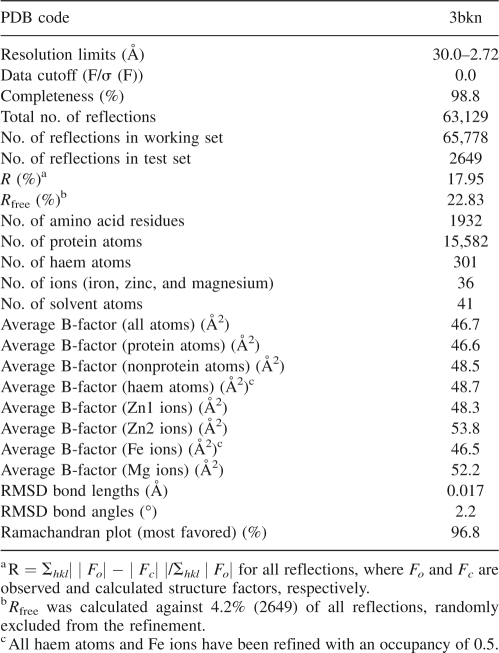
Abstract
Bacterioferritins, also known as cytochrome b 1, are oligomeric iron-storage proteins consisting of 24 identical amino acid chains, which form spherical particles consisting of 24 subunits and exhibiting 432 point-group symmetry. They contain one haem b molecule at the interface between two subunits and a di-nuclear metal binding center. The X-ray structure of bacterioferritin from Mycobacterium smegmatis (Ms-Bfr) was determined to a resolution of 2.7 Å in the monoclinic space group C2. The asymmetric unit of the crystals contains 12 protein molecules: five dimers and two half-dimers located along the crystallographic twofold axis. Unexpectedly, the di-nuclear metal binding center contains zinc ions instead of the typically observed iron ions in other bacterioferritins.
Keywords: bacterioferritin, Mycobacterium smegmatis, di-metal site, iron storage, haem
Iron is an essential element for the growth of all organisms. In the living world, iron exists as Fe(II) (ferrous) and Fe(III) (ferric) ions. Its main functions are to act as a catalyst in electron transport processes and to store and transport elemental oxygen. Despite the fact that iron is needed its presence may also cause undesired effects inside living cells. Just to serve as an example, Fe(II) can activate dioxygen and generate highly reactive hydroxyl radicals (Pryor et al. 2006). Under physiological conditions, Fe(III) exhibits very low solubility. Mammals evolved to deal with this by developing mechanisms of controlling the reversible transition between the hydrated Fe(II) in solution and the solid Fe(III) form (Theil 2001). The proteins responsible for this control are called ferritins, and apart from their main function of storing iron, they play a role in the detoxification of iron and dioxygen (Theil 2001). Due to the presence of ferritins, little or no free Fe(II) or Fe(III) exists within the cells.
After infecting their host, pathogenic bacteria must acquire iron from the host. Iron metabolism is therefore considered to play a central role in virulence, and a well-functioning mechanism to scavenge iron is a prerequisite for microbial pathogenicity. Bacteria developed a whole arsenal of tools to extract iron from their host. They utilize ferritins, but in addition they also express bacterioferritins (Bfrs, also known as cyt b 1, cyt b 557, cyt b 557.5, or cyt b 559) in order to make this process more efficient. As a matter of fact, one of the most abundant proteins present in in vivo grown Mycbacterium leprae is a Bfr (Hunter et al. 1990; Pessolani et al. 1994), suggesting an important role for this protein. It is also important to mention here that Bfrs are frequently used for the serological diagnosis, for example the one from Mycbacterium paratuberculosis, also known as antigen D, which can be used for the diagnosis of paratuberculosis in sheep (Brooks et al. 1988; Sugden et al. 1991). Antibodies to this protein were detected in high frequency in sera from animals with paratuberculosis. Additionally, the major membrane protein II (MMP-II) from M. leprae was identified as one of the antigenic cell membrane proteins (Maeda et al. 2005) and recognized as being identical to mycobacterial Bfr (Pessolani et al. 1994).
