Abstract
Isothiocyanates (ITC) are potentially anticarcinogenic phytochemicals formed from the metabolism of glucosinolates and are found in cruciferous vegetables as well as a select number of other foods. ITC are both substrates for and inducers of glutathione S-transferase (GST) phase II metabolizing enzymes involved in carcinogen detoxification as well as effectors of phase I pathways. Previous studies report mixed results on the interaction between cruciferous vegetable intake, GST polymorphisms, and risk of cancer. We conducted a study of 114 healthy human subjects between 18 and 50 y of age to examine the biologic mechanism underlying the associations, specifically, to assess whether GST genotype is associated with urinary ITC metabolites following a known dose of broccoli. After 48 h of abstaining from all sources of glucosinolates, participants provided a blood sample, consumed 1 meal containing 2.5 g broccoli/kg body weight, and collected urine for 24 h. ITC metabolites were measured in the urine using a HPLC cyclocondensation assay. DNA was extracted from blood samples, and GSTM1 deletion, GSTT1 deletion, GSTP1 Ile105Val, and GSTA1*A/*B were genotyped by matrix-assisted laser desorption/ionization time-of-flight. A chi-square test was used to compare high and low ITC excretion levels across genotypes. ITC levels were regressed on genotype, adjusting for gender. There were no substantial differences in ITC levels among genotypes, either individually or in combination. Contrary to our hypothesis, a higher proportion of GSTM1 null individuals had high ITC excretion (62%) compared with the proportion of GSTM1 present with high ITC excretion (39%) (P = 0.03). These results are in agreement with another feeding study, and lend support to the idea of alternative routes of ITC metabolism.
Introduction
Recent advances in the fields of metabolomics, proteomics, and genomics have improved our ability to study genetic susceptibility to disease and its interaction with nutritional and other environmental factors (1). Gene-diet interactions in cancer epidemiology hold promise by explaining inconsistent results observed in epidemiologic studies of diet and disease. Deepening our understanding also holds promise for improving the efficiency and efficacy of our interventions by identifying individuals who are most susceptible to the benefits of an intervention.
One gene-diet interaction that has been studied in relation to various cancers is that of cruciferous vegetable intake and polymorphisms in glutathione S-transferase (GST)6 genes. Cruciferous vegetables (e.g., broccoli, cabbage, and Brussel sprouts) are the primary dietary source of glucosinolates, whose major metabolic breakdown products, isothiocyanates (ITC) and indoles, have been shown to be anticarcinogenic in experimental models (2). ITC both induce and are substrates for the phase II metabolizing GST enzymes (2–4). ITC also affect phase I pathways, apoptosis, and cell cycle arrest (5). The GST are a family of enzymes that are polymorphic in a relatively large proportion of the population. For GSTM1 and GSTT1 genes, deletions result in no enzyme activity in an estimated 53 and 20% of Caucasian populations, respectively (6). For GSTP1, a single nucleotide polymorphism on exon 5 results in an amino acid substitution (Ile105Val) and has been associated with reduced specific activity toward the major ITC in broccoli, sulforaphane (7). This single nucleotide polymorphism is present in ∼38−49% (heterozygous) and 7−12% (homozygous) of different populations (8–10). For GSTA1, a haplotype is associated with reduced enzyme expression and ∼14% of the population is homozygous (11).
Several studies have examined the joint effects of cruciferous vegetable intake (or urinary ITC level as a biomarker for intake) and GST polymorphisms in relation to cancer risk [reviewed in (12)]. The null or less active GST genotypes have been suggested to be related to decreased metabolism and urinary excretion of ITC, thus increasing the body pool of ITC, offering more exposure to these protective phytochemicals, and reducing the risk of cancer through phase I, phase II, apoptotic, or cell cycle control processes. Studies from Asia, where the leading type of cruciferous vegetable consumed is cabbage, tend to support this hypothesis by finding the greatest risk reduction in consumers of cruciferous vegetables with the null or less active GST genotypes (13,14). Studies in the United States, however, where broccoli is the main cruciferous vegetable consumed, found the greatest risk reduction for high cruciferous vegetable intake among individuals with the most active or expressed genotypes (15–17).
