Abstract
Cytoskeletal proteins of the ezrin-radixin-moesin (ERM) family contribute to T cell activation in response to antigen, and also to T cell polarization in response to connective tissue matrix proteins and chemokine gradients. Previous work has shown that T cells from aged mice are defective in their ability to develop molecular linkages between surface macromolecules and the underlying cytoskeletal framework, both for proteins that move to the synapse and those that are excluded from the site of T cell/APC interaction. T cells from aged mice also show defective cytoskeletal rearrangements and lamellipodia formation when placed in contact with slides coated with antibodies to the TCR/CD3 complex. Here we show that aged CD4 T cells differ from young CD4 T cells in several aspects of ERM biochemistry, including ERM phosphorylation and ERM associations with CD44, CD43 and EBP50. In addition, CD4 T cells from old mice show defects in the Rho GTPase activities known to control ERM function.
Keywords: T-Lymphocytes, Aging, Cytoskeleton, Signal Transduction, Cellular Activation
Introduction
Cytoskeletal proteins participate in many aspects of T cell function, including formation of the immunological synapse and spreading of the T cell membrane (lamellipodia) adjacent to APC (1-4). Recent models of immunosynapse formation suggest that the initiation and maintenance of the immunosynapse involves the interactions of surface molecules with the cytoskeleton (5,6). The Ezrin-Radixin-Moesin family of cytoskeletal proteins (ERM) plays a key role in this remodeling, particularly with respect to the exclusion of glycoproteins (such as CD43) from the synapse area (4,7-154). The ERM family is also critical to other aspects of T cell function, including cell adhesion, formation of lamellipodia and membrane ruffling (14,156). ERM action often involves linkages between cell surface molecules (including CD44, CD43 and P-glycoprotein or MDR-1) and adaptor proteins such as Ezrin binding protein 50 (EBP50) with the plasma membrane (3,16-22). The regulation of these interactions is thought to involve the phosphorylation of ERM (p-ERM), specifically on Thr558 of moesin or Thr567 of ezrin, leading to opening of the FERM domain in the ERM molecules and therefore to interaction with surface molecules and adaptor proteins (23,24). In resting T cells from young mice, ERM proteins are usually phosphorylated (p-ERM), and dephosphorylation is induced by CD3 ligation and APC contact (6,9). The level or status of ERM phosphorylation may be important in the breakdown of microvilli, spreading of the cell membrane over adjacent surfaces, formation of lamellipodia, and polarization of the cell, including asymmetric distribution of internal and surface macromolecules (6,25,26) during the formation of the immunological synapse (6, 2,9,12,18,21,23,24). During aging there is a decline in the proportion of CD4 T cells that can translocate surface molecules and signaling adaptor proteins to the area of T cell-APC contact (27-30). Those T cells that are unable to form synapses are also unable to exclude CD43 from the area of APC contact. Aging also leads to defects in the association of TCR (CD3ζ) and CD43 to the cytoskeletal network of mouse CD4 T cells (28,29). We hypothesized that age-related alteration in cytoskeletal reorganization might be responsible for the defects in translocation of proteins during immunosynapse formation. In one series of experiments, we treated CD4 T cells with an O-sialoglycoprotein endopeptidase (OSGE), a bacterial enzyme that cleaves surface proteins (including CD43) that contain O-linked glycans. We found that OSGE treatment of CD4 T cells from aged mice fully restores the translocation of signaling molecules, including the TCR, PLCγ, LAT and Grb-2, to the immunosynapse (29,31,32). Treatment with OSGE did not, however, significantly repair the age-dependent decline in exclusion of CD43 from the area of T cell-APC contact, even in cells that formed a functional immunosynapse after OSGE treatment (29). Because exclusion of CD43 from the synapse is dependent on ERM function (8,33), we hypothesized that age-related defects in the ERM pathways might impair activation of aged T cells independent of the role of O-glycan containing surface proteins (29,34). Here we document age-dependent defects in activation of ERM cytoskeletal proteins of CD4 T cells, and present data to suggest that some of the upstream signaling pathways of the Rho family of GTPases, known to play important roles in regulation of the ERM pathways in T cells, are also affected by aging.
Material and Methods
Animals and cell culture
Breeding pairs of the AND line of TCR-transgenic mice, whose T cells respond to pigeon cytochrome C (PCC), were a generous gift from Drs. Susan Swain and Laura Haynes (Trudeau Institute, Saranac Lake, NY). Transgene-positive mice were aged in a specific pathogen-free colony at the University of Michigan. Specific-pathogen free male [BALB/c × C57BL/6]F1 (CB6F1) mice were purchased from the National Institute of Aging contract colonies at the Charles River Laboratories (Kingston, NJ) and at Harlan (Indianapolis, IN). Mice were given free access to food and water. Sentinel animals were examined quarterly for serological evidence of viral infection; all such tests were negative during the course of these studies. Mice that were found to have splenomegaly or macroscopically visible tumors at the time of sacrifice were not used for experiments. CB6F1 mice were used at 6-8 (young) or 22-24 (old) months of age; AND mice were used at 6-8 months or at 14-16 months of age.
Antibodies, reagents and cell preparations
Goat anti-CD43 (M19), rabbit anti-MDR and MoAb anti-CD45 (clone MB4B4) were purchased from Santa Cruz (www.scbt.com). Rabbit anti-RhoA, anti-Rac1, anti-pERM [anti-phosphoEzrin (Thr567)/phosphoMoesin (Thr558)], anti-moesin, anti-LAT and anti-phospho-LAT (Tyr191) came from Cell signaling (www.cellsignal.com). Rabbit anti-CD44 was purchased from Abcam (www.abcam.com). MoAb anti-ezrin (clone 3C12) was purchased from Sigma (www.sigma.com). MoAb anti-moesin (clone38/87) was from NeoMarkers (www.labvision.com). Rabbit anti-EBP50 was obtained from Affinity Bioreagents (www.bioreagents.com). The monoclonal antibodies for flow-cytometry and CD44 (clone IM7) were purchased from BD Biosciences (www.bdbiosciences.com). GST-Rhotekin and GST-Pak1 fusion proteins were acquired from Pierce (www.piercenet.com) CD4+ T cells were obtained and stimulated from transgenic or CB6F1 mice using the negative selection methods previously described in (35) and (30) respectively. Flow-cytometric analysis of a typical preparation showed it to be 90-95 % positive for both CD3 and CD4.
