Abstract
The results reported here for CBA/CaJ mice describe the effects of regular dosing with a common antiretroviral drug combination on outer hair cell (OHC) function using measures of 2f1-f2 distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs). Specifically, experimental mice were treated daily over a 3-mo period with the nucleoside reverse transcriptase inhibitors (NRTIs), zidovudine (ZDV) and lamivudine (3TC), dissolved in their drinking water, while their control counterparts received untreated water. DPOAE levels and ABR detection thresholds prior to and after 12 wk of NRTI treatment did not differ between experimental and control groups. To assess whether NRTI treatment potentiates the adverse effects of noise overexposure on OHC function, both experimental and control mice were exposed 1 wk later, while still on the drug regimen, to a 10-kHz octave-band noise (OBN) at 105 dB SPL for 1 h. A major outcome of the sound over-exposure episode was that the NRTI-pretreated mice showed significantly greater permanent OBN-induced reductions in DPOAE levels at 2 wk postexposure than were observed for the untreated control animals. These findings support the notion that a synergistic relationship exists between certain NRTIs and intense sounds in that such preexposure drug treatments produced greater noise-induced decreases in DPOAE activity than did noise exposure alone. This drug/noise interaction is consistent with the known harmful effects of NRTIs on cellular mitochondrial activity.
Keywords: CBA/CaJ mice, nucleoside reverse transcriptase inhibitors, zidovudine, lamivudine, distortion product otoacoustic emissions, octave-band noise exposure, auditory brainstem responses, synergistic effects
1. Introduction
According to the international acquired immunodeficiency syndrome (AIDS) charity AVERT (2007), at the end of 2006, there were an estimated 39.5 million adults and children worldwide, who were living with the human immunodeficiency virus (HIV), which is the virus that causes AIDS. During 2006, an estimated 4.3 million individuals became newly infected with HIV, and 2.9 million deaths occurred from AIDS. Since 1981, some 25 million patients have died of AIDS (WHO/UNAIDS, 2007). In high-income countries, the total number of individuals living with HIV continues to increase, largely due to widespread access to antiretroviral therapy, which prolongs the lives of HIV-positive (HIV+) patients (AVERT, 2007).
Current knowledge holds that HIV+ patients may develop AIDS as late as 20 y after they become infected. In these patients, the gradual depletion of their cellular immune systems results in a wide spectrum of morbidity, which may affect virtually any body process. Such symptoms are dominated by the opportunistic infections of relatively non-virulent organisms and the common occurrence of rare malignancies. Due to the nature of the disease, HIV+ patients are at a risk for developing hearing loss as well as the many other manifestations of the disease. It is believed that somewhere between 21–49% of HIV-infected patients develop a sensorineural hearing loss (SNHL), which predominantly involves the higher frequencies (Lalwani and Sooy 1992; Moazzez and Alvi 1998; Chandrasekhar et al. 2000; Mata Castro et al. 2000). Although hearing loss can also be seen in symptom-free HIV+ patients, the degree of SNHL is usually correlated with the severity of the disease (Chandrasekhar et al. 2000).
The hearing loss observed in HIV-infected patients has been attributed to a number of sources including a neurotrophism of the virus (Moazzez and Alvi 1998), neurological complications of AIDS-related opportunistic infections (Eng et al. 1986), direct invasion of the cochlea from local opportunistic infections or malignancies (Michaels et al. 1994), and drug use due to the accompanying infections (Nadol 1993). It can be hypothesized that a relatively significant percentage of the hearing loss observed in HIV+ patients is due to the use of ototoxic drugs such as the antibiotics commonly used to treat opportunistic infections that complicate the disease (e.g., aminoglycoside compounds and amphotericin B). However, in up to 50% of HIV-infected individuals with SNHL, no cause can be identified to explain the associated hearing loss (Lalwani and Sooy 1992).
The nucleoside reverse transcriptase inhibitors (NRTIs) continue to be the most common antiretroviral drug group used to treat HIV+ patients. NRTIs act as chain terminators of mitochondrial deoxyribonucleic acid (DNA) and as such cause damage to mitochondrial DNA. The NRTIs are phosphorylated intracellularly to active triphosphate forms and are then incorporated into new DNA strands synthesized by HIV reverse transcriptase (Carr and Cooper 2000). The medium- to long-term toxicities of NRTIs are thought to be secondary to the inhibition of mitochondrial DNA polymerase γ, resulting in impaired synthesis of the mitochondrial enzymes that generate adenosine triphosphate by oxidative phosphorylation (Carr and Cooper 2000). It seems reasonable to speculate that the resulting insufficient energy production within the cell may damage cochlear hair cells, or interact to augment hair cell damage, when these cells are metabolically stressed as, for example, during intense noise exposure (e.g., Henderson et al. 2006).
NRTIs constitute the only antiretroviral drug group that has been proposed in some case-study patient reports to cause hearing loss. Since all identified inherited mitochondrial anomalies cause hearing loss, it is very likely that NRTI-related ototoxicity, if it exists, is related to mitochondrial damage (Marra et al. 1997). As plasma NRTI concentrations do not reflect intracellular NRTI-triphosphate concentrations, attempts to monitor the progression of mitochondrial toxicity are ineffective (Carr and Cooper 2000). Currently, there are a number of NRTIs approved by the United States Food and Drug Administration to treat HIV. Zidovudine (ZDV), which was the first NRTI drug used in HIV therapy, is used orally and most commonly in combinations that incorporate from one to three NRTIs, and sometimes even include the addition of a non-NRTI antiretroviral drug as well.
The present study was designed to investigate initially the ototoxic potential of NRTIs in experimental mice compared to a comparable group of non-treated control mice using distortion product otoacoustic emissions (DPOAEs) and auditory brainstem evoked responses (ABRs). DPOAEs are well known to be reduced or eliminated by exposure to either ototoxic drugs or excessive noise stimulation due to their ability to assess the functional status of the OHC system, which represents the initial cochlear components that are injured by such harmful agents. Additionally, ABRs provide a sensitive measure of the normalcy of the cochlea’s overall input to the central auditory nervous system. Because, as noted above, NRTI use in HIV+ patients is usually in mixtures consisting of more than one NRTI, a relatively common preferred combination consisting of ZDV and lamivudine (3TC) was selected as the test pharmacological agents. Following this drug-alone comparison, the effects of a subsequent noise over-exposure episode were determined to establish whether treatment with NRTIs potentiates the adverse influences of sound overstimulation on DPOAE levels.