It is generally believed that both ferritins and Bfrs uptake Fe(II) into their di-nuclear ferroxidase center. There, the oxidation of Fe(II) by O2 takes place yielding H2O2 in the case of ferritins or H2O in the case of Bfrs (Bou-Abdallah et al. 2002). After oxidation, Fe(III) is transported to the inside of the protein shell, which has a diameter of ∼80 Å, and which may house up to 4500 Fe(III) ions (Harrison and Arosio 1996; Chasteen 1998). The core of mammalian ferritins contains a small amount of phosphate, and Fe(III) is stored mostly as ferrihydrite (Wade et al. 1993; Chasteen and Harrison 1999). In Bfrs, the level of phosphate is significantly higher, and Fe(III) is stored as an amorphous hydrate iron phosphate (Rohrer et al. 1990; Wade et al. 1993). Typically, ferritins are oligomeric, globular proteins consisting of 24 amino acid chains, which assemble into spherical particles displaying 432 point-group symmetry. However, there are also dodecameric ferritins, such as the one from Listeria innocua which exhibits 23 point-group symmetry (Ilari et al. 2000). Vertebrate ferritins are comprised of two different chains, the light (L) and the heavy (H) ones with molecular weights of 19 kDa and 21 kDa, respectively. In plants and bacteria, however, they only consist of the H type chain. The di-nuclear ferroxidase center is well conserved among all species; it is located within each subunit of a bacterial ferritin and each heavy subunit of the mammalian protein. This di-nuclear metal-binding site links together the four nearly parallel α-helices of the subunit and typically contains two iron ions. Each iron is ligated by one histidine and one glutamic acid, and in addition the two iron ions are bridged by two carboxylate groups of glutamic acid side chains. Bfrs contain both a di-nuclear iron center and a haem b prosthetic group. The single haem group is symmetrically bound between two subunits and its iron ion is bis-methionine ligated by Met52 (Mycbacterium smegmatis numbering). Bfr mutants with Met52 replaced by any other amino acid are haem free (Andrews et al. 1995). The role of haem in Bfrs is not clear, but it may be involved in mediating iron-core reduction and iron release from protein (Richards et al. 1996; Andrews 1998; Chasteen 1998). The tertiary and quaternary structures of Bfrs are very similar to those of ferritins, although their primary structures are rather dissimilar. To date, Escherichia coli is the only organism for which X-ray structures of both ferritin Ec-FtnA (PDB ID 1eum) (Stillman et al. 2001), and bacterioferritin Ec-Bfr (PDB IDs 1bcf) (Dautant et al. 1998), (2htn) (van Eerde et al. 2006), (1bfr) (Frolow et al. 1994) have been determined. A superposition of single subunits of Ec-FtnA and Ec-Bfr (chains A) yields an RMSD of 1.9 Å for 147 superimposed Cα pairs. When comparing the dimers AB of both proteins, the RMSD is 2.4 Å for 304 superimposed Cα pairs, and the whole 24-mer displays an RMSD of 2.5 Å for 1353 superimposed Cα pairs. The available structural information for Bfrs is not quite as rich as the one for ferritins. Three-dimensional structures of Bfrs from only four different sources have been determined: Bfr from Desulfovibrio desulfuricans (Dd-Bfr) in its reduced (PDB ID 1nf4) (Macedo et al. 2003), reoxidized (PDB ID 1nf6) (Macedo et al. 2003), and as-isolated (PDB ID 1nfv) (Macedo et al. 2003) form; Bfr from Rhodobacter capsulatus (Rc-Bfr) with a metal-free ferroxidase center and the haem iron in a crystallographically special position (PDB ID 1jgc) (Cobessi et al. 2002); Bfr from Azotobacter vinelandii (Av-Bfr) (PDB ID 1sof) (Liu et al. 2004), in its oxidized (PDB ID 2fl0) (Swartz et al. 2006), and reduced (PDB ID 2fkz) (Swartz et al. 2006) form; and Bfr from E. coli (Ec-Bfr), the most studied Bfr (PDB IDs 1bcf) (Frolow et al. 1994), (2htn) (van Eerde et al. 2006), and (1bfr) (Dautant et al. 1998). All mentioned structures except Dd-Bfr contain the Fe-protoporphyrin IX (haem) group at the intersubunit interface, while Dd-Bfr contains its natural cofactor Fe-coprotoporphyrin III (Macedo et al. 2003).
M. smegmatis is an acid-fast bacterial species in the genus Mycobacterium and is generally considered a nonpathogenic microorganism, although in some cases it can cause disease in humans (Wallace Jr. et al. 1988; Pierre-Audigier et al. 1997). The complete genome sequence of the best characterized M. smegmatis strain mc2155 is known and available from http://cmr.tigr.org/tigr-scripts/CMR/GenomePage.cgi?database=gms. M. smegmatis bacterioferritin Ms-Bfr (MSMEG_3564, UniProtKB/TrEMBL entry A0QY79) consists of 161 amino acids with a molecular weight of 18,482 Da. It exhibits 87% sequence identity to its homolog from the highly pathogenic bacterium M. tuberculosis (Rv1876, UniProtKB/Swiss-Prot entry P63697). Here, we report the 2.7 Å resolution structure of Ms-Bfr in its as-isolated form solved by molecular replacement. The crystals exhibit a new space group symmetry not yet observed in other bacterioferritin crystals. The asymmetric unit of the monoclinic C2 crystals contains 12 protein molecules: five dimers and two half-dimers located on the crystallographic twofold axis.