Associations between urinary ITC levels and GSTT1 in one study (18), and GSTP1 Ile105Val (19) in another study, were reported in observational (i.e., nonintervention) settings where past usual cruciferous vegetable intake was measured using food frequency questionnaires. Additionally, the relation between GSTM1 genotype and sulforaphane metabolism and excretion after broccoli and super broccoli intake in humans was recently reported in a controlled feeding study (20). Because the observational studies suggested associations with GSTT1, GSTP1 and GSTA1 as well, our objective was to examine, in humans, the relation among urinary ITC metabolite excretion after a known dose of broccoli and polymorphisms in 4 of the GST family of genes: GSTM1, GSTT1, GSTP1, and GSTA1.
Materials and Methods
Participants and protocol
The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, and all participants provided signed informed consent. Participants were recruited from greater Chapel Hill, North Carolina, through an e-mail advertisement to the University of North Carolina at Chapel Hill e-mail system. Participants were screened using an interviewer-administered screening questionnaire on the telephone. Eligibility criteria included being healthy and between the ages of 18−50 y. Furthermore, eligible individuals could not report any of the following: history of cancer, cardiovascular disease, diabetes, or gastrointestinal disorder; food allergies or intolerances, particularly to cruciferous vegetables; oral antibiotic use within the past 3 mo; body weight >150% of ideal; current drug therapy for a diagnosed disease; regular nonsteroidal anti-inflammatory drug use; alcohol intake >2 servings/d (i.e., >0.7 L beer, 0.3 L wine or 0.09 L distilled spirits); use of tobacco products; job-related exposure to smoke and/or organic solvents; regular overexposure to secondhand smoke; current or planned pregnancy; or no interest in participating in the study.
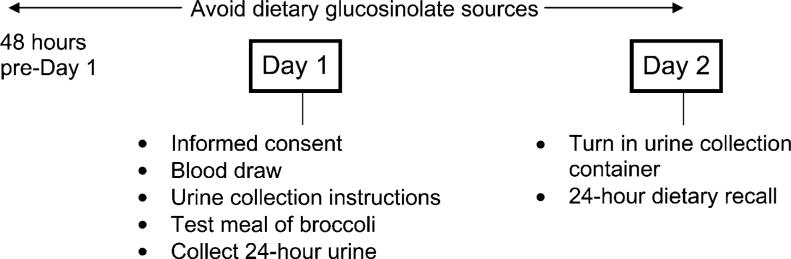
A total of 114 subjects participated in this pilot study, which involved 2 visits on consecutive days to the General Clinical Research Center (GCRC) at UNC Hospitals. Each participant received, by mail, a list of foods to avoid (all dietary sources of glucosinolates, including cruciferous vegetables) for 48 h prior to their first study visit on d 1 (see Fig. 1). Participants were allowed to time their 1st visit to accommodate their schedule. On the 1st visit (d 1) to the GCRC, subjects reviewed and signed the informed consent form. Then a trained and certified nurse at the GCRC collected a nonfasting blood sample from each participant. After collection, tubes were placed on ice and delivered to the Clinical Nutrition Research Center laboratory for immediate processing. DNA was extracted from whole blood and stored at −80°C.
Figure 1.
Study design and protocol.
Following the blood draw, subjects were instructed to void once, but not collect the sample, before consuming the test meal of broccoli. Subjects were given 2.5 g [frozen; wet weight, uncooked] broccoli per kg body weight based on body weight ranges (Table 1), as well as a main course of pasta and a beverage. All broccoli consumed by study participants originated from the same batch of frozen broccoli and was lightly microwaved before consumption. All participants consumed the entire portion of broccoli they were given.
TABLE 1.
Broccoli consumed by participant body weight
| Body weight ranges, kg | Broccoli amount, g |
|---|---|
| 45.0−49.9 | 119 |
| 50.0−54.4 | 131 |
| 54.5−58.9 | 142 |
| 59.0−63.4 | 153 |
| 63.5−67.9 | 164 |
| 68.0−72.4 | 176 |
| 72.5−76.9 | 187 |
| 77.0−81.4 | 198 |
| 81.5−85.9 | 209 |
| 86.0−90.4 | 221 |
| 90.5−94.9 | 232 |
| 95.0−99.4 | 243 |
| 99.5−103.9 | 254 |
| 104.0−108.4 | 266 |
| 108.5−112.5 | 276 |
Two grams of ascorbate were added to the urine collection container as a preservative. Following the test meal, all urine for the next 24 h was collected by the subject and returned to the GCRC the following day (d 2). A total volume measure was recorded and multiple 10-mL aliquots were stored in 15-mL tubes wrapped in aluminum foil and stored at −80°C. A 24-h dietary recall interview was administered to each participant following the return of their urine collection container to assess compliance with the dietary restrictions.
