Abstract
Rationale: In adult rats, bilateral ablation of pre-Bötzinger complex (preBötC) neurokinin 1–expressing (NK1R) neurons leads to a progressive and irreversible disruption in breathing pattern, initially during sleep, eventually resulting in an ataxic breathing pattern during wakefulness.
Objectives: Here we determine whether ablation of fewer preBötC NK1R neurons leads to a persistent pattern of disordered breathing during sleep but not during wakefulness.
Methods: Adult male Sprague-Dawley rats (n = 12) were instrumented to record diaphragmatic, abdominal, and neck EMG, and EEG. Fourteen days later, a second surgery was performed to stereotaxically microinject into the preBötC on one side the toxin saporin conjugated to substance P (SP-SAP), which selectively ablates NK1R neurons.
Measurements and Main Results: Postinjection, rats were monitored within a plethysmograph until they were killed (Days 21–51). At Days 6–9 post–unilateral SP-SAP injection, respiratory pattern during sleep, particularly REM sleep, became increasingly disordered, characterized by an increase in frequency of central sleep apnea and hypopneas (36.8 ± 7.4 episodes/h of REM vs. 6 ± 2.0 episodes/h in preinjection controls; P < 0.05), whereas breathing during resting wakefulness remained stable. Unlike bilateral SP-SAP–injected rats, an ataxic breathing pattern did not develop during wakefulness. Rats that were monitored up to 51 days post–SP-SAP injection continued to have sleep-disordered breathing; breathing during wakefulness remained relatively stable. Histologic analysis of the ventrolateral medulla confirmed that NK1R neurons within the preBötC on the injected but not on the contralateral side of the medulla were ablated.
Conclusions: Gradual loss of preBötC NK1R neurons may be an underlying factor of sleep-disordered breathing, in particular of central sleep apnea.
Keywords: respiratory control, apnea, ventrolateral medulla, saporin, neurokinin 1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
An essential component of the respiratory rhythm generating circuitry in mammals is the pre-Bötzinger complex (preBötC). Bilateral ablation of the preBötC leads to deterioration of breathing, initially during sleep, progressing into wakefulness, then death.
What This Study Adds to the Field
Unilateral ablation of the preBötC in rats leads to disrupted breathing during sleep but not wakefulness.
Failure to generate a reliable and robust respiratory rhythm compromises body function and can be fatal. In humans, perturbations in the rhythm are most commonly manifest as sleep-disordered breathing (SDB), as occurs during sleep apnea, congenital central hypoventilation syndrome, several neurodegenerative diseases, and, perhaps, sudden infant death syndrome. An essential component of the respiratory rhythm–generating circuitry in mammals is the pre-Bötzinger complex (preBötC) (1–3), of which approximately 300 neurons per side in the rat express high levels of the neurokinin 1 receptor (NK1R) (4). Bilateral ablation of preBötC NK1R-expressing neurons by the toxin saporin conjugated to substance P (SP-SAP) leads to an irreversible deterioration of breathing pattern, initially during sleep, progressing to an ataxic breathing pattern during wakefulness (4, 5). We propose that loss of preBötC NK1R neurons can underlie SDB in humans (5).
SDB affects a large proportion of the adult population (6) and has a detrimental impact on everyday life, from excessive daytime sleepiness (7) and impaired cognitive function (8), to increased prevalence of stroke (9) and mortality (10). SDB is characterized by hypopneas (i.e., periods of reduced ventilation) and apneas (i.e., periods of respiratory cessation resulting either from a transient occlusion of the upper airway [obstructive sleep apnea (OSA)] or loss in central respiratory drive [central sleep apnea (CSA)]). OSA is the most common type of apnea in young adults, with CSA becoming increasingly prevalent with age (11, 12). CSA is common during both non-REM (NREM) and REM stages of sleep in both humans and rats when serotonin and norepinephrine neurons are inactive throughout the brain (13). This sleep-related withdrawal of excitation combined with disruption of respiratory rhythm–generating mechanisms in the brainstem (e.g., by neurogeneration) may be an underlying cause of CSA and explain why this disorder is exacerbated in the elderly and in some patients with certain neurodegenerative diseases. Developing a model of CSA would greatly enhance our understanding of the neural mechanisms underlying SDB and aid in the development of preventative pharmacologic tools.