2. Methods
2.1 Subjects
Experimental animals were 20 female mice of the CBA/CaJ strain that were purchased from a commercial breeder (The Jackson Laboratory, Bar Harbor MA) at 5 wk of age. When no gender differences are expected, female mice are routinely employed in our laboratory to avoid difficulties often encountered when the more aggressive males are housed together as in the control group. After a 3-wk acclimation period, 8-wk old mice were used for the study. All functional measures were obtained with the animals placed in a sound proofed booth, while under anesthesia consisting of an intramuscular injection of a mixture of ketamine hydrochloride (Ketaset, Wyeth, Madison NJ) at 100 mg/kg and xylazine (Rompun, BayerHealth Care, Wuppertal Germany) at 4 mg/kg in equal volumes of bacteriostatic water. During the measurement period, mice were placed on a homoeothermic feedback-controlled heating table (Harvard Apparatus, Holliston MA) to maintain normal rectal temperature at 36.5±0.5°C. Supplemental injections of anesthetic agents at approximately half the induction dose were provided, when twitching of the vibrissae was observed typically at ~30–40 min following administration of the original dose. The Animal Care and Use Committee of the University of Colorado Health Sciences Center, where these data were collected, approved all experimental procedures.
2.2 Nucleoside Reverse Transcriptase Inhibitor (NRTI) Administration
The potential ototoxic effects of a combination of ZDV (Sigma-Aldrich, St Louis MO) and 3TC (GlaxoSmithKline, Research Triangle Park NC) were tested in this initial study of the influence of NRTIs on ear function. Toward this end, mice were treated orally over the 3-mo drug-study period by dissolving these pharmaceutical agents (1.5 mg/ml ZDV and 3.75 mg/ml 3TC) in drinking water. Mice in the experimental group (n=10) were housed in separate cages that allowed the volume of the drug consumed for each individual mouse to be roughly determined. The control group (n=10), which was housed in group cages consisting of five mice each, received normal drinking water. Average daily consumption of water was approximately 5 ml in the drug group. This resulted in a mean consumption amount of about 300 mg/kg body weight per day (bw/d) ZDV and 750 mg/kg bw/d 3TC. The control group consumed a similar amount of water per mouse each day. However, it was noted that the experimental mice were visibly thinner than the control mice, but this weight loss did not seem to be due to a lack of water consumption. In mouse studies, these drugs are frequently given orally, usually by gavage, but they can also be given in the drinking water (e.g., Ayers et al. 1997; Note et al. 2003). We chose the drinking-water approach, because of the ease of administration over the long 3-mo time frame. The dosages chosen were at the upper limits of these drugs when given to simulate lifetime exposures.
2.3 Distortion Product Otoacoustic Emission (DPOAE) Measures
The present study measured the levels of the 2f1-f2 DPOAE as the primary measure of cochlear function, i.e., of OHC activity, in particular. Details of the DPOAE-recording procedure used for mice have been described elsewhere (Jimenez et al. 1999, 2001; Martin et al. 2006). Briefly, the f1 and f2 primary tones were generated by a dual-channel synthesizer (Model 3326A, Hewlett-Packard, Palo Alto CA) and attenuated, under computer control, using customized software. The f1 and f2 primaries (f2/f1=1.25) were then presented over two separate ear-speakers (Radio Shack Realistic Dual Radial Horn Tweeters, Tandy Corp, Ft Worth TX) and delivered to the outer-ear canal through an acoustic probe (ER-10B+, Etymotic Research, Elk Grove Village IL), where they were allowed to mix acoustically. The probe was sealed securely into the outer ear canal via a small Silastic® tube attached to the tip of the probe. Earcanal sound pressure levels, which were measured by the ER-10B+ emissions-microphone assembly embedded in the probe, were sampled at 44,100 Hz, synchronously averaged, and analyzed using a 4096-point fast Fourier transform (FFT) for f2 frequencies ranging from 6.3 to 20.1 kHz by a computer-based digital-signal processor (DSP) board. Corresponding noise floors (NFs) were computed by averaging the levels of the ear-canal sound pressure for five frequency bins above and below the 2f1-f2 DPOAE frequency bin (i.e., ±54 Hz).
For test frequencies above 20.1 kHz, a computer-controlled dynamic-signal analyzer (Model 3561A, Hewlett-Packard, Palo Alto CA) was used. For these frequencies, the ear-canal signal was root-mean square averaged (n=8) over a frequency span of 500 Hz, which resulted in a bandwidth resolution of 1.88 Hz. The related NFs for these latter measures were estimated by averaging the levels of the ear-canal sound pressure for the two FFT frequency bins immediately below the 2f1-f2 DPOAE frequency (i.e., for 3.75 Hz below the DPOAE). No artifactual DPOAEs were measured in a hard-walled cavity that approximated the size of the mouse outer-ear canal, which was used to calibrate the tonal stimuli. DPOAEs were considered to be present when they were at least 3 dB above the NF. DPOAE levels were plotted as DP-grams, i.e., DPOAE levels as a function of the f2 frequencies. Specifically, DP-grams described emission levels in response to primary tones at L1=L2=55, 65, and 75 dB SPL as a function of the f2 frequencies, which ranged from 6.3-54.2 kHz, in 0.1-octave steps. One ear of each mouse was randomly selected as the test ear. A test session consisted of obtaining DP-grams at the three primary-tone levels. For the drug-administration protocol, DP-grams were collected weekly for both the experimental and control groups starting from before the first administration of drug until the termination of the ototoxicity study some 3 mo later.