Brij-58 treatments, immunoprecipitations and Rho activity assays
Approximately 10 × 106 CD4+T cells were treated with OSGE and stimulated for 5 min by crosslinking CD3ε with CD4 and CD28 as described previously (29,32); controls included cells that were not treated with OSGE and others that were not activated. The cells were lysed in 1 ml of 1% Brij-58, PBS, pH 7.4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium orthovanadate for 30 minutes on ice. The lysates were ultracentrifuged at 100,000×g for 1h at 4°C. The pellet (“Brij-resistant fraction”) was washed once with lysis buffer, centrifuged at 100,000×g for 10 min at 4°C, resuspended in 50 μl of SDS sample buffer and boiled for 15 min. The supernatant (“Brij-58 soluble fraction”) was used for total cell lysate analysis, for immunoprecipitation (for 6 hours) with specific antibodies pre-coupled to protein G Sepharose as previously described (28,35), or for the RhoA/Rac pull down assay using 25 μg/sample of GST-Rhotekin or GST-Pak1 fusion protein as previously described (36). Samples were fractionated in polyacrylamide gels, transferred to PVDF, and incubated with specific antibodies as described in the text; the bands were visualized by chemifluorescence and quantified as previously described (35).
Intracellular staining and flow-cytometric analysis
Intracellular staining of T cells was performed according to a procedure recommended by Becton-Dickinson (www.bd.com). Briefly, purified T cells were stained using Cy-5 labeled anti-CD4 and PE-labeled CD44, then fixed and treated with methanol at -20°C. After two washes, the cells were stained with rabbit polyclonal antibodies specific for the phosphorylated or unphosphorylated forms of the protein of interest, followed by goat anti-rabbit FITC. The cells were analyzed on a Becton-Dickinson FACSCalibur, with gating for CD4 T cells.
Statistical analyses
Unless otherwise indicated, results are presented as means ± SEM. Statistical significance was assessed using a Mann-Whitney test at p = 0.05.
Results
Effect of age in the distribution of molecules between the Brij-58 detergent resistant and Brij-58 detergent soluble fractions of CD4 T cells
We and others have shown that some membrane associated proteins can be resistant to Brij-58 detergent extraction under under specific conditions (28,29,37,38). We evaluated the effects of Brij extraction on ERM protein distribution in freshly isolated CD4 T cells. As shown in Figure 1, CD44, CD45, MDR-1, EBP50 and the majority of the moesin and ezrin are solubilized by Brij-58 treatment. In contrast, CD3ζ(p21ζ) can be found distributed between both soluble and resistance fractions. Furthermore, TCR stimulation of CD4 T cells from young mice increases the amount of CD3ζ associated with the Brij-58 resistant fraction, but does not have any significant effect on distribution of CD3ζ in CD4 T cells from aged mice. In addition, CD43 is divided between the detergent-resistant and detergent-soluble fractions. TCR stimulation of CD4 T cells from young mice increases the amount of CD43 found in the detergent-resistant fraction, while decreasing the amount in the soluble fraction. However, stimulation does not have this effect on CD43 of CD4 T cells from old donors. The age-related change in CD3ζ distribution and the lack of response of CD43 in old donors are in good agreement with our previously published data (6,29). In contrast to its effects on CD43 and CD3ζ localization, TCR stimulation of freshly isolated CD4 T cells does not alter the extractability of molecules that are known or hypothesized to be excluded from the immunosynapse, including ERM, CD44 and CD45, findings in line with results of other studies on T cells (39-42). The nature of this detergent resistance to extraction is not understood but we hypothesize that cytoskeleton or cortical actin interaction with membrane proteins may play a role. Therefore alteration in the Brij-58 extraction may reflect age-related changes in the interactions between surface molecules and cytoskeleton proteins.
Figure 1.
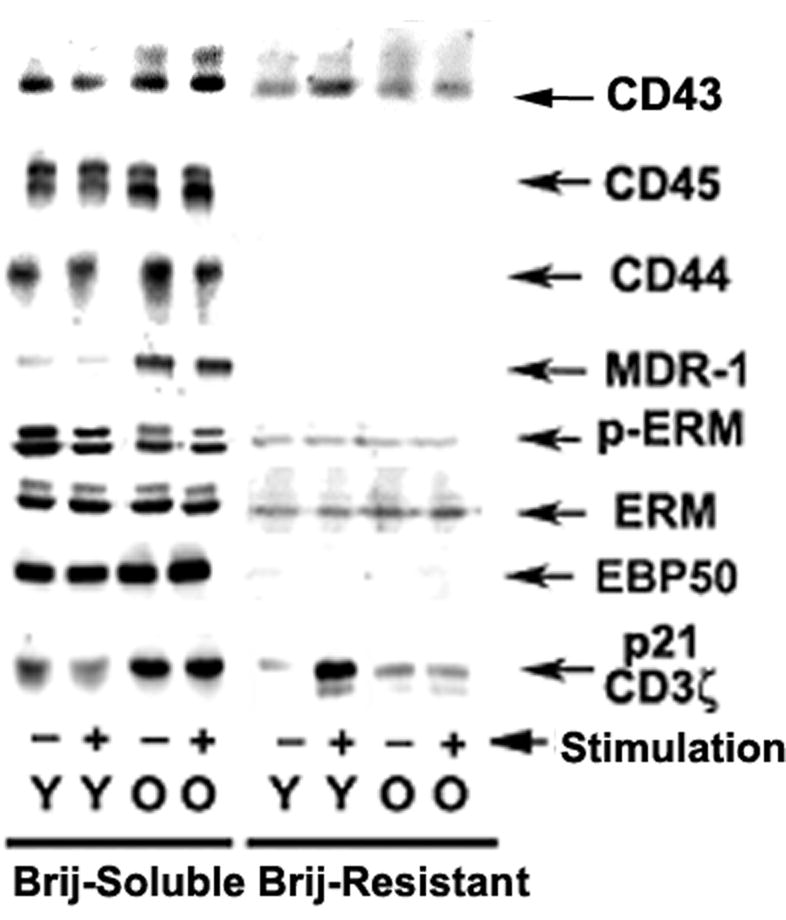
Distribution of proteins between the Brij-58 resistant (cortical actin) and Brij-58 soluble fractions in CD4 T cells from young and old mice. Purified CD4 T cells from young and old CB6F1 donors were stimulated (+) or not stimulated (-) with anti-CD3 plus anti-CD4 and anti-CD28 crosslinking for 5 min. The cells were lysed with 1% Brij-58 detergent and centrifuged. Samples from the pellet (Brij-Resistant) or soluble (Brij-Soluble) fractions were analyzed by western blots using specific antibodies. In the ERM and p-ERM doublets, moesin is the lower band and ezrin the upper band in each case. The figure is representative of 5 experiments.
During the formation of the immunological synapse, the dephosphorylation and rephosphorylation of the ERM participates in the exclusion of surface molecules from the T cell-APC contact area (6-9). This process can be partially mimicked by anti-CD3 stimulation, which triggers ERM dephosphorylation. As shown in Figure 1, anti-CD3 stimulation induces a decline in the ERM phosphorylation (see p-ERM arrow) in CD4 T cells from young donors; however ERM phosphorylation of CD4 T cells from old donors does not display such a change (for a more detailed study see Figure 3). These results suggest that alterations in ERM phosphorylation may be in part responsible for the lack of CD43 exclusion in the course of T cell-APC contact.
Figure 3.
