Abstract
The proline-rich designer antibacterial peptide dimer A3-APO is currently under preclinical development for the treatment of systemic infections caused by antibiotic-resistant Gram-negative bacteria. The peptide showed remarkable stability in 25% mouse serum in vitro, exhibiting a half-life of ∼100 min as documented by reversed-phase chromatography. Indeed, after a 30-min incubation period in undiluted mouse serum ex vivo, mass spectrometry failed to identify any degradation product. The peptide was still a major peak in full blood ex vivo, however, with degradation products present corresponding to amino-terminal cleavage. When injected into mice intravenously, very little, if any unmodified peptide could be detected after 30 min. Nevertheless, the major early metabolite, a full single-chain fragment, was detectable until 90 min, and this fragment exhibited equal or slightly better activity in the broth microdilution antimicrobial assay against a panel of resistant Enterobactericeae strains. The Chex1-Arg20 metabolite, when administered three times at 20 mg/kg to mice infected with a sublethal dose (over LD50) of an extended spectrum β-lactamase-producing Escherichia coli strain, completely sterilized the mouse blood, similar to imipenem added at a higher dose. The longer and presumably more immunogenic prodrug A3-APO, injected subcutaneously twice over a 3-wk period, did not induce any antibody production, indicating the suitability of this peptide or its active metabolite for clinical development.
Keywords: degradation, efficacy, Gram-negative bacteria, immunogenicity, metabolite, pharmacokinetics, resistant infection, serum
Antimicrobial peptides have historically been a primary focus of peptide research (Hancock and Scott 2000; Brogden 2005). Their size makes them amenable to detailed structure–activity studies, and the measurement of their cellular efficacy is relatively simple, even if the assay conditions have to be adjusted to fit peptide antibiotics (Cudic et al. 2002). Naturally, the most active derivates were considered viable alternatives to small molecules in antimicrobial drug therapy (Zasloff 2002). Resistance induction is rarely seen with peptide-based antimicrobials compared with traditional antibiotics (Ge et al. 1999), but their parenteral use is occasionally hampered by inadequate safety margins and frequently by rapid clearance, leaving them suitable only for topical applications (Bush et al. 2004). Recently, we introduced a designer proline-rich peptide dimer, A3-APO, which kills bacteria by a dual mode of action, and thus is able to kill multidrug-resistant clinical isolates of Enterobactericeae in vitro in concentrations acceptable for clinical development (Otvos Jr. et al. 2005). A3-APO, one of the most potent peptide antibiotics to date, appears to combine the positive features of nontoxic membrane-active antibacterial peptides (Chen et al. 2005) and those acting on intracellular targets (Cudic and Otvos Jr. 2002). The bacterial target of A3-APO, similar to many other native proline-rich antimicrobial peptides, is the C-terminal D-E helix of the 70-kDa bacterial heat-shock protein DnaK (Kragol et al. 2001; Otvos Jr. et al. 2005; Bikker et al. 2006).
The first steps for progressing from in vitro efficacy measurements to in vivo evaluation of activity and ensuing clinical development are the assessments of peptide stability in vitro and pharmacokinetics in vivo. Serum stability is considered the most important secondary screening assay in drug discovery because it can identify peptides that are unstable in body fluids, and thus will fail in the development process (Powell et al. 1993). Pharmacokinetics can identify a 1-h time period, needed for full bacterial killing of the proline-rich peptides with intracellular targets (Cudic et al. 1999), when the peptide concentration in serum exceeds 130% of the in vitro minimal inhibitory concentration (MIC) value, a minimal dose required for in vivo efficacy (Bush et al. 2004). However, as we show here, the extent of the decomposition of peptide A3-APO gradually increases from in vitro to ex vivo and in vivo in murine models, with different metabolites appearing in different blood preparations. On one hand, the data presented here identifies a significantly larger dose of A3-APO required for in vivo efficacy studies than would be calculated from the in vitro stability data. On the other hand, our findings generally question the validity of serum stability measurements to assess clinical suitability of peptides, at least for peptide antibiotics. Fortunately, the major in vivo metabolite of A3-APO effectively kills bacteria in vitro and protects mice from sublethal infection in vivo. In addition, the prodrug exhibits no immunogenic properties, allowing further clinical development.