2.4 Auditory Brainstem Response (ABR) Testing
ABR measurements were performed using a commercial real-time signal-processing system (System II, Tucker-Davis Technologies, Alachua FL) equipped with a differential amplifier and associated stimulus-generation modules. Stimuli were delivered through the same probe used to collect the DPOAEs. To elicit the ABR, tone-pips were generated with 1-ms rise/fall times and 2-ms plateaus. For recording, subdermal stainless-steel needle electrodes were placed at the vertex and ventrolateral to the left and right ears. ABR waveforms were recorded for 10 ms at a sampling rate of 45 kHz using bandpass filter settings of 100–3,000 Hz. Generally, waveforms based on 200 stimuli presented at a frequency of 12 Hz were averaged to compute the resulting evoked potential. ABR waveforms were recorded in 5-dB steps by decreasing stimulus intensity from the maximum tone-pip level of 80 dB SPL until no wave V could be visualized, which was the intensity setting that was designated as the threshold value. Waveforms were stored for off-line analysis to obtain average thresholds for tone-pip frequencies at 8, 12, 16, 24, 32, and 48 kHz based upon thresholds scored by three blinded observers. ABRs were collected before and after 12 wk of drug administration. Because of the increased probability of loosing mice due to the prolonged anesthesia required for combined DPOAEs and ABR testing, ABRs were not obtained for the noise exposure part of the study.
2.5 Noise Exposure
After the 12-wk NRTI-drug alone treatment phase was completed, the mice were maintained on the drug regimen for an additional 1 wk and were then exposed to an octave-band noise (OBN) centered at 10 kHz at a root-mean square level of 105 dB SPL for 1 h. Postexposure DPOAE levels were measured immediately after the noise exposure ended, and again at 2 d and 2 wk postexposure. The OBN was generated by filtering (Model 9002, Frequency Devices Inc, Ottawa IL) a broadband white-noise signal produced by a custom-made noise generator and amplifying it with a stereo amplifier (DRA-295, Denon Electronics, Mahwah NJ). The resulting OBN was presented by two direct-reflecting loudspeakers (Model 901, Bose Corp, Framingham MA) that were controlled by an associated active-sound equalizer. The resulting spectrum, when analyzed in 1/3-octave bands with a dynamic-signal analyzer (Model 3561A, Hewlett-Packard, Palo Alto CA), ranged from 8–15 kHz, with the maximum energy of 105 dB SPL at 10 kHz. A 1/2-in microphone (AC07013, ACO Pacific Inc, Belmont CA) in combination with a precision sound-level meter (Model 155, Quest Technologies Inc, Oconomowoc WI) was used to monitor the magnitude of the OBN. During the noise-exposure session, awake mice were placed a compartmentalized cage that rotated at 1 rpm to ensure that the noise stimulus was evenly distributed among the mice situated within the exposure chamber.
2.6 Statistical Analysis