Results and Discussion
Space group and crystal packing
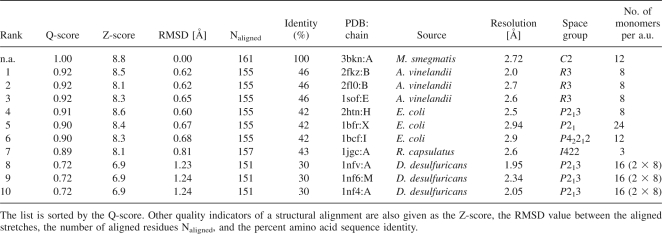
Bfrs crystallize mostly in high symmetry space groups such as P213 (Macedo et al. 2003; van Eerde et al. 2006), P42212 (Frolow et al. 1994), I422 (Cobessi et al. 2002), and R3 (Liu et al. 2004; Table 1) with three to 16 molecules in the asymmetric unit. The only exception is Ec-Bfr, which crystallized in the monoclinic space group P21 with the entire 24-mer present in the asymmetric unit (Dautant et al. 1998). Here, we present the first instance when a Bfr was crystallized in space group C2. The asymmetric unit of the M. smegmatis Bfr crystals contains half of the entire 24-mer characteristic for ferritins and Bfrs (Fig. 1), i.e., 12 individual amino acid chains. The chain pairs AB, CD, EF, GH, IJ form noncrystallographic dimers within the 24-mer, while the chains K and L form dimers via the crystallographic twofold axis (KK′ and LL′). The analysis of the solvent content in the various Bfr crystals shows rather large variation. The biological unit of a Bfr oligomer (24 subunits) exhibits a diameter of ∼120 Å and a volume of ∼900,000 Å3. Treating the inside volume (diameter 80 Å, volume 268,000 Å3) of the Bfr sphere as part of the Bfr volume, the solvent content ranges from 23% for Ec-Bfr (PDB ID 2htn) (van Eerde et al. 2006) to 44% for the tetragonal crystals of the same protein (PDB ID 1bcf) (Frolow et al. 1994). Ms-Bfr crystals contain 28% of solvent outside the protein shell, which is on the lower side of what was observed before, but still well within the range of values observed for Bfr other crystals.
Table 1.
Structures of other bacterioferritins identified by secondary structure matching (SSM)
Figure 1.
The overall structure of the functional 24-meric biological unit of Ms-Bfr. The color code used is the following: green for protein chain A, blue for protein chain B, dark red for the metal ions from the haem group and the ferroxidase center, red for the protoporphyrin IX cofactor, and gray for all remaining 22 protein chains. The figure was prepared in CueMol (http://cuemol.sourceforge.jp/en/index.php).
Overall fold and comparison of bacterioferritins
The overall structure of Ms-Bfr is similar to the one observed for Bfrs from other bacterial sources. This applies to the analysis of a single chain, but also to the analysis of the complete biological unit, the 24-mer. The protein exhibits the typical ferritin-like fold, which consists of four almost parallel, about 30 amino acid long α-helices. An additional short two-turn α-helix occurs close to the C terminus. This helix is almost perpendicular to the other four helices. It lines the fourfold channel, which will be described later. When comparing the structure of Ms-Bfr subunit (chain A) with the homologs from other bacteria it can be observed that the structure is relatively closely related to Ec-Bfr and Av-Bfr with RMSD of 0.60 and 0.62 Å, respectively, for 155 superimposed Cα-pairs (Table 1). The RMSD values to Rc-Bfr and Dd-Bfr are somewhat higher with 0.81 and 1.23 Å for 157 and 151 superimposed Cα pairs, respectively. Dd-Bfr is the most dissimilar, also from the amino acid sequence point of view, as it shows 30% identity to Ms-Bfr while the other representatives are ∼42%–45% identical to Ms-Bfr. The structure-based sequence alignment of Bfrs is presented in Figure 2 and the overlap of the monomeric Bfr structures in Figure 3. The close relationship between the three-dimensional structures is evident. Furthermore, all the amino acids that have previously been identified as bearing some functional relevance with respect to metal binding are conserved between the different Bfrs.