Laboratory methods
Genotyping
Genotyping of GSTM1, GSTT1, GSTP1, and GSTA1 was conducted at BioServe Biotechnologies in Laurel, Maryland, using Sequenom's high-throughput matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Each set of study assays included positive and negative control samples. A 12% random subset of samples was repeated; with 98% total concordance observed for the 4 polymorphisms.
High performance liquid chromatography
Concentrations of ITC and their dithiocarbamate metabolites in urine were measured by the benzenedithiol cyclocondensation procedure (21,22) by Craft Technologies. An aliquot (5 mL) of urine from each subject was centrifuged (200 × g for 5 min at 4°C) to remove particulate matter. Clarified urine samples were reacted with 1,2-benzenedithiol to form quantitatively 1,3-benzodithiole-2-thione (molar extinction coefficient 23,000 at 365 nm). The product was then analyzed by reversed-phase HPLC (using a BDSHypersil C18 column with a mobile phase of methanol/water, 85:15, v:v). Limits of detection for this assay were ∼40 nmol/L urine. A 10% random subset of repeats were performed, and the Pearson correlation of ITC levels between the 2 sets was 98%. Creatinine also was measured in urine samples [using the Jaffe color reaction (23) in microwell plates] and used to control for completeness of the 24-h urine collection.
Statistical analysis
ITC concentrations (μmol/L) were multiplied by the total volume of urine obtained to give total ITC in μmol units. Levels of ITC were classified as either low or high using the median as the cutoff point. A combined gene variable was created by summing the number of variants for each gene where variants were defined as GSTM1 deletion, GSTT1 deletion, GSTP1 105 presence of Val allele, and GSTA1 presence of *B allele. The summary of variable values ranged from 0 to 6, and due to the low frequency of individuals with >4 variant alleles, the variable was collapsed into a 3-level variable of 1) 0 or 1 variant, 2) 2 variants, and 3) ≥3 variants. The standard chi-square test was used to compare demographic characteristics of the participants by GST genotypes and to compare the level of urinary ITC by GST genotypes. ITC values, which were found to have non-normal distributions, were log-transformed and regressed on genotype with adjustment for gender. Medians ± SE are presented. Additionally, we divided ITC level by grams of broccoli consumed and conducted the analyses using this variable to account for differences in broccoli dosing by body weight. Differences were considered significant at P < 0.05. All analyses were performed using SAS, version 8.02 (SAS Institute).
Results
Fourteen subjects were excluded from data analysis because their total urine volume collected was <0.5 L and/or their measured urine collection completeness was too low or too high based on biologically plausible creatinine levels (creatinine cutoffs were <8.9 mmol/24 h for males and <4.9 mmol/24h for females). Data from one participant with a statistically influential outlying ITC value were excluded. For 7 individuals, ITC data were either not detectable (n = 2) or were not reported (n = 5) by the laboratory conducting the HPLC analyses. Genotyping data were missing for 4 individuals. A total of 88 individuals had complete data for all variables studied.
Demographic characteristics of the 88 participants did not differ by genotype (Table 2), with the exception of the gender and racial distributions. Prevalence of the null genotypes for GSTM1 and GSTT1 were 52 and 20%, respectively. Prevalence of heterozygote genotypes were 47 and 54%, and homozygous variant genotypes were 11% and 15% for GSTP1 and GSTA1, respectively. Urinary ITC excretions ranged from 0.8 μmol/24 h to 43.7 μmol/24 h. The median ITC excretion by females was 10.9 ± 1.1 μmol/24 h and for males, 11.9 ± 1.8 μmol/24 h.
TABLE 2.
