Abstract
Age of sexual maturity, daily and lifetime reproductive rates, and life span were recorded in a laboratory cohort of Mexican fruit flies consisting of over 1100 females maintained individually. The results revealed that, relative to the medfly, the Mexfly is slower maturing (14 vs 17 days), more fecund (1400 vs 650–1100 eggs/female), and longer lived (50 vs 35 days). The results reinforced the generality of several earlier findings on the medfly including the deceleration of mortality at older ages and the weakness of the correlation between the rate of egg laying at early ages and both subsequent reproduction and remaining longevity. Discussion includes perspectives on the role of artificial selection in shaping the demographic traits of the mass-reared strain of Mexfly used in this study, as well as the overall significance of large scale biodemographic studies in understanding aging and longevity.
Keywords: Biodemographic studies, Mexfly, Medfly
1. Introduction
Although the literature in biogerontology, biodemography and ecology contains life history information on a wide variety of invertebrate and vertebrate species, there appears to be published data on only three species (all invertebrates) in which life span and age-specific birth rates were derived from large numbers of individuals maintained in solitary confinement under controlled conditions. These include the nematode, Caenorhabditis elegans (Chen et al., 2005; Johnson et al., 2001), the fruit fly, Drosophila melanogaster (Clark and Guadalupe, 1995; Curtsinger et al., 1992; Promislow et al., 1996; Tatar et al., 1996), and the Mediterranean fruit fly, Ceratitis capitata (Carey, 2003). Remarkably, Homo sapiens is the only other species for which data on reproductive histories are available for large numbers of individuals (Wood, 1994).
There are several reasons why large-scale biodemographic studies of individuals are important. First, a large database on individual-level reproduction provides stronger statistical power for assessing the relationship between early reproduction and old-age mortality as well as for correlating lifetime reproduction and total longevity (Bell and Koufopanou, 1986; Chippindale et al., 1997; Partridge, 1987). The use of extraordinarily large initial numbers of individuals is particularly important for mortality estimates at advanced ages. Second, comparative biodemographic studies of closely-related species are possible when detailed birth and death data exists on large numbers of individuals of multiple species. For example, the results of large-scale biodemographic study on a new fruit fly species will shed light on whether many of the observations on the medfly concerning age patterns of reproduction and reproductive costs (Carey, 2003) are unique to the medfly or more general. Third, subtle age patterns of reproduction at the level of the individual shed important light on inter- and intra-individual aging. For example, a patterns of sporadic or intermittent egg laying at older ages are difficult to characterize when observed in a small number of older individuals but much easier and more definitive when these patterns are observed in larger numbers.
Because of the paucity of species upon which large-scale biodemographic studies have been conducted and thus the limited perspective on the relationship between birth and death rates at the level of the individual, we initiated a study to monitor age-specific reproduction and life span in over 1100 individual Mexican fruit flies (Anastrepha ludens), commonly known as Mexflies. Our broad objectives were to add a new species to the limited literature involving large-scale biodemographic studies and, because the Mexfly is closely related to the medfly, to create a database for use in making biodemographic comparisons of the two species, particularly comparisons between the species-specific relationship of longevity and reproduction. Our specific goals were to determine the age-specific patterns of reproduction in Mexfly subcohorts, to examine the cost of reproduction (i.e. relationship between reproduction at young ages and longevity), and to document individual life span and lifetime reproduction in a large cohort of flies.
2. Methods and materials
2.1. Background
The Mexican fruit fly, A. ludens, belongs to the dipteran family Tephritidae—a group of about 4000 species referred to as the ‘true’ fruit flies that is distributed throughout most of the world (Christenson and Foote, 1960). Members of this group (which includes the Mediterranean fruit fly, C. capitata, commonly known as the medfly) lay eggs in intact fruit using their sharp ovipositor rather than on decaying fruit as do their distant relatives the gnat-sized vinegar flies in the family Drosophilidae (also referred to as pomace flies).
The genus of the Mexfly Anastrepha is a large Neotropical group with over 190 known species (Norrbom and Foote, 1989) that are endemic to the American tropics and subtropics and distributed from Central American and the West Indies to Argentina and Chile. The distribution of A. ludens is restricted to Mexico and northern countries of Central America (Aluja, 1994). The life cycle of the Mexfly is typical of most tephritids (Bateman, 1972; Christenson and Foote, 1960; Fletcher, 1989) where adult mated females deposit their eggs within host fruit, the eggs hatch into larvae within 2–4 days and begin feeding on the fruit pulp. Within 1–2 weeks, the larvae complete their development and exit the fruit to pupate in the ground. After about 2 weeks, the adults emerge from the pupae and begin laying eggs in one to two weeks, depending on mating, host, dietary and climatic conditions. The literature on this species indicates that it lays between 800 and 1200 eggs/female over its lifetime and lives an average of 20–40 days (Baker, 1944; Berrigan, 1988; Berrigan et al., 1988; Liedo et al., 1992; Mangan, 2003).
2.2. Source of flies
The Mexflies used in the current study were reared from pupae taken from the fruit fly mass-production facility (Vargas, 1989) in Metapa, Mexico (Liedo et al., 1993; Vargas, 1989). The general history of the Mexfly strain currently used for mass rearing is similar to that for many tephritid species used in factory production (Leppla, 1989) (P. Liedo, personal communication) involving (i) colonization of flies several decades earlier (i.e. originally collected in the 1960s from infested fruit near Monterrey, Mexico); (ii) small-scale rearing for use in pilot studies for several decades; (from 1960 to 1980); (iii) periodical addition of wild-caught flies to increase vitality and enhance genetic diversity including introduction of wild-caught flies in 2003; and (iv) constant improvement in both rearing technology and increase in scale. The Mexfly used in the current study fits the criteria of a ‘domestic phenotype’ —that combination of phenotypic traits that enables an animal to adapt to man and the captive environment man provides for that species (Price, 1984). This strain can thus be considered a ‘periodically refreshed’ laboratory biotype several hundred generations removed from the wild adapted to adult crowding and for ovipositing into either an artificial medium (moist chamois-like material) or nylon mesh.
