Abstract
Assembly and guide–target interaction of an archaeal box C/D-guide sRNP was investigated under various conditions by analyzing the lead (II)-induced cleavage of the guide RNA. Guide and target RNAs derived from Haloferax volcanii pre-tRNATrp were used with recombinant Methanocaldococcus jannaschii core proteins in the reactions. Core protein L7Ae binds differentially to C/D and C′/D′ motifs of the guide RNA, and interchanging the two motifs relative to the termini of the guide RNA did not affect L7Ae binding or sRNA function. L7Ae binding to the guide RNA exposes its D′-guide sequence first followed by the D guide. These exposures are reduced when aNop5p and aFib proteins are added. The exposed guide sequences did not pair with the target sequences in the presence of L7Ae alone. The D-guide sequence could pair with the target in the presence of L7Ae and aNop5p, suggesting a role of aNop5p in target recruitment and rearrangement of sRNA structure. aFib binding further stabilizes this pairing. After box C/D-guided modification, target–guide pairing at the D-guide sequence is disrupted, suggesting that each round of methylation may require some conformational change or reassembly of the RNP. Asymmetric RNPs containing only one L7Ae at either of the two box motifs can be assembled, but a functional RNP requires L7Ae at the box C/D motif. This arrangement resembles the asymmetric eukaryal snoRNP. Observations of initial D-guide–target pairing and the functional requirement for L7Ae at the box C/D motif are consistent with our previous report of the sequential 2′-O-methylations of the target RNA.
Keywords: sRNA, snoRNA, guide RNA, pre-tRNA, RNP
INTRODUCTION
Most of the 2′-O-methylations and pseudouridylations of eukaryal ribosomal and other RNAs are guided by box C/D and box H/ACA small nucleolar RNAs (snoRNAs), respectively (Maxwell and Fournier 1995; Filipowicz and Pogacic 2002; Kiss 2002; Decatur and Fournier 2003; Henras et al. 2004; Tran et al. 2004; Yu et al. 2005; Reichow et al. 2007). Box C/D RNAs have a conserved box C/D motif near the termini of the RNA and a less conserved box C′/D′ motif located internally. The specificity of the residue in the target RNA to be modified is dictated by guide sequences (generally, 10–21 nucleotides [nt] long) upstream of the D and D′ boxes. It is precisely the fifth residue in the guide–target duplex that gets 2′-O-methylated. These guide RNAs function as part of ribonucleoprotein complexes (snoRNPs). In Eukarya, box C/D-guide RNAs are associated with fibrillarin (a methyltransferase), Nop56p, Nop58p, and 15.5K (Snu13p) proteins, to form the core snoRNP complex. Our understanding of eukaryal snoRNPs is limited because no active eukaryal complex has been reconstituted in vitro, and to date, only immunoprecipitated native RNP complexes containing tagged proteins have been shown to be active (Galardi et al. 2002).
Archaea also use box C/D and box H/ACA RNAs to guide 2′-O-methylation and pseudouridylation of their ribosomal and other RNAs (Gaspin et al. 2000; Omer et al. 2000; Tran et al. 2004; Baker et al. 2005; Charpentier et al. 2005; Dennis and Omer 2005; Tang et al. 2005; Yu et al. 2005; Meier 2006; Reichow et al. 2007). These RNAs are called small or snoRNA-like RNAs (sRNAs). Assembly of archaeal box C/D RNPs is initiated by the L7Ae protein (a 15.5K/Snu13p homolog), followed by aNop5p (a single polypeptide with homology with both Nop56p and Nop58p) and aFib (archaeal fibrillarin) (Omer et al. 2002; Tran et al. 2003). In vitro reconstituted box C/D RNP complexes using recombinant proteins from four different thermophilic archaea have provided vital clues about the mechanism, specificity and functional requirements of these complexes (Omer et al. 2002; Bortolin et al. 2003; Rashid et al. 2003; Tran et al. 2003; Singh et al. 2004).
The box C/D (and C′/D′) motifs form a specific RNA secondary structure called a kink-turn or K-turn (Watkins et al. 2000; Klein et al. 2001). Sometimes the K-turn associated with box C′/D′ is modified to the K-loop (Nolivos et al. 2005). Both L7Ae and 15.5K proteins recognize the K-turn motif, but only L7Ae can recognize the K-loop (Charron et al. 2004; Hamma and Ferre-D'Amare 2004; Moore et al. 2004; Cojocaru et al. 2005; Nolivos et al. 2005; Suryadi et al. 2005; Szewczak et al. 2005; Turner et al. 2005). Binding of L7Ae to the K-turn changes the conformation of the box C/D motif, and this altered RNA conformation is then recognized by aNop5p (Rashid et al. 2003). Structural probing of the sRNP complexes during assembly has shown that sRNA remodeling is critical for the assembly and functioning of these complexes (Gagnon et al. 2006). Although three archaeal core proteins assemble symmetrically at the two box motifs of sRNA, the complex formed at one of the two motifs may have a preference for modification (Rashid et al. 2003).
Intron-containing pre-tRNATrp of Haloferax volcanii and some other Euryarchaeota is unique in that both guide and target RNAs are present in the same molecule (Omer et al. 2000; Clouet d'Orval et al. 2001; Singh et al. 2004). The intron is a functional box C/D sRNA when present either as part of the pre-tRNA or as free excised RNA (in both linear and circular forms) (Salgia et al. 2003; Singh et al. 2004). It can guide in trans 2′-O-methylation of the two target residues positioned in the pre-tRNA's exons in a sequential manner (Singh et al. 2004). In the present work, we have structurally separated the guide and target features of the pre-tRNA from each other without affecting their respective functions. Using these RNAs we studied the temporal relationships of the RNA–protein and RNA–RNA interactions during sRNP assembly and the sequential 2′-O-methylations.
We show that L7Ae binds to both box C′/D′ and box C/D motifs, although the L7Ae concentrations needed to form stable complexes with these motifs are not the same. Interchanging the two motifs relative to the ends of the guide RNA does not affect this binding and functions of the sRNA. L7Ae binding sequentially exposes the respective guide sequences in the order in which the box motifs are occupied. Further binding of aNop5p and aFib somewhat masks the guide sequences that are exposed after L7Ae binding. The D-guide sequence of the incompletely assembled sRNP containing only L7Ae and aNop5p can pair with the target RNA, and this interaction appears to be stabilized by aFib binding. Asymmetric RNPs containing a single L7Ae at the intact box C/D motif are functional. Time course experiments reveal that D-guide–target interaction is initiated early to produce the first of two sequential methylations. After completion of box C/D-guided modification, target–guide pairing at this site is disrupted, and some rearrangement of proteins seems to occur.
