Abstract
Periosteal woven bone forms in response to stress fractures and pathological overload. The mechanical factors that regulate woven bone formation are poorly understood. Fatigue loading of the rat ulna triggers a woven bone response in proportion to the level of applied fatigue displacement. However, because fatigue produces damage by application of cyclic loading it is unclear if the osteogenic response is due to bone damage (injury response) or dynamic strain (adaptive response). Creep loading, in contrast to fatigue, involves application of a static force. Our objectives were to use static creep loading of the rat forelimb to produce discrete levels of ulnar damage, and subsequently to determine the bone response over time. We hypothesized that 1) increases in applied displacement during loading correspond to ulnae with increased crack number, length and extent, as well as decreased mechanical properties; and 2) in vivo creep loading stimulates a damage-dependent dose-response in periosteal woven bone formation. Creep loading of the rat forelimb to progressive levels of sub-fracture displacement led to progressive bone damage (cracks) and loss of whole-bone mechanical properties (especially stiffness) at time-zero. For example, loading to 60% of fracture displacement caused a 60% loss of ulnar stiffness and a 25% loss of strength. Survival experiments showed that woven bone formed in a dose-dependent manner, with greater amounts of woven bone in ulnae that were loaded to higher displacements. Furthermore, after 14 days the mechanical properties of the loaded limb were equal or superior to control, indicating functional repair of the initial damage. We conclude that bone damage created without dynamic strain triggers a woven bone response, and thus infer that the woven bone response reported after fatigue loading and in stress fractures is in large part a response to bone damage.
Introduction
Repetitive loading of bone causes fatigue, characterized by the formation and propagation of cracks and the progressive loss of strength and stiffness [7, 21]. Bone fatigue can lead to stress fractures, which are common in athletes and military recruits [1, 24]. Histologically, stress fractures are associated with localized intracortical remodeling and periosteal woven bone formation [16, 20], findings that are also observed in the rat ulna after a bout of damaging fatigue loading [2, 15, 25].
The factors that contribute to fatigue-induced woven bone formation are poorly understood, although recent studies using the rat ulna loading model have examined this issue. In this model, dynamic compressive (fatigue) loading is applied to the forelimb, causing bending of the ulna leading to gradients in mechanical strain [17]. Peak strains are located on the medial (compressive) surface, 1–3 mm distal to the midpoint of the ulna, corresponding with sites of fatigue crack formations [11, 25, 29] and maximal woven bone formation [17]. Woven bone formation is stimulated in areas of high strain [2, 17, 25]. Recently, we used this model to produce discrete levels of ulnar damage in vivo by controlling the level of peak displacement applied during fatigue loading [29]. We observed that 7 days after loading, there was a dose-response in the area of new periosteal woven bone formation, where the amount of new bone was proportional to the level of imposed damage [28]. This finding suggests that the woven bone response to fatigue loading is damage-dependent. However, because fatigue produces damage by application of dynamic loading, it is unclear if the osteogenic response is due to the effects of bone damage or to direct effects of dynamic strain.
Creep loading, in contrast to fatigue, involves application of a static force. As with fatigue loading, bone can sustain progressive displacement and damage under creep loading [5, 6]. Thus, static creep loading is a method that can be used to produce bone damage without dynamic loading and thereby provides a useful experimental tool to separate the effects of these two osteogenic stimuli. While non-damaging static loading is generally believed to be non-osteogenic [18, 22], to our knowledge the response of bone to creep loading that produces measurable bone damage has not been reported.
Our objectives were to use static creep loading of the rat forelimb to produce discrete levels of ulnar damage, and subsequently to determine the in vivo bone response to creep damage. By analogy to the results of recent fatigue experiments [28, 29], we hypothesized that 1) increases in applied displacement during loading correspond to ulnae with increased crack number, length and extent, as well as decreased mechanical properties; and 2) in vivo creep loading stimulates a damage-dependent dose-response in periosteal woven bone formation.
Methods
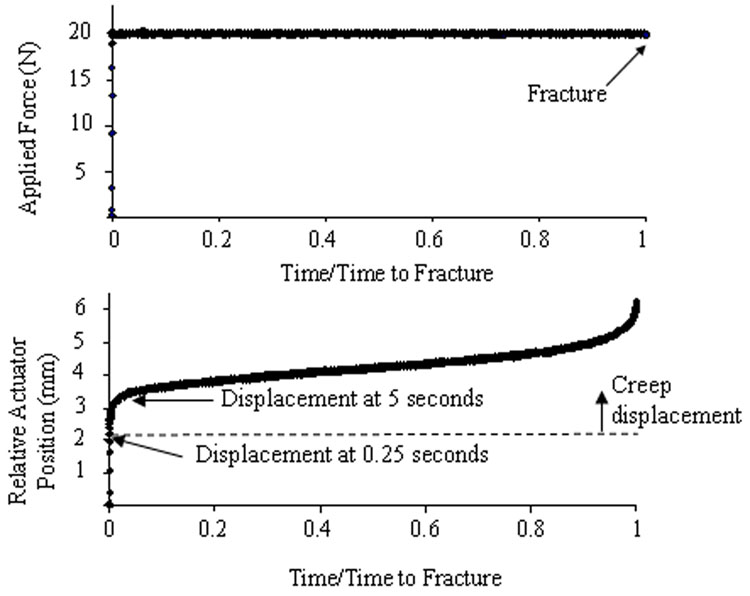
The forelimbs of 134 adult (4 ½ – 5 ½ month old) male Fischer 344 rats (Harlan) were loaded in axial compression based on an established rat forelimb loading model [2, 15, 25, 29], except rather than applying cyclic loading we applied static loading. Rats were anesthetized using 1–3% isoflorane and loading was applied across the carpus and olecranon process using a servohydraulic materials testing machine (Instron 1321/8500R or 8841). After application of a 0.3 N preload, a force-controlled single ramp (ramp time: 0.25 s) was applied to a predetermined force level, which was then held constant. Force and displacement data were recorded at 30 Hz (LabView 7.0). In an initial experiment, right and left forelimbs (n = 35) from 18 rats were creep loaded to failure at peak forces between 17 and 30 N. The creep curves were consistent with previous descriptions of bone creep behavior (Fig. 1). Time to failure (tf; minutes) correlated moderately well with force (F; newtons): F = −1.64Log(tf) + 25.0 (r2 = 0.66, p < 0.05). The peak force necessary to cause failure after an average of 1 hour of loading was estimated from the force-time to failure equation. Importantly, forelimbs fractured reproducibly (independent of force magnitude or time to failure) when the displacement increased by 2.32 ± 0.46 mm compared to the displacement at 5 seconds.