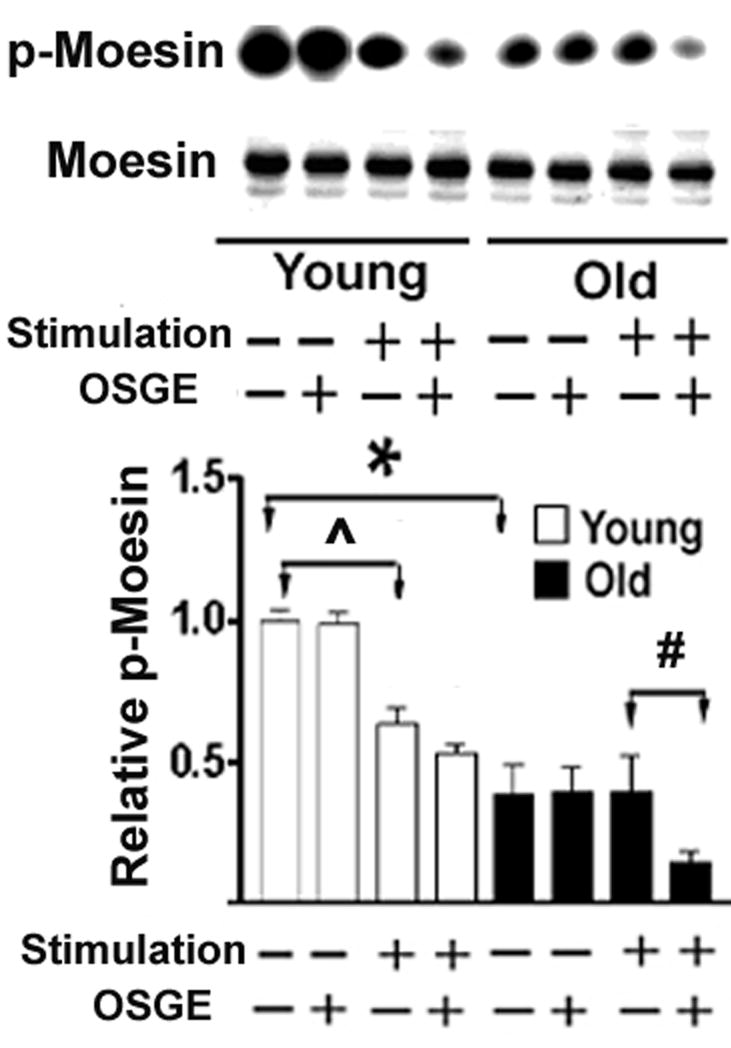
Analysis of the phosphorylation status of moesin in CD4 T cells from young and old donors. CD4 T cells from CB6F1 mice were treated with OSGE or left untreated, and aliquots were then stimulated through the TCR. Lysates were evaluated by immunoblotting using antibodies specific for p-moesin or for moesin and ezrin. The amount of moesin was used to normalize the amount of p-moesin in each sample. The bar plot represents the mean and SEM of 7 young (open bars) and 5 old (closed bars) mice from 5 independent experiments. Groups indicated by (* ˆ #) are different at a significance level of p < 0.05.
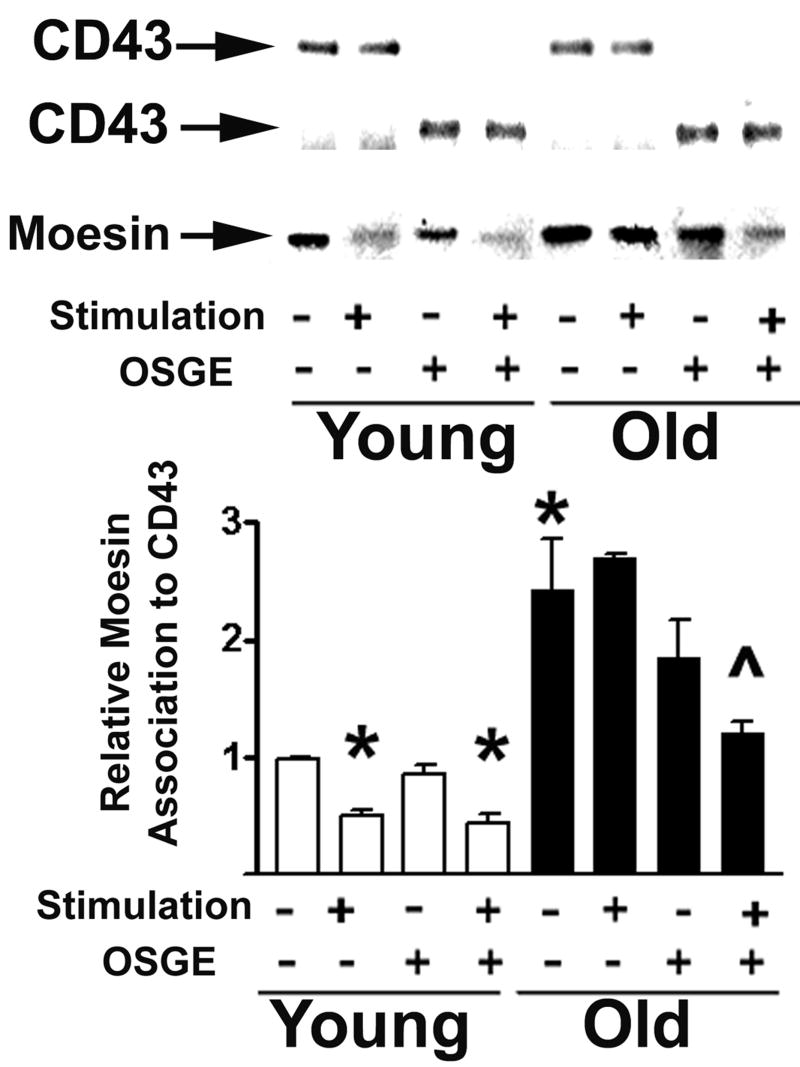
Age-related increases in the association of CD43 with moesin
During T cell-APC interaction there is an age-related decline in the number of CD4 T cells that can exclude CD43 from the T-cell APC contact area. The decline in CD43 exclusion can also be found in CD4 T cells from old donors that do form efficient immunosynapses (29). Treatment of old CD4 T cells with OSGE restores to youthful levels the fraction of cells that can form synapses, but does not increase the fraction that excludes CD43, suggesting that aged T cells might have a defect in cytoskeletal pathways required for CD43 translocation.
