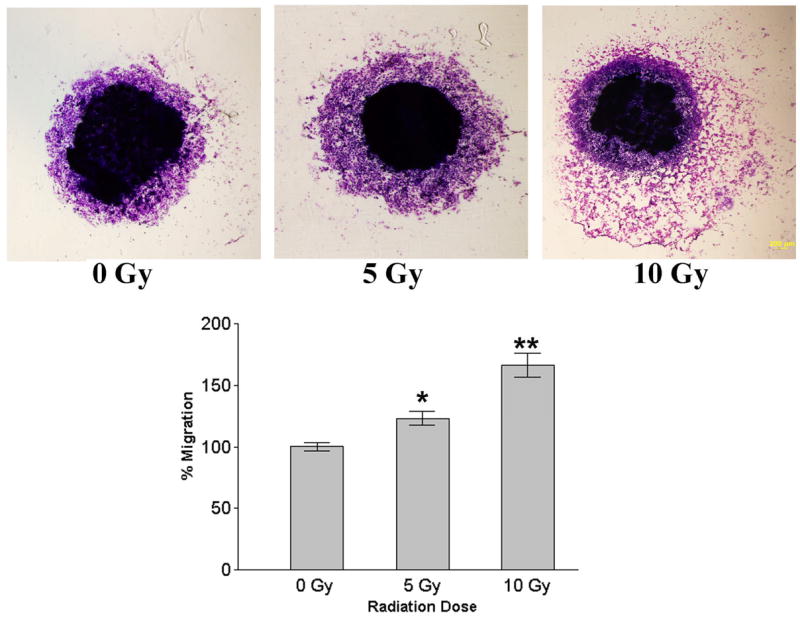
Spheroid migration assay. Single multicellular spheroids of human neuroblastoma cells SK-N-AS were irradiated with different doses (5Gy and 10Gy) and cultured for 48 h. Then, spheroids were fixed and stained with Hema-3 and cellular migration from the spheroids was assessed using light microscopy. Values are mean ± SD of four determinations. * p<0.05; * *p<0.01, significantly different from non-irradiated cells.
Figure 4.
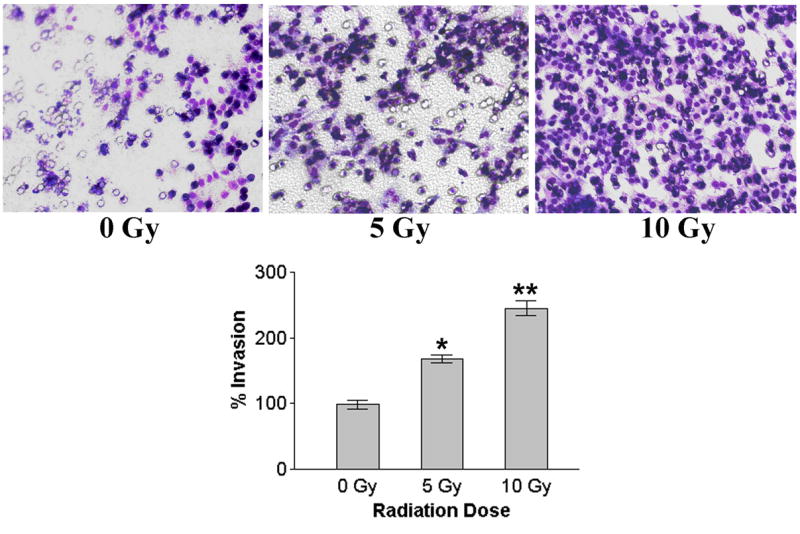
Matrigel invasion assay. Irradiated and nonirradiated human neuroblastoma cells SK-N-AS were added to Matrigel-coated cell culture inserts in Transwell chambers. After 24 h incubation period, cells on the matrigel-coated side of the filter were removed with a cotton swab and the migrated cells remaining on the bottom part of the filters were fixed, stained, counted, photographed and the percentage of cells that had migrated through the Matrigel was determined. The graphical representation (bottom right panel) of invasion data as mean ± SD of two experiments done in duplicate. * p<0.05; * *p<0.01, significantly different from non-irradiated cells.