The aim of this study was to develop a stable model of SDB and to determine if partial ablation of the preBötC leads to a disruption in breathing pattern that is exacerbated during sleep. We hypothesized that unilateral preBötC NK1R ablation would result in SDB, but unlike bilateral ablation of the preBötC would not lead to an ataxic breathing pattern during wakefulness. Some of the results of these studies have been previously reported in the form of an abstract (14).
METHODS
See the online supplement for details.
Surgical Procedures
All experimental procedures were approved by the Chancellor's Animal Research Committee (UCLA, Los Angeles, CA). Male Sprague-Dawley rats (n = 12, 250–350 g) were implanted with electrodes to record the following: diaphragm electromyography (EMG); neck EMG; and electroencephalography (EEG). Two weeks later, a second surgery was performed to stereotaxically microinject either the SP-SAP (100–150 nl; 6.7 ng; n = 9; Advanced Targeting Systems, San Diego, CA) or SP mixed with but not conjugated to SAP (unconjugated, control; n = 3), unilaterally into the left preBötC.
Data Acquisition and Analysis
Rats were housed in a room with a 12-h light/dark cycle. Data were acquired using Chart 5 software (Powerlab 16SP; AD Instruments, Colorado Springs, CO), amplified (Grass Model P511K; Grass Instrument Co., West Warwick, RI), filtered (EEG: 0.3–100 Hz, EMG: 10–100 Hz, ECG: 10–100 Hz), and sampled at 1,000 Hz. Data acquired within a 2-hour period (between 12:00 and 18:00, light cycle) every day postinjection were analyzed. Responses to hypercapnia and hypoxia were tested during wakefulness pre- and postinjection.
Sleep and wakefulness were determined from visual inspection, analysis of neck EMG activity, and the fast Fourier transform of the EEG signal in 10-second epochs at delta (0.3–5 Hz), theta (6–9 Hz), and sigma (10–15 Hz) frequency bands. Only data when the rat was asleep and in quiet wakefulness were analyzed.
Changes in breathing frequency and amplitude were measured on a breath-by-breath basis using whole body plethysmography (Buxco Electronics, Inc., New York, NY). A constant airflow (2 L/min) was delivered through the plethysmographic chamber and respiratory parameters quantified by recording pressure fluctuations, relative to a reference chamber, that are proportional to tidal volume (differential pressure transducer, DRAL501; Honeywell Data Instruments, Golden Valley, MN). Inspiratory amplitude was calculated from the plethysmographic pressure signal output (in mV); control (preinjection) data were normalized to 1 A.U. (arbitrary units) and all data acquired on subsequent days were rescaled relative to control. Spontaneous respiratory disturbances were defined as hypopnea when inspiratory amplitude was less than 0.5 A.U. of normalized control values and apnea when termination of breathing was greater than 2 seconds (∼3 missed breaths). Respiratory disturbances are expressed as number of episodes normalized to 1 hour each of wakefulness (WAKE), NREM, and REM. All data are expressed as mean ± SEM. Statistical analyses were performed using a two-way repeated measures analysis of variance, with post hoc Bonferroni analysis for individual comparisons (StatView software; Abacus Concepts, Berkeley, CA). Differences were regarded as significant if P values were less than 0.05.
Histology
Rats were transcardially perfused between Days 21–51 postinjection; the brainstem was sectioned at 40 μm and stained for one or two of the following primary antibodies: rabbit anti-NK1R (1:20,000; Chemicon, Temecula, CA). goat anti-choline acetyl transferase (ChAT 1:400; Chemicon), mouse anti-tyrosine hydroxylase (1:500; Chemicon). Under light microscopy (Zeiss, Axioplan2; Carl Zeiss, Thornwood, NY), the extent of the lesion was determined by counting NK1R immunopositive soma in a series of transverse sections representing approximately 20% of the preBötC, within a circle of 600 μm diameter representing the extent of the preBötC (4), and in a rectangle (1,600 × 1,070 μm) outside this circle to determine the extent of NK1R neuronal damage outside the preBötC. The mapped sections were at least 160 μm apart to eliminate double counting, beginning with the section in which the rostral border of the lateral reticular nucleus was identified, to the section that included the caudal end of the facial nucleus.