Demographic characteristics of the study population by GST genotype1
| GSTM1 | GSTM1 | GSTT1 | GSTT1 | GSTP1 | GSTP1 | GSTP1 | GSTA1 | GSTA1 | GSTA1 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | deletion | present | deletion | present | A/A | G/A | G/G | *A/*A | *A/*B | *B/*B | |
| n | 88 | 44 | 44 | 17 | 71 | 36 | 42 | 10 | 28 | 46 | 14 |
| Age, y | 30.1 ± 9.1 | 29.3 ± 8.5 | 30.8 ± 9.7 | 28.2 ± 8.5 | 30.5 ± 9.3 | 28.8 ± 9.0 | 31.7 ± 8.8 | 28.3 ± 10.5 | 31.4 ± 8.3 | 28.8 ± 9.5 | 31.9 ± 9.2 |
| Height, cm | 169.8 ± 9.2 | 170.4 ± 9.4 | 169.1 ± 9.1 | 167.3 ± 8.6 | 170.4 ± 9.3 | 168.5 ± 9.4 | 170.9 ± 8.9 | 169.6 ± 10.1 | 166.3 ± 8.5 | 172.3 ± 8.7 | 168.6 ± 10.5 |
| Weight, kg | 68.7 ± 12.8 | 69.2 ± 13.8 | 68.2 ± 11.9 | 67.3 ± 12.8 | 69.1 ± 12.8 | 67.3 ± 13.8 | 70.4 ± 12.4 | 66.6 ± 10.7 | 67.7 ± 13.6 | 70.6 ± 13.6 | 64.8 ± 6.2 |
| BMI, kg/m2 | 23.7 ± 3.6 | 23.8 ± 4.0 | 23.7 ± 3.3 | 24.0 ± 3.6 | 23.7 ± 3.7 | 23.6 ± 3.9 | 24.1 ± 3.7 | 23.0 ± 2.4 | 24.3 ± 3.6 | 23.7 ± 4.0 | 22.9 ± 2.3 |
| Race, % | |||||||||||
| White | 82 | 86 | 77 | 71 | 85 | 81 | 79 | 100 | 64 | 91 | 86 |
| Nonwhite | 19 | 14 | 23 | 29 | 15 | 19 | 21 | 0 | 36 | 9 | 14 |
| Gender, % | |||||||||||
| Male | 33 | 36 | 30 | 23 | 35 | 31 | 33 | 40 | 14 | 44 | 36 |
| Female | 67 | 64 | 70 | 76 | 65 | 69 | 67 | 60 | 86 | 56 | 64 |
Values are means ± SD or %, n = 88 individuals with complete data for all variables.
A greater proportion of individuals with the GSTM1 null genotype had high urinary ITC levels compared with those with the GSTM1 present genotype (P = 0.03, Table 3). There were no other differences in low levels compared with high levels of urinary ITC excretion between the genotypes (Table 3). Similarly, levels of ITC metabolites did not differ by genotype (Table 4), with the exception of a borderline difference between GSTT1 present and deletion genotypes. When examining the genes combined, the distribution or levels of ITC, compared with individuals with 0 or 1 variant to those with 2 or ≥3 variants, did not differ (Tables 3 and 4). Gender was a predictor of ITC levels (P = 0.04) and was included in each model.
TABLE 3.
Distribution of urinary ITC excretion following a broccoli meal in humans according to GST genotype
|
Urinary ITC1 |
|||
|---|---|---|---|
| Genotype | Low | High | P-value2 |
| GSTM1 | n (%) | ||
| Present | 27 (61) | 17 (39) | |
| Deletion | 18 (38) | 29 (62) | 0.03 |
| GSTT1 | |||
| Present | 36 (50) | 36 (50) | |
| Deletion | 10 (56) | 8 (44) | 0.67 |
| GSTP1 | |||
| A/A | 23 (59) | 16 (41) | |
| A/G | 17 (40) | 26 (60) | |
| G/G | 6 (60) | 4 (40) | 0.17 |
| GSTA1 | |||
| *A/*A | 12 (43) | 16 (57) | |
| *A/*B | 25 (51) | 24 (49) | |
| *B/*B | 9 (64) | 5 (36) | 0.42 |
| Combined genes | |||
| 0 or 1 variant | 14 (58) | 10 (42) | |
| 2 variants | 14 (50) | 14 (50) | |
| ≥3 variants | 17 (47) | 19 (53) | 0.69 |
Low urinary ITC is defined as < median cutpoint (11.4 μmol/24 h); high urinary ITC is defined as ≥ median cutpoint (11.4 μmol/24 h).
Chi-square P-values.
TABLE 4.
