Abstract
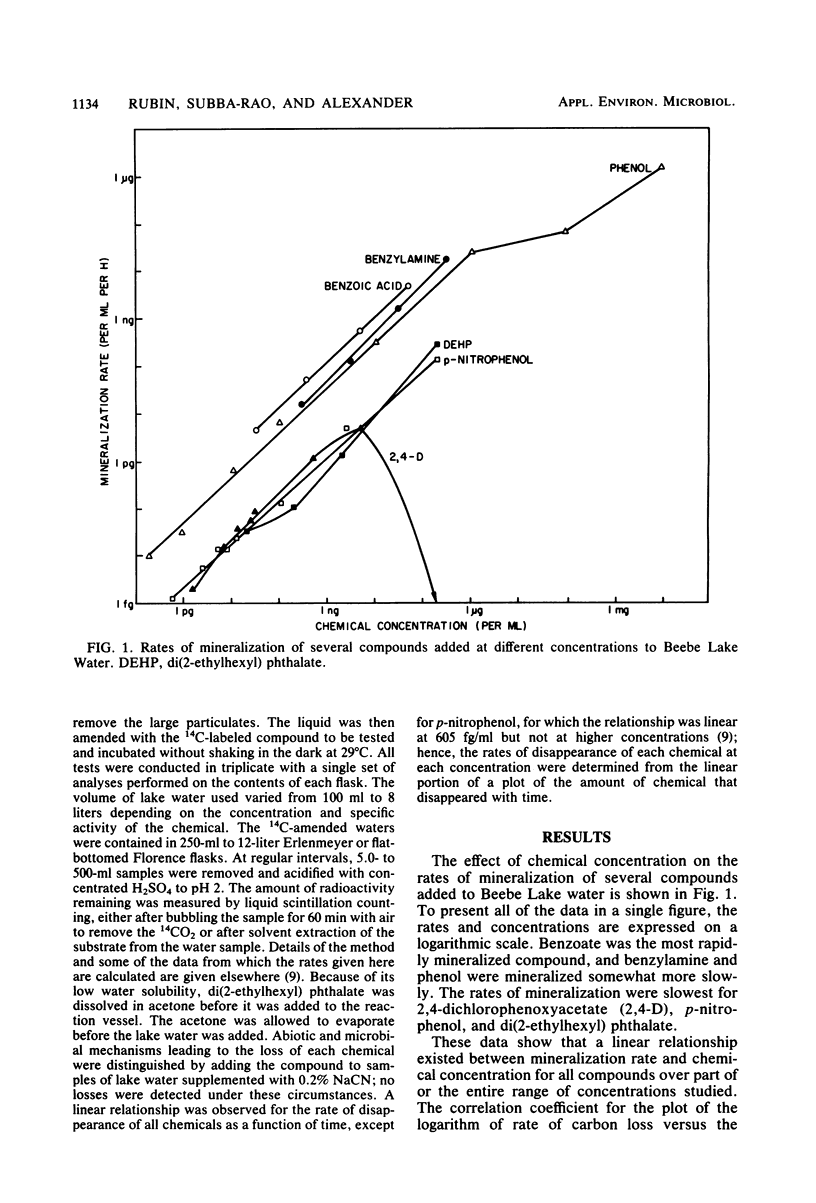
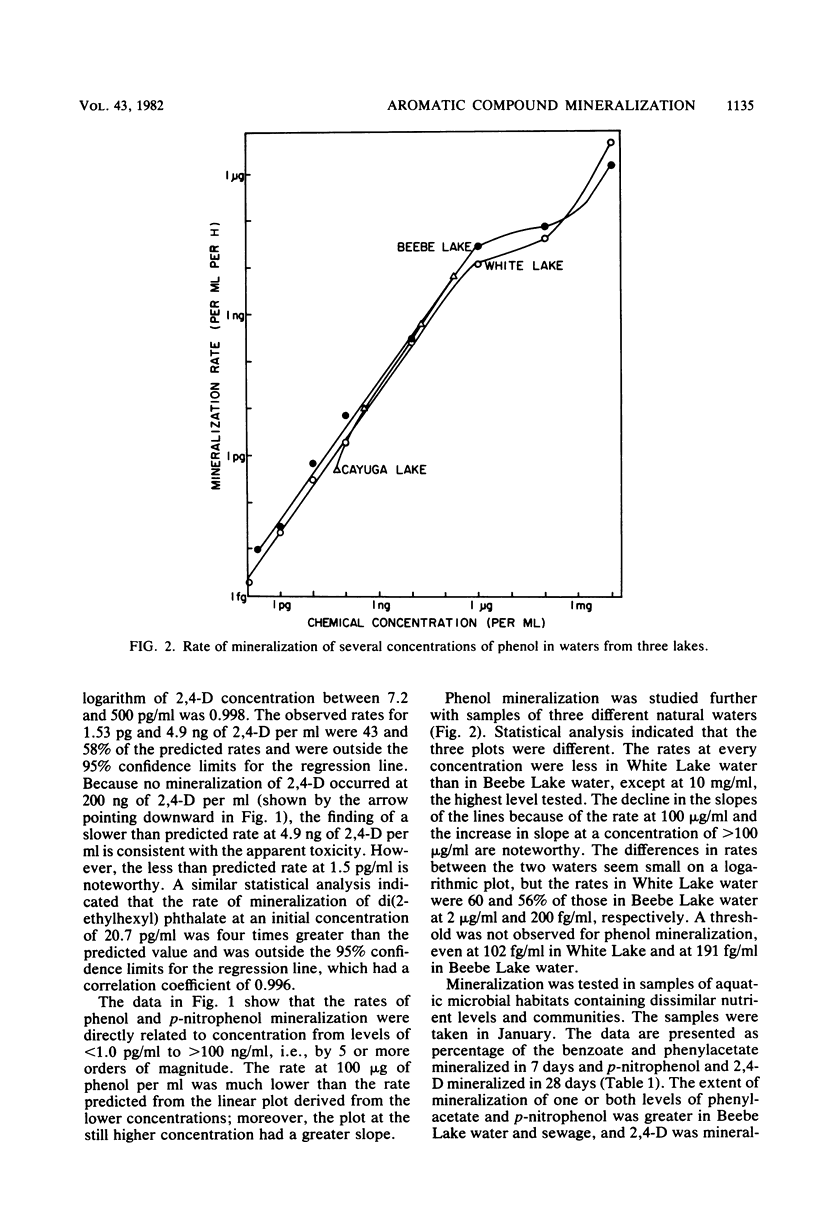
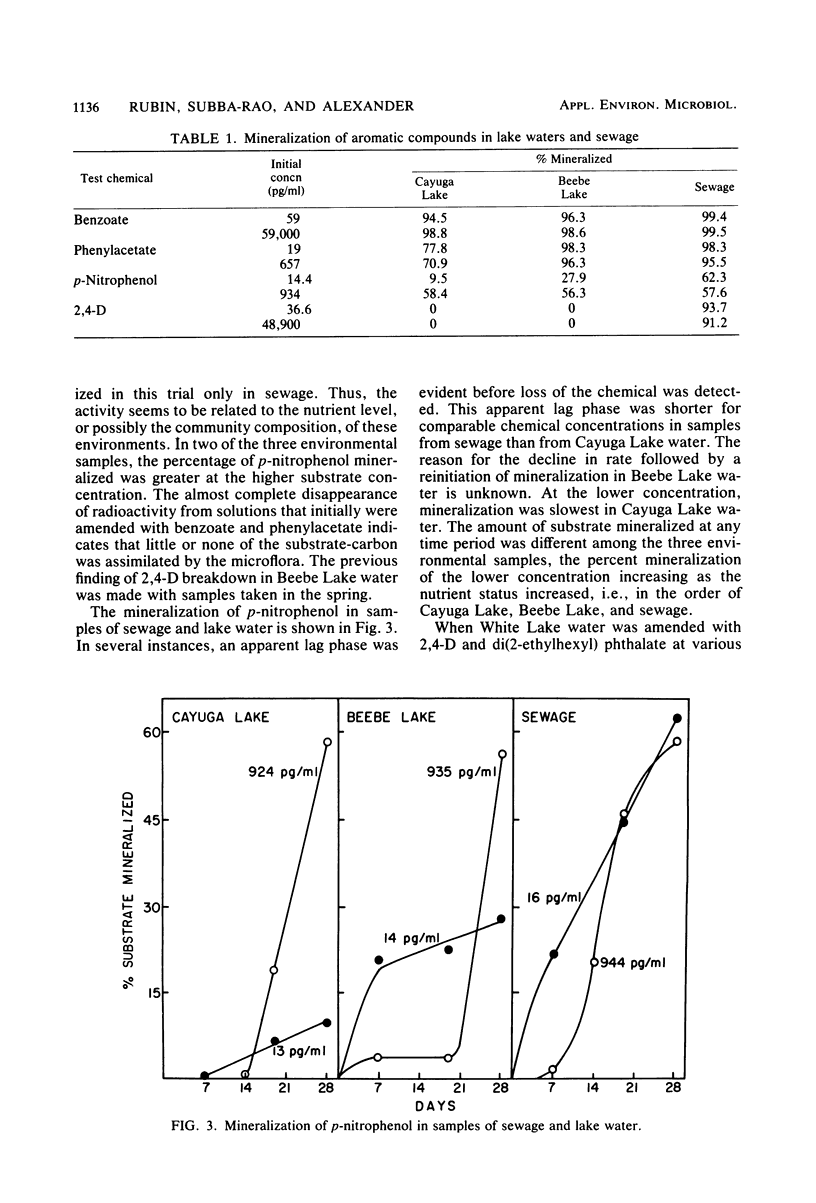
The rates of mineralization of phenol, benzoate, benzylamine, p-nitrophenol, and di(2-ethylhexyl) phthalate added to lake water at concentrations ranging from a few picograms to nanograms per milliliter were directly proportional to chemical concentration. The rates were still linear at levels of <1 pg of phenol or p-nitrophenol per ml, but it was less than the predicted value at 1.53 pg of 2,4-dichlorophenoxyacetate per ml. Mineralization of 2,4-dichlorophenoxyacetate was not detected in samples of lake water containing 200 ng of the chemical per ml. The slope of a plot of the rate of phenol mineralization in samples of three lakes as a function of its initial concentration was lower at levels of 1 to 100 μg/ml than at higher concentrations. In lake water and sewage supplemented with <60 ng of 14C-labeled benzoate or phenylacetate per ml, 95 to 99% of the radioactivity disappeared from solution, indicating that the microflora assimilated little or none of the carbon. The extent of mineralization of some compounds in samples of two lakes and sewage was least in the water with the lowest nutrient levels. No mineralization of 2,4-dichlorophenoxyacetate and the phthalate ester was observed in samples of an oligotrophic lake. These data suggest that mineralization of some chemicals at concentrations of <1 μg/ml is the result of activities of organisms different from those functioning at higher concentrations or of organisms that metabolize the chemicals at low concentrations but assimilate little or none of the substrate carbon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordy J. R., Alexander M. Microbial metabolism of N-nitrosodiethanolamine in lake water and sewage. Appl Environ Microbiol. 1980 Mar;39(3):559–565. doi: 10.1128/aem.39.3.559-565.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