RESULTS
Guide and target roles of H. volcanii pre-tRNATrp during box C/D RNP-mediated 2′-O-methylation can be separated
The C34 and U39 residues (mature tRNA positions) of the intron-containing H. volcanii pre-tRNATrp can be 2′-O-methylated when the pre-tRNA is incubated with Methanocaldococcus jannaschii box C/D core proteins and AdoMet (Singh et al. 2004). The pre-tRNA molecules function both as a guide and as a target, in trans, in these in vitro reactions. These two functions have been separated here by using two different derivatives of this pre-tRNA. Pre-tRNATrp intron (Fig. 1A) contains box C/D and C′/D′ motifs, and has previously been used as a guide RNA in 2′-O-methylation reactions (Singh et al. 2004). Pre-tRNATrpΔ67 (Fig. 1B) created here, as a target is a truncated version of pre-tRNATrp. It contains the two target sites, but not the box C/D and C′/D′ motifs required in the methylation reactions. Unlike the full-size pre-tRNATrp, the pre-tRNATrpΔ67 is unable to bind to the L7Ae protein (data not shown) and was not modified when incubated alone in the methylation reactions (Fig. 1D). However, the C34 and U39 residues of pre-tRNATrpΔ67 were 2′-O-methylated, when reactions were done in the presence of the pre-tRNATrp intron (Fig. 1E), although the amount and rate of U39 modification was less than that observed for the full-size pre-tRNA. These reactions are also sequential (data not shown) with C34 modification preceding U39 modification, as in the full-size pre-tRNATrp (Singh et al. 2004). Truncation in pre-tRNATrpΔ67 does not adversely affect the bulge-helix-bulge (BHB) motif, as the RNA was efficiently cleaved by the H. volcanii splicing endonuclease (Fig. 1C, lane 2).
FIGURE 1.
In vitro transcribed functional guide and target derivatives of H. volcanii pre-tRNATrp. Sequences and predicted secondary structures of the guide: pre-tRNATrp intron (A) and the target: pre-tRNATrpΔ67 (B) are shown. The C, D, C′, and D′ boxes are outlined in A and labeled, and guide residues (lowercase) are in white on black. GA sequences within the four boxes that have been changed to CC in mutant versions are in large letters. In B the tRNA anticodon sequence (CCA) is in large letters, target residues (C34 and U39) are in white on black, and exon–intron junctions, indicated by arrows, are located within the bulge–helix–bulge structure required for pre-tRNA splicing. The complementary guide and target sequences (in A and B, respectively) are indicated by thick (C/D motif) and thin (C′/D′ motif) lines. The complementary guide and target base pairs, g:C and a:U, are indicated by white in black squares (C/D motif) and black circles (C′/D′ motif), respectively. Radiolabeled pre-tRNATrpΔ67 was treated with H. volcanii splicing endonuclease. The products were resolved on 8% denaturing acrylamide gel (C). Lane 1: untreated RNA; lane 2: endonuclease treated RNA. (Note that the two exons and the intron, all being about the same size, are not resolved in lane 2.) TLC analyses of RNase T2-digested [α-32P]ATP-labeled pre-tRNATrpΔ67 that was either incubated alone (D) or with unlabeled pre-tRNATrp intron (E) in the methylation reactions as described in the Materials and Methods. Mononucleotide and dinucleotide products are designated. The appearance of radiolabeled CmCp and UmCp indicates methylation of C34 and U39, respectively.
L7Ae binds to two box motifs of the guide RNA and exposes corresponding guide sequences in a sequential manner
Electrophoretic mobility shift assays (EMSA) with pre-tRNATrp intron and increasing amounts of L7Ae protein showed the presence of two distinct RNP complexes (data not shown), indicating two L7Ae binding sites in this RNA. This is in agreement with other known cases of archaeal box C/D RNAs (Rashid et al. 2003; Tran et al. 2003).
To better understand the L7Ae binding and its effects on the structure of guide RNA, 3′ -end-labeled pre-tRNATrp intron alone and in complex with L7Ae was probed by lead (II)-induced cleavage. By this technique, regions of the RNA that are paired or protected by proteins show reduced cleavage, while unprotected single-stranded regions show increased cleavage. Very few chemical footprinting probes for RNA are available that are not base-specific, and can also detect formation or breakage of double-stranded regions produced in response to interaction with proteins but are beyond the regions protected by the bound protein. Representative lead (II)-cleavage patterns in the presence of varying amounts of the L7Ae protein and a fixed amount of WT intron and their graphic representations are shown in Figure 2B. (These cleavage patterns are repeatable and comparable.) Surprisingly, at lower concentrations of L7Ae only the C′ and D′ boxes were protected (Fig. 2B, lane 3 and plot iii), as shown by lead (II) undercutting. At higher L7Ae concentrations the C and D boxes were also protected (see protection of C box in Fig. 2B, lane 1 and plot i). The amount of lead (II)-induced cleavage, close to the labeled 3′-end of the RNA varies from one experiment to another, but the patterns of cleavage relative to the control in each experiment remain the same. Experiments with full-size pre-tRNATrp (where the D box is far from the labeled 3′ end) were also done to eliminate the possibility of lowered gel resolution near the ends, as the cause of the observed variability in the cleavage patterns. The footprint of full-size pre-tRNATrp in the presence of L7Ae showed a pattern similar to that of the intron; all boxes (including the D box) were protected, and guide sequences and the terminal loop were exposed (data not shown). Furthermore, as with the 3′-end-labeled intron, 5′-end-labeled intron (where the D box is far from the labeled end) also showed protection of the D box at higher L7Ae concentration (data not shown).
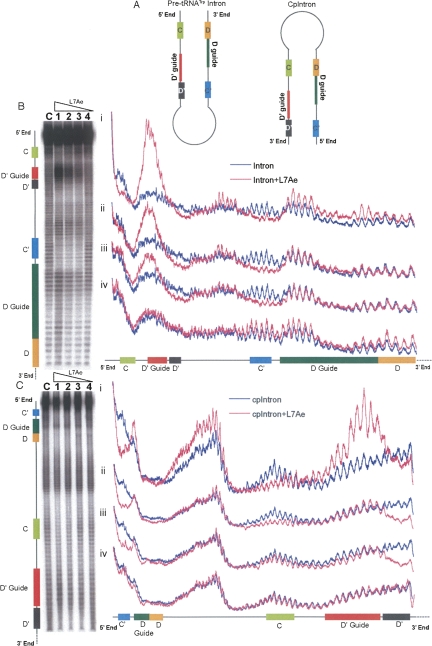
FIGURE 2.
Circular permutation of the two box motifs in the intron does not change their differential affinity to M. jannaschii L7Ae protein. Schematic representations of the pre-tRNATrp intron and CpIntron, its circularly permuted version, are shown in A. The positions of different box motifs and guide sequences are highlighted by colored blocks labeled C, D′ guide (for U39 modification), D′, C′, D guide (for C34 modification), and D that represent residues at positions 13–19, 27–34, 36–39, 64–70, 72–82, and 83–86, respectively, of the pre-tRNATrp intron as indicated in Figure 1A. Representative phosphorimager scans of denaturing 10% acrylamide gels showing footprints after lead (II)-induced cleavage of 3′-end labeled RNAs in the presence of increasing amounts of L7Ae protein are presented on the left in panel B for the pre-tRNATrp intron and in panel C for the CpIntron. Cleavage patterns of 3′ -end-labeled RNAs in the absence of L7Ae are shown in lane C of panels B and C. Lanes 1 through 4 show footprinting profiles of 3′ -end-labeled RNAs (final concentration 60 nM) in the presence of decreasing amounts of L7Ae protein (17 μM, 1.7 μM, 170 nM, and 17 nM). Lane profile analyses were done using ImageQuant software and are plotted at the right. Heights of the peaks represent the degree of lead (II)-induced cleavage of the RNA at that site. Plots i–iv are analyses of lanes 1–4 (pink lines), respectively, superimposed for comparison over lane C (dark blue line) of the same panel. Linear schematics indicate the specific regions of the RNA corresponding to the regions in the gel scans and peaks in the plots. The colored blocks correspond to those in panel A. (Note that the positions of the boxes and guide regions are reversed in the CpIntron relative to the pre-tRNATrp intron.)