in vitro capillary-like structure formation
The sprouting of endothelial cells and formation of capillary-like structures are crucial steps in the angiogenic process. To study the influence of irradiation of SK-N-AS cells on angiogenic process, conditioned medium from irradiated and nonirradiated human neuroblastoma SK-N-AS was added to HMECs and cells were monitored for capillary-like structure formation. Conditioned medium from irradiated cells caused increased capillary-like structure formation in HMECs compared to non-irradiated conditioned medium (Fig. 5)
Figure 5.
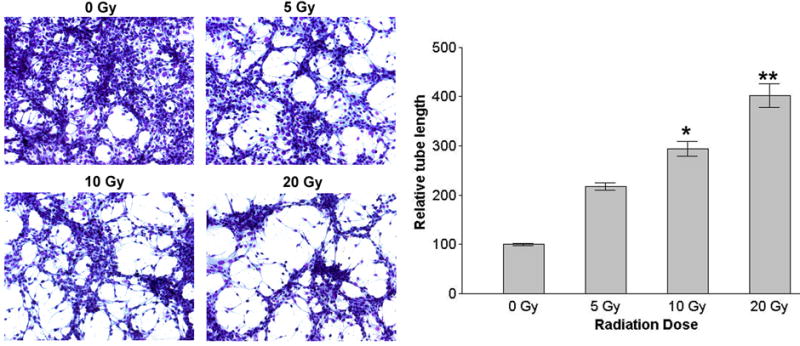
In vitro capillary-like structure formation. Capillary like structure formation in human microvascular endothelial cells (HMECs) was induced using conditioned medium from neuroblastoma tumor cells SK-N-AS. HMECs were allowed to grow in presence of conditioned media from irradiated and nonirradiated SK-N-AS cells for 16 h to measure the induction of cellular alignment into capillary-like structures. The graphical representative of three experiments done in duplicate expressed as mean ± SD. * p<0.05; * *p<0.01, significantly different from conditioned media of non-irradiated cells.
Endothelial Matrigel invasion assay
Here, we sought to analyze the altered invasiveness in HMECs by radiation induced soluble factors in SK-N-AS cells. In an effort to mimic in vivo conditions, we used a coculture system utilizing transwell chambers in which SK-N-AS tumor cells were placed in the bottom compartment and endothelial cells were placed in the top compartment, both separated by a Matrigel matrix. It is anticipated that enhanced release of angiogenic factors by radiation in tumor cells may activate and attract endothelial cells toward the tumor cell compartment. Compared with nonirradiated controls, SK-N-SH cells irradiated at dose of 10 Gy caused higher numbers of invading HMECs (Fig. 6).
Figure 6.
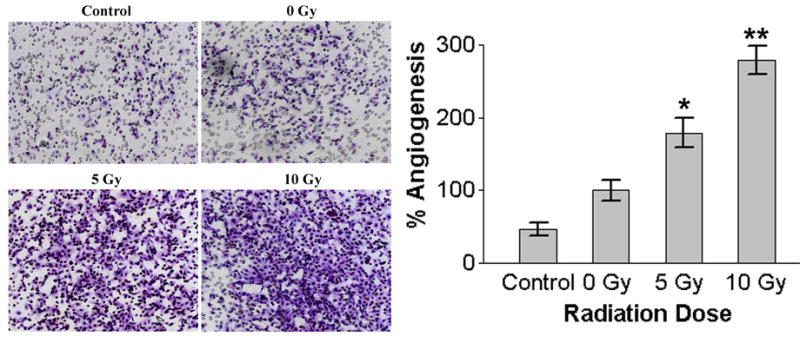
Endothelial Matrigel invasion assay. A modified coculture model of Matrigel invasion assay was performed to assess the effects on endothelial cells after selective radiation of tumor cells. Human neuroblastoma cells SK-N-AS were first seeded in 24-well plates. After irradiation of the neuroblastoma cells, Matrigel-coated 8-μm pore size transwell inserts with HMECs were added in the upper compartment and allowed to migrate toward the lower neuroblastoma compartment. After 24 of incubation, HMECs that had invaded into the underside of the membrane were fixed, stained and photographed. The graphical representation of invasion data as mean ± SD of two experiments done in duplicate. * p<0.05; * *p<0.01, significantly different from non-irradiated cells.