Figure 1.
Representative creep curves as a function of normalized time. The force is ramped up within 0.25 seconds and held constant for the duration of the test. Displacement changes after 0.25 seconds are considered to be creep displacement. The test shown here ended when fracture occurred (at 6.9 min). The relative actuator position tracks the displacement through three stages of creep [4, 5, 7]. The primary stage has an initial rapid increase in creep displacement followed by gradually decreasing slope. The secondary creep stage follows with a relatively constant creep rate. The tertiary creep stage begins with a rapid acceleration in creep rate leading to fracture. The average displacement to fracture was 2.32 ± 0.46 mm relative to the displacement at 5 seconds. (Five seconds was used as a reference time to match the reference time used in our previous fatigue studies [29]. Displacement in the first 5 seconds of the test is attributed to elastic deformation of the forelimb and soft tissue creep.) In subsequent sub-fracture experiments, actuator displacement was monitored and stopped at a percentage of the average fracture displacement.
The right forelimbs of the remaining rats were loaded until the displacement reached a target value less than the average displacement to fracture. Left limbs were not loaded and served as controls. The force applied to the right forelimbs was between 17.3–19.8 N (although constant for each rat; avg. 18.9 ± 0.7 N) resulting in average loading times less than 1 hour. Based on previous strain gage data, these forces were expected to generate initial compressive strains of 2330 – 2660 µε[29]. During loading of approximately one-half of the forelimbs, there were small sinusoidal variations in applied load (0.1 Hz, ±0.1 N) due to servo controller error. Subsequent strain gage tests were performed on three post mortem specimens; full methods were described previously [29]. Briefly, strain gages (SS-080-050-500P-SI; Micron Instruments) were attached near the midpoint of the ulna and the forelimb was creep-loaded. Fluctuations in strain corresponding to the small sinusoidal variations in applied load were recorded. Results indicated that the peak-to-peak strain associated with these variations was < 20 microstrain, or < 1% of the total ulnar strain. For pain relief after loading, rats were administered an intramuscular injection of Buprenex (1.67 mg/kg buprenorphine hydrochloride). All rats were euthanized by CO2 asphyxiation on day 0, 7 or 14 according to their experimental group. This study was approved by our institutional Animal Studies Committee.
Loading-induced damage
Seventy-four rats were used to investigate the effects of a single bout of creep loading on ulnar crack formation and mechanical properties. Rats were assigned to eight sub-fracture displacement groups (20, 30, 40, 50, 60, 70, 80 and 90%; n = 7–11 per group). Right forelimbs were loaded until the displacement reached the prescribed stopping displacement (X% of the average displacement to fracture). Forelimbs of two rats fractured during loading and they were excluded from the study. The loading time ranged from 0.22 min (a rat from the 20% displacement group) to 192.5 min (a rat from the 50% displacement group) (Table 1). Rats were euthanized immediately after loading.
Table 1.
Time-zero crack parameters of loaded (Right) ulnae measured using microCT (mean ± SD; n=7–11/group). The displacement groups were determined as a percentage of the average displacement to fracture (2.32 mm). Each displacement group had examples of ulna with a visible crack, although no group had a visible crack in every ulna.
| Displacement Group | Loading Time (min) | Visible crack (% of ulnae) | Crack extent (mm) | Cr.L.D (mm/mm2) | Cr.N.D. (#/mm2) | Crack midpoint1 (mm) |
|---|---|---|---|---|---|---|
| 20% | 1.05 ± 0.6 | 22 | 0.34 ± 0.19 | 0.32* ± 0.03 | 0.62* ± 0.04 | 0.30 ± 1.01 |
| 30% | 6.65 ± 9.9 | 33 | 0.74* ± 0.38 | 0.47* ± 0.15 | 0.60* ± 0.03 | 0.02 ± 0.40 |
| 40% | 19.33 ± 25.3 | 44 | 1.07* ± 0.66 | 0.58* ± 0.11 | 0.62* ± 0.07 | 0.08 ± 0.97 |
| 50% | 54.21z ± 65.8 | 45 | 0.52 ± 0.32 | 0.33*c ± 0.09 | 0.60* ± 0.06 | 0.29 ± 1.21 |
| 60% | 17.49 ± 18.1 | 66 | 0.58 ± 0.29 | 0.54*d ± 0.19 | 0.64* ± 0.05 | 0.64 ± 0.80 |
| 70% | 17.78 ± 15.8 | 56 | 0.92* ± 0.31 | 0.64*ad ± 0.11 | 0.61* ± 0.07 | −0.52e 0.82 |
| 80% | 10.04 ± 10.9 | 71 | 1.18*ade ± 0.43 | 0.75*abde ± 0.11 | 0.76*bcdef ± 0.08 | 0.21 ± 0.93 |
| 90% | 9.19 ± 14.2 | 56 | 1.32*ade ± 0.61 | 0.74*abde ± 0.26 | 0.78*abcdef ± 0.17 | −0.21 ± 0.78 |
P<0.05:
vs. zero;
vs. 20%;
vs. 30%;
vs. 40%;
vs. 50%;
vs. 60%;
vs. 70%;
vs. all displacement groups
The crack midpoint was measured in the distal direction from the midpoint of the ulna; negative midpoint values are proximal to the ulna midpoint.