RESULTS
As we previously reported, electrode implantation surgery did not change breathing frequency when compared with preelectrode implantation (115 ± 15 vs. 127 ± 14 breaths/min; P > 0.05) (5). After injection surgery, all rats appeared lethargic and had shallow breathing. In those rats that were injected unilaterally with SP mixed with but not conjugated to SAP (unconjugated, control), breathing frequency returned to preinjection levels by Day 3 postinjection as we have previously reported (121 ± 6 breaths/min; n = 3) (5), and respiratory disturbances (i.e., hypopneas and apneas) at Day 10 postinjection did not significantly differ from preinjection (WAKE, 4 ± 0.12; NREM, 4 ± 0.12; REM, 6 ± 2.0 episodes/h; P > 0.05).
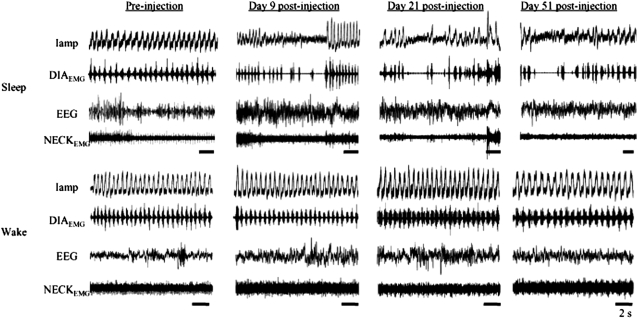
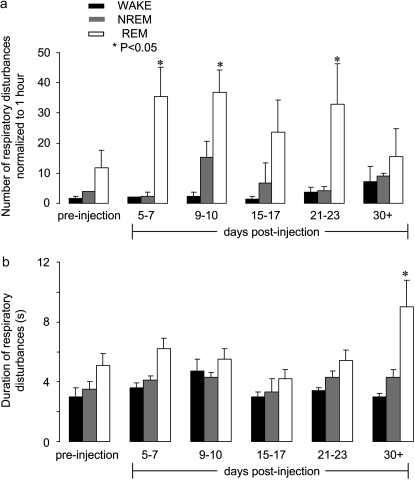
In rats injected unilaterally with SP-SAP, breathing patterns that had been shallow after injection surgery returned to preinjection levels by Day 3 postinjection (134 ± 23 breaths/min; n = 9; P > 0.05) and remained normal for up to 6 days after injection. Breathing disturbances became evident during sleep between Days 6–9 postinjection (Figure 1). These disturbances were most frequent during REM sleep (36.8 ± 7.4 episodes/h, 5.5 ± 0.7 s; Figure 2; n = 6; P < 0.004) but were also evident during NREM sleep (NREM: 15.3 ± 5.3 episodes/h, 4.3 ± 0.3 s; Figure 2; n = 6; P > 0.05) (5). The disturbances were characterized by hypopneas that occasionally led to short periods with neither airflow nor diaphragmatic EMG inspiratory activity (i.e., CSA). In some cases, the rats woke up at the end of the apnea and resumed breathing; in other cases, the rats resumed breathing while remaining asleep. Compared with preinjection, breathing was not disturbed during wakefulness; it remained stable with no change in frequency (preinjection, 115 ± 15, vs. Day 21 postinjection, 127 ± 27 breaths/min; P > 0.05).
Figure 1.
Breathing during sleep and wakefulness in the adult rat. Preinjection: breathing is regular and stable during sleep (4 upper panels of traces) and wakefulness (4 bottom panels of traces). Days 9–51 postinjection: breathing is disrupted during sleep (top panels), but there is no substantial change to breathing pattern during wakefulness (bottom panels). During sleep, breathing disorders are characterized by hypopnea and central sleep apnea. DIAEMG = diaphragmatic electromyogram; Iamp = inspiratory amplitude measured in arbitary units; NECKEMG = neck electromyogram. Time bar = 2 s.
Figure 2.
Number and duration of respiratory disturbances (central sleep apneas [CSAs] and hypopneas). (a) The number of respiratory disturbances significantly (P < 0.05) increase during REM sleep 5–7 days postinjection and continue to be greater in number, although variable, up to 30+ days postinjection compared with preinjection. During non-REM (NREM) sleep, there is a slight increase in the number of respiratory disturbances compared with preinjection. There is no significant increase during wakefulness (WAKE). (b) Preinjection: respiratory disturbances characterized as hypopneas and CSA are longest during REM sleep. Postinjection, duration of disturbances becomes variable during both stages of sleep and wakefulness, becoming significant during REM at Day 30 (P < 0.05).