All DPOAEs and their corresponding NFs were converted to ASCII text files using customized software and then databased in a commercially available spreadsheet (Excel:mac v.11.3.3, Microsoft Corp, Redmond WA). Specifically, raw data were averaged across mice for each determination for the experimental and control groups and plotted as mean DP-grams using standard spreadsheet software. ABR thresholds for each test frequency were also assembled in a similar spreadsheet file. For drug administration alone, all data were analyzed as raw DPOAEs levels or as ABR thresholds in dB SPL. For the noise-exposure portion of the study, difference DP-grams were constructed by subtracting preexposure DPOAE levels from post-noise exposure DPOAEs. This procedure resulted in difference DP-grams that had negative values below a zero ‘no-change’ baseline, if there was an effect of the noise exposure. Preexposure DPOAE levels were subtracted from post-noise exposure NFs so that the resulting negative NFs represent the maximum loss possible. The 12-wk drug-alone DPOAE levels served as the preexposure baseline for these transformations.
Statistical analyses were accomplished using commercially available software (StatView v.4.5, Abacus Concepts, Berkeley CA). The effects of drug administration were assessed by relating either DPOAEs levels or ABR thresholds between non-treated control mice as compared to their drug-treated counterparts. All analyses were based upon analysis of variance (ANOVA) with subject group (i.e., control versus drug) as the between factor and f2 (i.e., test frequency) as the within factor.
3. Results
3.1 NRTI Effects on DPOAEs and ABRs
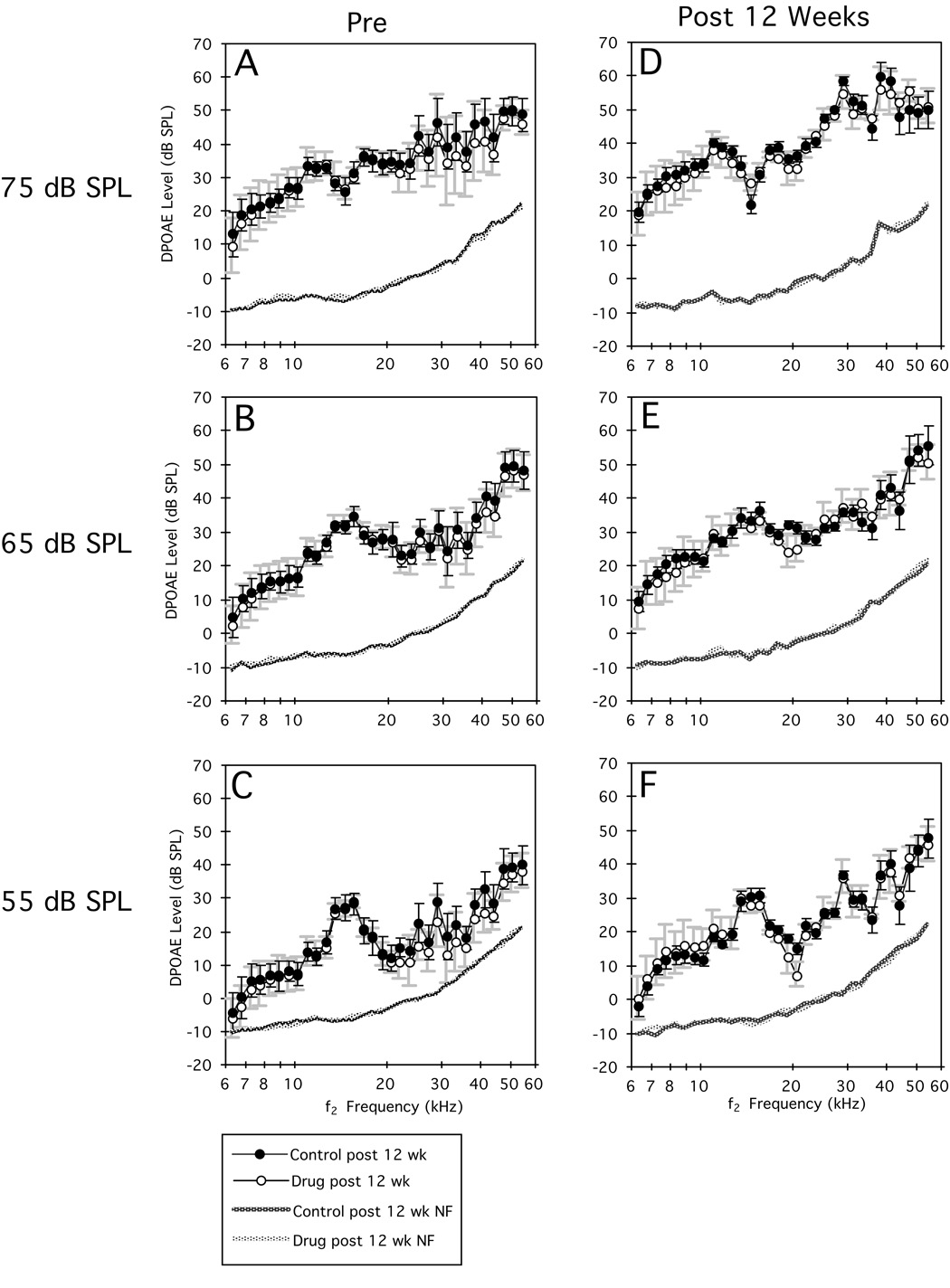
Figure 1 illustrates average DP-grams in response to each of the primary-tone levels [L1=L2=75 (A,D), 65 (B,E), and 55 dB SPL (C,F)] measured before (A–C) and after 12 wk of drug dosing (D–F). It is apparent from inspection of the plots in this figure that there were no mean DPOAE-level differences (A–C) between the control (open circles) and experimental groups (closed circles) prior to drug administration. It is also obvious from panels D–F that after 12 wk of drug dosing, on average, there were no measurable effects of NRTIs on DPOAEs levels. These visual impressions were confirmed by the lack of statistical significance for separate ANOVAs performed on data represented in each panel of the figure.
Figure 1.
Mean DP-grams (±1 SD) collected at three primary-tone levels before (A–C) and after (D–F) 12 wk of NRTI treatment for untreated control (solid circles) and drug-treated (open circles) mice. From inspection of these plots, it is clear that the two groups of mice exhibited similar pre-treatment (left panels) DPOAE levels, and that these measures were basically unchanged at the end of the drug-treatment period (right panels). Dotted lines at the bottom of each plot indicate the related NFs of the measurement system.
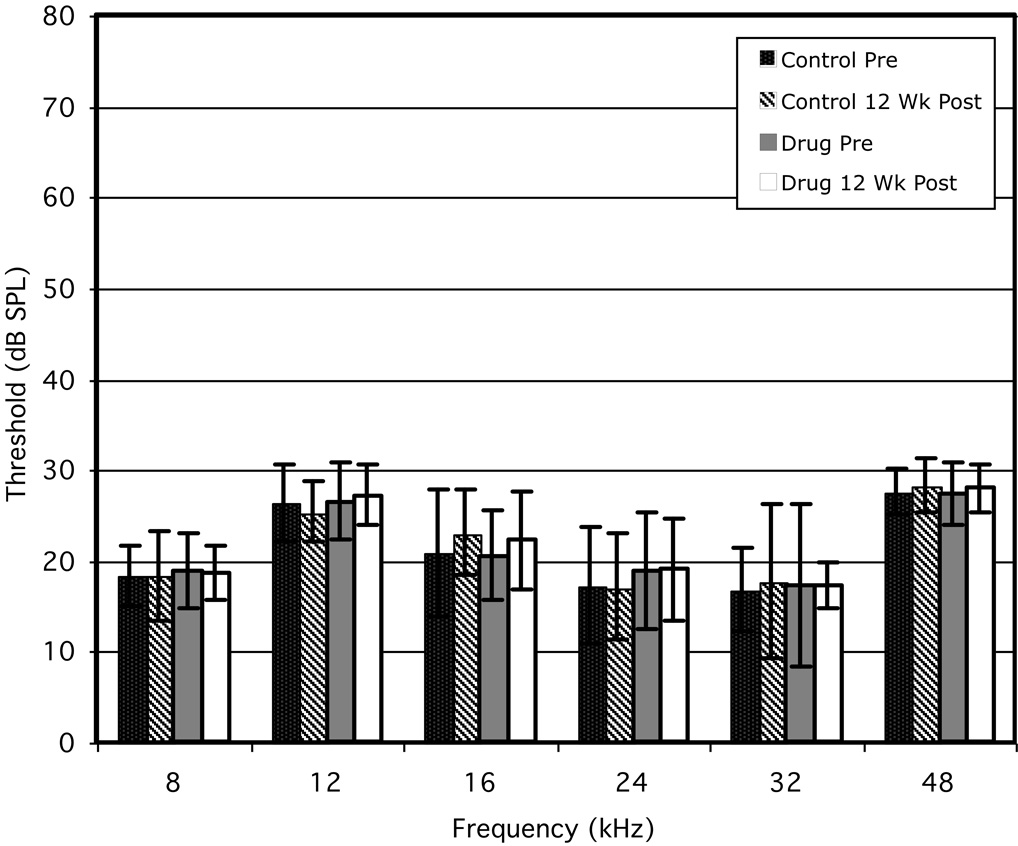
The corresponding ABR findings are presented in Fig 2 for six frequencies ranging from 8 to 48 kHz. Like the DPOAEs results presented in Fig 1, there were no differences pre-drug exposure between the control (black bars) and experimental (gray bars) groups. In addition, there was no evidence of an effect of NRTIs on either cochlear function (Fig 1) or more central auditory activity as indicated by no apparent differences between the 12-wk control (cross-hatched bars) and the drug-treated (white bars) groups. Again, statistical analyses confirmed the lack of any significant differences between the treated versus untreated mouse groups.
Figure 2.