Micro-computed tomography (microCT, Scanco Medical µCT40) was used to image and characterize cracks in excised ulnae before mechanical testing. The middle one-third of the ulna was scanned at 16 µm resolution (55 kVp, 172 mA, 200 ms) perpendicular to the long axis of the bone. First, the ulnae were assessed for presence of a crack. The overall crack extent (Cr.E) was measured along the length of the bone as the distance between the first and last transverse CT slice where the crack appeared. The position of the midpoint of the crack relative to the midpoint of the bone was calculated. Finally, using the manufacturer’s software (Eval v5.0) a single user determined the crack number density (Cr.N.D) and length density (Cr.L.D), averaged over four evenly spaced CT slices spanning the crack region.
The mechanical properties of loaded ulnae and non-loaded controls were assessed using three-point bending (Instron 8841). Ulnae were positioned on supports 15 mm apart and the displacement was applied on the medial surface at the midpoint of the ulnar length, producing bending in the same plane as during axial compression. A 0.5 N preload was manually applied followed by a 0.5 mm/s ramp to failure. Standard structural properties were determined from force-displacement curves (Labview 7.0). Ultimate force was calculated as the peak force; stiffness was calculated as the slope of the curve between 25 and 75% of the ultimate force; post-yield displacement was calculated as the displacement from the yield point to the fracture point; fracture energy was calculated as the area under the curve up to the fracture point.
Bone formation response
To investigate bone formation after damaging creep loading, survival rats (n=10/group) were assigned to three sub-fracture displacement groups: low (20% of the average displacement to fracture), medium (40%) or high (80%) displacement. The average loading times for the three displacement groups were 1.08 ± 0.4, 9.58 ± 9.4 and 42.12 ± 50.5 minutes, respectively. Rats were given intraperitoneal injections of 5 mg/kg calcein green (Sigma) on day 0 and 30 mg/kg alizarin-complexone (Sigma) on day 5 before being euthanized on day 7. The excised ulnae were imaged using microCT to discern cracks and calculate woven bone extent before being embedded in plastic and sectioned for histology.
Bone area (B.Ar) and bone mineral density (BMD) (calibrated to the manufacturer’s hydroxyapatite [HA] mineral phantom) were determined using microCT. Analysis was performed at eight locations spaced longitudinally along the bone from 6 mm proximal to the midpoint (P6) to 8 mm distal to the midpoint (D8) in 2 mm intervals. Using manufacturer’s software an average of six CT slices (96 µm total) at each location were analyzed for bone area and BMD (images manually segmented by a single user). Bone mineral content (BMC) was calculated as BMC = B.Ar × BMD. Ulnae were also analyzed for the presence of a visible crack and woven bone. If woven bone was present, the extent was computed by determining the beginning and ending CT slices where woven bone was visible. The density of the woven bone at the midpoint was computed for a subset of specimens (n=5).
Ulnae were then embedded in plastic (methylmethacrylate, Sigma) using standard procedures in preparation for measurement of bone formation parameters. Sections (100 µm thick) were cut at five locations along the length of the ulna from 5 mm proximal to the midpoint (P5) to 7 mm distal to the midpoint (D7) in 3 mm intervals using a diamond-tipped saw microtome (Leica Microsystems, SP 1600). Each section was mounted on a glass slide and visualized on an inverted microscope with a 100 W mercury-halogen light source at a 4x objective (DP-30, Olympus). Calcein was imaged using a fluorescein isothiocyanate (FITC) filter, while alizarin was imaged using a tetramethylrhodamine isothiocyanate (TRITC) filter. The images were overlaid using camera software (Olympus). The sections were analyzed (ImageJ) by a single user for woven bone area (Wo.B.Ar), bone area and labeled surfaces, including single-labeled surface, double-labeled surface and woven bone-labeled surface. Contralateral ulnae were pooled from each displacement group (n=4/group; 12 total) to form a common control group.
Recovery of mechanical properties
The recovery of mechanical properties was examined 14 days after loading. Rats (n=12) were loaded to high displacement (80% of the average displacement to fracture) and then allowed free cage activity for 14 days before being euthanized. The average loading time was 52.95 ± 35.4 minutes. Ulnae from nine rats were available for mechanical testing, as one forelimb did not reach the necessary displacement within the 3-hour time limit and two forelimbs fractured during loading. Excised ulnae were imaged using microCT before mechanical testing. Identical to the time-zero experiment, the mechanical properties of the loaded and control ulnae were assessed using three-point bending.
Statistics
Differences between right and left ulnae were assessed using paired t-tests, while one-way analysis of variance (ANOVA) was used to compare differences between displacement groups. Significance was defined at P<0.05.
Results
Loading-induced damage
Static creep loading of the forelimb created ulnar cracks visible on microCT at time-zero. Each displacement group had examples of loaded ulnae with visible cracks (usually one per ulna), in addition to ulnae with no visible cracks (Table 1). The average crack (including all displacement groups) was centered 0.11 ± 0.88 mm distal to the midpoint of the ulna. Generally, crack extent and crack length density increased with increasing displacement, with greater values in the 80 and 90% displacement groups than in most of the lower displacement groups. Left ulnae served as controls and did not have any visible cracks.