Figure 2.
Structure-based sequence alignment of bacterioferritins prepared using the SSM server (http://www.ebi.ac.uk/msd) (Krissinel and Henrick 2004). The sequence from Mtb-Bfr was aligned manually to the remaining sequences. The figure is color coded as follows: cyan for the haem coordinating residue Met52, red for the residues forming the ferroxidase center, yellow for the residues building up the fourfold channel, green for the residues building up the threefold channel, and magenta for the residues defining the B-site. The numbering of the residues is based on the Ms-Bfr sequence. The light red bars labeled as H1 to H5 at the top of the sequence alignment indicate the five α-helices present in the structure of the Bfrs. The alignment is underlined with the symbols “*”, “:”, and “.” describing different degrees of sequence conservation in descending order. The overall sequence identity values between Ms-Bfr and Mtb-Bfr, Ec-Bfr, Av-Bfr, Rc-Bfr, and Dd-Bfr are 87%, 41%, 46%, 42%, and 29%, respectively.
Figure 3.
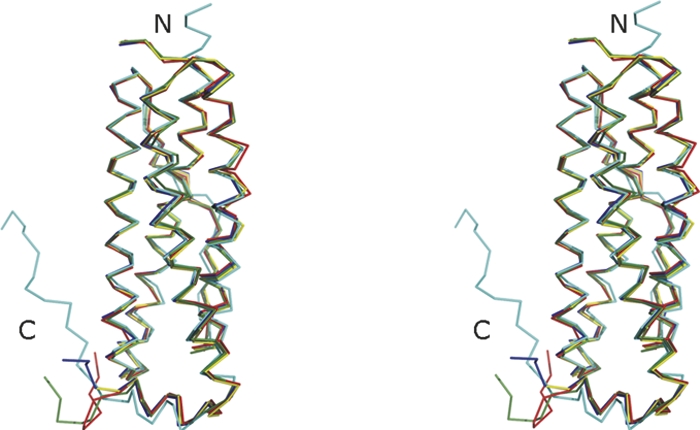
Stereoview of the overlap of Ms-Bfr monomer (chain A, shown in red) with monomers (always the chains denoted A in the respective PDB entries were used) of Rc-Bfr (PDB ID 1jgc; green), Dd-Bfr (PDB ID 1nfv; cyan), Av-Bfr (PDB ID 2fkz; yellow), and Ec-Bfr (PDB ID 2htn; navy blue). The figure was prepared with PyMOL (http://www.pymol.org).
Haem binding site
The Fe atom from the haem group is axially coordinated from both sides by the SD atom of Met52 from two adjacent monomers, with the Fe…S distance ranging from 2.30 to 2.50 Å (average 2.40 Å) (Fig. 4). These Met residues are absolutely conserved in the known Bfr structures. The haem groups of the two crystallographic dimers (KK′ and LL′) are located on the crystallographic twofold axis, as was also observed in the structure of Bfr from the photosynthetic organism Rhodobacter capsulatus (PDB ID 1jgc) (Cobessi et al. 2002), in which all haem groups are located along crystallographic symmetry elements. The sigmaA-weighted (2Fobs-Fcalc, αcalc)-electron density map contoured at 1σ is well defined for all haem groups except for the propionic acid side chains, which are disordered in most of the cases. Furthermore, the other Fe-protoporphyrin IX substituents, the vinyl and methyl groups, cannot be distinguished on the basis of the electron density; therefore, the two alternative orientations of the prosthetic groups were modeled with 0.5 occupancy, as was also done for Ec-Bfr (PDB ID 2htn) (van Eerde et al. 2006), and Dd-Bfr with Fe-coproporphyrin III (PDB IDs 1nf4, 1nf6, 1nfv) (Macedo et al. 2003). In contrast to this, the structure of Av-Bfr (Liu et al. 2004) was refined with the haem group in only one orientation, although no significant positive or negative peaks could be observed for the vinyl or methyl substituents. The haem groups in the crystallographic dimers KK′ and LL′ were treated differently since the crystallographic twofold axis automatically introduced the alternative haem orientations. Since the prosthetic group binding site exhibits twofold symmetry it may be that even in atomic resolution structures the orientation of the haem group remains ambiguous. The presence of iron in the protoporphyrin IX was confirmed by the examination of an anomalous difference Fourier electron density map calculated based on the data collected at the iron peak wavelength 1.739 Å (Fig. 4; Table 2B). All iron atoms inside the haem could be identified as peaks of 3.6–4.9 standard deviations above the mean of the map.