Results and Discussion
Degradation kinetics in diluted serum
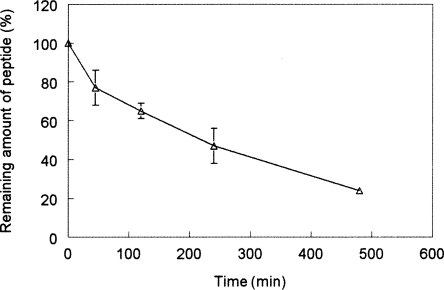
The stability of peptide A3-APO was first studied in commercially available pooled mouse serum diluted to 25% with distilled water. It has been shown that dilution of serum linearly reduces the degradation rate without changing any other proteolytic parameters (Powell et al. 1992). The reduced degradation rate makes the collection and workup of the parallels more manageable for ensuing reversed-phase chromatographic analysis of peptide content. Peptide A3-APO was remarkably stable in diluted mouse serum, exhibiting a half-life of ∼230 min (Fig. 1). Calculating back to undiluted serum, this indicated that within the first hour almost half of the original peptide amount remained intact. With a safe margin of 16 μg/mL MIC value, 1.8 mL total mouse blood volume and 20 g of mouse weight, our therapeutic dose should be no more than 100 μg/mouse or 5 mg/kg. Accordingly, all ensuing stability, pharmacokinetics, and immunization studies were done at 6 μg/100 μL in vitro or ex vivo or 5 mg/kg in vivo peptide doses.
Figure 1.
Degradation of peptide A3-APO in 25% pooled mouse serum. The peptide was incubated with the serum for different time periods and the remaining peptide amounts were determined by reversed-phase high-performance liquid chromatography.
Metabolic stability in full serum in vitro and blood ex vivo
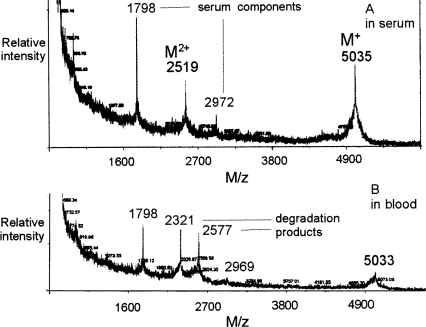
The next question we asked was how the peptide degrades in blood-derived medium. A3-APO was added to the undiluted mouse serum preparation and was incubated in full serum for 30 min. Then, the serum proteins were precipitated and the serum was submitted for mass spectroscopic analysis. The only peptide-related peaks that could be identified corresponded to the unmodified peptide at 5035 M/z, the double-charged species at 2519 M/z, and two serum components at 1798 and 2972 M/z (Fig. 2A). In our experience with peptide stability studies, these peaks always present themselves in murine blood products, regardless of whether test peptides are added or not. So far, we have not identified the source of these MS peaks. With this technology, we could not identify any of the early degradation products either. According to the kinetic analysis as presented in Figure 1, ∼35% of the peptide had to be degraded after the 30-min examination period. Most probably none of the early degradation products were stable enough to be detected by mass spectroscopy. In any event, it was clear that peptide A3-APO was unusually stable in mouse serum.
Figure 2.
Stability of peptide A3-APO in blood preparations. The peptide was incubated with commercially available 100% pooled mouse serum (A) or full blood retrieved from CD-1 mice locally (B) for 30 min. After removing cells (B) and plasma proteins (A,B), the blood preparations were submitted to matrix-assisted laser desorption/ionization flight-of-time mass spectroscopy analysis.