Mean ABR thresholds (±1 SD) obtained at six test frequencies before and following 12 wk of administering NRTIs to the experimental group. Mean ABR thresholds for the untreated control group some 12 wk later (diagnonal striped bars) showed no change from their baseline values (solid bars). Similarly, baseline ABR thresholds for the NRTI-treated mice (gray bars) exhibited no apparent changes at any of the test frequencies following the 12-wk treatment period (open bars), nor any differences from the untreated controls (open bars versus diagonal-striped bars)
3.2 Noise-Exposure Effects on DPOAEs
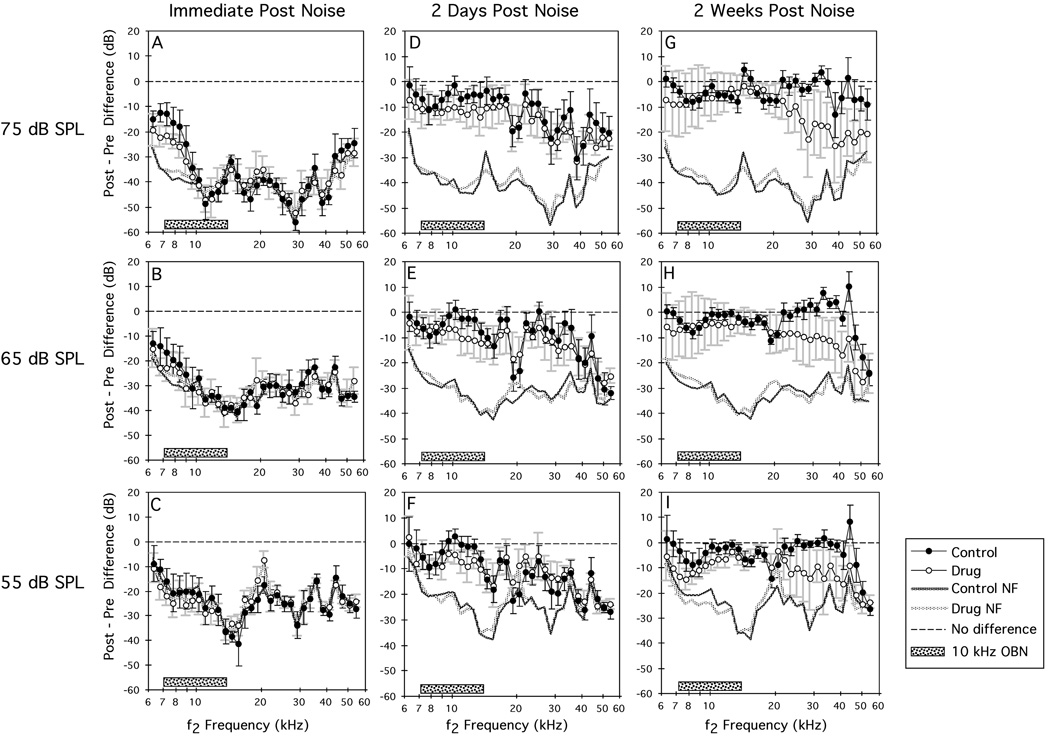
The effects of a 1-h OBN exposure on ‘control’ mice and mice exposed for 12 wk to the NRTI regimen are shown in Fig 3 as difference DP-grams at primary-tone levels of 75 (A), 65 (B), and 55 (C) dB SPL, for three postexposure time periods—immediately, 2 d, and 2 wk postexposure. Immediately postexposure (Figs 3A–C), regardless of primary-tone level, DPOAEs for both control (solid circles) and drug (open circles) groups were reduced to the measurement’s NF. However, by 2 d postexposure (Figs 3D–F), DPOAEs at all levels had recovered substantially, without any significant differences between the drug-treated versus untreated groups. However, by 2 wk postexposure (Figs 3G–I), while DPOAEs for the control group were essentially recovered to their ‘no-change’ baseline magnitudes (dashed line) for all primary-tone levels, DPOAEs for the NRTI-treated mice elicited by high-frequency primaries still exhibited the adverse effects of the noise exposure. Often, lower-level primaries, for example, 55 vs 75 dB SPL, tend to reflect more dysfunction following noise overexposure. In this case, there appears to be approximately equal amounts of DPOAE loss regardless of primary-tone level. However, that all levels reflect the DPOAE-loss pattern suggests that the emission changes were reliable.
Figure 3.
Mean difference DP-grams (±1 SD) computed by subtracting baseline DPOAE levels from their corresponding postexposure values. DPOAEs were elicited at three primary-tone levels and were obtained immediately (A–C), at 2 d (D–E), and at 2 wk (G–I) postexposure to a 1-h, 105-dB SPL, 10-kHz OBN for the control (solid circles) and NRTI-treated (open circles) mouse groups. Immediately postexposure, DPOAEs for both groups were reduced to the system’s NF. Two days later, both groups showed substantial recovery at all test levels. However, at 2 wk postexposure, the NRTI mice showed permanent DPOAE losses in the high-frequency test region thus suggesting an interaction of NRTIs with noise that enhanced the detrimental effects of the noise over-exposure. Dashed line at ‘0’ on the ordinate indicates no change from preexposure baseline values; heavy dotted lines without symbols designate the maximum possible DPOAE loss; stippled bar centered at 10 kHz indicates the frequency extent of the OBN exposure.
ANOVAs performed on the data represented in all the panels of Fig 3 revealed a significant control versus drug effect at 2 wk postexposure for the 65/65- (Fig 3H: df=1/13, p=0.02) and 55/55-dB SPL (Fig 3I: df=1/13, p=0.0009) primaries. These findings indicate that the DPOAEs for the drug-treated mice showed significantly less recovery from the OBN exposure. Additional ANOVAs performed on high-frequency DPOAEs ranging from 21.1–48.4 kHz demonstrated highly significant differences between the control- and drug-treated groups for all primary-tone levels, which is, again, indicative of larger permanent DPOAE losses for the NRTI-treated mice (75/75 dB SPL: df=1/13, p<0.001; 65/65 dB SPL: df=1/13, p=0.0009; 55/55 dB SPL: df=1/13, p=0.0009).
4. Discussion
The present study initially examined the effects of potentially ototoxic drugs and then intense sound exposures on auditory-system function in mice. That is, in the first experiment, functional measurements were obtained, which evaluated both the peripheral (DPOAE) and central (ABR) auditory nervous systems of mice that were consuming high-dose combinations of NRTI drugs. Although an NRTI combination was used that was considerably more potent than human therapeutic doses, the results showed no differences in either DPOAEs (see Fig 1) or ABRs (see Fig 2) between the drug-treated experimental and non-drug treated control groups after 12 wk of exposure to a common NRTI-treatment regimen.