SK-N-AS conditioned medium increases production of proangiogenic molecules in HMECs
In order to further support our findings concerning a possible angiogenic activity of irradiated neuroblastoma cells, we studied the effect of conditioned medium of irradiated cultures of neuroblastoma cells on the levels of proangiogenic factors in HMECs. Endothelial cells were grown in conditioned medium from irradiated and non-irradiated neuroblastoma cells for 24 h and then analyzed for enzyme activities of uPA, MMP-9 and protein levels of VEGF. As shown in Fig. 7 conditioned medium from irradiated cells induced activities of uPA and MMP-9 and VEGF protein in HMECs.
Figure 7.
Effect of irradiated neuroblastoma tumor cell conditioned medium on the expression of proangiogenic molecules in human microvascular endothelial cells. Human microvascular endothelial cells (HMECs) were grown in conditioned medium from irradiated and nonirradiated human neuroblastoma cells SK-N-AS for 24 h. Serum free conditioned medium of HMECs was collected and analyzed for uPA and MMP-9 activity by substrate gel zymography. Cells were extracted in a lysis buffer containing protease inhibitors and analyzed by SDS-PAGE. VEGF protein levels were determined by immunoblotting with VEGF monoclonal antibodies (Santa Cruz Biotechnology). For detection, the ECL detection system (Amersham Biosciences) was used according to the manufacturer's instructions. Equal loading of the gels was confirmed by reincubation of the membrane with a monoclonal antibody for β-actin (Abcam).
Discussion
Neuroblastoma patients with advanced stage disease have a poor outcome despite multimodality treatment with aggressive therapeutic regimens including radiation treatment (11, 12) at least partially attributable to an early pattern of dissemination. However, optimal administration of radiation, specifically dosage, and timing, is still elusive. Local as well as distant relapse are major components of treatment failure in several published clinical studies (12–14). The effectiveness of radiation is often limited by normal tissue tolerance and/or by tumor cell resistance to therapy. In the present study, we examined the effects of irradiation on proangiogenic molecules that determine the malignant progression of neuroblastoma tumors. The results have shown that ionizing radiation enhances the release/production of uPA, MMP-9 and VEGF in human neuroblastoma cells and these radiation-induced alterations are associated with an increase in migration and invasive potential of neuroblastoma cells. The results also showed that the angiogenesis, invasion and release/production of VEGF, uPA and MMP-9 could be induced in unirradiated HMECs by preincubation in conditioned medium from irradiated neuroblastoma cells.
Overexpression of VEGF, an important growth factor controlling angiogenesis, has been associated with tumor progression, metastasis, and reduced survival in neuroblastoma and other tumors (15–18). The induction of VEGF in tumor cells by irradiation has been previously reported (19–21). In keeping with these reports, we found an up-regulation of VEGF mRNA transcript and protein after irradiation in neuroblastoma cells analysed. The cancer cells also showed a dose-dependent increase in VEGF secretion after radiation. The irradiated neuroblastoma cells receiving a sublethal dose in clinical radiotherapy might induce VEGF expression and increase VEGF protein between fractions until the accumulated radiotherapy doses reach the tumoricidal level. The radiotherapy-induced VEGF could be a paracrine proliferative stimulus to accelerate the growth of microtumors not included in the radiotherapy field. Irradiation induced VEGF might increase tumor cell survival and thereby lead to a decreased response to irradiation in vivo in agreement with inhibition of VEGF-signalling results in the reversal of tumor resistance to radiotherapy (22).