ITC excretion in urine following a broccoli meal in humans according to GST genotype1
| n | ITC, μmol ITC/24 h | P-value2 | |
|---|---|---|---|
| GSTM1 | |||
| Present | 44 | 9.9 ± 1.4 | |
| Deletion | 47 | 14.0 ± 1.3 | 0.48 |
| GSTT1 | |||
| Present | 72 | 11.6 ± 0.9 | |
| Deletion | 18 | 9.9 ± 2.7 | 0.05 |
| GSTP1 | |||
| A/A | 39 | 9.9 ± 1.5 | |
| G/A | 43 | 14.8 ± 1.4 | |
| G/G | 10 | 10.8 ± 1.4 | 0.48 |
| GSTA1 | |||
| *A/*A | 28 | 13.2 ± 1.6 | |
| *A/*B | 49 | 11.0 ± 1.4 | |
| *B/*B | 14 | 9.9 ± 2.1 | 0.22 |
| Combined genes | |||
| 0 or 1 variant | 24 | 10.4 ± 1.8 | |
| 2 variants | 28 | 11.5 ± 1.7 | |
| ≥3 variants | 36 | 11.8 ± 1.5 | 0.30 |
Values are medians ± SE.
P-value from regression of log-transformed ITC values adjusted for gender.
We also reran the analyses using the variable of the ITC level divided by the gram amount of broccoli consumed, and there were no differences in the results obtained (data not shown). In addition, we conducted 2 separate analyses with a limited dataset: 1) we excluded women who reported hormonal contraceptive use (n = 18) and, 2) we excluded women aged 41−50 y (n = 12). The results of the analyses did not differ substantially from the analyses using the entire dataset (data not shown).
Discussion
Cruciferous vegetables contain glucosinolates that are metabolized by myrosinase into ITC, indoles, and other compounds. ITC have anticarcinogenic properties in cell culture and animal models (2), which may be partly explained by their ability to induce metabolizing enzymes such as the GST, a family of phase II metabolizing enzymes involved in the detoxification of carcinogens (4), as well as by their ability to affect phase I pathways, apoptosis, and cell cycle arrest (5). ITC are also substrates for GST. We examined whether the GST genotype was associated with urinary excretion of ITC by conducting a 1-meal feeding study in humans. Our results do not support the hypothesis that urinary ITC levels are decreased in individuals with the null or less-active or less-expressed genotypes for GSTM1, GSTT1, GSTP1 Ile105Val, and GSTA1*A and *B following the intake of a standardized dose of a cruciferous vegetable.
Joint effects of cruciferous-vegetable intake and GST polymorphisms have been studied in relation to cancer risk in several observational studies. For GSTM1, there appear to be contrasting associations depending upon the population being studied. As Gasper et al. (20) point out, studies from Asia have generally found greater lung cancer protection (13,14) with the increasing intake of cruciferous vegetables (or increasing urinary ITC level as a biomarker of intake) among GSTM1 null genotype carriers compared with GSTM1 positive individuals. The proposed biologic mechanism for these findings explains that the null or less-active GST genotypes may be related to decreased metabolism and urinary excretion of ITC, thus offering greater exposure to these protective phytochemicals. Another study of lung cancer conducted in central and eastern Europe supports this hypothesis (24), although a study of breast cancer (25) and one of colorectal cancer (26), both in Asia, found no evidence for differential effects of urinary ITC levels or dietary intake of ITC, respectively, by GSTM1 genotype.
In contrast, studies in the United States found a greater reduction in cancer risk from cruciferous-vegetable consumption among GSTM1-positive individuals compared with GSTM1-null individuals for prostate cancer (17) and lung cancer among smokers (15,16), or no evidence for differential effects of broccoli intake by genotype for colon cancer (27,28), breast cancer (29), or for cruciferous vegetable intake and head and neck cancer (30). Gasper et al. (20) suggest that differences in the type of cruciferous vegetable (and consequently, amounts of different ITC) consumed across countries may explain these contrasting results between Asia and the United States because the specificities of the GST vary for different ITC (31).
Similar contrasting effects were observed for the GSTT1 deletion polymorphism in modifying the association between cruciferous vegetable intake and cancer, with several studies in Asia or Europe finding the greatest protection from ITC exposure among GSTT1 null individuals (13,14,24,26,32), one study in the United States finding greatest protection among GSTT1 positive smokers (15), and other studies finding no difference in association across genotypes (16,25,30). In addition, several studies found the strongest risk reduction for high ITC exposure for combined GSTM1 and GSTT1 null genotype carriers compared with combined GSTM1 and GSTT1 positive individuals (13,14,24,26), or greater protection for the combined positive genotypes (15), whereas others found no differential effects for the combined genotypes (16,32,33).