L7Ae binding to the box motifs exposes the corresponding guide sequences (Fig. 2B). This effect is more apparent for the D′-guide (for U39 modification) region. Lead (II)-induced cleavage at this site increases with the increase in L7Ae concentration, and is greatly enhanced when the second L7Ae binds to the box C/D motif. This is because as the D-guide region opens up, the region at positions 21–27 (Fig. 1A), which pairs with a segment of this D guide, also becomes single stranded. This position 21–27 segment just precedes the D′ guide; thus, these two opened up segments showed a very high peak of lead (II)-induced cleavage (Fig. 2B, panel i). Formation and stabilization of the K-turn in the absence of protein has been shown to require divalent cations (Matsumura et al. 2003; Goody et al. 2004). However, increased lead (II)-induced cleavage of the D′-guide region in our studies does not appear to be due to an increase in the amount of lead (II) bound to the K-turn of box C′/D′ in the presence of L7Ae. Treatment of guide RNA with hydrazine followed by aniline cleavage (U-specific chemical reaction for the sequencing of RNAs) in the presence and absence of L7Ae showed the same effect (data not shown). Aniline cleavage of the D′-guide region (due to the presence of several Us) increases as the concentration of L7Ae is increased, as is the case for lead (II) cleavage (Fig. 2B). Opening of the D-guide region requires more L7Ae than that needed for the D′ guide. This may be due to the higher concentrations of L7Ae needed to either form a stable complex with the box C/D or break pairing between the D guide and the region at positions 21–27. These data suggest that as L7Ae binds to the two motifs sequentially, the corresponding guide sequences are also exposed sequentially. Binding of L7Ae also causes some exposure of the loop region between the D′ and C′ boxes. Overall, these data show that L7Ae binds the box C′/D′, exposing its D′-guide region more readily than the binding box C/D and exposing the D-guide region. These studies are consistent with our EMSA studies (not shown) where stable RNA-L7Ae complexes are formed with the box C/D mutant (where C′/D′ was intact) at much lower concentration of L7Ae than with the box C′/D′ mutant (with C/D intact). GA sequences of the box motifs involved in tandem sheared G:A pairs and L7Ae binding are replaced by CC sequences in these mutants.
Interchanging the terminal and internal locations of the two box motifs of the pre-tRNATrp intron does not change the patterns of L7Ae binding and methylation activity
CpIntron, a circularly permutated version of pre-tRNATrp intron, was created to check whether the internal positioning of the box C′/D′ motif is responsible for its apparent higher affinity toward L7Ae. This CpIntron (Fig. 2A) has the box C′/D′ motif near the termini and the box C/D motif positioned internally. Both WT pre-tRNATrp intron and CpIntron guide sequential modifications of C34 and U39 in the pre-tRNATrpΔ67 in the same manner (data not shown). This suggests that the positions of box motifs with respect to termini of the RNA have minimal or no effect in the archaeal dual-guide sRNA-mediated modifications.
Conformational changes for WT intron and CpIntron were similar after L7Ae binding (Fig. 2, cf. B and C). Box C′/D′ retained its higher affinity toward L7Ae even in CpIntron. Both WT intron and CpIntron showed similar protection of boxes and exposure of guide sequences upon binding of L7Ae. These results again suggest that the differences observed between cleavage patterns of box C/D and box C′/D′ in Figure 2B are not due to lowered resolution observed near the ends of the gel. A notable conformational change in CpIntron was the exposure of the new terminal loop between the C and D boxes at a high concentration of L7Ae (Fig. 2C, plot i).
Association of aNop5p and aFib during sRNP assembly somewhat masks the guide regions overexposed after L7Ae binding
The lead (II) cleavage profiles of the guide RNA incubated with the three core proteins and with L7Ae alone were significantly different (Fig. 3A). The two guide sequences that showed enhanced cleavage (overexposed) after L7Ae binding now show reduced cleavage or are somewhat masked, indicating some conformational change in the sRNA. As shown later, the guide sequences can still pair with the target RNA even after this apparent masking. Furthermore, the box motifs are more protected after binding of aNop5p and aFib. These data suggest that binding of these two proteins after L7Ae binding increases the size of the protein footprint on the guide RNA, mainly toward the D- and D′-guide regions.
FIGURE 3.
RNP assembly of WT and box mutant guide RNAs are different. Plots of lead (II)-induced cleavage of 3′ -end-labeled WT (A), and box C′/D′ (B) and C/D (C) mutant introns alone are superimposed over similar plots of RNPs assembled on these RNAs. Components present during the assembly of the RNPs are indicated in each panel. Plots and schematic representations are as for Figure 2. A dashed circle in C indicates the positions 21–27 of the guide RNA.
Asymmetric sRNP containing a single L7Ae at the intact box C/D motif is functional
As observed by others (Omer et al. 2002), we also could not observe modification of the pre-tRNATrpΔ67 or of full-size intron-containing pre-tRNATrp by the pre-tRNATrp intron even at high concentrations (28.5 μM each) of aNop5p and aFib, in the absence of L7Ae (data not shown). However, there is a reported observation of active archaeal box C/D complex formed at high concentrations of aNop5p and aFib but in the absence of L7Ae (Bortolin et al. 2003). To determine if L7Ae binding to both motifs is essential for functioning of the pre-tRNATrp intron sRNP, modifications guided by sRNP complexes reconstituted with decreasing amounts of L7Ae while keeping the other two protein concentrations constant, were assessed. As the concentration of L7Ae was lowered, the amount of modifications also decreased. Figure 4A is one such reaction where L7Ae was 0.1 μM (0.01× standard concentration). L7Ae alone at similar concentration binds only to the box C′/D′ motif (see Fig. 2B, plot iii). Surprisingly, the lead (II) cleavage pattern of the intron with 0.01× concentration of L7Ae and 1× concentration of the other two proteins was not different from that with 1× concentrations of all three proteins (data not shown). These lead (II) cleavage data and the activity patterns theoretically suggest that either asymmetric binding of only one L7Ae at box C′/D′ and other proteins at both motifs is sufficient for the activity of both sites of the WT intron or binding of aNop5p and aFib may facilitate the binding of second L7Ae to the box C/D motif at much lower L7Ae concentration, again making it a symmetric complex; the latter seems to be the case (see discussion).
FIGURE 4.
Methylation activity of RNPs assembled on WT and box mutant guide RNAs are not similar. TLC analyses of RNase T2-digested [α-32P]ATP-labeled pre-tRNATrpΔ67 after methylation by RNP containing wild-type or box mutant intron are shown in panels A–D. The guide RNAs and the concentrations of L7Ae used in the reactions are indicated in the panels. (Some extra spots appearing in panels B and C are due to incomplete RNase T2 digestion.) Concentrations of L7Ae are 0.01× in A, 0.1× in C, and 1× in B and D when compared to our standard (10 μM) methylation reactions. Concentrations of aNop5p and aFib were 1× (10 μM) in all of these reactions.
To further study this L7Ae concentration-dependent effect, box mutant introns that could bind L7Ae at only one site were used for the modification reactions at two different concentrations of L7Ae, keeping the other two proteins constant (at 1× concentration). The box C′/D′ mutant (where box C/D is WT) did guide some modification of both C34 and U39 at standard (Fig. 4B) but not at lower (Fig. 4C) concentrations of L7Ae. This is in contrast to the WT intron that modifies at much lower concentration of L7Ae (Fig. 4A). On the other hand, the box C/D mutant (box C′/D′ is WT) intron was unable to guide either C34 or U39 modification at a standard concentration of L7Ae (Fig. 4D). These data suggest that, as shown by others (Rashid et al. 2003; Tran et al. 2003), in our system too, at least one asymmetrically assembled box C/D RNP is functional, although at lower efficiency. The reason for this can be explained by sequential nature of the two modifications (see discussion).