Because ERM/CD43 interaction plays a role in the exclusion of CD43 (6,22), we postulated that age could alter CD43 association with ERM molecules. To test this hypothesis we immunoprecipitated CD43 using a goat antibody that recognizes an internal domain of CD43 (M19) and analyzed the amount of moesin/ezrin associated with CD43. Figure 2 shows a typical western blot of moesin (using a monoclonal antibody, 38/87, that also recognizes ezrin) after CD43 has been precipitated from CD4 T cells varying in donor age, treatment with OSGE, and stimulation through the TCR. None of the experiments showed any significant amount of ezrin associated with CD43. The bar plot in Figure 2 shows that unstimulated CD4 T cells from old donors have two-fold higher levels of moesin associated with CD43 (p=0.031) compared to young cells. As expected, stimulation of CD4 T cells from young donors leads to a significant decline in the amount of moesin associated with CD43 (p=0.036) (6,8,10,11). This decline is not seen in CD4 T cells from old donors. Western blot analysis of CD43 levels in the same lysates showed no significant effect of either age or stimulation (Figure 2 top panel; note that OSGE treatment reduces the MW of the CD43 molecule). The age-related changes in CD43-moesin association and the lack of CD43 exclusion from the immunosynapse in CD4 T cells from old donors (29) suggest that age might alter pathways regulating the function of ERM proteins. Because OSGE treatment restores the function of T cells from aged mice, we evaluated the effects of OSGE on CD43-moesin association. As shown in Figure 2, CD4 T cells from young donors show no significant effect of the OSGE treatment, consistent with the lack of effect of OSGE on immunosynapse formation of young CD4 T cells (29). In contrast, OSGE treatment of CD4 T cells from old donors, prior to stimulation, leads to a statistically significant decline in CD43-moesin association (p=0.004) compared to stimulated aged cells not exposed to OSGE. OSGE treatment also leads to a small decrease in CD43-moesin association in resting CD4 T cells from old mice, but this effect does not reach statistical significance (p=0.07). Taken together, these results suggest that OSGE treatment of T cells from aged mice can enhance some aspects of TCR signaling as related to the ERM pathway. OSGE treatment, however, does not fully restore the defects in CD43-moesin association, suggesting the presence of age-related defects in the ERM or in its signaling pathways that are independent of immunosynapse formation.
Figure 2.
Age-dependent increase in the association of CD43 with moesin in CD4 T cells. CD4 T cells from young and old CB6F1 donors were treated with OSGE (+) or left untreated (-), then stimulated (+) or left unstimulated (-) as described in the methods section. The cells were lysed and the CD43 immunoprecipitated using an antibody that recognizes the cytoplasmic domain. CD43 and associated moesin were then quantified by western blots. CD43 (arrows at top) was used as an internal control to normalize the amount of moesin of each sample; note that treatment with OSGE (+) decreases the molecular weight of CD43. The bar plot shows mean and SEM of normalized CD43-associated moesin for 5 young and 5 old mice from 4 independent experiments. (*) indicates statistical significance with respect to cells from young donors that were neither stimulated nor treated with OSGE. (ˆ) indicates statistical significance with respect to OSGE-treated, unstimulated cells from the old donors.
Age-related declines in the level of ERM phosphorylation of CD4 T cells
Because interactions between ERM and other proteins are regulated by phosphorylation, specifically Thr558 in moesin and Thr567 in ezrin (43,44), we measured the levels of p-ERM in resting and stimulated CD4 T cells from young and old CB6F1 donors, with and without OSGE treatment to restore synapse formation. The amount of p-ERM of each sample was assessed by western blots using an antibody that recognizes both phospho-Thr558 of moesin (p-moesin) and phospho-Thr567 of ezrin (p-ezrin). Figure 3 shows a typical digital image of a p-moesin blot, with corresponding levels of total moesin (using MoAb clone 37). Ezrin and p-ezrin are also present in the original images (see Figure 1), but at lower levels of detection in samples from old donors, making quantification difficult (data not shown). The bar plot in Figure 3 presents ratios of p-moesin to moesin from 5 independent experiments. Resting CD4 T cells from old mice have only half the level of moesin phosphorylation (p=0.0025) seen in cells from young controls. Stimulation of CD4 T cells from young mice induces a significant dephosphorylation of the moesin (p=0.003), in good agreement with published reports (6,9,16). In contrast, CD4 T cells from old donors do not show any decline in moesin phosphorylation after TCR stimulation. OSGE treatment enhances the dephosphorylation of moesin in CD4 T cells from old donors (p=0.047), consistent with our previous observations that OSGE has a preferential effect on some aspects of early T cell activation in T cells from old mice (29,32,34), without a parallel effect on young CD4 T cells.
To see if age effects on moesin phosphorylation reflect the shift from naïve to memory CD4 T cells with aging, we used flow cytometry to evaluate the ERM phosphorylation status using phospho-specific antibodies, together with anti-CD44 as a marker to distinguish naïve from memory cells. As controls, some aliquots were stained with anti-p-LAT (rabbit anti-phosphoTyr191) and anti-LAT (rabbit polyclonal) antibodies. Figure 4 shows typical distributions of p-moesin and moesin in populations gated either for all CD4 T cells or for CD4 naïve and memory subsets. Because the p-moesin antibody can also recognize p-ezrin, we refer to the signal as p-ERM in the figure. Both naïve and memory CD4 T cells show an age-dependent decline in p-ERM, but little or no change in total moesin levels. A series of four experiments showed a significant decline with age in cellular levels of p-moesin (p=0.0001, see Fig. 4), consistent with the immunoblotting data, but no change in the level of moesin itself. As in a previous report (30), we saw no significant age-dependent change in the level of LAT or p-LAT (see Fig. 4), suggesting that the data on p-moesin does not reflect changes in fixation or other staining artifacts. We also obtained results similar to the LAT data using antibodies to LCK and phospho-Tyr505 LCK (data not shown).
Figure 4.