The present study has also demonstrated that ionizing radiation enhances the production of uPA and MMP-9 but not of MMP-2 in human neuroblastoma cells. Plasminogen activators (PAs) and MMPs are a family of extracellular matrix-degrading enzymes associated with numerous physiological and pathological events such as malignant tumor cell migration and invasion (23–25). After irradiation, uPA expression was increased in association with an increased migration. uPA was overexpressed in high-risk, unfavorable tumor of neuroblastoma and that overexpression was associated with the ability of invasion, metastasis and a prognosis for neuroblastoma (26). MMPs are important in creating and maintaining an environment that initiates and maintains growth of primary and metastatic tumors (27). Among MMPs, neuroblastoma tumors secrete predominantly MMP-2 and MMP-9 (28, 29). MMP-9 is a rate-limiting extracellular protease involved in cell migration across basement membranes and triggers tumor angiogenesis (30, 31). In our study, we showed that irradiation increases the expression and the secretion of MMP-9 by neuroblastoma cells. Neuroblastomas have been reported both by in vitro and in vivo studies to utilize MMPs for invasive growth and spread (32–36). The increase in MMP-9 and uPA expression and secretion, after irradiation of neuroblastoma cells may be responsible for the induction of migration and invasion. It seems that increased expression of MMP-9 and VEGF by radiation can lead to increased angiogenesis.
Radiotherapy increased tumor invasiveness at doses used clinically as a fractionated irradiation, implying that sublethal doses of irradiation could promote tumor migration and distant metastasis. Earlier studies showed that sublethal doses of irradiation enhanced the migration and invasiveness of human glioblastoma cells in association with enhanced expression/activity of MMPs (37, 38). Radiotherapy induced an increase in invasive potential of pancreatic cancer cells via increased activity of MMPs (39). Consequently, pharmaceutical inhibitors of MMPs have successfully prevented radiation-induced tumor cell invasion (39, 40). Our results support previous data that show that sublethal irradiation enhances invasive capability of neuroblastoma cells via up-regulated expression of c-met and increased activity ECM degrading proteases (41). These results favor the concept that irradiation might promote expression of invasion-related genes and rationalizes the use of inhibitors that could interrupt these molecules concomitantly with radiotherapy in cancer treatment.
The increased invasiveness observed in the present study may contribute to dissemination of neuroblastoma cells after irradiation at sublethal doses. Rofstad et al (42) demonstrated that melanoma tumors regrowing after subcurative irradiation showed a higher frequency of lymph node metastasis than unirradiated tumors. The use of radiation to eradicate a primary Lewis lung carcinoma(LCC) has been found to cause accelerated growth of lung metastasis (43,44). Further, studies showed that radiotherapy to the transplanted C-1300 neuroblastoma in hind legs of syngenic mice caused liver metastasis where as no liver metastasis was found in non-irradiated mice (45). The mechanisms underlying the enhancement of metastasis following local tumor irradiation are still unknown.
We further studied the effect of irradiation of neuroblastoma cells on endothelial cells' invasion and angiogenesis in vitro. In our studies, tumor cell conditioned medium from radiation promotes endothelial cell invasion, as well as branching morphogenesis; these are all requisite steps in new vessel formation. In a coculture invasion model, endothelial invasion was enhanced by selectively irradiating the tumor cell compartment, suggesting that ionizing radiation has indirect angiogenic properties. This resulted, at least in part, from ionizing radiation-induced up-regulation of proangiogenic molecules in neuroblastoma cells. This model of elevated paracrine release of proangiogenic molecules may account for the in vivo observations that sublethal doses of ionizing radiation promote migration and invasiveness of tumor cells (37,46). These data suggest that subcurative radiation doses may induce many of the requisite phenotypes for angiogenesis. Likewise, the increase in proangiogenic factors in unirradiated HMECs preincubated in irradiated neuroblastoma conditioned medium could partially explain enhanced endothelial cell invasiveness. These data are supported by previous observations that suggest that the tumor cell compartment might play a role in stimulating its endothelial bed (47) and ionizing radiation–induced interplay between the tumor cell compartment and its endothelial environment may lead to increased tumor radioresistance (47–49). Therefore, the inhibition of radiation-induced secretion of proangiogenic factors might have strong clinical impact on the success rate of radiotherapy.