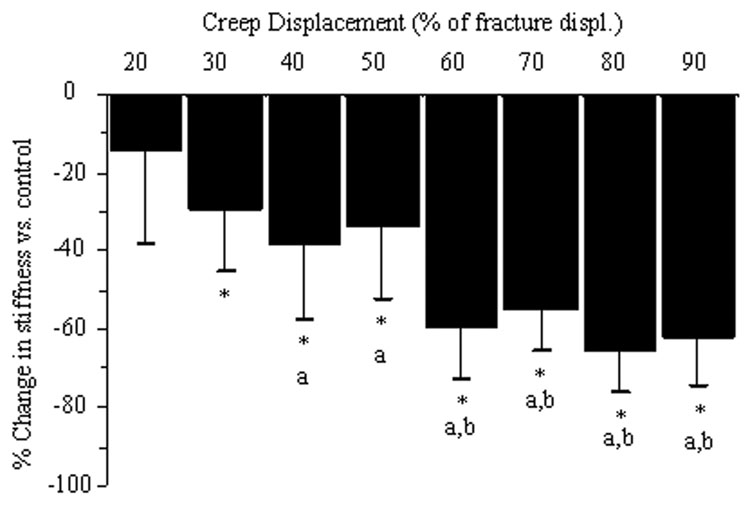
Static creep loading caused degradation of ulnar mechanical properties. Three-point bending tests revealed significant reductions in ulnar stiffness (loaded [Right] compared to control [Left]) in displacement groups higher than 20% (Fig. 2). For example, forelimbs loaded to 60% of fracture had a 60% loss of ulnar stiffness. Beyond 60% displacement there were no further reductions in stiffness. There were similar reductions in ultimate force, although of less magnitude (Table 2). Post-yield displacement and energy to fracture did not display a clear decreasing trend with higher displacement groups. Notably, reductions in mechanical properties were observed in both loaded ulnae with and without visible cracks on microCT.
Figure 2.
Increasing displacement caused progressive loss of time-zero bone stiffness. (The percent change in stiffness for loaded (R) versus control (L) limbs was calculated using (R-L)/ L*100.) For all displacement groups beyond 20%, loaded ulnae were significantly less stiff than controls. Beyond 60% displacement there were no further reductions in stiffness. (P<0.05: * vs. control; a vs. 20%; b vs. 30%, 40% and 50%)
Table 2.
Time-zero mechanical properties of isolated ulnae determined using three-point bending after in vivo creep loading of the right forelimb (mean ± SD; n=7–11/group). Data from the loaded (Right) limbs and control (Left) limbs were evaluated.
| Displacement Group | Stiffness (N/mm) |
Ultimate force (N) |
Fracture energy (Nmm) |
Post-yield displacement (mm) |
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| 20% | 19.8 ± 3.4 | 16.9 ± 4.8 | 13.3 ± 1.1 | 12.8 ± 1.6 | 19.7 ± 3.1 | 20.7 ± 4.2 | 1.24 ± 0.26 | 1.35 ± 0.28 |
| 30% | 20.9 ± 1.8 | 14.9* ± 4.2 | 13.5 ± 1.1 | 12.6 ± 1.5 | 24.3z ± 5.2 | 19.0 ± 3.8 | 1.66z ± 0.55 | 1.18 ± 0.31 |
| 40% | 21.0 ± 2.1 | 12.8*a ± 3.8 | 13.3 ± 1.0 | 11.9 ± 1.4 | 24.0 ± 3.8 | 18.1* ± 6.2 | 1.63z ± 0.27 | 1.12* ± 0.48 |
| 50% | 21.3 ± 2.3 | 13.9* ± 3.5 | 13.5 ± 1.3 | 12.7 ± 1.7 | 24.7z ± 6.1 | 21.4 ± 5.7 | 1.68z ± 0.46 | 1.39 ± 0.51 |
| 60% | 22.2z ± 2.9 | 8.90*abcd ± 2.5 | 13.8 ± 1.4 | 10.3*abcd ± 1.8 | 23.4 ± 3.0 | 14.7*ad ± 5.6 | 1.51 ± 0.19 | 0.90d ± 0.57 |
| 70% | 22.1z ± 2.1 | 9.86*abd ± 2.3 | 13.6 ± 1.0 | 11.1*abd ± 1.0 | 22.5 ± 4.6 | 14.2*ad ± 5.9 | 1.47 ± 0.29 | 0.79*ad ± 0.58 |
| 80% | 21.1 ± 1.7 | 7.27*abcd ± 1.9 | 13.5 ± 1.1 | 10.3*abd ± 1.2 | 24.4z ± 4.3 | 15.3*d ± 4.8 | 1.63z ± 0.27 | 0.82*ad ± 0.50 |
| 90% | 21.5 ± 2.3 | 8.06*abcd ± 2.4 | 13.4 ± 0.82 | 10.6*abd ± 1.9 | 23.4 ± 6.3 | 17.3 ± 6.5 | 1.58 ± 0.48 | 1.07 ± 0.62 |
P<0.05:
vs. control;
vs. 20%;
vs. 30%;
vs. 40%;
vs. 50%;
vs. 20% control group
Bone formation response
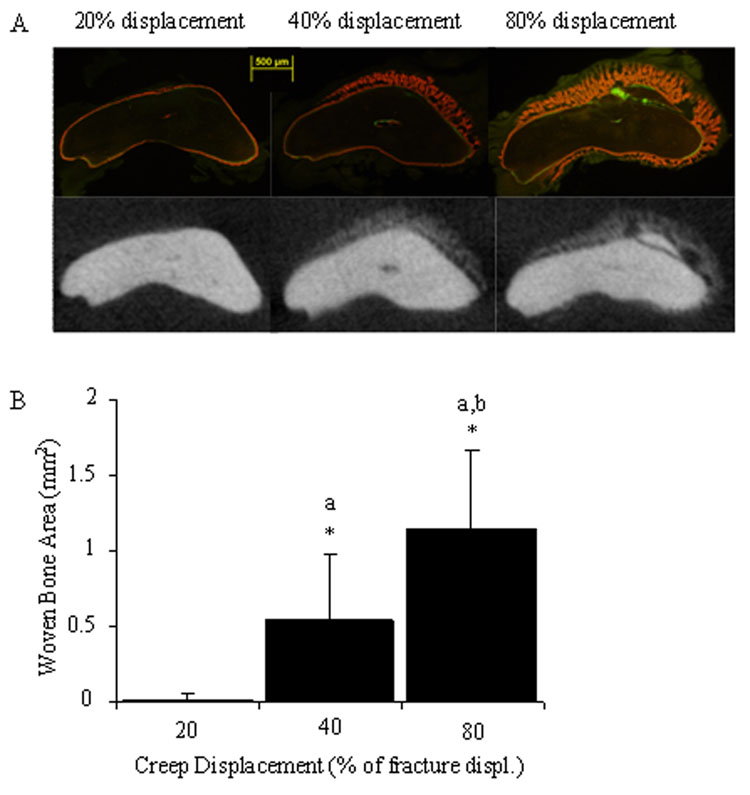
Micro-computed tomography of loaded ulnae 7 days after loading revealed a displacement-dependent woven bone response (Fig. 3a, Table 3). Total woven bone area (summed over five histomorphometry sections) increased progressively and was significantly different between each of the displacement groups (Fig. 3b). The same results were seen for woven bone extent. Woven bone was absent from all control specimens.