Rats that were monitored up to 51 days postinjection (n = 2) continued to have SDB, whereas breathing during wakefulness remained unchanged (Figure 1). Unlike bilaterally injected rats (4, 5), breathing pattern during sleep in unilateral injected rats did not substantially deteriorate after about Day 10. At no time did the rats develop a respiratory disturbance during every period of sleep. When a respiratory disturbance occurred, it was characterized by hypopnea that in most cases developed into a CSA upon transition to REM (15.4 ± 9.4 episodes/h, 5.9 ± 0.7s; Figure 2; n = 2; P < 0.05). CSA did not occur exclusively in REM but occurred more frequently in REM compared with NREM (Figure 2), and sleep pattern post–unilateral injection was more fragmented compared with preinjection. When the rats aroused from a CSA, it was usually a gentle transition to wakefulness, with slight head movement and very little body movement, rather than an abrupt arousal that is seen after bilateral injection when breathing pattern is highly disrupted due to frequent respiratory disturbances (4, 5).
Chemosensitivity
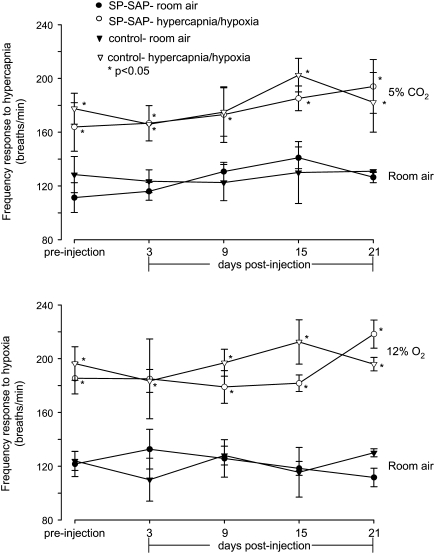
Bilateral ablation of preBötC NK1R neurons blunts the ventilatory response to hypercapnia and hypoxia during wakefulness (4). To see if this were the case after unilateral ablation, awake rats were exposed to hypercapnic and hypoxic inhaled gas mixtures. In unilateral SP-SAP–injected rats, there was no significant difference in the frequency response during wakefulness to hypercapnia when tested on Days 3, 9, 15, and 21 post–unilateral SP-SAP injection compared with preinjection (preinjection: 164 ± 18; Postinjection Day 3: 167 ± 13; Day 9: 173 ± 21; Day 15: 185 ± 9; Day 21: 201 ± 16; P > 0.05; Figure 3). When compared with the hypercapnic response of rats injected with SP mixed with but not conjugated to SAP (unconjugated, control), frequency at all time points was not significantly different (P > 0.05; Figure 3).
Figure 3.
Unilateral ablation of pre-Bötzinger complex NK1R neurons does not significantly alter responses to hypoxia and hypercapnia during wakefulness. In response to hypercapnia (5% CO2, 21% O2, 84% N2), there was a significant increase in respiratory frequency compared with that in room air both pre- and postinjection (P < 0.05); the responses postinjection were not significantly different (P > 0.05) from preinjection responses or from those of rats injected with substance P mixed with saporin (unconjugated, control). Similarly, hypoxia (12% O2, 88% N2) induced significant increases (P < 0.05) in frequency both pre- and postinjection compared with that in room air; these responses were not significantly different (P > 0.05) from preinjection responses or from control animals. *P < 0.05, significantly different from responses in room air. SP-SAP = saporin conjugated to substance P.
In response to hypoxia, there was no significant difference in the frequency response during wakefulness to hypoxia when tested on Days 3, 9, 15, and 21 post–unilateral SP-SAP injection compared with preinjection (preinjection: 185 ± 12; Postinjection Day 3: 185 ± 30; Day 9: 179 ± 12; Day 15: 182 ± 6; Day 21: 218 ± 10; P > 0.05; Figure 3). When compared with the hypoxic frequency response of rats injected with SP mixed with but not conjugated to SAP (unconjugated, control) there was no significant difference (P > 0.05; Figure 3).