A completely different picture emerged when the peptide was added to mouse blood ex vivo. We bled healthy CD-1 mice from the eye, from where the cleanest blood can be extracted, and added peptide A3-APO immediately after the blood was retrieved. After a 30-min incubation period, the cells and the plasma proteins were removed and the mass spectroscopy was repeated. In this case the unmodified peptide with an M/z of 5033 was still present, but in significantly lower quantities (Fig. 2B). The double-charged ion could not be detected. Present were the two serum components at 1798 and 2969 M/z and two new peaks at 2321 and 2577 M/z. These latter peptide species corresponded to stable early degradation products. The molecular weight (MW) of the N-terminal Chex1-Val19 fragment is 2318 Da, while the single-chain Chex1-Dab21 fragment has a MW of exactly 2577 Da (Fig. 3). Peptide cleavage around the C-terminal arginines and prolines did not seem surprising, as these residues are the major degradation sites of pyrrhocoricin and drosocin, the native ancestors of the designer peptide A3-APO (Hoffmann et al. 1999). Although the peak heights in mass spectroscopy are not linearly dependent upon the load of the analytes, Figure 2, panel B, undoubtedly suggests that the quantities of degradation products well exceed those of the intact peptide. The degradation rate of peptide A3-APO appeared to significantly increase as we went from in vitro serum to ex vivo blood preparations. It is possible that the peptide is cleaved by proteases residing in different cells of blood, such as the thimet oligopeptidase that preferentially cleaves peptides between valine, leucine, or other hydrophobic amino acids and positively charged residues (Dando et al. 1993), the exact sequence features of our ex vivo observed degradation products. These enzymes, of course, are not significantly present in the serum and plasma preparations used during the in vitro stability studies.
Figure 3.
A3-APO fragments detected in various blood preparations. Chex stands for 1-cyclohexane-carboxylic acid, and the C-terminal scaffold Dab stands for 2,4-diamino-butyric acid. It was not determined which amino group of Dab was substituted in the 2577 M/z cleavage product.
Pharmacokinetics in vivo
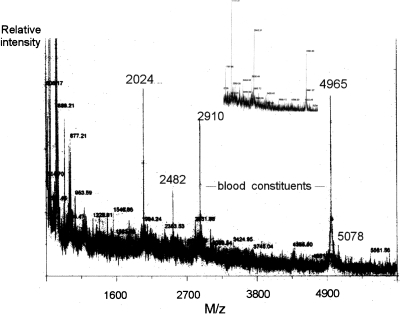
To complete the analysis of A3-APO stability, we injected the peptide into mice intravenously, and blood was taken before peptide inoculation and at 0, 5, 15, 30, 90, 120, and 240 min from three mice at each time point. The blood was worked up identically to that described for the ex vivo assay. RP-HPLC failed to identify unmodified A3-APO peptide in any of the samples, suggesting that the peptide completely decomposed in vivo, or was present only below our detection level of ∼0.5 μg in 50 μL of blood (<10% of the injected amount). By MS without peptide present, two peaks could be identified at 2910 and 4965 M/z. The double-charged peaks of these species could not be detected. Figure 4 shows the mass spectrum taken at 30 min after peptide administration. The peak at 4965 M/z could also be seen during the ex vivo assay, albeit at lower quantities (cf. with Fig. 2B). Interestingly, the 2910 M/z blood component is new for the PK assay and the 2969 M/z peak detected in serum and blood is no longer visible. The 1798 M/z blood component appeared at a later time point, at 90 min (Fig. 4, inset). At 30 min, the MS peak representing the unmodified peptide A3-APO was absent from the spectrum, unless the minor peak at 5078 M/z corresponded to the potassium adduct (calculated MW: 5074 Da). With all likelihood, the peptide indeed almost completely decomposed in vivo, confirming the chromatography data. Two new major peptide-derived peaks could be observed at 2024 and 2482 M/z (Figs. 3, 4). These were already present 5 min after peptide inoculation, and their quantities increased by the 30-min mark. By 90 min, the quantity of the 2024 M/z peak further increased, and that of the 2482 M/z decreased relative to the two blood-derived components (Fig. 4, inset). The 5078 M/z peak no longer was observable at 90 min, suggesting that it indeed corresponded to unmodified peptide A3-APO. None of the new A3-APO-derived peaks were observable at 120 or 240 min. Actually, in these mass spectra, only the blood components at 1798 (dominant) and 2910 (minor) were visible. Because the peptide was designed to pass cellular membranes, bacterial and eukaryotic alike, most probably the cellular shuffling made it available for any intracellular protease to degrade.