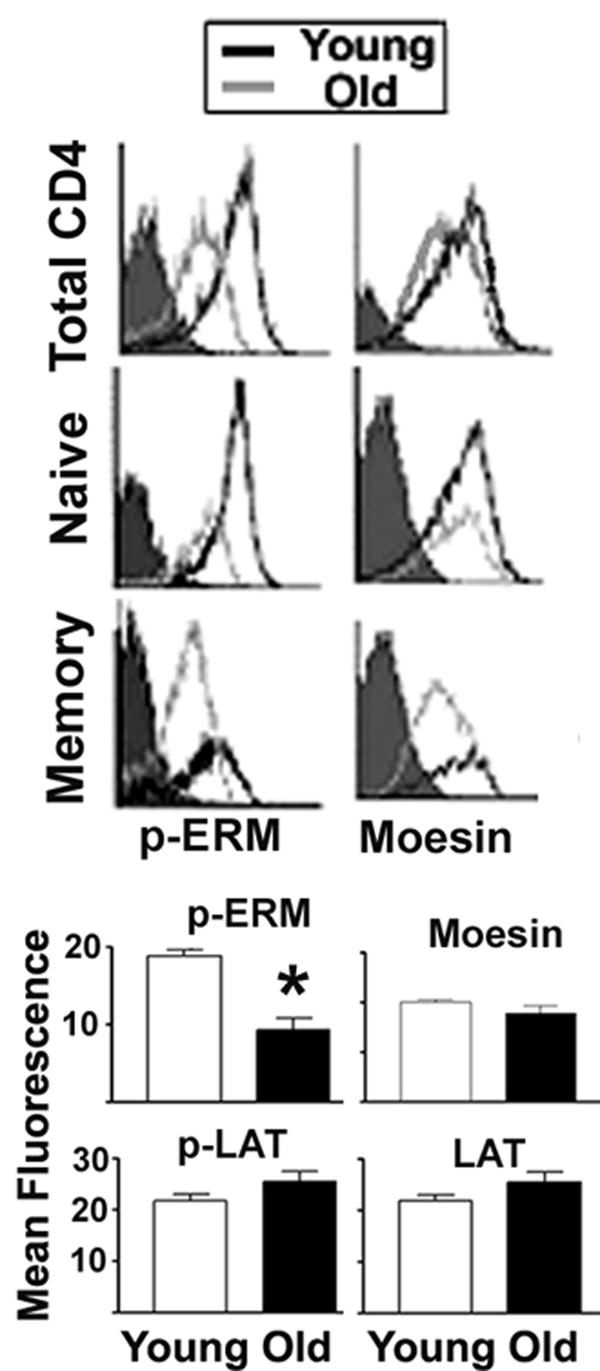
Intracellular staining of p-ERM in CD4 T cells from young and old CB6F1 donors. T cells were stained for CD4 and CD44 and fixed, followed by intracellular labeling for p-ERM, moesin, p-LAT or LAT, and then analyzed by flow cytometry. The cells were gated for CD4, and the CD44 signal was used to distinguish between naïve and memory subpopulations. The top panels show typical histograms for p-moesin or moesin in total, naïve or memory CD4 T cells from young and old donors. The lower panels show the mean and SEM for 15 young and 12 old donors from 4 independent experiments for p-ERM, moesin and controls (p-LAT and LAT) performed on the same cell preparation. (*) indicates statistically significant effects.
Age-related declines in CD44 and EBP50 association with ERM proteins
The decline with age in ERM phosphorylation in resting CD4 T cells could result in changes in the interaction of ERM proteins with T cell surface molecules and intracellular signaling proteins. To further test this hypothesis we immunoprecipitated CD44, a surface glycoprotein known to be associated with ERM (22), and measured the amount of associated ezrin and moesin. As shown in Figure 5A, we found significant amounts of both ezrin and moesin associated with CD44; this is in contrast to the CD43 immunoprecipitates, in which only moesin was easily detectable. The image, showing results from two young and two old mice, suggests an age-dependent decline in the levels of both moesin and ezrin associated with CD44. Statistical analysis of four independent experiments showed a significant age-related decline in the amount of moesin associated with CD44 (p=0.003). Ezrin showed a similar age-related decline, but the low levels of ezrin in resting CD4 T cells from old donors precluded its accurate quantification and statistical analysis.
Figure 5.
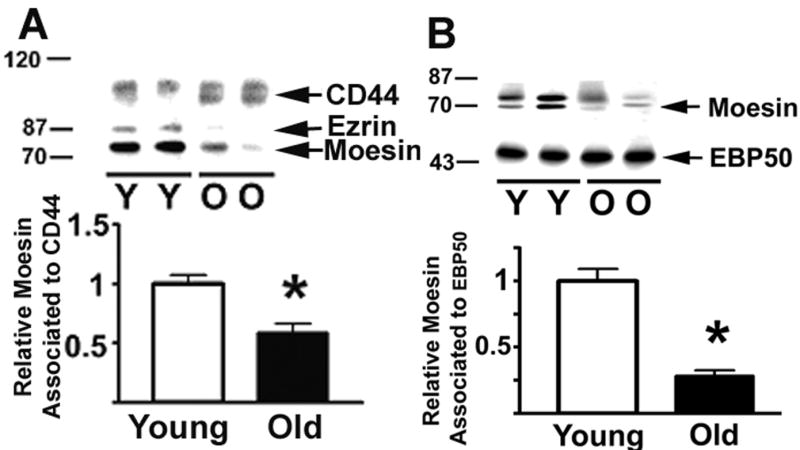
Age-related declines in the association of ERM to CD44 and EBP50. CD4 T cells from young and old CB6F1 donors were lysed, CD44 or EBP50 immunoprecipitated, and the associated ERM, CD44 and ERBP50 quantified by western blots. Panel A shows ERM associated with CD44; the bar plot shows mean and SEM of 13 young and 19 old mice tested in 4 experiments. Panel B shows the ERM levels immunoprecipitated by antibody to EBP50; the plot shows mean and SEM of 8 young and 14 old mice tested in 4 independent experiments. (*) indicates statistically significant effects.
ERM proteins can also interact with EBP50, an adaptor protein involved in the regulation of the Csk tyrosine kinase and TCR signaling (7,45-47). Figure 5B shows a typical image of the moesin and ezrin levels in EBP50 immunoprecipitates from CD4 T cells of young and old donors. Analysis of 4 independent experiments showed a significant age-related decline in the amount of moesin associated with EBP50 (p= 0.0004). As in the case of CD44, the lower level of ezrin in samples of old mice precluded accurate quantification. In addition, we found no evidence for age-associated change in the total amount of T cell moesin (see Figs. 4 and 5), ezrin (see Figure 7, below) or EBP50 (data not shown) that could explain the age-related changes.
Figure 7.