Figure 4.
The haem group located between the chains I (colored in cyan) and J (colored in yellow) of the Ms-Bfr structure. The protein chains are shown as tubes and the Met52 amino acid as well as the haem group as sticks colored by atom type. Superimposed on the figure is the anomalous difference Fourier electron density map contoured at 4σ, which identifies the position of the iron in the center of the haem group. The distances between the two Met52-SD atoms and the Fe ion are given in angstroms. The figure was prepared in CueMol (http://cuemol.sourceforge.jp/en/index.php).
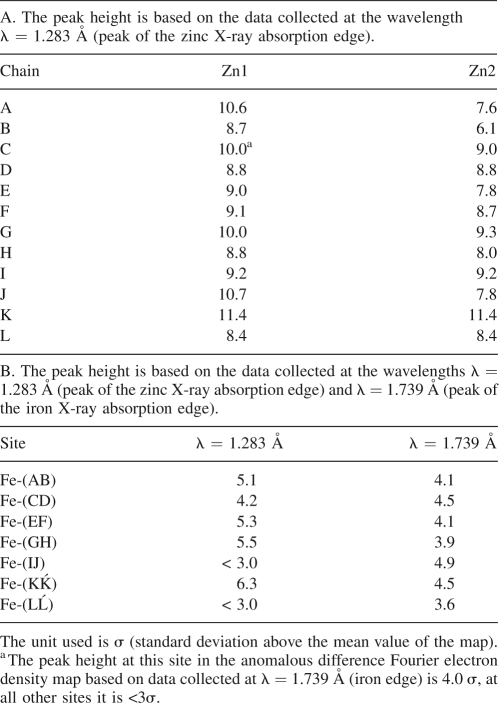
Table 2.
Peak height at the Zn and Fe positions, respectively, in the anomalous difference Fourier electron density map
Di-nuclear metal-binding site
The positions of two metal atoms in the di-nuclear metal-binding site are designated as Me1 and Me2 with the labeling being in accord to Macedo et al. (2003), where the two Me atoms had been identified as Fe. Surprisingly, in the structure of Ms-Bfr, no iron could be detected in this site, but zinc ions were unequivocally identified as occupying the positions of the two metal ions (Fig. 5). The anomalous difference Fourier synthesis based on data collected at the peak of the Fe K-edge revealed the presence of iron only at the haem site (see previous paragraph and Table 2), whereas the anomalous difference Fourier synthesis based on data collected at the peak of the Zn K-edge clearly indicated the presence of Zn at the di-metal site. Table 2 contains the list of all peaks stronger than 3σ found in the anomalous difference Fourier synthesis and the corresponding zinc and iron positions. No significant signal can be observed at the zinc positions in the map from the data collected at the iron edge, whereas the iron atoms still exhibit a significant signal in the map from the data collected at the zinc edge. This is in accord with the experimentally derived anomalous scattering lengths Δf″, which are 0.8 and 3.9 electrons for Zn at the two wavelengths (λ = 1.739 Å and λ = 1.283 Å), and 3.9 and 2.4 electrons for Fe at the same two wavelengths (Fig. 6). The fact that zinc is bound in the dimetal-binding site instead of iron is indeed surprising, because zinc was neither added during purification nor used for the crystallization of the protein. The only instance where zinc was added was during the growth of M. smegmatis cells in Middlebrook 7H9 medium. This medium contains 151 μM ammonium iron(III) citrate and 6.2 μM zinc sulphate. The protein was purified and crystallized in its as-isolated form, which means that most likely zinc was taken up from the cells. This does suggest that the affinity of Ms-Bfr is higher to zinc than to iron, which contradicts earlier observations made for other Bfrs, where iron has been found occupying the di-metal-binding site.
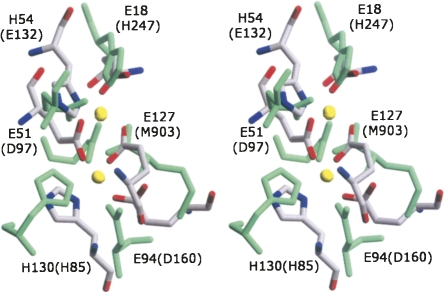
Figure 5.