Figure 4.
Processing of peptide A3-APO in mice. The peptide was injected into CD-1 mice intravenously, and after 30 min, blood was taken, the cells and plasma proteins were removed, and the peptide was submitted to matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy analysis. The main spectrum shows a representative sample selected from three parallels. The inset shows the blood content 90 min after peptide administration. In the inset, the main peaks correspond to blood-derived components at 1798, 2910, and 4965 M/z, and the shorter peptide degradation product at 2024 M/z. The in vivo active peptide metabolite at 2482 M/z is still clearly visible.
A careful examination of the A3-APO peptide-derived peaks once again identified degradation products expectable from the metabolic pathways of native pyrrhocoricin and drosocin. The calculated MW of the single-chain Chex1-Arg20 fragment was 2475 Da, which corresponded to the 2482 M/z peak within the experimental error of mass spectrometry in biological media, emphasizing the C-terminal instability of the dimer. The 2024 M/z product likely corresponded to the Arg7-Arg(-1) fragment (calculated mass 2014 Da). In addition to the cleavage site around the C-terminal arginines, major cleavage sites for both pyrrhocoricin and drosocin are located five to seven residues from the amino termini (Hoffmann et al. 1999). In spite of the sequence optimization process that resulted in improved cell penetrating and antimicrobial activity (Otvos Jr. et al. 2005), the conserved architecture of the proline-rich antimicrobial peptides apparently retained the major proteolytic degradation sites.
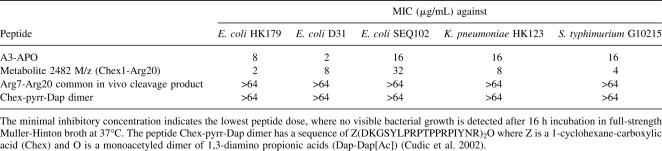
These data would suggest that A3-APO will be inactive in systemic protection studies in vivo, unless the major metabolite(s), which is present in blood for at least 90 min, is active in the in vitro antimicrobial assay. To investigate this possibility, we synthesized the Chex1-Arg20 single-chain unit and the Arg7-Arg20 common cleavage fragment of the two metabolites and studied their ability to kill Enterobactericeae strains in undiluted Muller-Hinton broth, a true measure of potential clinical viability (Otvos Jr. et al. 2005). As Table 1 shows, the single-chain complete peptide was overall twice as potent as A3-APO in killing resistant bacteria. The measured minimal bactericidal concentration (MBC) values were 2–32 μg/mL, 8 μg/mL, and 4 μg/mL against Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium isolates with IC50 values between 100 and 500 nM. Thus, the peptide is expected to be active in bacteremia models of E. coli, K. pneumoniae, and S. typhimurium infections, and the effective dose is equal to or better than the 10–20 mg/kg calculated for A3-APO. The shorter Arg7-Arg20 fragment exhibited MIC values >64 μg/mL against the test strains, supporting the previous findings regarding the importance of the N-terminal four to six residue fragments (Hoffmann et al. 1999). The negative control first generation pyrrhocoricin dimer (Chex-pyrr-Dap dimer) remained without significant activity in full-strength Muller-Hinton broth as expected (Cudic et al. 2003).
Table 1.