Histology
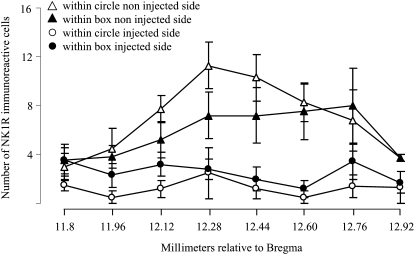
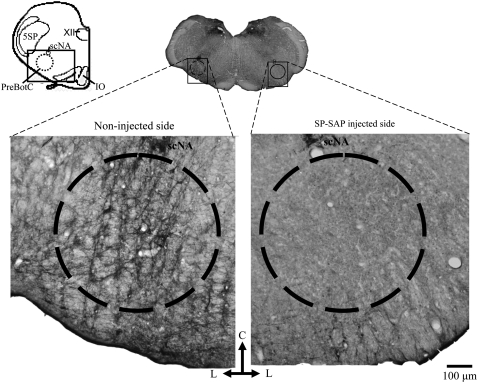
The accumulating bilateral loss of preBötC neurons leads to a progressive disturbance in breathing that is initially seen during sleep, eventually leading to an ataxic breathing pattern when more than 80% of preBötC NK1R neurons are ablated (4, 5). Here, unilateral injection limited the maximum destruction to 50% (∼300 preBötC NK1R neurons) and left the contralateral preBötC intact. In representative samples (see Methods and the online supplement), almost all NK1R neurons within the lesioned preBötC were ablated based on representative neuron counts (10 ± 0.2 NK1R+/side; n =5; P < 0.05), compared with the noninjected side (56 ± 0.2 NK1R+/side; Figures 4 and 5). Lesions were mostly confined to the preBötC (4), but in the surrounding rectangular area, NK1R staining was also reduced (20 ± 0.1 NK1R+/side) compared with the noninjected side (46 ± 0.3 NK1R+/side; P < 0.05) or SP mixed with but not conjugated to SAP (unconjugated, control; Figures 4 and 5). A small number of neurons within the nucleus ambiguous were damaged. There was no significant neuronal loss in other regions examined (e.g., solitary tract, motor nucleus of vagus, retrotrapezoid nucleus), similar to the results reported in our previous studies using the same injection protocol (4, 5). Also, there was no reduction in the number of tyrosine hydroxylase–expressing neurons that are intermingled with but do not express NK1R, thus confirming the selectivity of the toxin.
Figure 4.
Rostrocaudal distribution of NK1R immunoreactivity within the ventrolateral medulla. Neuronal profiles counted within a circle of 600 μm diameter representing the extent of the pre-Bötzinger complex (see Figure 5) were reduced in the side injected with saporin conjugated to substance P compared with the noninjected side. There was some damage to NK1R-expressing neurons located within a rectangular area (1,600 × 1,070 μm) outside this circle (see Figure 5). On the noninjected side, there was no damage to NK1R neurons, as determined by comparison to noninjected control rats.
Figure 5.
Saporin conjugated to substance P (SP-SAP) specifically ablates NK1R neurons within the pre-Bötzinger complex (preBötC). Schematic of transverse medullary slice denotes extent of preBötC (circle of 600 μm diameter) and surrounding area (rectangle, 1,600 × 1,070 μm) analyzed for NK1R-expressing neurons. Almost all neurons positively stained for NK1R within the preBötC were ablated after SP-SAP injection (right side of image) along with some neurons outside the preBötC. On the noninjected side (left side of image), there was no damage to NK1R neurons. IO = inferior olive; 5SP = trigeminal nucleus; ScNA = compact nucleus ambiguous; XII = hypoglossal nucleus. Scale bar, 100 μm.
DISCUSSION
Unilateral destruction of preBötC NK1R neurons leads to SDB that was stable for up to 7 weeks, whereas breathing during wakefulness was only very slightly affected. Unlike bilateral ablation of the preBötC, an ataxic breathing pattern did not develop (4, 5).
Transitions between sleep and wake states induce changes in ventilation, with SDB being common even in healthy individuals (15), and exaggerated in many diseases and in the elderly. SDB is a significant health problem in adults (6), greatly impacting quality of life (7, 8). CSA, characterized by an absence of respiratory effort, is less common in young adults but becomes increasingly prevalent with age (11, 12), even in elderly individuals who are relatively “healthy” (16). The etiology of CSA is presently unknown; however, we show here and have shown previously (5) that disruption of preBötC function may be an underlying or contributing cause. In humans, CSA regularly occurs as part of a periodic breathing pattern and has a comorbidity with congestive heart failure (17). The waxing and waning Cheyne-Stokes' pattern of respiration that is commonly seen in patients with congestive heart failure was not seen in the unilaterally injected rats. In all rats, only apneas of central origin were recorded, which were evident due to the lack of diaphragmatic EMG activity. Obstructive events were not observed, likely because the upper airway anatomy and sleeping position of rats is not conducive for OSA. We concede that some CSA may be representative of mixed apnea commonly seen in humans (18). A mixed apnea can begin as central but becomes obstructive upon the restoration of diaphragmatic contraction. The close relationship between CSA and OSA highlights the need to understand the underlying mechanisms and participation of CSA in the manifestation of SDB, even if CSA accounts for only a small number of SDB events in the healthy, nonelderly adult.