2.3. Experimental framework
The biodemographic study of Mexfly females was conducted at the fruit fly mass rearing facility near Metapa, Chiapas, Mexico. Rearing conditions throughout this period were 26 ± 2 °C, 80 ± 10% relative humidity and 12:12 light: dark cycle. The Mexfly adults used in this study were obtained from the regular rearing process and maintained in 4 × 4 × 10 cm plastic cages. The eggs were laid on a 1.5 cm wide black silicon stripe applied over mesh covering the front of each cage. Water and ad libitum food (3:1 sugar-yeast dry mixture dissolved in water) were provided as droplets on slides each day. At eclosion a single adult of each sex was placed in each individual cage. Males were replaced with same-aged, virgin males if the male died before the female. A total of 34 successive cohorts of 10, 25, 50 or 100 pairs were set up at irregular intervals (number varied according to time available to technicians) and daily egg production and age at death were recorded from a total of 1151 females.
3. Results
3.1. Survival and mortality
Life table analysis supports the findings in previous small-scale studies that the Mexfly is a long-lived tephritid with its longevity mean, median, mode, and maximum of 49.1, 51, 55, and 96 days, respectively. The mean is lower than the median and modal ages of death because of the ‘premature’ deaths of females at younger ages. The age of death in the oldest female in the cohort was nearly twice that of the average female.
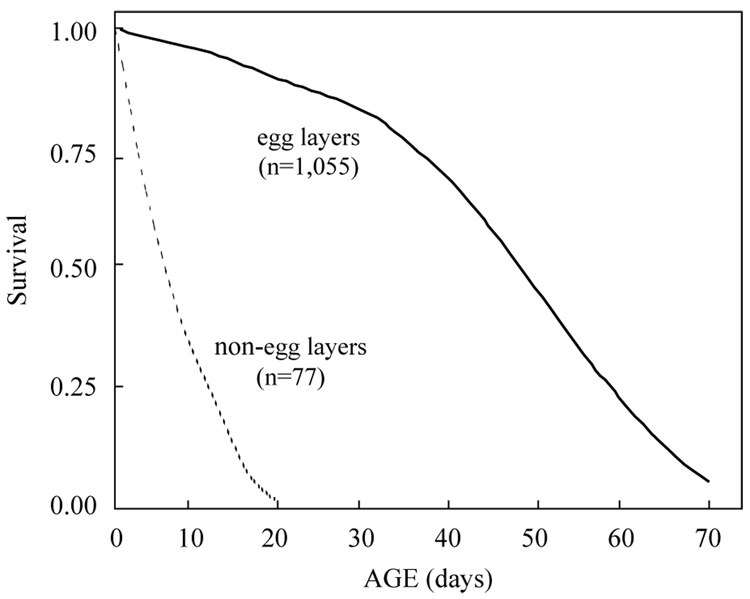
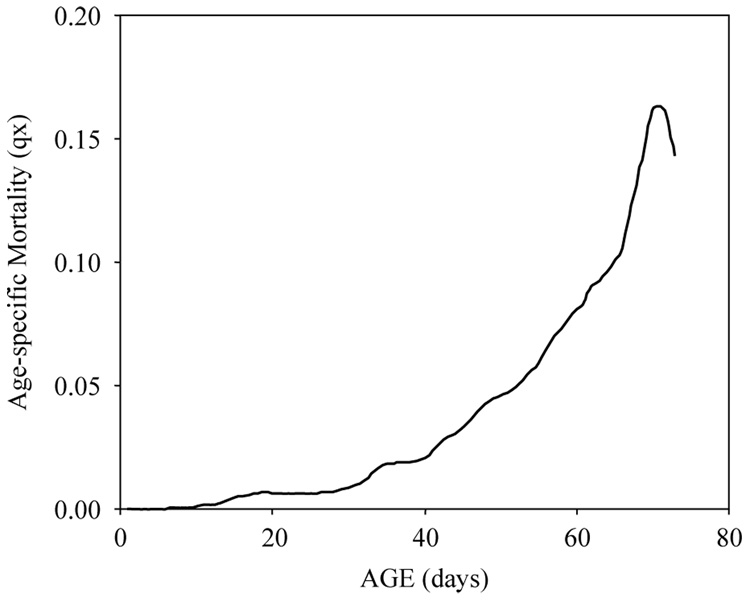
The age-specific survival schedules for both non-egg laying and egg laying females are shown in Fig. 1 which reveals the rapid, near-linear decline in survival for females that laid no eggs during their life time and the reverse sigmoidal decline in survival of egg laying females—a gentle decline through the first month followed by more rapid decrease in survival. This pattern is the outcome of low mortality during the first month followed by rapidly-increasing mortality for the following 50 days as shown in Fig. 2. Note that mortality decelerated at around 70 days and decreased thereafter. The life expectancy of non-egg layers was substantially less than the life expectancy of egg layers because (i) flies that die before maturation (by definition) will be non-egg layers; and (ii) the lack of egg laying in flies that lived long enough to mature eggs was likely a reflection of poor overall condition rather than merely sterility. That no infecund flies lived beyond 3 weeks indicates that sterility was a marker of frailty.
Fig. 1.
Age-specific survival for Mexican fruit fly females in non-egg laying and egg-laying subcohorts.
Fig. 2.
Smoothed age-specific mortality rates for Mexican fruit fly females maintained in the laboratory.