Stereoview of the di-metal center of Ms-Bfr. The traces of the protein (chain A) are indicated as cyan tubes. The two zinc ions (denoted Zn1 and Zn2) are shown as yellow spheres and the Zn-binding amino acid side chains as sticks colored by atom type. The Zn-ligand distances are given in Table 5. The anomalous difference Fourier electron density map calculated from the data collected at the peak of the Zn-absorption edge contoured at 8σ is superimposed on the figure. This map unequivocally identifies the positions of the two zinc ions. The figure was prepared in CueMol (http://cuemol.sourceforge.jp/en/index.php).
Figure 6.
The X-ray fluorescence spectra collected at the absorption edges of zinc (upper panel) and iron (lower panel). In addition, the values of Δf′ and Δf″ are given for both elements.
In Ms-Bfr, both zinc atoms are ligated by one histidine and one glutamic acid (His54 and Glu18 for Zn1 and His130 and Glu94 for Zn2). In addition they are bridged by two carboxylate groups of glutamic acids (Glu51 and Glu127). These residues are highly conserved in bacterioferritins, as is shown in the structure-based sequence alignment (Fig. 2). The average Me1…Me2 distance differs in the available Bfr structures depending on the oxidation state of the metal ions. The best example showing this difference is bacterioferritin from A. vinelandii, for which structures of oxidized and reduced form are known (Swartz et al. 2006). In Av-Bfr, the iron atoms are separated by 3.5 Å in the oxidized protein (PDB ID 2fl0), while, when reduced, the distance increases to 4.0 Å (PDB ID, 2fkz). A similar situation was observed for the R2 protein of E. coli ribonucleotide reductase (RNR-R2), which contains the same di-iron center as Bfrs. In RNR-R2, the Fe1…Fe2 distance changes from 3.9 to 3.4 Å upon conversion from the reduced to the oxidized form (Nordlund and Eklund 1995). The structure of Dd-Bfr brings an additional interesting feature of the di-iron center (Macedo et al. 2003). In the reduced crystals (PDB ID 1nf4) the iron atoms are separated by 4 Å, while, when reoxidized, Dd-Bfr loses an ion from its Fe1 position. For the same protein in its as-isolated form the Fe…Fe distance is 3.7 Å (PDB ID 1nfv) (Macedo et al. 2003). The distance between the two zinc ions in the presented Ms-Bfr structure ranges from 3.85 to 4.15 Å in the 12 subunits (average 4.0 Å), which is close to the Me…Me distance found in the reduced form of the other Bfrs.
An important observation is that the anomalous signal observed for the Zn2 site in the Ms-Bfr structure is almost always weaker (Table 2) than the one for Zn1. This indicates that the Zn2 position may not be fully occupied. Similar observations have been made previously for other Bfrs containing iron (PDB IDs 1sof) (Liu et al. 2004), (2htn) (van Eerde et al. 2006), and (2fl0 and 2fkz) (Swartz et al. 2006). Moreover, some of the metal-coordinating side chains of His130 display weak negative difference electron density (at the −3σ level). Positive difference density (at the +3σ level) next to them indicates a potential second conformation of this side chain (the density, however, was too weak to model a second conformer with confidence). A similar situation occurs for Tyr25, which resides in the neighborhood of the di-metal-binding site and which forms a strong hydrogen bond with Glu94. When His130 adopts its alternative conformation, the Tyr25 side chain may have to flip or rotate away to make room for it. Such a conformational change was observed for Av-Bfr in its reduced form (PDB ID 1fkz) (Swartz et al. 2006) and in the Av-Bfr crystals accidentally obtained during crystallization attempts of anaerobically purified Cr-containing nitrogenase component I (PDB ID 1sof) (Liu et al. 2004). In the latter structure, the side chain of His130 is moved, and the average distance between Fe2 and the amino nitrogen ND1 of His130 increased to 3.5 Å (Liu et al. 2004) when compared to the 2.3 Å average distance in the oxidized form (PDB ID 2fl0) (Swartz et al. 2006).