The second part of the study was designed to evaluate a possible interaction between NRTIs and exposure to intense noise on OHC function using DPOAE measures. The results showed that immediately after noise exposure, or at 2 d post-noise exposure, there were no apparent differences in the reduced DPOAE levels between the control and NRTI-treated mice. However, after 2 wk of postexposure recovery, the drug-treated group showed permanent DPOAE losses that were confined to the higher-test frequencies from ~21–48 kHz (see Figs 3G–I), which represents the classic high-frequency effects of ototoxic compounds.
The synergistic effect of noise and drugs is well established (e.g., Dayal et al. 1971; Jauhiainen et al. 1972; Eddy and Morgan 1976; Hawkins et al. 1976; Vernon et al. 1977; Bombard et al. 2005), along with the interaction of various industrial chemicals and noise (e.g., Barregard and Axelsson 1984; Fechter et al. 2002; Morata et al. 2002; Schaper et al. 2003; Chang et al. 2006). Thus, in combination with the lack of any effect on auditory function of NRTIs alone, the above findings provide a convincing demonstration of a noise/NRTI synergistic interaction. It is also well documented that in most risky situations the basal or high-frequency end of the cochlea is most vulnerable to various insults (Billett et al. 1989; Sha et al. 2001). The fact that mice exposed to NRTIs showed permanent high-frequency DPOAE deficits reinforces the notion that these drugs are capable of potentiating the damaging effects of excessive noises, particularly, for the most sensitive, high-frequency region of the cochlea.
Although research has shown that some HIV patients have elevated hearing levels, reported cases of hearing loss attributed to NRTI use are relatively rare (Powderly et al. 1990; Monte et al. 1997; Christensen et al. 1998; Vogeser et al. 1998; Simdon et al. 2001). Curiously, the majority of these particular cases were reported to develop hearing loss immediately after commencement of, or changing to, a treatment protocol that included one or more NRTIs. Despite this small number of cases, the occurrence of an abrupt hearing loss suggests a probable ototoxic susceptibility, just like that seen in patients with mitochondrial mutations that make them susceptible to aminoglycoside ototoxicity (Prezant et al. 1993). In addition, most of the patients described in these case-study reports were older than the average HIV+ patients, and/or had preexisting hearing problems such as noise-induced hearing loss (Smidon et al. 2001). It is then reasonable to speculate that the complex histories of many HIV patients may also result in an underreporting of NRTI-related cases of ototoxicity, because of the difficulty in identifying the real cause of hearing loss in a patient group that is vulnerable to a number of risk factors.
As noted previously, NRTIs represent the most commonly used antiretroviral drugs against HIV. Mitochondrial toxicity of the nucleoside analogues was initially confirmed from in vitro studies (Brinkman et al. 1998; Medina et al. 1998). For example, Medina et al. (1998) demonstrated that within a few days of exposure to antiretroviral drugs, the mitochondrial DNA content of cells decreased. Each cell contains hundreds of mitochondria, which serve important metabolic functions, with the most significant function being the synthesis of the nucleotide, adenosine 5’-triphosphate (ATP), by oxidative phosphorylation. The fact that NRTIs were capable of interacting synergistically with the loud sounds associated with the noise exposure of the present study is consistent with the known effects of mitochondrial defects on hearing. Specifically, mitochondrial DNA mutations have been linked to a number of different causes of hearing loss including syndromic deafness, non-syndromic deafness, presbycusis, and a genetic susceptibility to ototoxicity (Higashi 1989; Hu et al. 1991; Hutchin and Cortopassi 2000; Fischel-Ghodsian 2003; Xhing et al. 2007).
Mitochondrial changes are just one possible mechanism that could explain the influence of NRTIs on DPOAEs following noise. It is certainly possible that other cellular processes in the cochlea besides the mitochondria were affected by the drug treatment and consequently were responsible for the observed effects. One possibility is that the stria vascularis may have been compromised in some manner by the NRTI administration. However, it is well known that DPOAEs elicited by low-level primaries (e.g., L1=L2=55-dB SPL) are sensitive to treatments that affect the stria. For example, ethacrynic acid administration cause DPOAEs evoked by 55-dB SPL primaries to be dramatically reduced, whereas DPOAEs elicited by higher-level primary tones are relatively unchanged (Whitehead et al. 1992). Thus, if strial damage was present in the current study, reduced DPOAEs in response to the lower-level primaries would have been detected, which was not the case. In addition, NRTIs could also have affected various other aspects of the OHC, such as the protein cadherin, which influences susceptibility to noise exposure in mice with age-related hearing loss (e.g., Noben-Trauth et al. 2003). But, until more detailed studies can be performed, exactly how NRTIs interact with intense noise remains speculative. All in all, however, mitochondrial changes would seem to be a likely possibility.
In humans, it is clear that most of the SNHL encountered in HIV-infected patients is multifactorial. In such patients, hearing loss may present as a unilateral or bilateral sudden hearing loss (Real et al. 1987; Kwartler et al. 1991; Grimaldi et al. 1993; Meynard et al. 1997). However, the majority of reported patient cases appear to develop hearing loss gradually in a subacute or chronic manner (Hariharan et al. 1996). In fact, several investigators have described in detail the audiological deterioration of HIV+ patients. For example, Birchall et al. (1992) noted abnormalities with respect to either auditory evoked-responses or pure-tone audiometry in one-third of their patients. Similarly, in a study by Chandrasekhar et al. (2000), 29% of their HIV-infected patients were discovered to have hearing losses. Marra et al. (1997) also reported hearing loss in 29% of their patients. Moreover, these latter investigators noted as well that patients, who were older than 35 y and who had received antiretroviral agents within the previous 6 mo, were more likely to exhibit auditory deficiencies. Marra et al. (1997) presumed that such abnormalities were due to mitochondrial DNA damage caused by the NRTIs. Because it is well established that aging is associated with mitochondrial DNA mutations (e.g., Cortopassi 2002), it may be that the coexistence of the aging and NRTI-drug factors facilitate the development of clinically distinguishable hearing problems.