3.2. Reproduction
3.2.1. Event history graph of reproduction
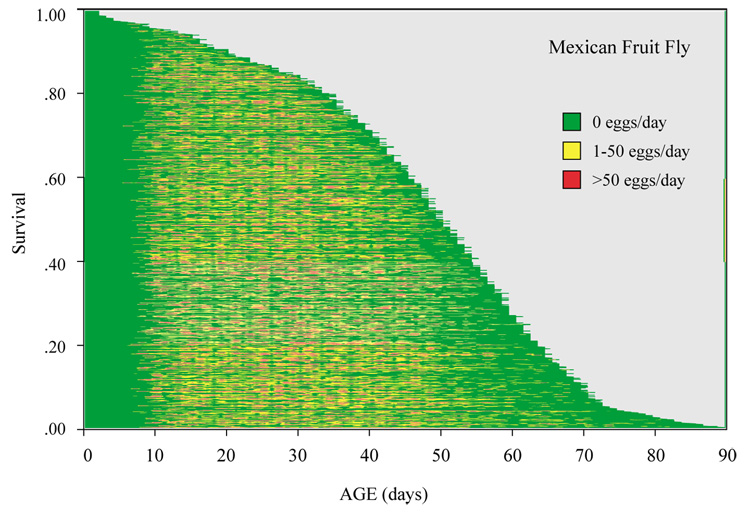
An event-history diagram (Carey et al., 1998b) showing age-specific reproductive patterns and heterogeneity in the Mexfly cohort is given in Fig. 3. Mexfly maturation rates, determined by the age at which the first egg an individual laid, averaged 12.2 days (SD=3.90) with a range of from 6 to 59 days. This pattern can be seen in Fig. 3 as the left-most green-coded region and, with the exception of the first 10% of flies to die (top of graph), shows no relationship to either egg laying intensity or of individual life span. These pre-oviposition fly-days accounted for 23.1% of all fly-days. Non-oviposition days lived by mature females (i.e. days after they began ovipositing) accounted for 21.3% of all fly-days. Thus, the percentage of a female’s life spent not laying eggs once egg laying commenced was nearly as great as during her pre-oviposition period. The average Mexfly female laid at least some eggs every other day (i.e. 56% of the time), and over 50 eggs approximately one day per week (red-coded ages in Fig. 3). In short, the Mexfly is an extraordinarily fecund species because of the frequency, intensity and duration of egg laying.
Fig. 3.
Event-history diagrams (Carey et al., 1998b) visualizing the demographic response of individual Mexican fruit fly females. Each horizontal line denotes a female ‘life line’, the length of which is proportional to her life span. The age-specific egg laying intensity of each of the 1151 flies corresponds to the shading: green=zero eggs/day; yellow=1–50 eggs/day; red=>50 eggs/day. Graphic depicts information on 1.6 million Mexfly eggs distributed over 31,000 egg-laying days. (For interpretation of the reference to colour in this legend, the reader is referred to the web version of this article.)
3.2.2. Daily and age-specific reproduction
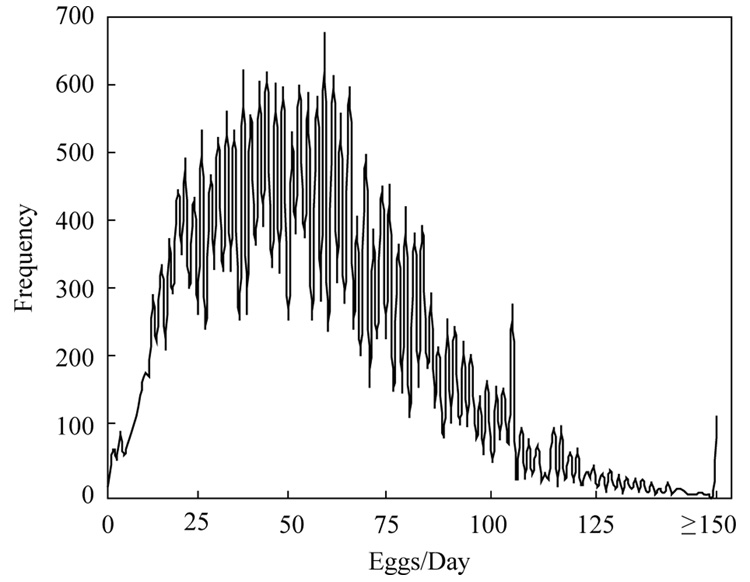
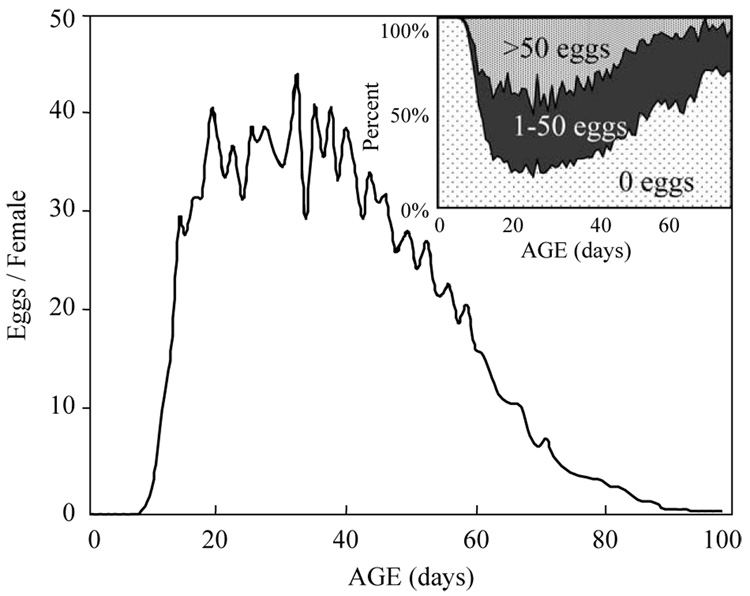
Daily egg production over all ages (lifetimes) and Mexfly females ranged from 0 to over 150 eggs (Fig. 4). Females laid between 25 and 60 eggs half of the days in which they laid eggs and produced over 100 eggs/day nearly 6% of the days that they laid eggs. Age patterns of reproduction given in Fig. 5 show that per capita egg production in the cohort was greatest between 3 and 6 weeks when the average female produced 35–50 eggs each day. This peak production rapidly declined after 6 weeks due primarily to an increase in the proportion of females, which produced zero eggs accounts (Fig. 5 inset). The highest proportion of females that laid eggs was greatest around 30 days when over three quarters of females laid some eggs and was lowest at the older ages (> 70 days) when nearly this same fraction of females laid no eggs. The highest levels of egg laying occurred between 20 and 50 days when 40–50% of the females laid over 50 eggs per day (Fig. 5 inset). However, the fraction of heavy egg layers in cohort tapered off at older ages (> 60 days) when less than 5% of living females laid over 50 eggs per day.