In humans, respiratory disturbances can result from medullary damage (19) or lesions of the bulbospinal pathway (20); such patients breathe spontaneously during wakefulness, but without mechanical ventilation they can have a fatal apnea during sleep. Adults with syringobulbia (with brainstem symptoms or magnetic resonance imaging evidence of medullary syrinx [21]), or children with congenital central hypoventilation syndrome (22), breathe relatively normally during wakefulness, but during sleep their breathing pattern is severely disrupted by CSA or severe hypopnea. This is in contrast to patients with rostrolateral medullary lesions (23) who have SDB characterized by OSA rather than CSA. CSA or severe hypopnea is common in patients with some neurodegenerative diseases, especially amyotrophic lateral sclerosis (24, 25), multiple systems atrophy (MSA) (26, 27), or Parkinson's disease (PD) (28, 29). In all of these patients, breathing during wakefulness is considered relatively normal. Here, adult rats with unilateral preBötC NK1R lesions breathed normally during wakefulness but had hypopneas and CSA during sleep. We suggest that human SDB characterized by hypopnea and CSA can result from dysfunction of preBötC NK1R neurons within the ventrolateral medulla (30) that are homologous to the rodent and goat preBötC (31). If preBötC neurons are vulnerable to neurodegeneration (5, 30), then this would explain the high incidence of SDB in patients with PD, MSA, or amyotrophic lateral sclerosis.
As with bilateral lesions, unilateral SP-SAP–induced respiratory disturbances were first evident during REM sleep, then also in NREM sleep; however, unlike bilateral lesions, unilateral preBötC ablation did not progress to an ataxic breathing pattern during wakefulness (4, 5). In rats, and humans (particularly the elderly), irregular breathing and spontaneous CSA occur during both REM (12, 32, 33) and NREM, but are rarely seen during wakefulness. Other than case studies of patients with medullary lesions, ataxic breathing, a highly irregular pattern in which randomly occurring deep and shallow breaths are interspersed with CSA, is rarely seen in awake humans (34). In humans, the sleep/wake cycle is accompanied by a large number of changes in respiratory control that together likely account for the increased fragility of breathing during sleep. The “wakefulness drive” to breathe (35) is diminished during sleep, ventral medullary respiratory neuron activity is significantly reduced during REM sleep (36), ventilatory responses to hypercapnia and hypoxia are reduced (37, 38), and there is an alteration in neurotransmitter levels throughout the brain. Neurons that release histamine, which plays a role in arousal, and serotonin and norepinephrine, which play roles in the control of muscle tone and arousal, are relatively inactive during REM sleep, whereas those releasing acetylcholine (ACh) are more active (13). Because serotonin and norepinephrine excite preBötC neurons (39, 40), any decrease in their release would disfacilitate preBötC neurons, which may compromise breathing stability during REM sleep. In the presence of further insults, such as SP-SAP lesions, neurodegeneration, or brainstem damage, severe SDB could result.
The preBötC is a bilateral cluster of functionally heterogeneous neurons, including glutamatergic (41) neurons that are anatomically defined by high levels of NK1R expression (42) and somatostatin mRNA (43), located just ventral to the nucleus ambiguous in the rostroventrolateral medulla (42, 43). In this study, there was slight damage to the nucleus ambiguous; however, the pathologic changes in breathing observed here (see also References 4 and 5) cannot be attributed to a small amount of upper airway motoneuronal damage within the nucleus ambiguous. Neither can the observed changes be attributed to the destruction of inspiratory premotor neurons that are also located within and adjacent to this area (41) because only a small number of these bulbospinal neurons express NK1R. The unilateral injections of SP-SAP specifically ablated NK1R neurons within the preBötC on the injected side only; there was no reduction in NK1R neurons in the contralateral preBötC. A similar protocol in rats performed by Wang and colleagues (44) reported no change in basal respiratory rate during wakefulness following unilateral preBötC ablation.