Activity of antimicrobial peptides against multidrug resistant clinical isolates
When the ex vivo blood stability studies were repeated on the Chex1-Arg20 in vivo metabolite, the peptide was almost completely stable within the entire 120-min examination period. The only degradation product that could be detected, at about one-third of the peak height of the Chex1-Arg20 starting material, was at 2321 M/z, representing a fragment with the C-terminal arginine missing. Apparently, this cleavage product is the Chex1-Val19 fragment we observed for peptide A3-APO when the stability studies were done in blood ex vivo (cf. with Figs. 2B, 3). Taken together, the Chex1-Arg20 single-chain fragment represents the major degradation product in blood preparations and is stable in the biological environment, where peptide A3-APO has to exert its activity against infections caused by resistant enteric bacteria.
In vivo efficacy of the major metabolite
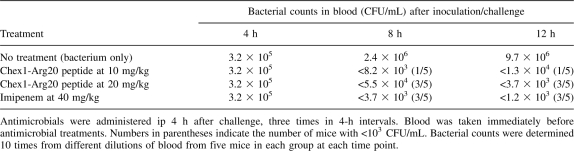
To ultimately prove that the Chex1-Arg20 fragment is the real in vivo metabolite of the prodrug A3-APO, and that it is suitable for the eradication of resistant Enterobactericeae, we performed in vivo efficacy studies on an animal model of systemic infections. As peptide drugs, including antimicrobial peptides, are predominantly cleared through the kidneys (Klootwijk et al. 1997) and the kidney functions of mice are 20-fold faster than those of humans, we pretreated the mice with 18 mg/kg cisplatin. This exercise reduces the kidney clearance rate of mice to the levels found in humans (Mathe et al. 2006). Blood bacterial counts amounted to 105 CFU/mL (CFU: colony-forming units) by 4 h after challenge and exceeded 107 CFU/mL after 12 h in untreated mice (Table 2). The Chex1-Arg20 peptide reduced the bacterial counts in a dose- and time-dependent manner. While at 10 mg/kg, the bacterial counts were reduced by approximately three log10 units, with only one mouse showing no bacteria at all after two doses of 20 mg/kg peptide at 3.5 log10 units; bacterial count reduction was observed, with 60% of the mice having blood bacterial levels below our detection limits. This efficacy level is similar to that produced by an identical imipenem treatment at 40 mg/kg doses. It needs to be mentioned that due to the arbitrary, more than zero bacterial counts assigned to mice with bacteria below the 1000 CFU/mL detection limit and to single outlier mice with high CFU counts, the bacterial loads were only approximate, and in reality, were lower than the numerical values given in Table 2 (indicated by the < sign).
Table 2.
Effect of the antimicrobial peptide metabolite on blood bacterial counts of mice pretreated with 18 mg/kg cisplatin for 3 d and challenged intraperitoneally with 1 × 108 CFU/g of extended spectrum β-lactamase-producing E. coli 5770 strain
A gross necropsy of two to three surviving mice per group 3 d post-infection showed no signs of toxicity at either peptide dose. The skin was smooth, and healthy liver, lungs, spleen, and kidneys were registered. No signs of internal bleeding (hemolysis), a frequent side reaction of antimicrobial peptides in systemic use, were detected. The favorable pharmacokinetic parameters together with the excellent in vivo efficacy data suggest that the Chex1-Arg20 metabolite will be suitable for ensuing preclinical development against systemic infections caused by strains of Enterobactericeae.
Immunogenicity
According to the observations above, peptide A3-APO or the Chex1-Arg20 derivative are ready for controlled preclinical development unless they show immunogenic properties. Antibody production is always a concern for not only antimicrobial peptides, but any peptide therapeutic. In our previous experience, we did not find native proline-rich peptides immunogenic at all. To isolate the precursor protein with the help of an anti-peptide monoclonal antibody, we repeatedly immunized mice with the 82-mer peptide diptericin, but all of our attempts failed to generate antibodies (Cudic et al. 1999). In the current study, peptide A3-APO was inoculated subcutaneously at 5 mg/kg twice in a 3-wk interval, and 4 wk after the second immunization the blood was analyzed for antibody production. Neither IgG1, IgG2a, IgG3, IgM, nor IgE presence was observed by enzyme-linked immunosorbent assay (ELISA) (ELISA readings between 0.09 and 0.13 AUFS units). The only antibody type with elevated levels in the post-immune sera was IgG2b (ELISA reading of 0.21 AUFS compared with 0.12 AUFS in the pre-immune sera), but this IgG variant is known to unspecifically bind antigens well above any other immunoglobulin subclass (Harley et al. 2002). In fact, all positive controls except IgG3 and IgE showed absorbance values over 0.23 AUFS (0.23–0.62). Therefore, peptide A3-APO, just like its family member diptericin, remained without any immunogenic activities.