In a recent prospective study, Schouten et al. (2006) reported audiometric findings for 33 HIV+ patients treated with ZVD and/or didanosine. In this study, there were no significant changes in audiometric measures following 32 wk of NRTI drug therapy. However, the patients’ hearing levels were already poor at the beginning of the Schouten et al. (2006) study in that 22 of the 33 subjects had hearing levels >25 dB at one or more frequencies, in one or both ears. In addition, 16 had at least one ear with hearing levels >40 dB, and seven patients had at least one test frequency with a hearing level >60 dB. Interestingly, the patients’ hearing levels improved somewhat after the cessation of the drug regimen, although none exhibited a statistically significant improvement. The authors suggested that hearing might improve in some patients and be correlated with an NRTI-induced improvement of their immune status, thus, masking any ototoxicities.
In the present study, there was no evidence in mice for any adverse effects on cochlear function from NRTIs administered alone. This outcome is consistent with the relatively low incidence of hearing loss reported for HIV+ patients taking NRTIs. However, the noise-exposure results suggest that there may be a potential for patients taking NRTIs to have an increased risk of hearing loss when exposed to high levels of noise. Considering the number of patients affected worldwide and the duration of their treatment, this risk factor presumably involves a significant number of individuals. In this respect, there is a need for studies evaluating HIV+ patients taking NRTIs, who are exposed to high levels of noise, especially in the workplace. The present findings are limited since only one of many possible combinations of anti-HIV drugs was evaluated. Clearly, further studies using different drug combinations along with various cochlear insults such as noise or aminoglycoside antibiotic administration are needed to better understand the effects of NRTIs on hearing in HIV+ patients.
Acknowledgements
This work was supported by the NIH (DC000613, DC003114). The authors thank Luba Nemanov for her assistance in collecting the mouse DPOAE and ABR data and for assisting with the databasing of these measures. The authors also thank three anonymous reviewers for their helpful comments.
Abbreviations
- 3TC
antiretroviral drug lamivudine
- ABR
auditory brainstem response
- AIDS
acquired immunodeficiency syndrome
- ATP
nucleotide adenosine 5’-triphosphate
- bw/d
body weight per day
- °C
degrees Celsius
- DNA
deoxyribonucleic acid
- DSP
digital signal processor
- DPOAE
distortion product otoacoustic emission
- DP-gram
DPOAE levels collected as a function of f2 frequency
- f1
lower-frequency primary tone
- f2
higher-frequency primary tone
- FFT
fast Fourier transform
- HIV
human immunodeficiency virus
- L1
level of lower-frequency primary tone
- L2
level of higher-frequency primary tone
- OBN
octave-band noise
- mo
month(s)
- NF
noise floor
- NRTI
nucleoside reverse transcriptase inhibitor
- OHC
outer hair cell
- rpm
rotations per minute
- SD
standard deviation
- SNHL
sensorineural hearing loss
- wk
week(s)
- y
year(s)
- ZVD
antiretroviral drug zidovudine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AVERT. 2007 http://www.avert.org/worldstats.htm (last viewed 10/30/07)
- Ayers KM, Torrey CE, Reynolds DJ. A transplacental carcinogenicity bioassay in CD-1 mice with zidovudine. Fundam Appl Toxicol. 1997;38:195–198. doi: 10.1006/faat.1997.2342. [DOI] [PubMed] [Google Scholar]
- Barregard L, Axelsson A. Is there an ototraumatic interaction between noise and solvents? Scand Audiol. 1984;13:151–155. doi: 10.3109/01050398409043054. [DOI] [PubMed] [Google Scholar]
- Billett TE, Thorne PR, Gavin JB. The nature and progression of injury in the organ of Corti during ischemia. Hear Res. 1989;41:189–197. doi: 10.1016/0378-5955(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Birchall MA, Wight RG, French PD, Cockbain Z, Smith SJ. Auditory function in patients infected with the human immunodeficiency virus. Clin Otolaryngol Allied Sci. 1992;17:117–121. doi: 10.1111/j.1365-2273.1992.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Bombard F, Campo P, Lataye R. Combined effects of noise and gentamicin on hearing in the guinea pig. Noise Health. 2005;7:29–39. doi: 10.4103/1463-1741.31631. [DOI] [PubMed] [Google Scholar]
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. Aids. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar SS, Connelly PE, Brahmbhatt SS, Shah CS, Kloser PC, Baredes S. Otologic and audiologic evaluation of human immunodeficiency virus-infected patients. Am J Otolaryngol. 2000;21:1–9. doi: 10.1016/s0196-0709(00)80117-9. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Chen CJ, Lien CH, Sung FC. Hearing loss in workers exposed to toluene and noise. Environ Health Perspect. 2006;114:1283–1286. doi: 10.1289/ehp.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LA, Morehouse CR, Powell TW, Alchediak T, Silio M. Antiviral therapy in a child with pediatric human immunodeficiency virus (HIV): case study of audiologic findings. J Am Acad Audiol. 1998;9:292–298. [PubMed] [Google Scholar]
- Cortopassi GA. Fixation of deleterious alleles, evolution and human aging. Mech Ageing Dev. 2002;123:851–855. doi: 10.1016/s0047-6374(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Dayal VS, Kokshanian A, Mitchell DP. Combined effects of noise and kanamycin. Ann Otol Rhinol Laryngol. 1971;80:897–902. doi: 10.1177/000348947108000616. [DOI] [PubMed] [Google Scholar]
- Eddy LB, Morgan RJ, Carney HC. Hearing loss due to combined effects of noise and sodium salicylate. ISA Trans. 1976;15:103–108. [PubMed] [Google Scholar]