Enough studies have not been conducted regarding the modifying effects of GSTP1 and GSTA1 polymorphisms on the association between cruciferous vegetable intake and cancer risk to compare results across countries for these genes and to account for differences in dietary intakes of Brassica vegetables. Contrary to the hypothesis of cruciferous vegetables being protective, one study found the greatest risk for colorectal adenoma among high cruciferous vegetable consumers with the low GSTP1 and GSTA1 capacity genotypes (33), whereas 2 other studies found no modification of the effect across GSTP1 genotypes for breast cancer (25) and colorectal cancer (26). Another study of the GSTA1 *A/B polymorphism found the highest breast cancer risk in individuals with low cruciferous vegetable intake and with the low activity genotype (34).
Two previous observational studies have examined the association between urinary ITC levels and GSTM1, GSTT1, and GSTP1 Ile105Val genotypes. Seow et al. (18) found an association between urinary ITC levels and GSTT1 genotype, whereas Fowke et al. (19) found marginally increased concentrations of urinary ITC in carriers of the null GSTT1 genotype and the GG genotype for GSTP1 105 compared with the wildtype geno-types. Contrary to the results from these 2 studies, both of which found no association between GSTM1 and urinary ITC levels, we found a significant association, but in a direction opposite to that of the hypothesis. In our study, a higher proportion of individuals with the null GSTM1 genotype had high urinary ITC levels compared with GSTM1 present genotype. This is in agreement with a recent broccoli feeding study in humans that compared sulforaphane metabolism between GSTM1-positive and GSTM1-null individuals (20). Contrary to the proposed hypothesis, Gasper et al. (20) found that GSTM1-null individuals excreted more sulforaphane metabolites in the 24 h following consumption and excreted the metabolites more rapidly in the first 6 h than GSTM1-positive individuals. They speculate, that for GSTM1-positive individuals, some of the ingested sulforaphane was retained in the body and alternatively metabolized.
The strength of the present study includes its design, which allowed for ITC comparisons across genotypes while holding cruciferous vegetable intake constant at relatively high levels. The study was powered for a sample size of n = 100 to be able to examine an association for individual polymorphisms with high prevalence of the variant, such as with GSTM1, where >50% of participants had the null genotype. We oversampled to n = 114 to make up for missing data that we anticipated would be due to incomplete 24-h urine collections and from the laboratory error, and we had enough broccoli from the original batch to do so. However, the small sample size limited our ability to examine combinations of genotypes across multiple levels of ITC excretion. If compensation by GST exists (e.g., in an individual, if having the GSTM1 null genotype is compensated for functionally by having the GSTT1 present genotype), then we may not expect to find an association between individual genotypes and ITC metabolite excretion. Theoretically, variants in multiple GST genes resulting in no or reduced activity or expression for several of the GST enzymes could lead to reduced metabolism of the ITC, with concomitant reductions in levels of ITC excreted in the urine even if individual genes alone have no association. This was not supported by our combined gene analyses, which found no association between ITC levels and the number of variants in the 4 polymorphisms studied.
One limitation of this study is that the genotyping assay used for GSTM1 and GSTT1 did not discriminate between homozygous wildtype (present) and heterozygotes; therefore, these were examined jointly in the analyses. New technology exists that distinguishes between homozygous wildtype and heterozygotes (35,36). In 1 study that reanalyzed samples previously analyzed for the dichotomous present/deletion GSTM1 genotype, an increased risk of breast cancer was observed for GSTM1 homozygous wildtype (present) carriers compared with GSMT1 homozygous variant (null) carriers, which had not been evident in the prior analyses when homozygous wildtype and heterozygote genotypes were combined (35). Another study found significantly increased erythrocyte enzyme activity for individuals with 2 GSTT1 active alleles compared with 1 null allele (36), suggesting an allele dose-response effect.