Materials and Methods
Peptides
The design, synthesis, and analysis of peptide A3-APO and control Chex-pyrr-Dap dimer were described earlier (Cudic et al. 2002; Otvos Jr. et al. 2005). Chex1-Arg20 and Arg7-Arg20 were made on a CEM Liberty automated synthesizer with standard Fmoc chemistry. The peptides were detached from the resin while still in the microwave chamber, and were purified by RP-HPLC. MALDI-MS verified the accuracy of the sequences and their high purity.
Antibacterial assay
Antibacterial growth inhibition assays were performed using sterile 96-well polypropylene plates (Nunc F96 microtiter plates) in a final volume of 100 μL as described previously (Cudic et al. 2002). The cell concentrations were estimated by measuring the ultraviolet absorbance at 600 nm and applying the formula CFU/mL = A600(3.8 × 108), where CFU is the number of colony-forming units. Briefly, 50 μL of a suspension of mid-logarithmic phase bacterial cultures diluted to 5 × 105 CFU/mL in Muller-Hinton broth (MHB) were added to 50 μL of serially diluted peptides also dissolved in MHB. The final peptide concentration was 128 μg/mL. Cultures were then incubated at 37°C, 5% CO2 for 16–20 h without shaking, and growth inhibition was measured by recording the absorbance at 600 nm using a microplate reader. MBCs were identified as the lowest antimicrobial doses when the 600 nm absorbance did not exceed that of the negative control medium-only values.
In vitro and ex vivo stability studies
(1) Peptide stability in diluted mouse serum. Ninety microliters of pooled sterile mouse serum (Equitech-Bio) were added to 10 μL of peptide A3-APO dissolved in distilled water at 1.28 mg/mL concentration. After incubation periods of 0, 15, 30, 60 or 90, 120, and 240 min at 37°C, 20 μL of 15% trichloracetic acid (TCA) were added, the mixture stored at 4°C for 20 min, and centrifuged at 13,000 rpm. The supernatant was submitted to a determination of the remaining amount of unmodified A3-APO peptide by MS and RP-HPLC. (2) Peptide stability ex vivo. Female CD-1 mice of 8 wk were retro-orbitally bled and 6 μg of peptide, dissolved in 10 μL of phosphate-buffered saline (PBS), were immediately added to ∼100 μL retrieved blood. After 30 min incubation at 37°C, the cells were removed by 10 min centrifugation at 2000 rpm, 50 μL PBS were added to 50 μL of the plasma, followed by addition of 45 μL of 15% TCA. The rest of the analysis was done as described for the serum stability. Control blood was treated identically without peptide addition.
In vivo pharmacokinetics (PK) and immunization
One-hundred micrograms of peptide A3-APO (5 mg/kg) dissolved in 200 μL of sterile PBS (pH 7.2) were injected either intravenously to the tail vein (PK) or subcutaneously (dorsally, around the shoulder blade, immunization) into healthy CD-1 mice (Charles River Laboratories) using three mice for each time point. For the PK studies, about 100 μL of blood was taken from the eye at 0 (right after peptide administration), 5, 15, 30, 90, 120, and 240 min. Each animal was used only at two time points. Cells were centrifuged and 20 μL of 15% TCA were added per 100 μL of plasma. After repeated centrifugation, 10 μL of supernatant were loaded to both a Voyager DE matrix-assisted laser ionization/desorption time-of-flight (MALDI-TOF) mass spectrometer (Applied Biosystems) and a C18 narrowbore HPLC column (2.1 mm/20 cm) that had previously been calibrated with 10, 30, and 100 ng of peptide A3-APO dissolved in PBS. Absorbance was measured at 214 nm. For the immunization studies, the mice were dosed with the peptides once again 3 wk after the first inoculation, and were bled 4 wk later. After removal of the cells as described above, an ELISA kit (BioRad, complete with a rat-anti-mouse IgE from BD Biosciences) was used for the identification of the presence of peptide-specific IgG1, IgG2a, IgG2b, IgG3, IgM, and IgE antibodies. The immunoglobulin levels were compared with those found in the blood of the same animals prior to peptide inoculation.