Fig. 4.
Frequency distribution of daily egg production derived from data on 31,221 egg-laying days in a cohort of 1151 Mexfly females.
Fig. 5.
Age-specific schedule of reproduction for 1151 Mexfly females (lifetime reproduction=1416.4 eggs/female). Inset: proportion of females at each age which laid either 0, 1–50, or>50 eggs/day.
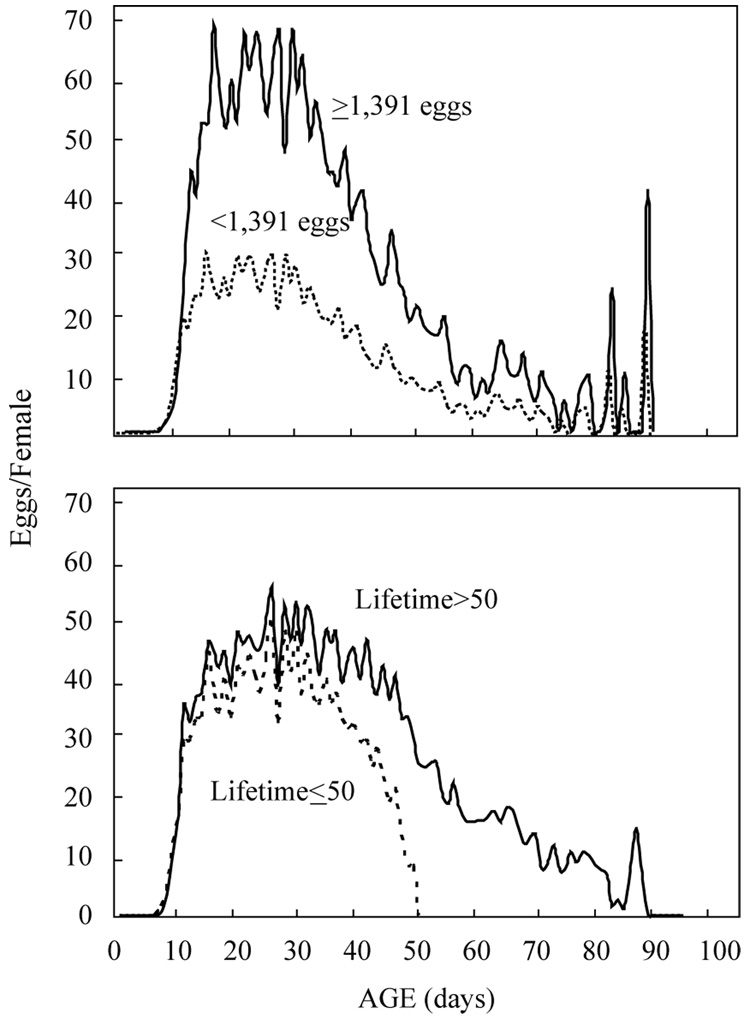
Schedules of age-specific reproduction for two subcohorts of females (high and low lifetime reproduction) reveal that (Fig. 6): (1) there is no sharply-defined peak period of egg production for either high or low lifetime egg producers. Rather egg production is maintained at relatively high levels starting at around 2 weeks and extending through 5–7 weeks, depending upon the subcohort; (2) although the general timing of maximal daily egg production is roughly the same for the two subcohorts, the intensity differs substantially—approximately 30 eggs/female in the lower lifetime egg production subcohort vs over 60 eggs/female in the higher lifetime egg production subcohort; and (3) egg production in both subcohorts continues to the oldest ages (90 days). The daily egg laying shown in Fig. 6 (lower panel) for the female cohort disaggregated by lifetime reveals virtually identical egg-laying patterns between the shorter- and longer-lived individuals at the younger ages ( < 30 days). Also the graphs show that the rate of decrease in egg production falls off very rapidly in the shorter-lived flies than in the longer-lived flies after 30 days.
Fig. 6.
Age schedules for subcohorts of Mexfly females disaggregated according to either total lifetime eggs (top) or lifetime (bottom). Solid lines depict reproduction in females that produced≥1391 eggs in their lifetime (top) or lived>50 days (bottom). Dashed lines depict the same relationships except for females that laid<1391 eggs or lived≤50 days, respectively. Note that the age patterns (e.g. peaks) for each of the subcohorts in both the top and bottom graphs are similar.