Lead (II) cleavage patterns of the RNPs assembled with box mutant introns suggest that the conformations of these two mutant introns in the RNPs are different (Fig. 3B,C). As expected, the L7Ae binding to the mutant introns differs from the binding to the WT intron (Fig. 3A–C, pink lines) in that L7Ae mainly shows footprints on the box motif that is left intact in the mutants. In the box C′/D′ mutant (Fig. 3B) L7Ae mainly binds to the C box (and the D box near the 3′ end is also not exposed), while in the box C/D mutant (Fig. 3C) it binds to the C′ and D′ boxes. Binding of L7Ae exposes guide regions, but the patterns are somewhat different from the WT. This was also observed in L7Ae binding to the box C/D mutant intron (Fig. 3C, pink line). Here the D′-guide sequence was exposed but the sequence at positions 21–27 that pairs with the D-guide sequence was not exposed (Fig. 3C, the pink peak [circled] is more asymmetric due to less cleavage at positions 21–27, when compared to the corresponding peak for the WT intron in Fig. 3A). Lead (II) cleavage patterns in the presence of all three proteins indicate that the D′-guide region was not as exposed in the mutants as in the WT introns. These lead (II) cleavage and methylation data with box mutant introns suggest that a functional archaeal sRNP can have an asymmetric distribution of L7Ae.
The overall results of Figures 3 and 4 can be interpreted as the following for our dual-guide RNA (pre-tRNATrp intron). Although L7Ae seems to have a higher affinity for the box C′/D′ motif, binding of L7Ae to any one of the two motifs is sufficient to recruit aNop5p and aFib to both motifs. However, binding of L7Ae to the box C/D motif (along with aNop5p and aFib) is necessary and sufficient to make the sRNP functional in guiding methylation by both box C/D and box C′/D′ motifs.
Recruitment of target RNA can occur in the RNP-complex containing L7Ae and aNop5p and guide–target interactions are stabilized in the presence of aFib
We wanted to determine the stage of the sRNP assembly where stable guide–target interaction can occur. Therefore, the lead (II) cleavage pattern of the guide RNA was determined after coincubation of target RNA (unlabeled pre-tRNATrpΔ67) with the components of the sRNP complex. As shown before, binding of L7Ae to the two boxes of the guide RNA exposed the guide sequences (Figs. 2B,3A). However, these exposed guide sequences did not pair with the target in presence of L7Ae alone (Fig. 5A). Lead (II) cleavage plots for intron+L7Ae and intron+target+L7Ae in this figure are nearly superimposable. Identical plots were obtained for guide RNAs, with and without target but in the absence of L7Ae (data not shown).
FIGURE 5.
Guide–target interactions in presence of various core proteins. Plots of lead (II)-induced cleavage of 3′ -end-labeled pre-tRNATrp intron in various RNP complexes assembled in the presence and absence of unlabeled target (pre-tRNATrpΔ67) are compared. Components present during the assembly of the RNPs are indicated in each panel. D-guide (C34-guide) regions of the plots are enlarged in the insets. Plots and schematic representations are as for Figure 2. Dashed circles indicate the positions 21–27 of the guide RNA.
Lead (II) cleavage patterns of guide RNA in the presence of L7Ae and aNop5P with and without target were also nearly superimposable except in the D-guide region (Fig. 5B). The target suppresses cleavage in this region, suggesting some base pairing between the D guide and the corresponding target regions. This pairing is expected to occur first, i.e., before the D′-guide–target pairing, since in the sequential reactions, the D-guided C34 residue of the target RNA is modified before the D′-guided U39. Therefore, we infer that intermolecular pairing of target RNA with the D-guide region can start as soon as L7Ae and aNop5p have assembled in the RNP, and aNop5p may play a role in recruitment of target RNA as well as producing conformational changes in the guide RNA (see Discussion).
The patterns of lead (II) cleavage of the guide RNA in the presence of the target in the reactions containing aFib with L7Ae and aNop5p were similar to those without aFib, except that cleavage in the D-guide region was further suppressed and at positions 21–27 was somewhat enhanced (Fig. 5, cf. B and C). A small amount of 2′-O-methylation always occurs in our control reactions in which we do not add AdoMet. This suggested that recombinant aFib preparations contain some bound AdoMet. As such, the guide–target interaction observed in Figure 5C may reflect a combination of pre- and post-modification of the C34 residue. Therefore, to determine the effect of aFib on guide–target interaction before modification of C34, lead (II) cleavage reactions of guide RNA with and without target were done in the presence of the three core proteins and AdoHcy. AdoHcy is a general inhibitor of AdoMet-dependent methyltransferases. It did inhibit modification of C34 and U39 in our 2′-O-methylation reactions (not shown). The experiments with AdoHcy suggest that the target–guide interactions that occur in the presence of aNop5p are indeed enhanced by addition of aFib (Fig. 5B,D, cf. D guide and positions 21–27). These data suggest that probably aFib provides stability to the guide–target interactions after initial recruitment of target into sRNP by aNop5p.
Dynamic interactions of guide and target in the functioning sRNP complex dictate sequential 2′-O-methylations
We analyzed the conformational changes in the guide RNA (pre-tRNATrp intron) in the sRNP complexes carrying out 2′-O-methylation reactions in the presence of the target (pre-tRNATrpΔ67) and AdoMet over a time course of 2 h (Fig. 6). We hypothesized that lead (II) cleavage patterns would reflect the conformation of the guide RNA at various stages of the reaction and after completion of reaction. Saturation would occur first for the D-guided C34 modification. The controls for these reactions were done in the presence of AdoHcy to inhibit any initial modification due to the AdoMet present in aFib preparations. The results showed that the C, C′, and D′ boxes remained protected throughout this time course, but the D guide and D box gradually became exposed (Fig. 6). This gradual exposure of the D-guide region and extreme exposure of the D box at the 2-h time point was consistently observed in four independent experiments. These time course reactions suggest that after modification of C34 (D guided), the target is removed from the D-guide region and some rearrangement (or removal) of proteins occurs at the D box. However, even at 2-h time point we see only slight change in the cleavage pattern of the D′-guide region (Fig. 6), which is consistent with very little U39 modification by this time.
FIGURE 6.
Conformational changes in guide RNA during sequential methylation reaction. Standard 2′-O-methylation reactions were done with 3′ -end-labeled pre-tRNATrp intron for 5 min (A), 15 min (B), 30 min (C), 60 min (D), and 120 min (E). Plots of the lead (II)-induced cleavage of the intron in the sRNP complex at the end of the reaction are presented in these panels. The plot for the control reaction (orange line) was generated by incubating intron and target with three core proteins and AdoHcy (instead of AdoMet) for 15 min at 68°C. Circles indicate the D-guide regions. TLC analyses of the RNase T2 digested products of the methylation reactions for the same time points that contained [α-32P]ATP-labeled pre-tRNATrpΔ67 are presented as panels i–v next to A–E. Plots and schematic representations are as for Figure 2 and the TLC analyses are as for Figure 1.
DISCUSSION
In this work, we have established an in vitro 2′-O-methylation system after separating the guide and target components of the intron containing H. volcanii pre-tRNATrp onto separate RNA molecules, while maintaining their respective properties including sequential nature of modifications.