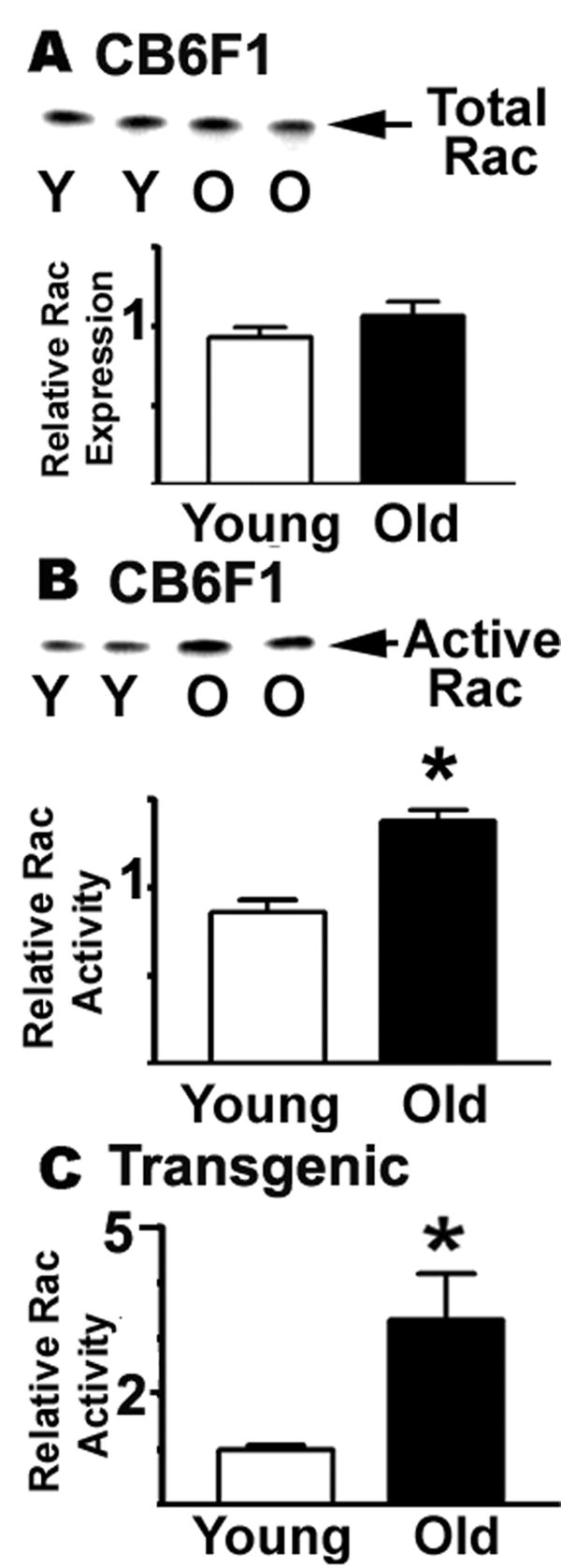
Rac GTPase activity increases with age. Resting CD4 T cells from young and old CB6F1, or from AND transgenic mice, were lysed and the aliquots tested for total Rac expression and for active Rac using a GST-Pak1 fusion protein in a pull-down protocol. The Rac was quantified by western blots. Panel A shows the total expression levels of Rac in the CB6F1 strain. Panel B shows a typical image for Rac levels; the bars show mean and SEM for 3 experiments totaling 7 young and 6 old donors from the CB6F1 mice strain. Panel C shows the mean and SEM of active Rac in 3 experiments using 6 young and 3 old donors from the AND transgenic strain.
Age-dependent changes in RhoA and Rac GTPase activities
It has been reported (17,48-52) that many aspects of cytoskeletal reorganization during TCR signaling, including the phosphorylation status of ERM proteins, are under the control of members of the Rho family of GTPases (RhoA, Rac, cdc42). Although details of the regulatory pathways are not yet well understood, it has been shown that RhoA and Rac may be upstream regulators of ERM phosphorylation (17,25,51-54). To see if age-related changes in GTPase activity of RhoA and Rac proteins could be implicated in the decline in ERM phosphorylation, we measured both the total RhoA protein level and the amount of active RhoA in CD4 T cells from young and old CB6F1 mice. Figure 6 shows typical experimental results for total RhoA and active RhoA in CB6F1 CD4 T cells, as well as mean values for 12 young and 6 old donors from 6 separate experiments. We found no significant age-related change in the expression of total RhoA (Figure 6A). Using an assay for active RhoA based upon a GST-Rhotekin fusion protein construct that binds RhoA only when it is in its GTP-bound configuration, we found (Figure 6B) a significant age-related decline in the amount of active RhoA (p=0.0001). To see if in the shift from the naïve to memory phenotype with age could explain the alteration in RhoA activity, we evaluated CD4 T cells from AND mice; flow-cytometric analysis of CD44 expression (not shown) indicated that approximately 80 % of the CD4 T cells in these mice were naïve at the age of 15 months. Figure 6C shows a significant decline in active RhoA in CD4 T cells from old AND mice (p=0.028). These results confirm that, at least in the naïve phenotype, there is an age effect on RhoA activity.
Figure 6.
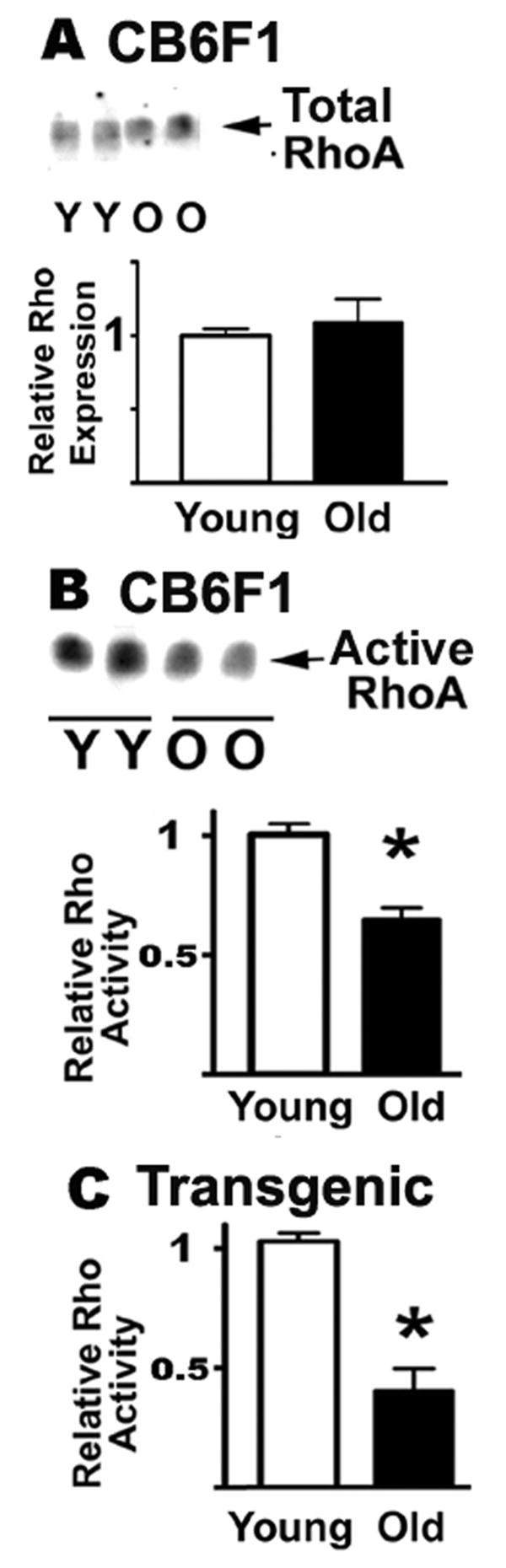
RhoA GTPase activity declines with age. Resting CD4 T cells from young and old CB6F1, or from AND transgenic mice, were lysed and the aliquots tested for total RhoA expression and for active RhoA using a GST-Rhotekin fusion protein in a pull-down protocol. The RhoA was quantified by western blots. Panel A shows the total expression levels of RhoA in the CB6F1 strain. Panel B shows a typical image for RhoA levels; the bars show mean and SEM for 6 experiments totaling 10 young and 10 old donors from the CB6F1 mice strain. Panel C shows the mean and SEM of active RhoA in 3 experiments using 4 young and 4 old donors from the AND transgenic strain.