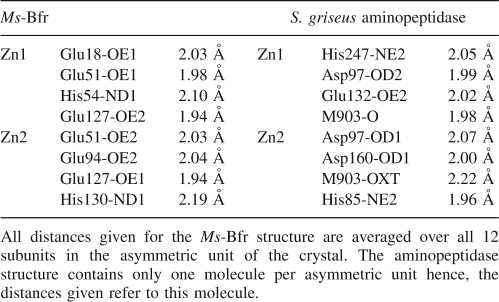
There are other protein structures containing di-nuclear zinc-binding sites with very similar architecture to the one found in Ms-Bfr. These include three aminopeptidases from Streptomyces griseus (PDB ID 1qq9) (Gilboa et al. 2000), Aeromonas proteolytica (PDB ID 1lok) (Desmarais et al. 2002), and Lactobacillus delbrueckii (PDB ID 1lfw) (Jozik et al. 2002). In these structures the architecture of the highly conserved active site shows the two zinc ions separated by 3.45–3.85 Å and coordinated by His and Glu/Asp, and in addition by a bridging Asp. On the opposite side from the bridging Asp residue, a second bridge is formed by a water molecule or the substrate/product molecule. Figure 7 shows the overlap of the di-metal-binding site from Ms-Bfr and the active site of the aminopeptidase from S. griseus. Although the similarity of the two sites is apparent, they differ dramatically in their accessibility. The di-metal site in Ms-Bfr is not accessible for any molecule bigger than metal ions. Thus, an enzymatic role of Ms-Bfr can be excluded. However, if zinc is indeed the natural ligand for Ms-Bfr in this site, the role of the di-nuclear metal-binding site can only be structural. With zinc instead of iron present, a redox reaction such as the one necessary to convert Fe(II) to Fe(III) cannot occur. We conclude therefore, that Ms-Bfr picked up zinc by chance into the di-metal-binding site. In vivo, two iron ions will probably be bound at this site. However, at this point, it cannot not be precluded that in M. smegmatis the ferritin (MSMEG_6422, UniProtKB/TrEMBL entry A0R647) is the major protein responsible for iron storage, whereas bacterioferritin may serve another, hitherto unknown function.
Figure 7.
Stereoview of the superposition of the di-metal sites of Ms-Bfr (chain A) and of Streptomyces griseus aminopeptidase (PDB ID 1qq9) (Gilboa et al. 2000). The latter structure contains a methionine product analog (denoted M903 in the figure). The zinc ions are shown as yellow spheres, the amino acid atoms of Ms-Bfr are in atom type colors, and the ones of aminopeptidase in light green. All zinc-ligating amino acids are labeled. The labels in parentheses correspond to the aminopeptidase residues. All Zn-ligand distances are detailed in Table 5. The figure was prepared in CueMol (http://cuemol.sourceforge.jp/en/index.php).
Threefold channels, fourfold channels, and the B-sites
The interior cavity of the biological unit of the Bfr particle is connected to the outside via eight channels along the threefold axes of the protein shell, the so-called threefold channels, and via six channels along the fourfold axes of the protein shell, the so-called fourfold channels. In addition, 24 pores termed the B-site according to Macedo et al. (2003), are located halfway in between the threefold and fourfold channels. For ferritins, it has been proven experimentally that the threefold channels play an important role in iron uptake and release (Ford et al. 1984; Harrison et al. 1986). In all animal ferritins, the threefold channel is hydrophilic and lined with highly conserved negatively charged residues (Asp and Glu). Mutations of those residues exhibit a significant decrease in the rate of iron uptake (Treffry et al. 1993; Levi et al. 1996). Moreover, crystallographic studies revealed the presence of divalent cations inside the threefold channel of ferritins (Hempstead et al. 1997), which lends further support to the notion of defined cation binding sites existing in the channel. Douglas and Ripoll (1998) and Takahashi and Kuyucak (2003) employed computational methods based on the Poisson-Boltzmann equation to obtain additional proof for the role of threefold channel in the Fe(II) deposition and release pathways in ferritin. In bacterioferritins, the situation is likely to be different than in ferritins since the threefold channels are composed of both positively and negatively charged residues. In the case of Ms-Bfr the channel is ∼21 Å long and it consists of five layers. The outer entrance of the channel is lined by the three partially disordered Glu110 residues, which form an ∼9 Å diameter entry to the funnel (Fig. 8). Following that, three Arg109 side chains interact with the three Glu121 residues to form a constriction site with a diameter of ∼6 Å. The electron density is very well defined for all of these six residues. The narrowest part of the constriction site between the Arg side chains is only ∼4 Å in diameter, which is just enough for unsolvated ions to pass through. However, the fact that Arg is positively charged strongly suggests that this path is not a path for cations. The inner part of the threefold channel contains two more layers of residues exhibiting relatively poor density. Here, the channel is getting wider again (∼7 Å diameter at Arg118 and ∼9 Å diameter at Gln122). In the Ms-Bfr structure, there is no indication of any metal or water molecule bound in the inside of this channel. In the other bacterioferritins, Av-Bfr, Ec-Bfr, and Rc-Bfr, the threefold channel is also strongly hydrophilic and always contains Arg residues in the position equivalent to Arg109 in Ms-Bfr. Rc-Bfr additionally contains Lys in the narrowest part of the channel. In the case of Dd-Bfr the threefold channel is mostly positively charged, the outer entrance to the channel consists of the 14 Å-diameter ring buildup of Lys111 and Glu115, then the narrowest part is lined with Lys114, Glu126, Arg123, and Arg127. It seems rather unlikely for cations to pass through a channel exhibiting such a positive overall charge. In accord with this is the observation that in the threefold-channel of Dd-Bfr (1nfv) (Macedo et al. 2003), a bound sulphate ion was observed. The main part of the threefold channel in M. tuberculosis seems to be unchanged, as clearly seen from the sequence alignment in Figure 2, in the sense that the overall positive charge distribution of the channel is conserved. However, the inner part of it has a slightly different character, with Arg118 and Gln122 in Ms-Bfr being mutated to Val and Lys in the Mtb-Bfr, respectively.