Generally, halophilic proteins have a high proportion of acidic amino acid residues and most of the halophilic enzymes are inactivated when Na+ or K+ concentrations in the solution decrease to <2M (Madern et al. 2000), making it difficult to work with recombinant proteins of halophiles. L7Ae and aFib proteins of H. volcanii and of thermophiles used by different workers are similar. H. volcanii aNop5p is somewhat similar to Archaeoglobus fulgidus aNop5p, which has also been used in studies with pre-tRNATrp (Rashid et al. 2003). Both aNop5p have a shorter coiled-coil domain and lack certain C-terminal residues, when compared to aNop5p of M. jannaschii, Sulfolobus solfataricus, and Pyrococcus abyssi used by others. Functional assays at high temperatures using recombinant proteins of thermophiles and at 37°C using H. volcanii cell extracts have provided comparable results (Omer et al. 2002; Bortolin et al. 2003; Rashid et al. 2003; Tran et al. 2003; Singh et al. 2004).
L7Ae induced structural rearrangements in sRNAs
L7Ae is the first protein that binds to the box C/D-guide RNA during RNP assembly. Unlike its eukaryal homolog 15.5K protein, one copy each of L7Ae has been proposed to bind symmetrically to each of the two box (C/D and C′/D′) motifs in Archaea. These motifs can form K-turn (or K-loop) structures, which are the binding sites for L7Ae. We show that L7Ae binds box C′/D′ exposing its D′-guide region more readily than the binding box C/D and exposing the D-guide region. Our analyses indicate that L7Ae forms a stable complex with the box C′/D′ motif at lower concentration than what is needed for the box C/D motif. This pattern of L7Ae binding and conformational changes in the RNA is not due to the internal location of box C′/D′ within the guide RNA as observed by analyses of CpIntron (see Fig. 2). Similar patterns of L7Ae binding and methylation activity were also observed with the sR1 guide RNA of Sulfolobus acidocaldarius and its circular permuted version that was prepared like our CpIntron (Omer et al. 2006). Even in the eukaryal system, preferential binding of the human 15.5K protein to the terminal box C/D of the Xenopus U25 snoRNA was not changed by circularly permuting the RNA (Szewczak et al. 2005). The similarity between pre-tRNATrp intron and its circularly permuted version was not unexpected, since the intron is normally spliced out as circular RNA and can be detected inside the cells (Salgia et al. 2003), and both linear and circular versions of this intron can guide 2′-O-methylation (Singh et al. 2004). Gel-shift analyses have indicated that only one L7Ae binds to archaeal box C/D-guide RNAs at a lower protein:RNA ratio (Rashid et al. 2003; Tran et al. 2003; Gagnon et al. 2006). However, it has been difficult to specifically assign this asymmetric binding of L7Ae to the box C/D or box C′/D′ motif. Lead (II) cleavage data presented here suggest that L7Ae binds to the box C′/D′ motif at low concentrations. However, it should be noted that in the pre-tRNATrp intron, the sequences of the C′ and D′ boxes (UUGACGA and CUGA, respectively) are more like the consensus sequences (RUGAUGA and CUGA) than those of the C and D boxes (GGGACGA and CCGA). Furthermore, in this guide RNA the box C′/D′ motif shows a K-turn with stem I of eight base pairs instead of the more common K-loop. A database search of the archaeal sRNAs has shown that some other sRNAs can also form similar structures.
Binding of L7Ae to a box motif exposes the adjacent guide sequence (Fig. 2B). This exposure of the two guide sequences occurs in sequential manner, i.e., the D′ guide opens up before the D guide. Note that the order in which these sequences are exposed, i.e., D′ guide first, is the reverse of the order in which methylation occurs, which is D-guided first. We believe that the reason for the absence of correlation between the orders of these two events is that the L7Ae induces structural changes in the sRNA to make it flexible/dynamic, such that stable guide–target pairing cannot be sustained. Both L7Ae and aNop5p are needed to detect the initial guide–target pairing in our system.
sRNP assembly and target recruitment
The two asymmetrically assembled sRNPs that contain the three core proteins and one or other box mutant intron are not functionally equivalent. The box C/D mutation containing sRNPs are not functional, but the ones with the box C′/D′ mutation are functional. Others have also shown that mutations of box C/D, but not of C′/D′, make archaeal dual guide RNAs nonfunctional (Bortolin et al. 2003; Rashid et al. 2003; Tran et al. 2003). This can be explained by the sequential nature of the 2′-O-methylations, where box C/D-guided modification of C34 (hence, the presence of the intact box C/D) is required before box C′/D′-guided modification of U39 can occur (Singh et al. 2004). Sequential modification can be observed specifically in our system because the two targets are present in the same RNA, not in two different RNAs (oligonucleotides). Although asymmetric archaeal RNPs can be formed with only one L7Ae in our system, a functional asymmetric RNP requires L7Ae to be present at the box C/D motif. Modification of both C34 and U39, albeit less efficiently, can occur with this functional asymmetric RNP. Even though a second L7Ae at the box C′/D′ motif is not needed, its presence does increase the efficiency of modification. Our functional box C′/D′ mutant guide containing sRNP (where L7Ae is present at the box C/D) is somewhat similar to the asymmetric eukaryal box C/D snoRNP (Cahill et al. 2002; Szewczak et al. 2002).
Binding of aNop5p and aFib after that of L7Ae creates two large footprints on the guide RNA. These footprints not only cover the box motifs but also extend over the guide sequences that are exposed by L7Ae binding. These changes may position aNop5p and aFib in correct orientation for the initiation of 2′-O-methylation reaction. Both of these footprints are observed even if only one L7Ae can bind to the guide RNA, as is the case for box mutant guide RNAs (see Figs. 3B,C). This suggests that binding of only one L7Ae to any one of the two box motifs is sufficient to recruit aNop5p and aFib proteins to both box motifs of the pre-tRNATrp intron. Furthermore, it is also possible that incorporation of aNop5p-aFib in the assembling sRNP facilitates the stable binding of second L7Ae to the box C/D motif at much lower concentrations than those required in their absence. The results that suggest this are: (a) normally L7Ae alone forms a stable complex with the box C/D motif at much higher concentrations than what is needed for the box C′/D′ motif as observed by lead (II) cleavage (Fig. 2B) and gel-shift analyses using box mutant guide RNAs (data not shown), (b) a functional asymmetric sRNP (containing aNop5P-aFib) requires a high concentration of L7Ae and intact box C/D (but not intact box C′/D′) (Fig. 4B), while (c) a functional symmetric sRNP (containing aNop5P-aFib) requires very low concentration of L7Ae (Fig. 4A).
Studies of guide–target interactions in the presence of core proteins provided some unexpected results. Although binding of L7Ae to the guide RNA exposed both D- and D′-guide sequences, these sequences could not form stable pairs with the target sequences in the presence of L7Ae. However, lead (II) cleavage of the D-guide sequence was suppressed in the presence of target RNA when both aNop5p and L7Ae were present. This suggested that the D-guide sequence of the L7Ae and aNop5p-containing complex could pair with the corresponding target sequence. No such pairing was observed with the D′-guide sequence, which is consistent with the sequential nature of the D-guided reaction occurring before the D′-guided reaction. This also suggested that aNop5p plays a crucial role in recruitment of the target RNA. Indeed, 4-thiouridine containing target RNAs has been shown to UV cross-link to aNop5p (Appel and Maxwell 2007; K. Gagnon and E.S. Maxwell, unpubl.). The first of the two guide–target interactions may possibly occur before complete assembly of the guide sRNP, i.e., before addition of aFib, in case aNop5p and aFib are not recruited together as a heterodimer. Furthermore, initial pairing of only the D guide with the target in the presence of aNop5p and its further stabilization by aFib in our system is consistent with the results of others, which suggest that only one active site is productively used at a time in the wild-type complex (Hardin and Batey 2006).