Acknowledgments
This work was supported in part by Children’s Miracle Network, Peoria, IL and NINDS Grant NS-051625 (to S.M.).
References
- 1.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 2.Escobar MA, Grosfeld JL, Powell RL, West KW, Scherer LR, 3rd, Fallon RJ, Rescorla FJ. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006;41:377–381. doi: 10.1016/j.jpedsurg.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt ML, Lukens JN, Seeger RC, Brodeur GM, Shimada H, Gerbing RB, Stram DO, Perez C, Haase GM, Matthay KK. Biologic factors determine prognosis in infants with stage IV neuroblastoma: A prospective Children's Cancer Group study. J Clin Oncol. 2000;18:1260–1268. doi: 10.1200/JCO.2000.18.6.1260. [DOI] [PubMed] [Google Scholar]
- 4.Laprie A, Michon J, Hartmann O, Munzer C, Leclair MD, Coze C, Valteau-Couanet D, Plantaz D, Carrie C, Habrand JL, Bergeron C, Chastagner P, Defachelles AS, Delattre O, Combaret V, Benard J, Perel Y, Gandemer V, Rubie H. Neuroblastoma Study Group of the French Society of Pediatric Oncology. High-dose chemotherapy followed by locoregional irradiation improves the outcome of patients with international neuroblastoma staging system Stage II and III neuroblastoma with MYCN amplification. Cancer. 2004;101:1081–1089. doi: 10.1002/cncr.20453. [DOI] [PubMed] [Google Scholar]
- 5.Escobar MA, Grosfeld JL, Powell RL, West KW, Scherer LR, 3rd, Fallon RJ, Rescorla FJ. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006;41:377–381. doi: 10.1016/j.jpedsurg.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 6.von Allmen D, Grupp S, Diller L, Marcus K, Ecklund K, Meyer J, Shamberger RC. Aggressive surgical therapy and radiotherapy for patients with high-risk neuroblastoma treated with rapid sequence tandem transplant. J Pediatr Surg. 2005;40:936–941. doi: 10.1016/j.jpedsurg.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Jadhav U, Chigurupati S, Lakka SS, Mohanam S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int J Oncol. 2004;25:1407–1414. [PubMed] [Google Scholar]
- 8.Mohanam S, Chandrasekar N, Yanamandra N, Khawar S, Mirza F, Dinh DH, Olivero WC, Rao JS. Modulation of invasive properties of human glioblastoma cells stably expressing amino-terminal fragment of urokinase-type plasminogen activator. Oncogene. 2002;21:7824–7830. doi: 10.1038/sj.onc.1205893. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22:392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- 10.Yanamandra N, Gumidyala KV, Waldron KG, Gujrati M, Olivero WC, Dinh DH, Rao JS, Mohanam S. Blockade of cathepsin B expression in human glioblastoma cells is associated with suppression of angiogenesis. Oncogene. 2004;23:2224–2230. doi: 10.1038/sj.onc.1207338. [DOI] [PubMed] [Google Scholar]
- 11.O'Reilly R, Cheung NK, Bowman L, Castle V, Hoffer F, Kapoor N, Kletzel M, Lindsley K, Shamberger R, Tubergen D. NCCN pediatric neuroblastoma practice guidelines. The National Comprehensive Cancer Network Oncology. 1996;10:1813–1822. [PubMed] [Google Scholar]
- 12.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 13.Kushner BH, Wolden S, LaQuaglia MP, Kramer K, Verbel D, Heller G, Cheung NK. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 14.Bradfield SM, Douglas JG, Hawkins DS, Sanders JE, Park JR. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer. 2004;100:1268–1275. doi: 10.1002/cncr.20091. [DOI] [PubMed] [Google Scholar]