In vivo efficacy studies
CD-1 female mice of 15–20 g were pretreated with 18 mg/kg cisplatin for 3 d to impair kidney clearance similar to the levels observed in humans before they were challenged intraperitoneally (ip) with 108 CFU per/g mouse of an extended-spectrum β-lactamase producing an E. coli strain (designation 5770). Four, 8, and 12 h after challenge 10 or 20 mg/kg peptide Chex1-Arg20 or 40 mg/kg imipenem were administered ip to 10 mice in each group. Prior to drug administration at all three time points, blood was taken from the tail vein of five mice for determining blood bacterial counts.
Conclusions
While serum stability in vitro is the most widely used assay for predicting in vivo stability for peptide drug candidates, for the proline-rich antimicrobial peptide dimer we investigated, it gave misleading results. Peptide degradation was not noticeably visible in serum, but became prominent in blood ex vivo. Pharmacokinetics almost completely failed to identify the presence of the original peptide at any time after drug administration. Nevertheless, an active in vivo metabolite exhibiting equal or slightly better antimicrobial properties than A3-APO itself could be easily identified by mass spectroscopy, and its blood level could provide suggestions for the active dose in animal models. Indeed, at 20 mg/kg, the Chex1-Arg20 metabolite sterilized the mouse blood after a lethal E. coli infection. Peptide A3-APO lacked immunogenic properties when injected subcutaneously, and this finding may stem from the fast proteolytic degradation in mice. As shorter peptides are less immunogenic than long ones, the truncated, but active primary metabolite may be the best lead molecule for antimicrobial drug development.
Acknowledgments
This work was supported by the Sbarro Health Research Organization and grant OTKA T46186 from the Hungarian Department of Health (to F.R.).
Footnotes
Reprint requests to: Laszlo Otvos Jr., Temple University, 1900 North 12th Street, Philadelphia, PA 19122, USA; e-mail: Otvos@temple.edu; fax: (215) 204-4021.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.034330.108.
References
- Bikker, F.J., Kaman-van Zanten, W.E., de Vries-van der Ruit, A.M., Voskamp-Visser, I., van Hooft, P.A., Mars-Groenendijk, R.H., de Visser, P.C., Noort, D. Evaluation of the antibacterial spectrum of drosocin analogues. Chem. Biol. Drug Des. 2006;68:148–153. doi: 10.1111/j.1747-0285.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Bush, K., Macielag, M., Weidner-Wells, M. Taking inventory: Antibacterial agents currently at or beyond phase I. Curr. Opin. Microbiol. 2004;7:466–476. doi: 10.1016/j.mib.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Mant, C.T., Farmer, S.W., Hancock, R.E., Vasil, M.L., Hodges, R.S. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005;280:12316–12329. doi: 10.1074/jbc.M413406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudic, M., Otvos L., Jr Intracellular targets of antibacterial peptides. Curr. Drug Targets. 2002;3:101–106. doi: 10.2174/1389450024605445. [DOI] [PubMed] [Google Scholar]
- Cudic, M., Bulet, P., Hoffmann, R., Craik, D.J., Otvos L., Jr Chemical synthesis, antibacterial activity and conformation of diptericin, an 82-mer peptide originally isolated from insects. Eur. J. Biochem. 1999;266:559–565. doi: 10.1046/j.1432-1327.1999.00894.x. [DOI] [PubMed] [Google Scholar]