Figure 3.
A clear dose-response was seen in the total amount of woven bone formed after creep loading. (A) Histological and microCT images illustrate increasing amounts of periosteal woven bone formation 1 mm distal to the midpoint of the bone for low (20%), medium (40%) and high (80%) displacement groups. (B) Quantification of woven bone area (totaled from all histomorphometry slides at five locations) demonstrates significant differences between displacement groups. (P<0.05: * vs. control; a vs. 20%; b vs. 40%)
Table 3.
MicroCT (n=5–10/group) and histological (n=8–10/group) parameters (mean ± SD) measured 7 days post loading. The last four parameters (BMC, B.Ar, Wo.B.Ar and LS/BS) were measured 2 mm proximal to the midpoint of the ulna. The loaded (Right) ulnae are compared to a pooled control (Left) ulnae group.
| Displacement Group | Visible Crack (% of ulnae) | Visible Wo.B. (% of ulnae) | Wo. B. Extent (mm) | BMC (mg/cm) | B.Ar (mm2) | Wo.B.Ar (mm2) | LS/BS (%) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0.00 ±0.00 | 1.85 + 0.09 | 1.74 + 0.06 | 0.00 ±0.00 | 66 ±17 |
| 20% | 0 | 30 | 0.37 ±0.88 | 1.85 ±0.05 | 1.79 ±0.04 | 0.00 ±0.00 | 89* ±9 |
| 40% | 50 | 90 | 3.70*a ±1.7 | 2.01*a ±0.17 | 2.11*a ±0.33 | 0.10*a ±0.13 | 92* ±8 |
| 80% | 80 | 100 | 5.25*ab ±0.98 | 2.25*ab ±0.29 | 2.71*ab ±0.65 | 0.18*a ±0.12 | 96* ±4 |
P<0.05:
vs. control;
vs. 20%,
vs. 40%
Woven bone formed near the midpoint of the ulnae in each of the three displacement groups. Sections taken 1 mm distal to the midpoint (D1) showed a clear dose-response with progressively larger amounts of woven bone in proportion to initial displacement. The dose-response at P2 was similar to that of D1, but without a significant increase from medium to high displacement (P = 0.053). Furthermore, there was a dose-response in woven bone labeled surface at D1 and P2. MicroCT data confirmed the changes in woven bone area seen using histology.
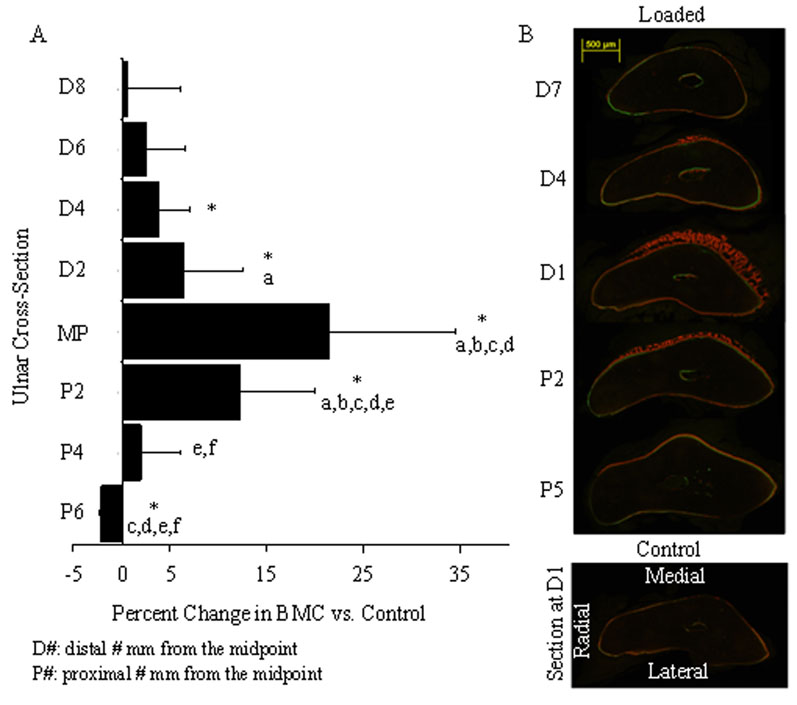
The distribution of bone formation varied along the length of the ulna (Fig. 4). The largest amount of woven bone formed in the area of highest strain [17], near the midpoint (MP) of the ulna. Bone mineral content and mineral density were significantly different from control at the midpoint and 2 mm proximal to the midpoint (P2) for the 40 and 80% displacement groups (data not shown). Outside of the region of high strain (away from the midpoint) woven bone transitioned into lamellar bone. Woven bone was absent in sections 5 mm proximal to the midpoint (P5) and comprised less than 1% of the total bone area at sections 4 and 7 mm distal to the midpoint (D4 and D7, respectively). Loaded ulnae (from all displacement groups) had significantly more labeled surface compared to controls at all locations with the exception of the low displacement group (20% displacement) at P5. In the low displacement group there were no significant changes in BMC or BMD over the entire region of interest (P6-D8).
Figure 4.