Using similar methods, we also evaluated the effect of aging on Rac levels and activity. Figure 7 shows typical experimental results for total Rac and active Rac in CB6F1 CD4 T cells, as well as mean values for 7 young and 6 old donors from 3 separate experiments. As in the case of RhoA, we found no statistically significant difference in the level of Rac expression between young and old mice (see Figure 7A). We used a GST-Pak1 construct to measure Rac activity (Figure 7B), and noted an age-dependent increase in Rac function. Analysis of results from 7 young and 6 old mice examined in 3 separate experiments revealed a 50% increase in Rac activity (p=0.0012). To see if these changes were due to a shift from a naïve to a memory phenotype with age, we analyzed Rac activity in the PCC transgenic mice from 6 young and 3 old donors and found a statistically significant three-fold increase in active Rac (p=0.023) without any change in total Rac expression (Figure 7C). These results confirm that naïve CD4 cells, at least, show age effects in activity of both RhoA and Rac, within the family of Rho GTPases that regulate ERM cytoskeletal proteins.
Discussion
The current model of immunosynapse formation suggests that the initial interactions between T cells and APCs induce formation of lamellipodia by relaxation of the T cell membrane and reorganization of the cytoskeletal network. These changes include increases in F-actin polymerization and translocation of TCR signaling molecules to the area of T cell contact with the APC, as well as exclusion of CD43 from the area of interactions. The experimental evidence shows that the ERM proteins are responsible for the exclusion of CD43 and contribute to negative regulation of TCR signaling (6,9,16). These transitions seem to involve the dephosphorylation and rephosphorylation of ERM proteins and physical segregation of ERM, with its associated molecules including CD43 and CD44, away from the F-actin network in areas of microclusters and at the T cell APC interaction area (9,16). The Brij-58 extraction experiments in figure 1 shows that much of the ERM protein, as well as the ERM-associated molecules CD44, MDR-1, and EBP50 are detergent soluble. However, the distribution of CD43 between the detergent-soluble and –resistant fraction (see figure 1) suggest that in the particular case of CD43, the extracellular or intracellular domains of CD43 may interact with other surface proteins or cytoskeleton proteins in an ERM-independent manner. The nature of the CD43 resistance to detergent extraction is not know, but we hypothesize that this insoluble fraction may be associated with the F-actin cytoskeleton in freshly isolated CD4 T cells from young and age mice.
If lamellipodia formation is dependent on the ERM (16), the age-related changes in ERM phosphorylation shown in Figures 3 and 4 suggest a model where age-related alterations in ERM signaling pathways are involved in the defects of lamellipodia formation found in CD4 T cells from old mice. In addition, the immunoprecipitation data shown in Figure 5 suggest age-related alterations in the association of proteins with the ERM: CD4 T cells from old mice show significant declines in the association of CD44 and EBP50 with ERM proteins. Altered ERM association with CD44 and EBP50 might be attributable to changes in phosphorylation of ERM sites that trigger its open conformation. In good agreement with this hypothesis, we found no age-related alteration in the levels of moesin and ezrin proteins in CD4 T cells, but a significant decline in ERM phosphorylation detected both by immunoblotting and flow cytometry (see Figures 3 and 4). A decline in the proportion of moesin and ezrin able to interact with CD44, EBP40 and other regulators could contribute to age-dependent declines in T cell activation. These changes appear to be independent of the naïve to memory cell shift seen in aging mice.
Previous work on CD43 biochemistry in T cell lines and from structural studies would predict that age-related changes in moesin phosphorylation would lead to coordinated changes in moesin association with CD43, CD44 and EPB50. Our data, based on CD4 T cells freshly isolated from mice, are in good agreement with respect to CD44 and EBP-50, but are at odds with the prediction with respect to CD43. There are, however, several aspects of ERM biology in freshly isolated CD4 cells that do not conform to previous reports on cell lines, including unexpectedly low levels of moesin, and virtual absence of ezrin, in CD43 immunoprecipitates (compared to CD44 immunoprecipitates). In addition, we note that CD43 is associated, partly, with the Brij-58 insoluble fraction, whereas CD44 and EPB50 are entirely Brij-soluble. We do not have a convincing molecular model that explains differences in CD43 coprecipitation from the data on CD44 and EPB50 coprecipitation, but these data do suggest that models that predict parallel behavior of CD43, CD44, and EBP50 may be incomplete with respect to freshly isolated T cells and it is possible that an ERM-independent mechanisms may contributed to the exclusion or retention of CD43 and other TCR negative regulator molecules from the immunosynapse in CD4 T cells from old donors (55, 56). Lastly, we can point to the previously reported age-related increase in glycosylation of CD43 (34), and can suggest that this age-dependent alteration might lead to increased association between CD43 and other moesin-associated surface proteins.
Studies using T cell lines and normal T cells from young donors are only gradually beginning to clarify relationships among kinases and phosphatases that control ERM phosphorylation. At present we do not know the molecular mechanism responsible for the age-related decline of ERM phosphorylation, but there is evidence that GTPases of the Rho family are among the upstream regulators of the ERM phosphorylation, with several studies showing that the Rho family of molecules, including Rac and RhoA, are critical for regulation of ERM signaling and lamellipodia formation (15,17,48,50-54). It is possible that the age-related changes in activation of one or more Rho-family GTPases may contribute to defects in ERM activation and changes in ERM phosphorylation. CD4 T cells from old mice show no significant change in the expression levels of RhoA and Rac, but do show a significant decline in the level of active RhoA (figure 6B) and a significant increase in active Rac (figure 7B). It is not likely that these changes are the result of the age-related shift from naïve to memory, because we found similar age-related declines in active RhoA and increases in active Rac using the PCC specific AND mouse strain, where most of the CD4 T cells remain in a naïve stage even late in life (55,56). Studies using transfected cell lines suggest that increases in the activity of Rac are directly linked with a decline in ERM phosphorylation (53). We found this to be the case for CD4 T cells from old donors (Figures 3 and 7). This, as well as the decline in active RhoA, could well contribute to age-related deficits in lamellipodia formation and polarization of T cells and to the exclusion of CD43 and other proteins from the TCR immunosynapse. However, additional studies are needed to directly test if GTPases are a key element in the age-related decline of CD4 T cell function.
Acknowledgments
We wish to thank Lynn Winkelman, Maggie Vergara, Jessica Sewald, Bill Kohler, and Melissa Han for technical assistance.
This work was supported by NIH grants AG19619 and AG024824.