- 15.Komuro H, Kaneko S, Kaneko M, Nakanishi Y. Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol. 2001;127:739–743. doi: 10.1007/s004320100293. [DOI] [PubMed] [Google Scholar]
- 16.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 17.Marcus K, Johnson M, Adam RM, O'Reilly MS, Donovan M, Atala A, Freeman MR, Soker S. Tumor cell-associated neuropilin-1 and vascular endothelial growth factor expression as determinants of tumor growth in neuroblastoma. Neuropathology. 2005;25:178–187. doi: 10.1111/j.1440-1789.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukata S, Inoue K, Kamada M, Kawada C, Furihata M, Ohtsuki Y, Shuin T. Levels of angiogenesis and expression of angiogenesis-related genes are prognostic for organ-specific metastasis of renal cell carcinoma. Cancer. 2005;103:931–942. doi: 10.1002/cncr.20887. [DOI] [PubMed] [Google Scholar]
- 19.Hovinga KE, Stalpers LJ, van Bree C, Donker M, Verhoeff JJ, Rodermond HM, Bosch DA, van Furth WR. Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in glioblastoma multiforme cell lines--a clue to radioresistance? J Neurooncol. 2005;74:99–103. doi: 10.1007/s11060-004-4204-7. [DOI] [PubMed] [Google Scholar]
- 20.Brieger J, Schroeder P, Gosepath J, Mann WJ. Vascular endothelial growth factor and basic fibroblast growth factor are released by squamous cell carcinoma cells after irradiation and increase resistance to subsequent irradiation. Int J Mol Med. 2005;16:159–164. [PubMed] [Google Scholar]
- 21.Chung YL, Jian JJ, Cheng SH, Tsai SY, Chuang VP, Soong T, Lin YM, Horng CF. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2706–2715. doi: 10.1158/1078-0432.CCR-05-2721. [DOI] [PubMed] [Google Scholar]
- 22.Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, Hallahan DE. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 23.Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types. APMIS. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 24.Adair JC, Baldwin N, Kornfeld M, Rosenberg GA. Radiation-induced blood-brain barrier damage in astrocytoma: relation to elevated gelatinase B and urokinase. J Neurooncol. 1999;44:283–289. doi: 10.1023/a:1006337912345. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K, Tanaka Y, Mimori K, Inoue H, Mori M. Differential expression of MMP and uPA systems and prognostic relevance of their expression in esophageal squamous cell carcinoma. Int J Cancer. 2004;110:201–207. doi: 10.1002/ijc.20067. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Gao Y, Ji Z, Zhang X, Xu Q, Li G, Guo Z, Zheng B, Guo X. Role of urokinase plasminogen activator and its receptor in metastasis and invasion of neuroblastoma. J Pediatr Surg. 2004;39:1512–1519. doi: 10.1016/j.jpedsurg.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 28.Ribatti D, Surico G, Vacca A, De Leonardis F, Lastilla G, Montaldo PG, Rigillo N, Ponzoni M. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 correlate with progression in human neuroblastoma. Life Sci. 2001;68:1161–1168. doi: 10.1016/s0024-3205(00)01030-4. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura Y, Shimada H, Seeger RC, Laug WE, DeClerck YA. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 30.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 32.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 33.Ribatti D, Alessandri G, Vacca A, Iurlaro M, Ponzoni M. Human neuroblastoma cells produce extracellular matrix-degrading enzymes, induce endothelial cell proliferation and are angiogenic in vivo. Int J Cancer. 1998;77:449–454. doi: 10.1002/(sici)1097-0215(19980729)77:3<449::aid-ijc22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999;10:353–367. [PubMed] [Google Scholar]