The magnitude and type of bone formed in response to creep loading varies longitudinally, similar to the pattern observed after fatigue loading [28]. (Data from 40% displacement group shown; other groups had similar distributions) (A) The percentage change in BMC peaks at the midpoint (MP) and decreases both in the proximal and distal directions away from the midpoint. (B) Histological images illustrate labeled surfaces at five locations along the ulna. Woven bone is greatest 1 mm distal to the midpoint (D1) on the medial side of the bone. (P<0.05: * vs. control; a vs. D8; b vs. D6; c vs. D4; d vs. D2; e vs. MP; f vs. P2)
Recovery of mechanical properties
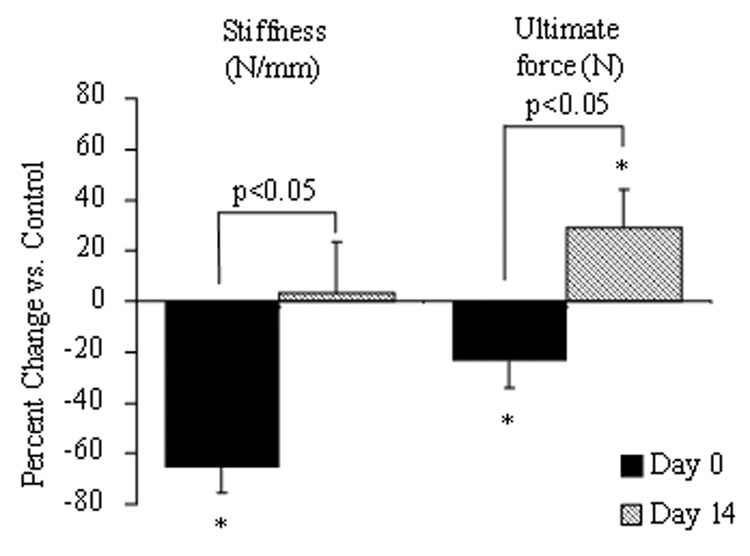
Ulnae from forelimbs loaded to high (80%) displacement demonstrated improvement in several mechanical properties compared to controls at 14 days (Table 4, Fig. 5). Three-point bending tests revealed that the loaded ulnae had significantly greater values of ultimate force, post-yield displacement and fracture energy than control. The stiffness of loaded limbs recovered to the level of control. Micro-computed tomography data showed visible cracks in 8 out of 9 ulnae. The woven bone density significantly increased between 7 and 14 days (7 days: 394 ± 21.5 mg HA/mm3; 14 days: 710 ± 32 mg HA/mm3; P < 0.0001), while woven bone extent was unchanged (7 days: 5.25 ±0.98 mm; 14 days: 5.34 ±0.94 mm; P = 0.84).
Table 4.
Day 14 mechanical properties of isolated ulnae determined using 3-point bending after in vivo creep loading of the right forelimb (mean ± SD; n = 9). There were significant increases in the loaded (Right) ulnae in ultimate force, fracture energy and post-yield displacement compared to control (Left).
| Displacement Group | Stiffness (N/mm) |
Ultimate force (N) |
Fracture energy (Nmm) |
Post-yield isplacement (mm) |
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| 80% | 23.9 ± 2.9 | 24.5 ± 3.7 | 14.9 ± 1.6 | 19.2* ± 1.5 | 21.1 ± 3.4 | 33.9* ± 6.0 | 1.2 ± 0.2 | 1.6* ± 0.3 |
P<0.05 vs. control
Figure 5.
Initial significant decreases in stiffness and ultimate force were recovered 14 days after loading. Percentage change is based on comparison to contralateral controls. There were significant increases over the 14 day recovery period in both of these parameters. The ultimate force of the loaded (Right) limb was significantly higher compared to control (Left) at 14 days post loading. (*P<0.05 vs. control)
Discussion
Our objectives were to use static creep loading of the rat forelimb to produce discrete levels of ulnar damage and subsequently to determine the in vivo bone response to creep damage. In support of our first hypothesis, microCT and mechanical testing indicated that a single bout of creep loading can lead to reduced structural properties at time-zero. Generally, increased displacement produced a higher percentage of visible cracks, along with increased crack length and extent and greater loss of stiffness. In support of our second hypothesis, creep damage stimulated a dose-response in woven bone formation. This woven bone response led to an enhancement of mechanical properties 14 days after loading. These findings demonstrate that even without dynamic strain, bone damage triggers a woven bone response that leads to a functional repair of whole-bone strength.
Previous studies of bone response to creep loading have reported mixed findings. Positive osteogenic effects associated with static loading were reported in dog femora [19] and rabbit calvariae [13]. These studies both used long term loading protocols that involved invasive surgical procedures to implant continuous loading devices. The bone growth seen in these models could be due to an injury response, rather than an adaptation response [13]. Our study produced an osteogenic response using a short-term non-invasive, damaging loading protocol. More commonly, studies have shown no osteogenic response to static loading, although these studies used loading protocols that probably did not produce bone damage. Comparisons between static versus dynamic loading protocols applied to the rabbit tibia [14], the turkey ulna [18] and the rat ulna [22] demonstrated that static loading produced no osteogenic response whereas dynamic loading was osteogenic. In growing rats, brief-duration static loading had an inhibitory effect on appositional bone formation while dynamic loading triggered an adaptive formation response [22]. In addition, dynamic loading did not stimulate bone formation at frequencies below 0.5 Hz in the rat tibia [27]. Our findings clearly demonstrate that when static loading produces measurable bone damage, woven bone formation is activated. While we also observed lamellar bone formation, this occurred between sites where woven bone was activated and sites where there was no response. We hypothesize that this lamellar formation is part of a coordinated damage response rather than a primary response to short-term static loading.