Dynamic guide–target interactions modulate structure and function of sRNP
Lead (II) cleavage of the guide RNAs going through the 2′-O-methylation reactions provided another interesting result. As the D-guided modification of C34 reached completion, the D-box and D-guide sequences became exposed. Perhaps D-guide exposure is due to the separation of the target region after modification of the target residue (C34). Exposure of D box can be the result of either removal or rearrangement of some proteins from this position after completion of the reaction. These changes may be required for an alternate conformation of the complex needed for the modification of U39. Recently it has been shown that alternate conformations of the aNop5p–aFib complex can exist in two different organisms (Oruganti et al. 2007). It has been proposed that these conformational differences reflect structural flexibility in aNop5p that would exist in complexes of any one organism as well.
The D-guide–target interaction occurring before addition of aFib, and the exposure of D-box and D-guide sequences after completion of the D-guided methylation, have implications for the turnover of box C/D-guide RNP. Possibly (at least some), components of the RNP disassemble after the 2′-O-methylation reaction(s) and a new functional RNP is assembled for the next reaction. The newly assembled RNP may contain some components of the old RNP. This phenomenon is not unusual. Similar cases of newly assembled functional macromolecules have been observed, for example, ribosomes and spliceosomes. Furthermore, target RNA may also play a role in this turnover. It has been observed that in the sRNA-guided 2′-O-methylation system, when the length of the target is increased, the turnover of the sRNP is also increased (Appel and Maxwell 2007).
Based on our results, a model for the assembly of a sequential 2′-O-methylating archaeal box C/D RNP can be proposed (Fig. 7). Initially, one L7Ae binds to the box C′/D′ motif overexposing the corresponding guide region. This is followed by sequential binding (box in Fig. 7) of the second L7Ae to the box C/D motif, overexposing both D- and D′-guide regions as well as the loop region connecting the C′ and D′ boxes. Binding of L7Ae facilitates the sequential binding of aNop5p and aFib. (Alternatively, aNop5p and aFib may bind as heterodimers. In principle, the second L7Ae can also bind to the box C/D motif after binding of aNop5p-aFib.) Binding of aNop5p–aFib changes the conformation of the sRNA to make it more ordered (especially in the guide regions), as well as recruits the target RNA. Initially, the D-guide region pairs with the corresponding target region. In vitro, this pairing can occur before binding of aFib, but may also occur after. The time of this pairing relative to aFib association may be unique to each guide–target pair. Binding of aNop5p, aFib, and the target RNA also induces some change in the loop between the C′ and D′ boxes, masking some of the distortions caused by L7Ae binding (shadowed in Fig. 7). After the methylation reaction, pairing between the D-guide–target region is disrupted (probably in vitro by high temperature and in vivo by a helicase) and a new D′-guide–target pairing would occur. At this time at least some of the core proteins at the box C/D motif are either removed or are shifted in position (question mark in Fig. 7). This model would suggest that for the guide RNA to participate in the second set of methylation reactions, either a new RNP is assembled or a significant rearrangement of the proteins occurs within the existing RNP.
FIGURE 7.
A model for the assembly of a functional archaeal dual–guide box C/D RNP complex. The arrows indicate a stepwise assembly of a functional RNP and its interaction with the two target sites containing RNA. The two alternate steps indicate that the interaction of target RNA may occur either before or after incorporation of aFib in the RNP. The clear box containing the shaded arrow indicates sequential stable binding of L7Ae to the second site (box C/D motif). The shaded box suggests unspecified changes in the region of RNA between the D′ and C′ boxes. The question mark suggests that proteins may or may not be present at the D-guide region at this stage.
MATERIAL AND METHODS
Site-directed mutagenesis
Mutations in H. volcanii pre-tRNATrp gene containing the plasmid pVT9P11 (Salgia et al. 2003) were introduced using the Quickchange site-directed mutagenesis kit from Stratagene using appropriately designed primers. GA54CC/GA124CC and GA77CC/GA105CC substitutions produced double box C/D and C′/D′ mutants, respectively (see Fig. 1A for the mutation sites). Deletion of residues from pre-tRNATrp positions 52–118 (Singh et al. 2004) produced pre-tRNATrpΔ67 (Fig. 1B).
DNA template construction and in vitro transcription
PCR-amplified templates were used for in vitro transcription. Primer pair 1 (5′-TAATACGACTCACTATAGGGGCTGTGGCCAAGC-3′) and 2 (5′-TGGGGCCGGAGGGATTTGAAC-3′) with the mutant pVT9P11 plasmid carrying the pre-tRNATrpΔ67 gene was used to produce a template for target RNA transcription. Templates for all pre-tRNATrp introns (WT or mutants) were produced by using primer pair 3 (5′-TAATACGACTCACTATAGGCTTGGCGCCCGGGA-3′) and 4 (5′-ATCTCCGGTGGGCACCT-3′) with pVT9P11 or the above-mentioned mutant gene-carrying plasmids. A typical 100 μL transcription reaction performed at 37°C for 3–4 h contained 40 mM Tris-Cl, pH 7.9, 6 mM MgCl2, 10 mM DTT (dithiothreitol), 2 mM spermidine, 20 μCi of [α-32P]ATP (MP Biomedicals, sp. act. 3000 Ci/mmol), unlabeled rNTPs (0.6 mM of ATP and 1.0 mM of each of the other nucleotides), varying amounts of PCR amplified DNA, and about 50 units of T7 RNA polymerase; 1 mM of each NTP was used to produce unlabeled transcripts. Transcripts were purified and quantitated as described before (Singh et al. 2004).
Splicing reactions and circular permutation of pre-tRNATrp intron
Splicing reactions to produce purified circular pre-tRNATrp intron and other products were done as described before (Zofallova et al. 2000; Salgia et al. 2003). The circular intron was used as a template in reverse transcriptase (RT) reactions using MMLV RT (Promega) and a reverse primer (5′-CGGAATTCAGTCGGTCGCTCAGTATATCAGC-3′). The cDNA obtained was used as a template in the PCR reaction with the reverse primer and a forward primer (5′-CCATCGATCATCGGTCGTGTTGACG-3′). This RT-PCR product was digested by EcoRI and ClaI and cloned into the pBluescript KS+ vector. This construct and primer pairs 5 (5′-TAATACGACTCACTATAGATCATCGGTCGTGTTGACG-3′) and 6 (5′-AGTCGGTCGCTCAGTATATCAGC-3′) were used to produce a template for transcription of CpIntron (circularly permuted intron).