- 35.Bjornland K, Bratland A, Rugnes E, Pettersen S, Johansen HT, Aasen AO, Fodstad O, Ree AH, Maelandsmo GM. Expression of matrix metalloproteinases and the metastasis-associated gene S100A4 in human neuroblastoma and primitive neuroectodermal tumor cells. J Pediatr Surg. 2001;36:1040–1044. doi: 10.1053/jpsu.2001.24735. [DOI] [PubMed] [Google Scholar]
- 36.Sartor L, Negro A, Barletta E, Mugnai G, Garbisa S. Modulation of proteolytic potential and differentiation by CNTF and BDNF in two mouse neuroblastoma clones: relation to invasion. Clin Exp Metastasis. 2002;19:709–716. doi: 10.1023/a:1021302802297. [DOI] [PubMed] [Google Scholar]
- 37.Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001;61:2744–2750. [PubMed] [Google Scholar]
- 38.Wick W, Wick A, Schulz JB, Dichgans J, Rodemann HP, Weller M. Prevention of irradiation-induced glioma cell invasion by temozolomide involves caspase 3 activity and cleavage of focal adhesion kinase. Cancer Res. 2002;62:1915–1919. [PubMed] [Google Scholar]
- 39.Qian LW, Mizumoto K, Urashima T, Nagai E, Maehara N, Sato N, Nakajima M, Tanaka M. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res. 2002;8:1223–1227. [PubMed] [Google Scholar]
- 40.Kaliski A, Maggiorella L, Cengel KA, Mathe D, Rouffiac V, Opolon P, Lassau N, Bourhis J, Deutsch E. Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol Cancer Ther. 2005;4:1717–1728. doi: 10.1158/1535-7163.MCT-05-0179. [DOI] [PubMed] [Google Scholar]
- 41.Schweigerer L, Rave-Frank M, Schmidberger H, Hecht M. Sublethal irradiation promotes invasiveness of neuroblastoma cells. Biochem Biophys Res Commun. 2005;330:982–988. doi: 10.1016/j.bbrc.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 42.Rofstad EK, Mathiesen B, Galappathi K. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res. 2004;64:13–18. doi: 10.1158/0008-5472.can-03-2658. [DOI] [PubMed] [Google Scholar]
- 43.Wei LH, Lai KP, Chen CA, Cheng CH, Huang YJ, Chou CH, Kuo ML, Hsieh CY. Arsenic trioxide prevents radiation-enhanced tumor invasiveness and inhibits matrix metalloproteinase-9 through downregulation of nuclear factor kappaB. Oncogene. 2005;24:390–398. doi: 10.1038/sj.onc.1208192. [DOI] [PubMed] [Google Scholar]
- 44.Camphausen K, Moses MA, Beecken WD, Khan MK, Folkman J, O'Reilly MS. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61:2207–2211. [PubMed] [Google Scholar]
- 45.Iwakawa M, Kaneko M, Ikebulkuro K. Enhanced metastasis after local therapy in a murine model using C-1300 neuroblastoma. J Pediatr Surg. 1999;34:1645–1646. doi: 10.1016/s0022-3468(99)90635-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhai GG, Malhotra R, Delaney M, Latham D, Nestler U, Zhang M, Mukherjee N, Song Q, Robe P, Chakravarti A. Radiation enhances the invasive potential of primary glioblastoma cells via activation of the Rho signaling pathway. J Neurooncol. 2006;76:227–237. doi: 10.1007/s11060-005-6499-4. [DOI] [PubMed] [Google Scholar]
- 47.Brown CK, Khodarev NN, Yu J, Moo-Young T, Labay E, Darga TE, Posner MC, Weichselbaum RR, Mauceri HJ. Glioblastoma cells block radiation-induced programmed cell death of endothelial cells. FEBS Lett. 2004;565:167–170. doi: 10.1016/j.febslet.2004.03.099. [DOI] [PubMed] [Google Scholar]
- 48.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 49.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]