- Cudic, M., Condie, B.A., Weiner, D.J., Lysenko, E.S., Xiang, Z.Q., Insug, O., Bulet, P., Otvos L., Jr Development of novel antibacterial peptides that kill resistant clinical isolates. Peptides. 2002;23:271–283. doi: 10.1016/s0196-9781(02)00244-9. [DOI] [PubMed] [Google Scholar]
- Cudic, M., Lockatell, V., Johnson, D.E., Otvos L., Jr In vitro and in vivo activity of an antibacterial peptide analog against uropathogens. Peptides. 2003;24:807–820. doi: 10.1016/s0196-9781(03)00172-4. [DOI] [PubMed] [Google Scholar]
- Dando, P.M., Brown, M.A., Barret, A.J. Human thimet oligopeptidase. Biochem. J. 1993;294:451–457. doi: 10.1042/bj2940451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, Y., MacDonald, D.L., Holroyd, K.J., Thornsberry, C., Wexler, H., Zasloff, M. In vitro properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 1999;43:782–788. doi: 10.1128/aac.43.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, R.E., Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, R., Gruffydd-Jones, T.J., Day, M.J. Non-specific labeling of mast cells in feline oral mucosa–a potential problem in immunohistochemical studies. J. Comp. Pathol. 2002;127:228–231. doi: 10.1053/jcpa.2002.0583. [DOI] [PubMed] [Google Scholar]
- Hoffmann, R., Bulet, P., Urge, L., Otvos L., Jr Range of activity and metabolic stability of synthetic antibacterial glycopeptides from insects. Biochim. Biophys. Acta. 1999;1426:459–467. doi: 10.1016/s0304-4165(98)00169-x. [DOI] [PubMed] [Google Scholar]
- Klootwijk, W., Sleddens-Linkels, E., de Boer, R., Jansen, C.A., Autar, R., de Herder, W.W., Boeve, E.R., Visser, T.J., de Greef, W.J. Renal clearance of the thyrotropin-releasing hormone-like peptide pyroglutamyl-glutamyl-prolineamide in humans. J. Clin. Endocrinol. Metab. 1997;82:3068–3073. doi: 10.1210/jcem.82.9.4219. [DOI] [PubMed] [Google Scholar]
- Kragol, G., Lovas, S., Varadi, G., Condie, B.A., Hoffmann, R., Otvos L., Jr The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry. 2001;40:3016–3026. doi: 10.1021/bi002656a. [DOI] [PubMed] [Google Scholar]
- Mathe, A., Kimka, K., Forczig, M., Szabo, D., Anderlik, P., Rozgonyi, F. The effect of different doses of cisplatin on the pharmacokinetic parameters of cefepime in mice. Lab. Anim. 2006;40:296–300. doi: 10.1258/002367706777611514. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr, Wade, J.D., Lin, F., Condie, B.A., Hanrieder, J., Hoffmann, R. Designer antibacterial peptides kill fluoroquinolone-resistant clinical isolates. J. Med. Chem. 2005;48:5349–5359. doi: 10.1021/jm050347i. [DOI] [PubMed] [Google Scholar]
- Powell, M.F., Grey, H., Gaeta, F., Sette, A., Colon, S. Peptide stability in drug development: A comparison of peptide reactivity in different biological media. J. Pharm. Sci. 1992;81:731–735. doi: 10.1002/jps.2600810802. [DOI] [PubMed] [Google Scholar]
- Powell, M.F., Stewart, T., Otvos L., Jr, Urge, L., Gaeta, F.C.A., Sette, A., Arrhenius, T., Thomson, D., Soda, K., Colon, S.M. Peptide stability in drug development. II. Effect of single amino acid substitution and glycosylation on peptide reactivity in human serum. Pharm. Res. 1993;10:1268–1273. doi: 10.1023/a:1018953309913. [DOI] [PubMed] [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]