In vitro 2′-O-methylation reactions and detection of modified nucleotides
Recombinant M. jannaschii L7Ae, aNop5p, and aFib proteins were prepared as described before (Tran et al. 2003; Singh et al. 2004). In vitro modification reactions were done in a 20 μL volume in the presence of 0.4 pmol appropriate unlabeled guide RNA, 0.9 pmol [α-32P]ATP labeled pre-tRNATrpΔ67 RNA, 0.05 mM AdoMet, and three recombinant core proteins (10 μM each under standard methylation conditions) in a buffer containing 20 mM Na-HEPES at pH 7.0, 150 mM NaCl, 0.75 mM DTT, 1.5 mM MgCl2, 0.1 mM EDTA and 10% glycerol. Concentrations of aNop5p and aFib were increased to 28.5 μM for modification assays in the absence of L7Ae. Modification results were similar whether or not the guide RNAs used in these reactions were heated and cooled first. After incubation at 68°C for 2 h (except in time course experiments), the RNA was extracted by phenol:chloroform and ethanol precipitated. Modification inhibition reactions were done at 68°C for 15 min in the presence of 0.84 mM AdoHcy. The RNA samples were digested with RNase T2, resolved by two-dimensional thin layer chromatography (TLC), and phosphorimaged as described before (Singh et al. 2004).
Footprinting and analyses of RNP complexes
For these reactions, 3′-end-labeled WT or specific mutant pre-tRNATrp intron was heated in water at 85°C for 3 min and gradually cooled to room temperature. Using 2 pmol of this RNA, a 36 μL reaction was carried out at 68°C for 15 min in 20 mM Na-HEPES, pH 7.0, 150 mM NaCl, 0.75 mM DTT, 1.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol in the presence of 1 μg of Escherichia coli tRNA, and 17 μM (under standard footprinting conditions) of one or more recombinant proteins. Typically, 20 pmol of unlabeled pre-tRNATrpΔ67 were added to the reaction for footprinting done in the presence of target RNA. AdoMet (0.1 mM) or AdoHcy (0.42 mM) was also included, as needed. Reactions were snap chilled on ice. Then 4 μL of freshly prepared lead acetate (120 mM) were added and the reactions were carried out at room temperature for 10 min. The reactions were stopped by addition of 5 μL of 0.5 M EDTA followed by phenol:choloroform extraction and ethanol precipitation of RNA. In time course experiments, samples were taken out at specific time points and treated with lead acetate as described above. U-specific sequencing reactions were done by previously published protocols using hydrazine followed by aniline treatment (Peattie 1979; Gupta 1984). RNA fragments were resolved on 10% denaturing acrylamide gels, and radioactivity was detected by a PhosphorImager. Lane analyses of footprint gels were done by ImageQuant software. Signal values from the lane analyses were exported to Microsoft Excel and linear plots were generated after normalizing the signals (Dang et al. 2006). Correlation of bands in the footprint profiles with specific positions in the RNAs was done by aligning the bands of lead (II)-induced cleavage with alkaline hydrolysis (for uniform cleavage at each residue) and U-specific sequencing reactions (not shown). 3′-Exon derived from splicing endonuclease treatment of 3′-end-labeled pre-tRNATrp was used as a size marker in these gels. The accuracy of the correlation was within one base because of the 3′-end heterogeneity of T7 RNA polymerase run-off transcripts. Two major 3′-end-labeled products differing in size by one base are produced after any cleavage of these transcripts.
ACKNOWLEDGMENTS
We thank E. Stuart Maxwell, Keith Gagnon, and David Clark for helpful discussions and review of the manuscript. This work was supported by National Institutes of Health Grant GM55045 to R.G.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1003308.
REFERENCES
- Appel C.D., Maxwell E.S. Structural features of the guide:target RNA duplex required for archaeal box C/D sRNA-guided nucleotide 2′-O-methylation. RNA. 2007;13:899–911. doi: 10.1261/rna.517307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.L., Youssef O.A., Chastkofsky M.I., Dy D.A., Terns R.M., Terns M.P. RNA-guided RNA modification: Functional organization of the archaeal H/ACA RNP. Genes & Dev. 2005;19:1238–1248. doi: 10.1101/gad.1309605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolin M.L., Bachellerie J.P., Clouet-d'Orval B. In vitro RNP assembly and methylation guide activity of an unusual box C/D RNA, cis-acting archaeal pre-tRNATrp . Nucleic Acids Res. 2003;31:6524–6535. doi: 10.1093/nar/gkg860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill N.M., Friend K., Speckmann W., Li Z.H., Terns R.M., Terns M.P., Steitz J.A. Site-specific cross-linking analyses reveal an asymmetric protein distribution for a box C/D snoRNP. EMBO J. 2002;21:3816–3828. doi: 10.1093/emboj/cdf376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier B., Muller S., Branlant C. Reconstitution of archaeal H/ACA small ribonucleoprotein complexes active in pseudouridylation. Nucleic Acids Res. 2005;33:3133–3144. doi: 10.1093/nar/gki630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron C., Manival X., Clery A., Senty-Segault V., Charpentier B., Marmier-Gourrier N., Branlant C., Aubry A. The archaeal sRNA binding protein L7Ae has a 3D structure very similar to that of its eukaryal counterpart while having a broader RNA-binding specificity. J. Mol. Biol. 2004;342:757–773. doi: 10.1016/j.jmb.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Clouet d'Orval B., Bortolin M.L., Gaspin C., Bachellerie J.P. Box C/D RNA guides for the ribose methylation of archaeal tRNAs. The tRNATrp intron guides the formation of two ribose-methylated nucleosides in the mature tRNATrp . Nucleic Acids Res. 2001;29:4518–4529. doi: 10.1093/nar/29.22.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru V., Nottrott S., Klement R., Jovin T.M. The snRNP 15.5K protein folds its cognate K-turn RNA: A combined theoretical and biochemical study. RNA. 2005;11:197–209. doi: 10.1261/rna.7149605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W., Kagalwala M.N., Bartholomew B. Regulation of ISW2 by concerted action of histone H4 tail and extranucleosomal DNA. Mol. Cell. Biol. 2006;26:7388–7396. doi: 10.1128/MCB.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatur W.A., Fournier M.J. RNA-guided nucleotide modification of ribosomal and other RNAs. J. Biol. Chem. 2003;278:695–698. doi: 10.1074/jbc.R200023200. [DOI] [PubMed] [Google Scholar]
- Dennis P.P., Omer A. Small noncoding RNAs in Archaea. Curr. Opin. Microbiol. 2005;8:685–694. doi: 10.1016/j.mib.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Pogacic V. Biogenesis of small nucleolar ribonucleoproteins. Curr. Opin. Cell Biol. 2002;14:319–327. doi: 10.1016/s0955-0674(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Gagnon K., Zhang X., Agris P., Maxwell E.S. Assembly of the archaeal box C/D sRNP can occur via alternative pathways and requires temperature-facilitated sRNA remodeling. J. Mol. Biol. 2006;362:1025–1042. doi: 10.1016/j.jmb.2006.07.091. [DOI] [PubMed] [Google Scholar]
- Galardi S., Fatica A., Bachi A., Scaloni A., Presutti C., Bozzoni I. Purified box C/D snoRNPs are able to reproduce site-specific 2′-O-methylation of target RNA in vitro. Mol. Cell. Biol. 2002;22:6663–6668. doi: 10.1128/MCB.22.19.6663-6668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspin C., Cavaille J., Erauso G., Bachellerie J.P. Archaeal homologs of eukaryotic methylation guide small nucleolar RNAs: Lessons from the Pyrococcus genomes. J. Mol. Biol. 2000;297:895–906. doi: 10.1006/jmbi.2000.3593. [DOI] [PubMed] [Google Scholar]