3.2.3. Relationship of reproduction and longevity
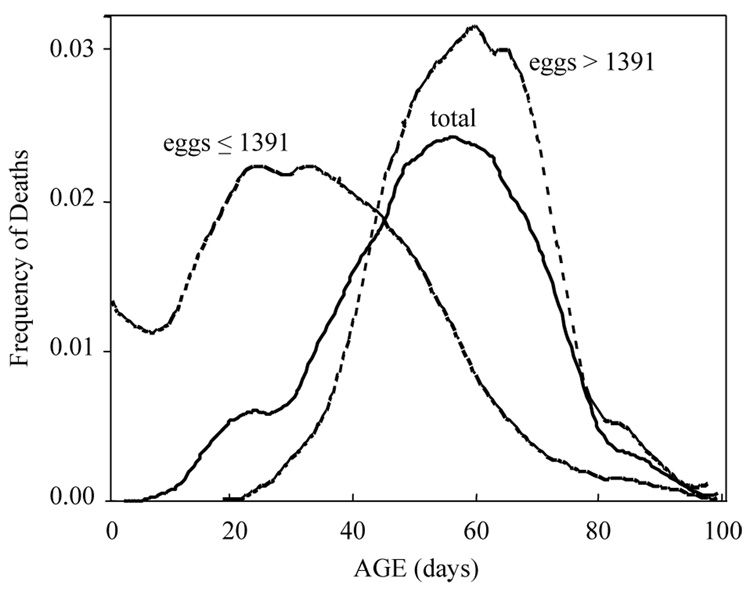
The density estimations of the lifetimes for females that laid either more than 1391 eggs or less than or equal to 1391 eggs as well as the density estimation for the whole cohort are presented in Fig. 7. The distribution of remaining lifetimes for the subgroup of flies that laid over 1391 eggs is skewed farther to the right than is the subgroup that laid less than this lifetime number of eggs. The shape and thus the heights of the two distributions also differ. This difference in the lifetime distributions reflects the correlation between life time and reproduction—given constraints on daily egg production, the longer-lived females have more days in which to produce eggs.
Fig. 7.
Frequency distributions of death in 1151 Mexfly females including total deaths and deaths disaggregated by subcohorts distinguished by lifetime egg production.
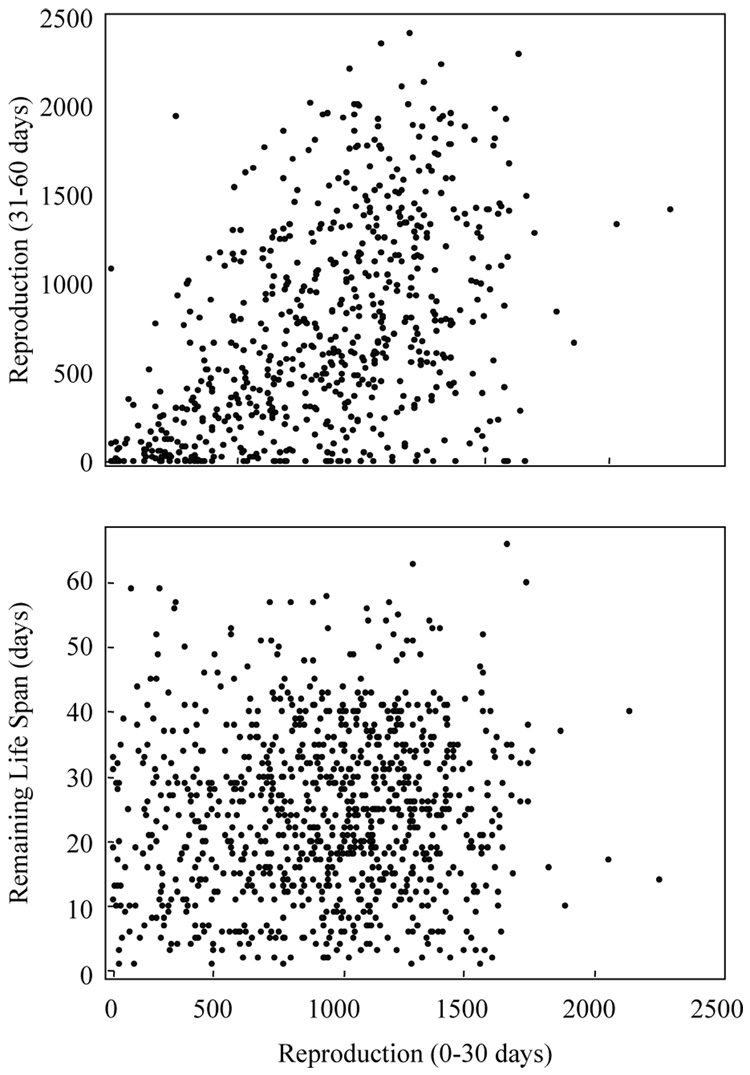
The number of eggs laid by Mexfly females at young ages (0–30 days) was only weakly correlated (r=0.498) with the number of eggs that females laid at older ages (31–60 days) and uncorrelated (r=0.083) with remaining life span as shown graphically in Fig. 8. The main point is this—knowledge of early egg laying provides no information for predicting either remaining life span or the number of eggs laid during the subsequent one-month period. Thus, at the level of the individual in non-manipulative studies such as the current one, there appears to be no obvious cost of reproduction. This lack of cost was also observed in a similar non-manipulative study of the medfly (Carey et al., 1998a) and may have a simple explanation—because food is available in unlimited supply, there is no demand and therefore there is no cost (Costa and Sinervo, 2004).
Fig. 8.
Relationship between the number of eggs laid by Mexican fruit fly females from eclosion (age 0) through 30 days and (a) eggs laid in the interval 31–60 days (r-value=0.498); and (b) remaining life span (r-value=0.083). The wide scatter in both plots reveals low of predictive power of knowledge of early egg laying for number of eggs laid in the next one-month interval (a) or remaining life span (b).
There are more data-points in the lower graph (n=953) than in the top one (n=621) because data were used from those individuals who lived to age 30 in the lower graph but to age 60 in the top graph—a criteria required for comparing egg-laying in all females which were alive in both 30-day intervals (i.e. 0–30 and 31–60). The next section contains the results of a more formal statistical analyses of the relationship between Mexfly mortality and reproduction.