- Eng RH, Bishburg E, Smith SM, Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986;81:19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Chen GD, Johnson DL. Potentiation of noise-induced hearing loss by low concentrations of hydrogen cyanide in rats. Toxicol Sci. 2002;66:131–138. doi: 10.1093/toxsci/66.1.131. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24:303–313. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- Grimaldi LM, Luzi L, Martino GV, Furlan R, Nemni R, Antonelli A, Canal N, Pozza G. Bilateral eighth cranial nerve neuropathy in human immunodeficiency virus infection. J Neurol. 1993;240:363–366. doi: 10.1007/BF00839968. [DOI] [PubMed] [Google Scholar]
- Hariharan R, Yao SK, Fred HL. Rapidly progressive deafness in a young woman. Hosp Pract (Minneap) 1996;31:159–160. doi: 10.1080/21548331.1996.11443369. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Higashi K. Unique inheritance of streptomycin-induced deafness. Clin Genet. 1989;35:433–436. [PubMed] [Google Scholar]
- Hu DN, Qui WQ, Wu BT, Fang LZ, Zhou F, Gu YP, Zhang QH, Yan JH, Ding YQ, Wong H. Genetic aspects of antibiotic induced deafness: mitochondrial inheritance. J Med Genet. 1991;28:79–83. doi: 10.1136/jmg.28.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin TP, Cortopassi GA. Mitochondrial defects and hearing loss. Cell Mol Life Sci. 2000;57:1927–1937. doi: 10.1007/PL00000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen T, Kohonen A, Jauhiainen M. Combined effect of noise and neomycin on the cochlea. Acta Otolaryngol. 1972;73:387–390. doi: 10.3109/00016487209138956. [DOI] [PubMed] [Google Scholar]
- Kokotas H, Petersen MB, Willems PJ. Mitochondrial deafness. Clin Genet. 2007;71:379–391. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- Kwartler JA, Linthicum FH, Jahn AF, Hawke M. Sudden hearing loss due to AIDS-related cryptococcal meningitis--a temporal bone study. Otolaryngol Head Neck Surg. 1991;104:265–269. doi: 10.1177/019459989110400219. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Sooy CD. Otologic and neurotologic manifestations of acquired immunodeficiency. Otolaryngol Clin North Am. 1992;25:1183–1197. [Google Scholar]
- Marra CM, Wechkin HA, Longstreth WT, Jr, Rees TS, Syapin CL, Gates GA. Hearing loss and antiretroviral therapy in patients infected with HIV-1. Arch Neurol. 1997;54:407–410. doi: 10.1001/archneur.1997.00550160049015. [DOI] [PubMed] [Google Scholar]
- Mata Castro N, Yebra Bango M, Tutor de Ureta P, Villarreal Garcia-Lomas M, Garcia Lopez F. Hearing loss and human immunodeficiency virus infection. Study of 30 patients. Rev Clin Esp. 2000;200:271–274. [PubMed] [Google Scholar]
- Medina DJ, Tung PP, Nelson CJ, Sathya B, Casareale D, Strair RK. Characterization and use of a recombinant retroviral system for the analysis of drug resistant HIV. J Virol Methods. 1998;71:169–176. doi: 10.1016/s0166-0934(97)00212-7. [DOI] [PubMed] [Google Scholar]
- Meynard JL, el Amrani M, Meyohas MC, Fligny I, Gozlan J, Rozenbaum W, Roullet E, Frottier J. Two cases of cytomegalovirus infection revealed by hearing loss in HIV-infected patients. Biomed Pharmacother. 1997;51:461–463. doi: 10.1016/s0753-3322(97)82326-8. [DOI] [PubMed] [Google Scholar]
- Michaels L, Soucek S, Liang J. The ear in the acquired immunodeficiency syndrome: I. Temporal bone histopathologic study. Am J Otol. 1994;15:515–522. [PubMed] [Google Scholar]
- Moazzez AH, Alvi A. Head and neck manifestations of AIDS in adults. Am Fam Physician. 1998;57:1813–1822. [PubMed] [Google Scholar]
- Monte S, Fenwick JD, Monteiro EF. Irreversible ototoxicity associated with zalcitabine. Int J STD AIDS. 1997;8:201–202. doi: 10.1258/0956462971919723. [DOI] [PubMed] [Google Scholar]
- Morata TC, Campo P. Ototoxic effects of styrene alone or in concert with other agents: A review. Noise Health. 2002;4:15–24. [PubMed] [Google Scholar]
- Nadol JB., Jr Hearing loss. N Engl J Med. 1993;329:1092–1102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Note R, Maisonneuve C, Letteron P, Peytavin G, Djouadi F, Igoudjil A, Guimont MC, Biour M, Pessayre D, Fromenty B. Mitochondrial and metabolic effects of nucleoside reverse transcriptase inhibitors (NRTIs) in mice receiving one of five single- and three dual-NRTI treatments. Antimicrob Agents Chemother. 2003;47:3384–3392. doi: 10.1128/AAC.47.11.3384-3392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powderly WG, Klebert MK, Clifford DB. Ototoxicity associated with dideoxycytidine. Lancet. 1990;335:1106. doi: 10.1016/0140-6736(90)92686-c. [DOI] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Real R, Thomas M, Gerwin JM. Sudden hearing loss and acquired immunodeficiency syndrome. Otolaryngol Head Neck Surg. 1987;97:409–412. doi: 10.1177/019459988709700414. [DOI] [PubMed] [Google Scholar]
- Schouten JT, Lockhart DW, Rees TS, Collier AC, Marra CM. A prospective study of hearing changes after beginning zidovudine or didanosine in HIV-1 treatment-naive people. BMC Infect Dis. 2006;6:28. doi: 10.1186/1471-2334-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Simdon J, Watters D, Bartlett S, Connick E. Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: a report of 3 possible cases and review of the literature. Clin Infect Dis. 2001;32:1623–1627. doi: 10.1086/320522. [DOI] [PubMed] [Google Scholar]
- Vernon J, Brummett R, Brown R. Noise trauma induced in the presence of loop-inhibiting diuretics. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1977;84:407–413. [PubMed] [Google Scholar]
- Vogeser M, Colebunders R, Depraetere K, Van Wanzeele P, Van Gehuchten S. Deafness caused by didanosine. Eur J Clin Microbiol Infect Dis. 1998;17:214–215. doi: 10.1007/BF01691123. [DOI] [PubMed] [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK. Evidence for two discrete sources of 2f1-f2 distortion-product otoacoustic emission in rabbit. II: Differential physiological vulnerability. J Acoust Soc Am. 1992;92:2662–2682. doi: 10.1121/1.404382. [DOI] [PubMed] [Google Scholar]
- WHO/UNAIDS. 2007 www.who.int/hiv/en/(last viewed 10/30/07)
- Xing G, Chen Z, Cao X. Mitochondrial rRNA and tRNA and hearing function. Cell Res. 2007;17:227–239. doi: 10.1038/sj.cr.7310124. [DOI] [PubMed] [Google Scholar]