Other limitations are the lack of multiple doses of cruciferous vegetables and the short duration of the feeding component of the study. We chose 2.5 g broccoli per kg body weight as the dose, which was based on a previous study that found urinary ITC levels predicted intake of Brassica vegetables when the intake averaged ∼150 g/d but not when intake averaged ∼200 /d (37). Seow et al. (18) found an association between GST genotypes and urinary ITC levels only among the consumers in the highest tertiles of cruciferous vegetables intake and not the lowest tertile (tertile intake not reported, but mean intake in the entire population was 41 g/d). Also, it is unclear whether 1 dose of broccoli is enough to examine an association, or whether longer-term feeding is needed to “turn on” the genes and be able to observe ITC variability between genotypes. Chronic exposure to factors that affect enzyme activity may influence how individuals respond to a single dose (20). Thus, it would have been interesting to examine the results stratified by usual or habitual intake of cruciferous vegetables, but we did not measure this information in our study. Given the large differences in type and amount of Brassica intake between populations and the potential for disease prevention, this would be a fruitful area for future research.
We required participants to avoid all sources of dietary glucosinolates for 48 h prior to the feeding intervention and during the 24-h time period following the broccoli meal. We relied on participant recall to monitor compliance with the dietary restriction. If some participants consumed glucosinolates outside the study protocol, this would increase the amount of ITC recovered in their urine. We would expect this to be at random in relation to the GST genotypes. It is therefore unreasonable to assume that this would bias the results of this study substantially. A similar issue is that the cyclocondensation assay used to measure total ITC metabolites in the urine is not specific to sulforaphane, the major ITC in broccoli. Thus, the presence of other compounds in the urine that react with 1,2-benzenedithiol in this assay may have created variability in the urinary ITC levels that is unrelated to broccoli intake or GST genotype. Smokers were excluded from the study in an effort to control for 1 source of these compounds. Other sources include environmental or occupational exposure through pesticides, manufacturing of natural and synthetic rubber articles, and the use of disulfiram, an aversion therapy for alcoholism (21), which cannot be excluded but are not likely to have been present in this study population.
There are other sources of variation in the excreted ITC that were not accounted for in our study and may confound the results. For example, we did not measure glucosinolates in the broccoli consumed by each individual. Although all broccoli used in the study came from the same batch, there could still be variation in the glucosinolate content of the broccoli consumed between individuals that would result in varying ITC levels excreted in the urine. Additionally, the enzyme myrosinase is virtually destroyed during the processing of frozen broccoli. Thus, the majority of the breakdown of glucosinolates would have been due to microbial thioglucosidase activity in the colon of subjects, and this activity may be variable among individuals. It would have been more desirable for subjects to consume fresh raw broccoli to maintain myrosinase activity within the broccoli. However, glucosinolate content of broccoli grown under different conditions is variable, and recruitment and enrollment of all subjects took place over several months, which eliminated our ability to use fresh raw broccoli from the same batch for each subject.
In conclusion, our results for GSTM1 are in agreement with those from another feeding study (20) and suggest that ITC may undergo alternative routes of metabolism than conjugation by GST. We examined 4 of the most commonly studied polymorphisms in the GST family of genes, but it is possible that other genes and functional polymorphisms within these genes that are associated with urinary ITC levels may be identified in the future. Future studies in this area might be improved by measuring heterozygotes for GSTM1 and GSTT1, examining associations stratified by sex and race, and conducting longer-term feeding studies with varying amounts of cruciferous vegetables.
Footnotes
This work was supported by a Pilot and Feasibility Award from the University of North Carolina (UNC) at Chapel Hill Clinical Nutrition Research Center grants DK56350 and ES10126, the UNC-Hospitals General Clinical Research Center grant RR000046, and a NIH grant 1K07CA102640. General Mills Bell Institute of Health and Nutrition provided the broccoli used in this study.
Abbreviations used: GCRC, General Clinical Research Center; GST, glutathione S-transferase; ITC, isothiocyanate.