3.2.4. Statistical models
The interplay of current egg-laying and of cumulative eggs laid in their effect on mortality was examined using the Cox hazard regression model. Different combinations of three time dependent predictors were considered in modeling survival of Mexflies including the number of eggs laid at time t by the ith fly, pti(t), the number of cumulative eggs laid to time t by the ith fly, cti(t), and the relative growth rate of the egg output at time t, pcti(t); i.e. {0, if pti(t)=0; pti(t)/cti(t), if pti(t)≠0}. The Cox model, log hi(t) = α(t) + β1pti(t) + β2cti(t)/100. where hi(t) is the hazard of the ith fly at time t was found to be the model that fits best to the data with smallest AIC, BIC and −2 log(likelihood) values among all alternatives. Here AIC stands for the Akaike Information criterion, with penalty=2p, where p is the number of parameters in the model, while BIC is the Bayesian Information Criterion with penalty=p log(n), where n is the number of subjects. Minimizing the values of either AIC or BIC are commonly used model selection criteria (Shibata, 1981). The interaction between pt and ct/100 has not been included in the model, since it is not found significant. The models have been fitted to the data using the Proc Phreg procedure of SAS. The output from SAS revealed that the time-varying predictors of daily egg-laying (pt) and cumulative egg laying counts normalized by 100 (ct/100) are of significant predictive value (p-values <0.0001).
The statistical models yielded two main findings: (1) at a given time t, the current eggs/day {pt(t)} is found to have a negative effect, so declines in egg-laying are associated with increased mortality; and (2) the cumulative number of eggs to time t {ct(t)}, is found to have a positive effect, so increasing accumulated numbers of eggs is also associated with increasing mortality. Thus, the highest mortality occurs for flies which have laid a lot of eggs but currently lay few eggs, and lowest mortality for those who have not laid a lot of eggs cumulatively but currently are laying many eggs. In short, the dual status of a female Mexfly’s cumulative and current egg production reveals her level of mortality risk at a given age.
4. Discussion
The demographic details that we observed in this study of the Mexfly are consistent with the specific findings from several earlier (but smaller-scale) studies on this species (Berrigan, 1988; Berrigan et al., 1988; Liedo et al., 1992) as well as with respect to tephritid life history traits in general (Bateman, 1972; Christenson and Foote, 1960; Fletcher, 1989) including rapid maturation time, high fecundity, and extended longevity. The medfly is the only other generalist tephritid on which comparable large-scale biodemographic data has been gathered and, therefore, on which valid biodemographic comparisons can be made (Carey, 2003). These comparisons include: (1) Longevity. Both species of tephritids are relatively long lived with the life expectancy of the Mexfly (females-only) in the current study at slightly less than 50 days and in the medfly in previous studies ranging from 33 to 42 days. Thus, the longevity of Mexfly under laboratory conditions is approximately 20–50% greater than in the medfly. (2) Mortality deceleration. Slowing of mortality at older ages has been exhaustively documented in the medfly (Carey, 2003; Carey et al., 1992) and was also observed in an earlier large-scale study of the Mexfly (Vaupel et al., 1998) as well as in the current study. (3) Lifetime fecundity. Both tephritid species are capable of laying 30–50 eggs per day during peak reproductive ages and 10–20 eggs per day at many of the older ages. Thus, the observed lifetime egg production in both these species is extraordinarily high with fecundity in the medfly ranging from 640 to 1150 eggs/female (Carey, 2003) and in the Mexfly in the current study at 1400 eggs/female, a fecundity higher than any lifetime rate observed in earlier studies on the medfly. (4) Reproduction and longevity. Studies of reproduction in both species revealed that the correlation between the intensity of early reproduction (i.e. numbers of eggs laid) and subsequent reproduction as well as remaining life span was either weak or non-existent (Carey et al., 1998a). However, two analyses of medfly data reveal relationships between reproduction and longevity that may also be present in the Mexfly including: (i) the rate of decrease in egg laying for individual medflies at young ages was positively correlated with their probability of death at subsequent ages (Müller et al., 2001); and (ii) individual-level reproduction can be characterized in a three-stage pattern: Stage I—reproductive stage (maturation); Stage II—a maturity stage characterized by a constant rate of production; and Stage III—a reproductive senescent stage with exponentially -decreasing pattern of egg production with age (Noveoseltsev et al., 2004; Novoseltsev et al., 2004). The quantitative characteristics (e.g. duration; rate of change) can be used in comparative contexts such as between medfly and Drosophila or, in the current context, between the medfly and the Mexfly.
The demographic characteristics of the Mexfly strain used in this study were determined partly by the genetic traits of the original founding stock (Roderick, 1996) and partly as a result of the selection process that existed in the mass-rearing factory including genetic modifications during colony establishment, growth and maintenance (Leppla, 1989; McDonald, 1976, 1983; Vargas, 1989). Whereas the wild Mexflies from which the factory stock was created were required to forage for food, water, mates and hosts in order to survive and reproduce, the mass-reared strain on which the study was conducted had neither the opportunity nor the need to forage. Thus, environmental conditions in the production facility favored individuals that invested maximally in growth, maturation and egg production, rather than in behaviors associated with foraging or in adaptations required for extending longevity as would be present in their free-living counterparts (Price, 1984). Selection for these demographic traits was intensified by protocols in more recent rearing procedures specifying that all adults be replaced (and thus killed) with newly-emerged adults after 14 days. Consequently the biodemographic traits of the mass-reared Mexflies used in the current study (Carey and Vargas, 1985; Hurtado et al., 1988; Liedo and Carey, 1994; Liedo et al., 1992) were shaped by intense selection for individuals with the ability to mature rapidly, mate quickly and reproduce in large numbers in two weeks or less with zero variation on this age limit. Thus, despite having been subjected to a long-term selection regime that would favor individuals that exhibited neither extended longevity nor egg laying beyond relatively young ages, both reproduction and survival at older ages in the factory-selected flies were quite high. This observation is consistent with the results of selection studies on both the medfly (Carey et al., 1998a) and Drosophila melanogaster (Arking et al., 2002; Rose, 1984; Tatar et al., 1996) where, even though only the youngest individuals were allowed to breed each generation, cohort longevity and reproduction at older ages remained extremely high. This is remarkable in light of the extremely young ages at which all flies were killed for scores of generations. This general finding across all three species underscores the operational constraints of life history traits selection as well as the importance of understanding the weak correlation (and thus weak selection) between levels of reproduction at early and late ages.