In addition to its essential role in respiratory rhythmogenesis, the preBötC plays a role in ventilatory responses to hypoxia and hypercapnea (4, 5). There are a number of chemoresponsive sites within the brain, including glutamatergic neurons within the retrotrapezoid nucleus of the ventral medulla (45), serotonergic neurons of the midline raphe and medullary surface (46), in addition to the nucleus of the solitary tract, fastigial nucleus, and locus ceruleus. The sensitivity of some of these areas is state dependent (2, 47, 48). After unilateral preBötC ablation, there was no significant difference in the responses to hypercapnia or hypoxia during wakefulness; however, impaired chemosensitivity is a symptom of bilateral preBötC ablation (4) and may reflect a role for the preBötC in integrating chemoresponsive information. Notably, patients with MSA or PD also have impaired chemosensitivity to hypoxia (49, 50), and in patients with MSA there is a depletion of glutamatergic and serotonergic neurons, putative chemoresponsive neurons, within the ventral medullary surface (51).
The increase of SDB in the elderly is a concern in an aging society, and highlights the need for preventative treatment. At present, patients who have CSA occurring with OSA are usually treated with nasal continuous positive airway pressure. Pharmacologic treatments with acetazolamide and theophylline attenuate CSA, but large-scale clinical trials on the effectiveness and side effects of these drugs are required. The heterogeneity of CSA means that treatment needs to be individualized; this can be very time consuming and expensive. Understanding the central mechanisms underlying dysfunction of preBötC respiratory control during sleep may pave the way for a generic treatment and/or prevention of CSA.
Conclusions
Unilateral preBötC ablation results in SDB, whereas breathing during resting wakefulness is only very slightly affected, and in most cases, is relatively normal. Over time, breathing during sleep does not improve, whereas breathing during wakefulness remains stable. We hypothesize that disruption of preBötC function in the brainstem is an underlying cause of CSA (5); if correct, this mechanism could explain why CSA is exacerbated in the elderly and in some patients with neurodegenerative diseases. We further speculate that SDB can go unnoticed because breathing during wakefulness is relatively stable. We have developed a model of SDB in the rodent that, together with clinical observations and postmortem analysis, will permit a greater understanding of the role of the preBötC in SDB.
Supplementary Material
Acknowledgments
The authors thank Wiktor Janczewski for technical support and discussion, Matt Knedel for assistance with data collection, and Grace Li for assistance with histology.
Supported by National Institutes of Health grant HL70029.
This article has an online supplement, which is accessible from the issue's table of content at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200712-1901OC on April 17, 2008
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 2006;7:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 2003;26:239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 1991;254:726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 2001;4:927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci 2005;8:1142–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 7.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000;118:372–379. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Zion M, Stepnowsky C, Marler T, Shochat D, Kripke F, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc 2001;49:1622–1627. [DOI] [PubMed] [Google Scholar]
- 9.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Kripke DF, Klauber MR, Fell R, Stepnowsky C, Estline E, Khazeni N, Chinn A. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep 1996;19:277–282. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144–148. [DOI] [PubMed] [Google Scholar]
- 12.Krieger J, Turlot JC, Mangin P, Kurtz D. Breathing during sleep in normal young and elderly subjects: hypopneas, apneas, and correlated factors. Sleep 1983;6:108–120. [DOI] [PubMed] [Google Scholar]
- 13.Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry 2004;65:4–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Mckay LC, Feldman JL. Unilateral ablation of NK1R neurons within the preBötC in adult rats disrupts breathing during sleep but not during wakefulness. Atlanta, GA: Society for Neuroscience; 2006.
- 15.Dempsey JA, Smith CA, Harms CA, Chow C, Saupe KW. Sleep-induced breathing instability. Sleep 1996;19:236–247. [PubMed] [Google Scholar]
- 16.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997;20:654–675. [DOI] [PubMed] [Google Scholar]
- 17.Bradley TD, Floras JS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation 2003;107:1822–1826. [DOI] [PubMed] [Google Scholar]
- 18.Badr MS. Central sleep apnea. Prim Care 2005;32:361–374. [DOI] [PubMed] [Google Scholar]
- 19.Plum F, Leigh RJ. Abnormalities of central mechanisms. In: Hornbein TF, editor. Regulation of breathing: lung biology in health and disease. New York: Marcel Dekker; 1981. pp. 989–1067.