Creep displacement is a component of the total displacement that occurs during fatigue loading in cortical [6, 10] and trabecular [3] bone. Moreover, it is well established that static loading by itself leads to progressive creep of bone [4, 5]. While there are several reports on the degradation of bone mechanical properties during fatigue (dynamic) loading both in vivo and ex vivo, with reported decreases in modulus [21], strength [7] and stiffness [7, 11, 29], similar data for in vivo creep (static) loading are lacking. Fondrk et al [12] demonstrated bone stiffness degradation during tensile creep, but they used multiple cycle tests. Our results indicate that while creep loading of the rat ulna often produced visible cracks on microCT (i.e., a stress fracture) and reductions in mechanical properties, there were important differences between creep and fatigue damage. (Note that these comparisons are being made between studies done in the same lab, using the same age and sex of rats, and the same microCT and mechanical testing protocols. In both studies actuator displacement was used to produce discrete levels of damage based on a percentage of the displacement to fracture.) Fatigue loading more consistently created ulnar cracks that were visible on microCT [29], whereas creep-loaded ulnae did not always have a visible crack even at high values of displacement. Overall, there were fewer cracks and lower crack length density for creep compared to fatigue loading. Reductions in ultimate force, although significant for creep loading, were smaller than those reported for fatigue loading (P<0.001) [29]. The two types of loading produced similar reductions in stiffness, and in both cases stiffness loss was detected in the absence of large cracks. We hypothesize that diffuse damage or cracks not visible at 16 µm resolution contribute to the loss of stiffness in both creep and fatigue. We further hypothesize that the loss of strength (ultimate force) is more strongly related to the formation of large cracks than to diffuse damage or microcracks. In summary, creep and fatigue loading of the rat ulna both produce bone damage but there are some notable differences between the two loading models.
Our motivation for examining the response of bone to damaging creep loading was to follow up on previous studies that showed woven bone formation after damaging fatigue loading [9, 15, 25, 28]. Consistent with fatigue loading [28], there is a dose-response in woven bone formation after creep loading whereby woven bone area increases with increasing damage. This strongly suggests that the damage component of fatigue is the predominant stimulus for the woven bone formation we and others have observed. Direct comparisons between the amount of woven bone formed for fatigue versus creep are difficult because of the differences in damage between the two loading modes as discussed above. Nonetheless, some qualified comparisons are worth considering. The “low” (20%), “medium” (40%) and “high” (80%) creep displacement groups were chosen for the survival experiments in this study because they produced equivalent loss of stiffness as the 30, 45 and 65% fatigue displacement groups [29] (~15, 40 and 60% loss of ulnar stiffness, respectively). If stiffness loss is taken as the measure of structural damage, our data indicate that creep produced a diminished woven bone response compared to damage-equivalent fatigue loading. The center of woven bone formation was similar between the two loading modes, at the ulnar midpoint (creep) or 1 mm distal to the midpoint (fatigue). Yet microCT analysis revealed consistently less woven bone extent for bones loaded in creep – 4 mm shorter (92% decrease) for the low-displacement group, and 2 mm shorter (35% and 25% decrease) for the medium- and high-displacement groups. In addition, total woven bone area (summed over five histology slides from P5 to D7) was reduced 94, 59 and 27% compared to fatigued loaded ulnae for low, medium and high displacement groups, respectively. By day 14 the mechanical properties of ulnae loaded in creep had recovered, similar to the response to fatigue loading [9, 15, 28], indicating a functional repair of the structural damage in both cases. There are two possible explanations for the relatively greater woven bone formation after fatigue loading compared to creep. First, the more severe crack formation in fatigue may have stimulated a greater repair response. Second, the dynamic strain that occurs with fatigue may have an additive effect on top of damage. The latter possibility is consistent with the fact that supraphysiological levels of strain can stimulate woven bone formation in the absence of damage [27], and the likelihood that the dynamic strain in the region of fatigue damage is supraphysiological.
There are several limitations to this study. First, our servohydraulic loading machines did not always hold a constant force when the forelimb was displacing (creeping) slowly. Thus, during creep loading, some forelimbs experienced a small, superimposed sinusoidal variation in strain (less than 1% of the total strain). We do not believe this influenced the creep response, as mouse forelimbs statically loaded with superimposed vibration were not stimulated to form new cortical bone [8], and the frequency of the superimposed variation (0.1 Hz) is less than that required to stimulate an adaptive bone response [27]. Second, the applied force was not equal for all animals. We found there were variations between different batches of rats and the time it took them to reach their pre-determined displacement, so we adjusted the force magnitude in an attempt to keep loading time in a target range of 10–60 minutes. Although this may have added variance to our data, post hoc statistical analysis revealed no force dependence on any of the parameters of interest. Third, it is possible that dynamic strain produced during normal cage activities in the days following the bout of creep loading may have contributed to the bone formation response. Future studies are planned to examine this possibility. Fourth, the resolution of microCT is not high enough to detect true microcracks nor can it detect diffuse damage. Additional studies are needed to better characterize and distinguish the microdamage associated with creep versus fatigue loading. Finally, the bone formation response and mechanical property assessment tests were not run at both 7 and 14 days. Previous work in our lab has demonstrated that the total woven bone area does not increase from 7 to 14 days [28], thus 7 days is a suitable time to characterize bone formation. Moreover, full recovery of mechanical properties after fatigue loading takes 12–14 days [15, 28]; thus we chose 14 days to assess mechanical properties.
The results of our study may have clinical implications. We have demonstrated that creep loading can lead to progressive bone damage, which suggests that the accumulation of bone microdamage with age [23] or disease may not be entirely due to dynamic loading. Static loading, especially in the axial skeleton, may contribute importantly to bone deformation and damage, as has been suggested previously [4]. Regardless of the mechanical loading history that produces damage, our results indicate that when the damage leads to measurable structural degradation, a woven bone repair response is activated.
In summary, creep loading results in decreased mechanical properties at time-zero and a robust woven bone response 7 days after loading. We observed a clear dose-response with progressively larger amounts of woven bone in proportion to initial creep displacement (damage). This study provides an assessment of the bone damage response in the (near) absence of dynamic strain. Comparisons between creep and fatigue demonstrate that the effects of creep loading are similar to fatigue loading. We conclude that the woven bone response seen after the creation of a stress fracture is largely a response to localized bone damage.