The current study enhances the biogerontology literature by increasing to four, the number of non-human species for which birth and death rates are available for large sample sizes. Although tephritid fruit flies such as the medfly and Mexfly are less well suited than are Drosophila spp. for certain kinds of basic research (e.g. genetics), species in this family of dipterans are ideal models for biodemographic research since the adults are large and robust, their sex can easily be determined by visual inspection, all stages are adaptable to mass-rearing, and females readily lay eggs in artificial ovipositional devices. The results of the current study reveals similarities and differences between the Mexfly and the medfly, reinforces earlier claims that the results of the medfly studies are general including the weak correlation between early egg laying and remaining life span and the slowing of mortality at older ages. More generally the results of the study provide a baseline for future research on persistent questions about the nature of aging at the level of the whole organism including costs of reproduction (Carey, 2003), dietary restriction (Carey et al., 2002), behavioral gerontology (Papadopoulos et al., 2002; Papadopoulos et al., 2003), and aging in natural populations (Müller et al., 2004).
Acknowledgements
The authors thank A. Oropeza, R. Bustamante, E. de Leon, S. Salgado, R. Rincón, A. Villela and G. Rodas for technical assistance and the Moscamed-MoscaFrut program in Mexico for allowing us use their facilities in Metapa. This research was supported by grants from the National Institute on Aging (P01-AG022500-01; P01-AG08761-10).
References
- Aluja M. Bionomics and management of Anastrepha. Annual Review of Entomology. 1994;39:155–178. [Google Scholar]
- Arking R, Novoselteva J, Hwangbo D-S, Novoseltev V, Lane M. Different age-specific demographic profiles are generated in the same normal-lived Drosophila strain by different longevity stimuli. Journal of Gerontology: Biological Sciences. 2002;57A:B390–B398. doi: 10.1093/gerona/57.11.b390. [DOI] [PubMed] [Google Scholar]
- Baker AC. A review of studies on the Mexican fruit fly and related Mexican species. USDA Miscellaneous Publication. 1944;531:1–157. [Google Scholar]
- Bateman MA. The ecology of fruit flies. Annual Review of Entomology. 1972;17:493–518. [Google Scholar]
- Bell G, Koufopanou V. The cost of reproduction. In: Dawkins R, Ridley M, editors. Oxford Surveys in Evolutionary Biology. Oxford: Oxford University Press; 1986. pp. 83–131. [Google Scholar]
- Berrigan D. Masters Thesis. Davis: University of California; 1988. Clutch size and host preference of the Mexican fruit fly, Anastrepha ludens (Diptera: Tephritidae) p. 111. [Google Scholar]
- Berrigan D, Carey JR, Aguilar JG, Hurtado HC. Age and host effects on clutch size in Anastrepha ludens. Entomologia Experimentalis et Applicata. 1988;47:73–80. [Google Scholar]
- Carey JR. Longevity: The Biology and Demography of Life Span. Princeton: Princeton University Press; 2003. [Google Scholar]
- Carey JR, Vargas R. Demographic analysis of insect mass rearing: case study of three tephritids. Journal of Economic Entomology. 1985;78:523–527. [Google Scholar]
- Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Chiou J-M. Relationship of age patterns of fecundity to mortality, longevity, and lifetime reproduction in a large cohort of Mediterranean fruit fly females. Journal of Gerontology: Biological Sciences. 1998a;53A:B245–B251. doi: 10.1093/gerona/53a.4.b245. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Functional Ecology. 1998b;12:359–363. [Google Scholar]
- Carey JR, Liedo P, Harshman L, Müller H-G, Partridge L, Wang J-L, Zhang Y. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Carey JR, Caswell-Chen EP. Longevity extension is independent of reproductive life span in Caenorhabditis elegans: comparative biodemography of wild-type, Clk-1 and Daf-2. Aging Cell. submitted for publication. [Google Scholar]
- Chippindale AK, Leroi AM, Saing H, Borash DJ, Rose MR. Phenotypic plasticity and selection in Drosophila life history evolution. 2. Diet, mates and the cost of reproduction. Journal of Evolutionary Biology. 1997;10:269–293. [Google Scholar]
- Christenson LD, Foote RH. Biology of fruit flies. Annual Review of Entomology. 1960;5:171–192. [Google Scholar]
- Clark AG, Guadalupe RN. Probing the evolution of senescence in Drosophila melanogaster with P-element tagging. Genetica. 1995;96:225–234. doi: 10.1007/BF01439576. [DOI] [PubMed] [Google Scholar]
- Costa DP, Sinervo B. Field physiology: physiological insights from animals in nature. Annual Review of Physiology. 2004;66:209–238. doi: 10.1146/annurev.physiol.66.032102.114245. [DOI] [PubMed] [Google Scholar]
- Curtsinger JW, Fukui HH, Townsend DR, Vaupel JW. Demography of genotypes: failure of the limited life-span paradigm in Drosophila melanogaster. Science. 1992;258:461–463. doi: 10.1126/science.1411541. [DOI] [PubMed] [Google Scholar]
- Fletcher BS. The biology of Dacine fruit flies. Annual Review of Entomology. 1989;32:115–144. [Google Scholar]