- 20.Nathan PW. The descending respiratory pathway in man. J Neurol Neurosurg Psychiatry 1963;26:487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogues M, Gene R, Benarroch E, Leiguarda R, Calderon C, Encabo H. Respiratory disturbances during sleep in syringomyelia and syringobulbia. Neurology 1999;52:1777–1783. [DOI] [PubMed] [Google Scholar]
- 22.Commare MC, Francois B, Estournet B, Barois A. Ondine's curse: a discussion of five cases. Neuropediatrics 1993;24:313–318. [DOI] [PubMed] [Google Scholar]
- 23.Morrell MJ, Heywood P, Moosavi SH, Guz A, Stevens J. Unilateral focal lesions in the rostrolateral medulla influence chemosensitivity and breathing measured during wakefulness, sleep, and exercise. J Neurol Neurosurg Psychiatry 1999;67:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthlen GM, Lange DJ. Unexpectedly severe sleep and respiratory pathology in patients with amyotrophic lateral sclerosis. Eur J Neurol 2000;7:299–302. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson KA, Strong MJ, Ahmad D, George CF. Sleep-disordered breathing in amyotrophic lateral sclerosis. Chest 1996;110:664–669. [DOI] [PubMed] [Google Scholar]
- 26.Cormican LJ, Higgins S, Davidson AC, Howard R, Williams AJ. Multiple system atrophy presenting as central sleep apnoea. Eur Respir J 2004;24:323–325. [DOI] [PubMed] [Google Scholar]
- 27.Munschauer FE, Loh L, Bannister R, Newsom-Davis J. Abnormal respiration and sudden death during sleep in multiple system atrophy with autonomic failure. Neurology 1990;40:677–679. [DOI] [PubMed] [Google Scholar]
- 28.Apps MC, Sheaff PC, Ingram DA, Kennard C, Empey DW. Respiration and sleep in Parkinson's disease. J Neurol Neurosurg Psychiatry 1985;48:1240–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maria B, Sophia S, Michalis M, Charalampos L, Andreas P, John ME, Nikolaos SM. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med 2003;97:1151–1157. [DOI] [PubMed] [Google Scholar]
- 30.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Depletion of ventromedullary NK-1 receptor-immunoreactive neurons in multiple system atrophy. Brain 2003;126:2183–2190. [DOI] [PubMed] [Google Scholar]
- 31.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Botzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol 2004;97:1620–1628. [DOI] [PubMed] [Google Scholar]
- 32.Guilleminault C. Sleep and breathing. In: Guilleminault C, editor. Sleeping and waking disorders, indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 155–182.
- 33.Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol 1990;259:R282–R287. [DOI] [PubMed] [Google Scholar]
- 34.Plum F, Posner JB. The diagnosis of stupor and coma. Contemp Neurol Ser 1972;10:1–286. [PubMed] [Google Scholar]
- 35.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol 1961;16:15–20. [DOI] [PubMed] [Google Scholar]
- 36.Orem J. Medullary respiratory neuron activity: relationship to tonic and phasic REM sleep. J Appl Physiol 1980;48:54–65. [DOI] [PubMed] [Google Scholar]
- 37.Douglas NJ, White DP, Weil JV, Pickett CK, Martin RJ, Hudgel DW, Zwillich CW. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis 1982;125:286–289. [DOI] [PubMed] [Google Scholar]
- 38.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982;126:758–762. [DOI] [PubMed] [Google Scholar]
- 39.Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol 1994;72:2538–2541. [DOI] [PubMed] [Google Scholar]
- 40.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 2002;22:11055–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 2002;22:3806–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 1999;286:1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J Comp Neurol 2003;455:499–512. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 2002;22:3755–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 2004;7:1360–1369. [DOI] [PubMed] [Google Scholar]
- 46.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 2004;5:449–461. [DOI] [PubMed] [Google Scholar]
- 47.Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 2001;90:1247–1257. [DOI] [PubMed] [Google Scholar]
- 48.Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol 2002;92:2119–2130. [DOI] [PubMed] [Google Scholar]
- 49.Onodera H, Okabe S, Kikuchi Y, Tsuda T, Itoyama Y. Impaired chemosensitivity and perception of dyspnoea in Parkinson's disease. Lancet 2000;356:739–740. [DOI] [PubMed] [Google Scholar]
- 50.Tsuda T, Onodera H, Okabe S, Kikuchi Y, Itoyama Y. Impaired chemosensitivity to hypoxia is a marker of multiple system atrophy. Ann Neurol 2002;52:367–371. [DOI] [PubMed] [Google Scholar]
- 51.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Depletion of putative chemosensitive respiratory neurons in the ventral medullary surface in multiple system atrophy. Brain 2007;130:469–475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.