Literature Cited
- 1.Zeisel SH, Freake HC, Bauman DE, Bier DM, Burrin DG, German JB, Klein S, Marquis GS, Milner JA, et al. The nutritional phenotype in the age of metabolomics. J Nutr. 2005;135:1613–6. doi: 10.1093/jn/135.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768S–74S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- 3.Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, et al. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev. 2000;9:787–93. [PubMed] [Google Scholar]
- 4.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–33S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 5.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–48. [PubMed] [Google Scholar]
- 7.Lin HJ, Johansson AS, Stenberg G, Materi AM, Park JM, Dai A, Zhou H, Gim JS, Kau IH, et al. Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione transferase P1–1. Biochim Biophys Acta. 2003;1649:16–23. doi: 10.1016/s1570-9639(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 8.Millikan R, Pittman G, Tse CK, Savitz DA, Newman B, Bell D. Glutathione S-transferases M1, T1, and P1 and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:567–73. [PubMed] [Google Scholar]
- 9.Gudmundsdottir K, Tryggvadottir L, Eyfjord JE. GSTM1, GSTT1, and GSTP1 genotypes in relation to breast cancer risk and frequency of mutations in the p53 gene. Cancer Epidemiol Biomarkers Prev. 2001;10:1169–73. [PubMed] [Google Scholar]
- 10.Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Vainio H, Uusitupa M, Hirvonen A. Glutathione S-transferase M1, M3, P1, and T1 genetic polymorphisms and susceptibility to breast cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:229–36. [PubMed] [Google Scholar]
- 11.Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH, Green B, Lang NP, Kadlubar FF. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics. 2001;11:663–9. doi: 10.1097/00008571-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: an epidemiological perspective. Mutat Res. 2005;592:58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Yu MC. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–9. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, et al. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–7. [PubMed] [Google Scholar]
- 15.Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:1017–20. [PubMed] [Google Scholar]
- 16.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of cruciferous vegetables, glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15:977–85. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 17.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50:206–13. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 18.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–81. [PubMed] [Google Scholar]
- 19.Fowke JH, Shu XO, Dai Q, Shintani A, Conaway CC, Chung FL, Cai Q, Gao YT, Zheng W. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1536–9. [PubMed] [Google Scholar]
- 20.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–91. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 21.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–7. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 23.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 24.Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Mates D, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a Mendelian randomisation approach. Lancet. 2005;366:1558–60. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 25.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 26.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–61. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 27.Slattery ML, Kampman E, Samowitz W, Caan BJ, Potter JD. Interplay between dietary inducers of GST and the GSTM-1 genotype in colon cancer. Int J Cancer. 2000;87:728–33. [PubMed] [Google Scholar]
- 28.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:647–52. [PubMed] [Google Scholar]
- 29.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 30.Gaudet MM, Olshan AF, Poole C, Weissler MC, Watson M, Bell DA. Diet, GSTM1 and GSTT1 and head and neck cancer. Carcinogenesis. 2004;25:735–40. doi: 10.1093/carcin/bgh054. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206:748–55. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 32.Turner F, Smith G, Sachse C, Lightfoot T, Garner RC, Wolf CR, Forman D, Bishop DT, Barrett JH. Vegetable, fruit and meat consumption and potential risk modifying genes in relation to colorectal cancer. Int J Cancer. 2004;112:259–64. doi: 10.1002/ijc.20404. [DOI] [PubMed] [Google Scholar]
- 33.Tijhuis MJ, Wark PA, Aarts JM, Visker MH, Nagengast FM, Kok FJ, Kampman E. GSTP1 and GSTA1 polymorphisms interact with cruciferous vegetable intake in colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2943–51. doi: 10.1158/1055-9965.EPI-05-0591. [DOI] [PubMed] [Google Scholar]
- 34.Ahn J, Gammon MD, Santella RM, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Eng SM, et al. Effects of glutathione S-transferase A1 (GSTA1) genotype and potential modifiers on breast cancer risk. Carcinogenesis. 2006;27:1876–82. doi: 10.1093/carcin/bgl038. [DOI] [PubMed] [Google Scholar]
- 35.Roodi N, Dupont WD, Moore JH, Parl FF. Association of homozygous wild-type glutathione S-transferase M1 genotype with increased breast cancer risk. Cancer Res. 2004;64:1233–6. doi: 10.1158/0008-5472.can-03-2861. [DOI] [PubMed] [Google Scholar]
- 36.Sprenger R, Schlagenhaufer R, Kerb R, Bruhn C, Brockmoller J, Roots I, Brinkmann U. Characterization of the glutathione S-transferase GSTT1 deletion: discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics. 2000;10:557–65. doi: 10.1097/00008571-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Fowke JH, Fahey JW, Stephenson KK, Hebert JR. Using isothiocyanate excretion as a biological marker of Brassica vegetable consumption in epidemiological studies: evaluating the sources of variability. Public Health Nutr. 2001;4:837–46. doi: 10.1079/phn2000113. [DOI] [PubMed] [Google Scholar]