- Hurtado H, Liedo P, Aluja M, Guillen J, Berrigan D, Carey JR. Demography of three Mexican tephritids: Anastrepha ludens, A. obliqua and A. serpentina. Florida Entomologist. 1988;71:110–120. [Google Scholar]
- Johnson TE, Wu D, Tedesco P, Dames S, Vaupel JW. Age-specific demographic profiles of longevity mutants in Caenorhabditis elegans show segmental effects. Journal of Gerontology: Biological Sciences. 2001;56A:B331–B339. doi: 10.1093/gerona/56.8.b331. [DOI] [PubMed] [Google Scholar]
- Leppla NC. Laboratory colonization of fruit flies. In: Robinson AS, Hooper G, editors. World Crop Pests. Fruit Flies: Their Biology, Natural Enemies and Control. Amsterdam: Elsevier; 1989. pp. 91–103. [Google Scholar]
- Liedo P, Carey JR. Mass rearing of Anastrepha (Diptera: Tephritidae) fruit flies; a demographic analysis. Journal of Economic Entomology. 1994;87:176–180. [Google Scholar]
- Liedo P, Carey JR, Celedonio-Hurtado H, Guillen-Aguilar J. Size specific demography of three species of Anastrepha fruit flies. Entomologia Experimentalis et Applicata. 1992;63:135–142. [Google Scholar]
- Liedo P, Zavala JL, Orozco D, Fredersdorff C, Schwarz AJ. Ten years of successful medfly sterile mass production at Metapa, Chiapas, Mexico. In: Aluja M, Liedo P, editors. Fruit Flies: Biology and Management. New York: Springer; 1993. pp. 269–275. [Google Scholar]
- Mangan R. Adult diet and male—female contact effects on female reproductive potential in Mexican fruit fly (Anastrepha ludens loew) (Diptera: Tephritidae) Journal of Economic Entomology. 2003;96:341–347. doi: 10.1603/0022-0493-96.2.341. [DOI] [PubMed] [Google Scholar]
- McDonald JF. Ecological genetics and the sampling of insect populations for laboratory colonization. Environmental Entomology. 1976;5:816–820. [Google Scholar]
- McDonald JF. The molecular basis of adaptation: a critical review of relevant ideas and observations. Annual Review of Ecology and Systematics. 1983;14:77–102. [Google Scholar]
- Müller HG, Carey JR, Wu D, Vaupel JW. Reproductive potential determines longevity of female Mediterranean fruit flies. Proceedings of the Royal Society, London B. 2001;268:445–450. doi: 10.1098/rspb.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wang J-L, Carey JR, Caswell-Chen EP, Chen C, Papadopoulos N, Yao F. Demographic window to aging in the wild: constructing life tables and estimating survival functions from marked individuals of unknown age. Aging Cell. 2004;3:125–131. doi: 10.1111/j.1474-9728.2004.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrbom AL, Foote RH. The taxonomy and zoogeography of the genus Anastrepha (Diptera: Tephritidae) In: Robinson AS, Hooper G, editors. Fruit Flies. Their Biology, Natural Enemies and Control. 1989. pp. 15–26. [Google Scholar]
- Novoseltsev VN, Carey JR, Novoseltseva JA, Papadopoulos NT, Blay S, Yashin AI. Systemic mechanisms of individual reproductive life history in female medflies. Mechanisms of Ageing and Development. 2004;125:77–87. doi: 10.1016/j.mad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Noveoseltsev VN, Arking R, Carey JR, Novoseltseva JA, Yashin AI. Hidden trade-offs and individual fecundity in Drosophila and medflies. Aging Cell. doi: 10.1196/annals.1297.108. submitted. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA, Müller H-G, Liu X. Supine behaviour predicts time-to-death in male Mediterranean fruit flies. Proceedings of the Royal Society of London: Biological Sciences. 2002;269:1633–1637. doi: 10.1098/rspb.2002.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Carey JR, Müller H-G, Zhang Y. High sexual calling rates predict extended life span in male Mediterranean fruit flies. Oecologia. 2003;138:127–134. doi: 10.1007/s00442-003-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Is accelerated senescence a cost of reproduction? Functional Ecology. 1987;1:317–320. [Google Scholar]
- Price EO. Behavioral aspects of animal domestication. The Quarterly Review of Biology. 1984;59:1–32. [Google Scholar]
- Promislow DEL, Tatar M, Khazaeli AA, Curtsinger JW. Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality. Genetics. 1996;143:839–848. doi: 10.1093/genetics/143.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick GK. Geographic structure of insect populations: gene flow, phylogeography and their uses. Annual Review of Entomology. 1996;41:325–352. doi: 10.1146/annurev.en.41.010196.001545. [DOI] [PubMed] [Google Scholar]
- Rose MR. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Shibata R. Optimal selection of regression variables. Biometrika. 1981;68:45–54. [Google Scholar]
- Tatar M, Promislow DEL, Khazaeli AA, Curtsinger JW. Age-specific patterns of genetic variance in Drosophila melanogaster. II. Fecundity and genetic covariance with age-specific mortality. Genetics. 1996;143 doi: 10.1093/genetics/143.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas RI. Mass production of tephritid fruit flies. In: Robinson AS, Hooper G, editors. World Crop Pests. Fruit Flies: Their Biology, Natural Enemies and Control. Amsterdam: Elsevier; 1989. pp. 141–152. [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Wood JW. In: Dynamics of Human Reproduction: Biology, Biometry, Demography. Hrdy SB, editor. New York: Aldine De Gruyter; 1994. [Google Scholar]