References
- 1.da Cruz LA, Penfold S, Zhang J, Somani AK, Shi F, McGavin MK, Song X, Siminovitch KA. Involvement of the lymphocyte cytoskeleton in antigen-receptor signaling. Curr Top Microbiol Immunol. 2000;245:135. doi: 10.1007/978-3-642-57066-7_4. [DOI] [PubMed] [Google Scholar]
- 2.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 3.Penninger JM, Crabtree GR. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- 4.nton van der MP, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol. 2000;12:5. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 6.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Sakakibara M, Yamasaki S, Takeuchi A, Arase H, Miyazaki M, Nakajima N, Okada M, Saito T. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J Immunol. 2002;168:541. doi: 10.4049/jimmunol.168.2.541. [DOI] [PubMed] [Google Scholar]
- 8.Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 9.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 10.Savage ND, Kimzey SL, Bromley SK, Johnson KG, Dustin ML, Green JM. Polar redistribution of the sialoglycoprotein CD43: implications for T cell function. J Immunol. 2002;168:3740. doi: 10.4049/jimmunol.168.8.3740. [DOI] [PubMed] [Google Scholar]
- 11.Sperling AI, Sedy JR, Manjunath N, Kupfer A, Ardman B, Burkhardt JK. TCR signaling induces selective exclusion of CD43 from the T cell-antigen-presenting cell contact site. J Immunol. 1998;161:6459. [PubMed] [Google Scholar]
- 12.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, utry-Varsat A, Alcover A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 14.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 15.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 16.Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987. doi: 10.2741/1940. [DOI] [PubMed] [Google Scholar]
- 17.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Legg JW, Isacke CM. Identification and functional analysis of the ezrin-binding site in the hyaluronan receptor, CD44. Curr Biol. 1998;8:705. doi: 10.1016/s0960-9822(98)70277-5. [DOI] [PubMed] [Google Scholar]
- 19.Luciani F, Molinari A, Lozupone F, Calcabrini A, Lugini L, Stringaro A, Puddu P, Arancia G, Cianfriglia M, Fais S. P-glycoprotein-actin association through ERM family proteins: a role in P-glycoprotein function in human cells of lymphoid origin. Blood. 2002;99:641. doi: 10.1182/blood.v99.2.641. [DOI] [PubMed] [Google Scholar]
- 20.Serrador JM, onso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sanchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11:109. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- 24.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 25.Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood. 2003;102:3890. doi: 10.1182/blood-2002-12-3807. [DOI] [PubMed] [Google Scholar]
- 26.Manes S, Gomez-Mouton C, Lacalle RA, Jimenez-Baranda S, Mira E, Martinez A. Mastering time and space: immune cell polarization and chemotaxis. Semin Immunol. 2005;17:77. doi: 10.1016/j.smim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 28.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 29.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 30.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 31.Berger SB, Sadighi Akha AA, Miller RA. A glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17:983. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- 32.Berger SB, Sadighi Akha AA, Miller RA, Garcia GG. CD43-independent augmentation of mouse T-cell function by glycoprotein cleaving enzymes. Immunology. 2006;119:178. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, Sperling AI. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med. 2004;199:1277. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- 35.Garcia GG, Miller RA. Increased Zap-70 association with CD3zeta in CD4 T cells from old mice. Cell Immunol. 1998;190:91. doi: 10.1006/cimm.1998.1394. [DOI] [PubMed] [Google Scholar]
- 36.Ren XD, Schwartz MA. Determination of GTP loading on Rho. Methods Enzymol. 2000;325:264. doi: 10.1016/s0076-6879(00)25448-7. [DOI] [PubMed] [Google Scholar]
- 37.Bacso Z, Nagy H, Goda K, Bene L, Fenyvesi F, Matko J, Szabo G. Raft and cytoskeleton associations of an ABC transporter: P-glycoprotein. Cytometry A. 2004;61:105. doi: 10.1002/cyto.a.20081. [DOI] [PubMed] [Google Scholar]
- 38.Gombos I, Bacso Z, Detre C, Nagy H, Goda K, Andrasfalvy M, Szabo G, Matko J. Cholesterol sensitivity of detergent resistance: a rapid flow cytometric test for detecting constitutive or induced raft association of membrane proteins. Cytometry A. 2004;61:117. doi: 10.1002/cyto.a.20080. [DOI] [PubMed] [Google Scholar]
- 39.del Pozo MA, Nieto M, Serrador JM, Sancho D, Vicente-Manzanares M, Martinez C, Sanchez-Madrid F. The two poles of the lymphocyte: specialized cell compartments for migration and recruitment. Cell Adhes Commun. 1998;6:125. doi: 10.3109/15419069809004468. [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Lebrero JL, Serrador JM, Dominguez-Jimenez C, Barreiro O, Luque A, del Pozo MA, Snapp K, Kansas G, Schwartz-Albiez R, Furthmayr H, Lozano F, Sanchez-Madrid F. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95:2413. [PubMed] [Google Scholar]
- 41.Serrador JM, Nieto M, onso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, Gonzalez-Amaro R, Sanchez-Mateos P, Sanchez-Madrid F. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91:4632. [PubMed] [Google Scholar]
- 42.Serrador JM, Nieto M, Sanchez-Madrid F. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol. 1999;9:228. doi: 10.1016/s0962-8924(99)01553-6. [DOI] [PubMed] [Google Scholar]
- 43.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110(Pt 24):3011. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 44.Chambers DN, Bretscher A. Ezrin mutants affecting dimerization and activation. Biochemistry. 2005;44:3926. doi: 10.1021/bi0480382. [DOI] [PubMed] [Google Scholar]
- 45.Brdickova N, Brdicka T, Andera L, Spicka J, Angelisova P, Milgram SL, Horejsi V. Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 2001;507:133. doi: 10.1016/s0014-5793(01)02955-6. [DOI] [PubMed] [Google Scholar]
- 46.Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- 47.Fouassier L, Yun CC, Fitz JG, Doctor RB. Evidence for ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem. 2000;275:25039. doi: 10.1074/jbc.C000092200. [DOI] [PubMed] [Google Scholar]
- 48.Bardi G, Niggli V, Loetscher P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett. 2003;542:79. doi: 10.1016/s0014-5793(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 49.Chavrier P, Gorvel JP, Bertoglio J. An immunologist’s look at the Rho and Rab GTP-binding proteins. Immunol Today. 1993;14:440. doi: 10.1016/0167-5699(93)90247-I. [DOI] [PubMed] [Google Scholar]
- 50.Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell. 2001;12:265. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deckert M, Moon C, Le BS. The immunological synapse and Rho GTPases. Curr Top Microbiol Immunol. 2005;291:61. doi: 10.1007/3-540-27511-8_5. [DOI] [PubMed] [Google Scholar]
- 52.Woodside DG, Wooten DK, Teague TK, Miyamoto YJ, Caudell EG, Udagawa T, Andruss BF, McIntyre BW. Control of T lymphocyte morphology by the GTPase Rho. BMC Cell Biol. 2003;4:2. doi: 10.1186/1471-2121-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nijhara R, van Hennik PB, Gignac ML, Kruhlak MJ, Hordijk PL, Delon J, Shaw S. Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J Immunol. 2004;173:4985. doi: 10.4049/jimmunol.173.8.4985. [DOI] [PubMed] [Google Scholar]
- 54.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linton PJ, Haynes L, Tsui L, Zhang X, Swain S. From naive to effector--alterations with aging. Immunol Rev. 1997;160:9. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]