Acknowledgements
This work was supported by the National Institutes of Health/NIAMS Grant AR050211
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck TJ, Ruff CB, Mourtada FA, Shaffer RA, Maxwell-Williams K, Kao GL, Sartoris DJ, Brodine S. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11(5):645–653. doi: 10.1002/jbmr.5650110512. [DOI] [PubMed] [Google Scholar]
- 2.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23(3):275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 3.Bowman SM, Guo XE, Cheng DW, Keaveny TM, Gibson LJ, Hayes WC, McMahon TA. Creep contributes to the fatigue behavior of bovine trabecular bone. J Biomech Eng. 1998;120(5):647–654. doi: 10.1115/1.2834757. [DOI] [PubMed] [Google Scholar]
- 4.Bowman SM, Keaveny TM, Gibson LJ, Hayes WC, McMahon TA. Compressive creep behavior of bovine trabecular bone. J Biomech. 1994;27(3):301–310. doi: 10.1016/0021-9290(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Caler WE, Carter DR. Bone creep-fatigue damage accumulation. J. Biomech. 1989;22(67):625–635. doi: 10.1016/0021-9290(89)90013-4. [DOI] [PubMed] [Google Scholar]
- 6.Carter DR, Caler WE. A cumulative damage model for bone fracture. J Orthop Res. 1985;3(1):84–90. doi: 10.1002/jor.1100030110. [DOI] [PubMed] [Google Scholar]
- 7.Carter DR, Hayes WC. Compact bone fatigue damage - I: residual strength and stiffness. J Biomech. 1977;10:325–337. doi: 10.1016/0021-9290(77)90005-7. [DOI] [PubMed] [Google Scholar]
- 8.Castillo AB, Alam I, Tanaka SM, Levenda J, Li J, Warden SJ, Turner CH. Low-amplitude, broad-frequency vibration effects on cortical bone formation in mice. Bone. 2006;39(5):1087. doi: 10.1016/j.bone.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Colopy SA, Benz-Dean J, Barrett JG, Sample SJ, Lu Y, Danova NA, Kalscheur VL, Vanderby R, Jr, Markel MD, Muir P. Response of the osteocyte syncytium adjacent to and distant from linear microcracks during adaptation to cyclic fatigue loading. Bone. 2004;35(4):881–891. doi: 10.1016/j.bone.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Cotton JR, WK, Zioupos P, Taylor M. Damage rate is a predictor of fatigue life and creep strain rate in tensile fatigue of human cortical bone samples. J Biomech Eng. 2005;127(2):213–219. doi: 10.1115/1.1865188. [DOI] [PubMed] [Google Scholar]
- 11.Danova NA, Colopy SA, Radtke CL, Kalscheur VL, Markel MD, Vanderby R, McCabe RP, Escarcega AJ, Muir P. Degradation of bone structural properties by accumulation and coalescence of microcracks. Bone. 2003;33(2):197–205. doi: 10.1016/s8756-3282(03)00155-8. [DOI] [PubMed] [Google Scholar]
- 12.Fondrk M, Bahniuk E, Davy DT, Michaels C. Some viscoplastic characteristics of bovine and human cortical bone. J Biomech. 1988;21(8):623–630. doi: 10.1016/0021-9290(88)90200-x. [DOI] [PubMed] [Google Scholar]
- 13.Hassler CR, Rybicki EF, Cummings KD, Clark LC. Quantification of bone stresses during remodeling. J Biomech. 1980;13:185–190. doi: 10.1016/0021-9290(80)90192-x. [DOI] [PubMed] [Google Scholar]
- 14.Hert J, Liskova M, Landgrot B. Influence of the long-term continuous bending on the bone. Folia Morphol (Praha) 1969;17:389–399. [PubMed] [Google Scholar]
- 15.Hsieh YF, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. J Orthop Res. 2002;20(4):764–771. doi: 10.1016/S0736-0266(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LC, Stradford HT, et al. Histogenesis of stress fractures. J. Bone Jt. Surg. [Am] 1963;45(7):1542. [Google Scholar]
- 17.Kotha SP, Hsieh YF, Strigel RM, Muller R, Silva MJ. Experimental and finite element analysis of the rat ulnar loading model-correlations between strain and bone formation following fatigue loading. J Biomech. 2004;37(4):541–548. doi: 10.1016/j.jbiomech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17(12):897. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 19.Meade JB, Cowin SC, Klawitter JJ, Van Buskirk WC, Skinner HB. Bone remodeling due to continuously applied loads. Calcif Tissue Int. 1984;36 Suppl.:S25–S30. doi: 10.1007/BF02406130. [DOI] [PubMed] [Google Scholar]
- 20.Mori S, Li J, et al. The histological appearance of stress fractures. In: Burr DB, Milgrom C, editors. Musculoskeletal fatigue and stress fractures. Boca Raton: CRC Press; 2001. pp. 151–159. [Google Scholar]
- 21.Pattin CA, Caler WE, Carter DR. Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech. 1996;29(1):69. doi: 10.1016/0021-9290(94)00156-1. [DOI] [PubMed] [Google Scholar]
- 22.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29(2):105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 23.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17(6):521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 24.Shaffer RA. Incidence and prevalence of stress fractures in military and athletic populations. In: Burr DB, Milgrom C, editors. Musculoskeletal fatigue and stress fractures. CRC Press; 2001. pp. 1–14. [Google Scholar]
- 25.Tami AE, Nasser P, Schaffler MB, Knothe Tate ML. Noninvasive fatigue fracture model of the rat ulna. J Orthop Res. 2003;21(6):1018–1024. doi: 10.1016/S0736-0266(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 26.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periosteal pressure. Calcif Tissue Int. 1994;54(3):241–247. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 27.Turner CH, Forwood MR, Otter MW. Mechanotransduction in bone: do bone cells act as sensors of fluid flow? FASEB J. 1994;8(11):875–878. doi: 10.1096/fasebj.8.11.8070637. [DOI] [PubMed] [Google Scholar]
- 28.Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: Stress fracture healing by rapid formation and densification of woven bone. J Bone Miner Res. 2007;22(10):1548–1556. doi: 10.1359/jbmr.0070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uthgenannt BA, Silva MJ. Use of the rat forelimb compression model to create discrete levels of bone damage in vivoq. J Biomech. 2007;40(2):317–324. doi: 10.1016/j.jbiomech.2006.01.005. [DOI] [PubMed] [Google Scholar]