- Goody T., Melcher S., Norman D., Lilley D. The kink-turn motif in RNA is dimorphic, and metal ion dependent. RNA. 2004;10:254–264. doi: 10.1261/rna.5176604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J. Biol. Chem. 1984;259:9461–9471. [PubMed] [Google Scholar]
- Hamma T., Ferre-D'Amare A.R. Structure of protein L7Ae bound to a K-turn derived from an archaeal box H/ACA sRNA at 1.8 Å resolution. Structure. 2004;12:893–903. doi: 10.1016/j.str.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Hardin J.W., Batey R.T. The bipartite architecture of the sRNA in an archaeal box C/D complex is a primary determinant of specificity. Nucleic Acids Res. 2006;34:5039–5051. doi: 10.1093/nar/gkl644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henras A.K., Dez C., Henry Y. RNA structure and function in C/D and H/ACA s(no)RNPs. Curr. Opin. Struct. Biol. 2004;14:335–343. doi: 10.1016/j.sbi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Kiss T. Small nucleolar RNAs: An abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109:145–148. doi: 10.1016/s0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- Klein D.J., Schmeing T.M., Moore P.B., Steitz T.A. The kink-turn: A new RNA secondary structure motif. EMBO J. 2001;20:4214–4221. doi: 10.1093/emboj/20.15.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madern D., Ebel C., Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4:91–98. doi: 10.1007/s007920050142. [DOI] [PubMed] [Google Scholar]
- Matsumura S., Ikawa Y., Inoue T. Biochemical characterization of the kink-turn RNA motif. Nucleic Acids Res. 2003;31:5544–5551. doi: 10.1093/nar/gkg760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell E.S., Fournier M.J. The small nucleolar RNAs. Annu. Rev. Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- Meier U.T. How a single protein complex accommodates many different H/ACA RNAs. Trends Biochem. Sci. 2006;31:311–315. doi: 10.1016/j.tibs.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T., Zhang Y., Fenley M.O., Li H. Molecular basis of box C/D RNA-protein interactions; Cocrystal structure of archaeal L7Ae and a box C/D RNA. Structure. 2004;12:807–818. doi: 10.1016/j.str.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Nolivos S., Carpousis A.J., Clouet-d'Orval B. The K-loop, a general feature of the Pyrococcus C/D guide RNAs, is an RNA structural motif related to the K-turn. Nucleic Acids Res. 2005;33:6507–6514. doi: 10.1093/nar/gki962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer A.D., Lowe T.M., Russell A.G., Ebhardt H., Eddy S.R., Dennis P.P. Homologs of small nucleolar RNAs in Archaea. Science. 2000;288:517–522. doi: 10.1126/science.288.5465.517. [DOI] [PubMed] [Google Scholar]
- Omer A.D., Ziesche S., Ebhardt H., Dennis P.P. In vitro reconstitution and activity of a C/D box methylation guide ribonucleoprotein complex. Proc. Natl. Acad. Sci. 2002;99:5289–5294. doi: 10.1073/pnas.082101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer A., Zago M., Chang A., Dennis P. Probing the structure and function of an archaeal C/D-box methylation guide sRNA. RNA. 2006;12:1708–1720. doi: 10.1261/rna.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oruganti S., Zhang Y., Li H., Robinson H., Terns M.P., Terns R.M., Yang W., Li H. Alternative conformations of the archaeal Nop56/58-fibrillarin complex imply flexibility in box C/D RNPs. J. Mol. Biol. 2007;371:1141–1150. doi: 10.1016/j.jmb.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Peattie D.A. Direct chemical method for sequencing RNA. Proc. Natl. Acad. Sci. 1979;76:1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid R., Aittaleb M., Chen Q., Spiegel K., Demeler B., Li H. Functional requirement for symmetric assembly of archaeal box C/D small ribonucleoprotein particles. J. Mol. Biol. 2003;333:295–306. doi: 10.1016/j.jmb.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Reichow S.L., Hamma T., Ferre-D'Amare A.R., Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35:1452–1464. doi: 10.1093/nar/gkl1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia S.R., Singh S.K., Gurha P., Gupta R. Two reactions of Haloferax volcanii RNA splicing enzymes: Joining of exons and circularization of introns. RNA. 2003;9:319–330. doi: 10.1261/rna.2118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Gurha P., Tran E.J., Maxwell E.S., Gupta R. Sequential 2′-O-methylation of archaeal pre-tRNATrp nucleotides is guided by the intron-encoded but trans-acting box C/D ribonucleoprotein of pre-tRNA. J. Biol. Chem. 2004;279:47661–47671. doi: 10.1074/jbc.M408868200. [DOI] [PubMed] [Google Scholar]
- Suryadi J., Tran E.J., Maxwell E.S., Brown B.A., 2nd The crystal structure of the Methanocaldococcus jannaschii multifunctional L7Ae RNA-binding protein reveals an induced-fit interaction with the box C/D RNAs. Biochemistry. 2005;44:9657–9672. doi: 10.1021/bi050568q. [DOI] [PubMed] [Google Scholar]
- Szewczak L.B., DeGregorio S.J., Strobel S.A., Steitz J.A. Exclusive interaction of the 15.5 kDa protein with the terminal box C/D motif of a methylation guide snoRNP. Chem. Biol. 2002;9:1095–1107. doi: 10.1016/s1074-5521(02)00239-9. [DOI] [PubMed] [Google Scholar]
- Szewczak L.B., Gabrielsen J.S., Degregorio S.J., Strobel S.A., Steitz J.A. Molecular basis for RNA kink-turn recognition by the h15.5K small RNP protein. RNA. 2005;11:1407–1419. doi: 10.1261/rna.2830905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T.H., Polacek N., Zywicki M., Huber H., Brugger K., Garrett R., Bachellerie J.P., Huttenhofer A. Identification of novel noncoding RNAs as potential antisense regulators in the archaeon Sulfolobus solfataricus . Mol. Microbiol. 2005;55:469–481. doi: 10.1111/j.1365-2958.2004.04428.x. [DOI] [PubMed] [Google Scholar]
- Tran E.J., Zhang X., Maxwell E.S. Efficient RNA 2′-O-methylation requires juxtaposed and symmetrically assembled archaeal box C/D and C′/D′ RNPs. EMBO J. 2003;22:3930–3940. doi: 10.1093/emboj/cdg368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E., Brown J., Maxwell E.S. Evolutionary origins of the RNA-guided nucleotide-modification complexes: From the primitive translation apparatus? Trends Biochem. Sci. 2004;29:343–350. doi: 10.1016/j.tibs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Turner B., Melcher S.E., Wilson T.J., Norman D.G., Lilley D.M. Induced fit of RNA on binding the L7Ae protein to the kink-turn motif. RNA. 2005;11:1192–1200. doi: 10.1261/rna.2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins N.J., Segault V., Charpentier B., Nottrott S., Fabrizio P., Bachi A., Wilm M., Rosbash M., Branlant C., Luhrmann R. A common core RNP structure shared between the small nucleoar box C/D RNPs and the spliceosomal U4 snRNP. Cell. 2000;103:457–466. doi: 10.1016/s0092-8674(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Yu Y.T., Terns R.M., Terns M.P. Mechanisms and functions of RNA-guided RNA modification. In: Grosjean H., editor. Fine-tuning of RNA functions by modification and editing. Springer; New York: 2005. pp. 223–262. [Google Scholar]
- Zofallova L., Guo Y., Gupta R. Junction phosphate is derived from the precursor in the tRNA spliced by the archaeon Haloferax volcanii cell extract. RNA. 2000;6:1019–1030. doi: 10.1017/s1355838200000613. [DOI] [PMC free article] [PubMed] [Google Scholar]