Figure 8.
The views along the path of the three- and fourfold channel as well as of the B-site of Ms-Bfr from the outside to the inside of the Bfr-sphere. For the threefold channel (A), chains A, D, and K are shown and for the fourfold channel (B) chains A, C, G, and I. (C) B-site of Ms-Bfr (chains A, C, and D) with the bound magnesium ion. The different subunits are shown in different colors. The residues lining the three- and fourfold channels are labeled in only one subunit in order to keep the figure clear. The figure was prepared in CueMol (http://cuemol.sourceforge.jp/en/index.php).
For the fourfold channel, the situation presents itself somewhat differently. Douglas and Ripoll (1998) and Takahashi and Kuyucak (2003) were able to demonstrate for ferritin that the fourfold channel cannot be involved in the transport of iron ions due to its highly hydrophobic character. On the other hand, Levi et al. (1996) showed that iron deposition in ferritin was altered by mutations of amino acid residues lining this channel. This means that these residues must play some role in the mineralization process. A possible functional role of the fourfold channels may be that they participate in the transfer of protons, which are released when Fe(II) ions are converted into ferrihydrite inside the cavity (Takahashi and Kuyucak 2003). In contrast to ferritins, the fourfold channels in Bfrs often contain hydrophilic residues. In Ms-Bfr, the fourfold channel consists of three layers: The outer one, which is hydrophobic, is lined with the side chains of the four Leu148 residues, and exhibits a diameter of ∼5.5–5.7 Å, the central one is composed of Gln152 (diameter ∼7.8 Å), and the inner one is built up of the positively charged side chains of Arg156 (Fig. 8). The length of the fourfold channel is ∼13 Å. In this respect, the channel is most similar to the one observed in Ec-Bfr, although the channel in Ec-Bfr is much more hydrophilic. The Ec-Bfr fourfold channel also possesses a three-layer structure, with Asn148 forming the outer layer, Glu151 the central one, and Arg155 the inner layer. The inner layer is wide open with a diameter of 10–11 Å. It is lined more by the hydrophobic part of the Arg155 side chain rather than the positively charged part. In the structures of Bfr from R. capsulatus, D. desulfuricans, and A. vinelandii, the fourfold channel consists of only two layers, the outer and the inner layer. These consist of Gly148 and Leu151 in Rc-Bfr, of Thr152 and Lys156 in Dd-Bfr, and of Asn148 and Gln151 in Av-Bfr, respectively. In the highly hydrophilic fourfold channels of Av-Bfr a binding site for Ba and Fe ions was detected (Liu et al. 2004; Swartz et al. 2006). Neither the most hydrophobic fourfold channel of Rc-Bfr nor the one of Ms-Bfr shows any indication of metal binding inside the channel. In Ms-Bfr, an anomalous difference Fourier electron density map calculation shows no sign of metal binding, but a difference electron density calculation revealed a prominent peak in each of the three asymmetric fourfold channels. This could be interpreted as a water molecule, coordinated by Gln152-ND2 (Gln152-OD1 is involved in the interaction with Arg156). In summary, the role of the fourfold channels in M. smegmatis Bfr still remains unclear. It may be assumed that, due to its hydrophobic character at the entrance and the positively charged exit, it may play a role in proton transfer as in mammalian ferritins (Takahashi and Kuyucak 2003), or just water rather than in metal transport.
Each three adjacent, independent subunits in the bacterioferritin 24-mer form a pore other than threefold channel, termed the B-site according to Macedo et al. (2003). In all Bfrs, the B-site is lined with hydrophilic residues. In both structures of Av-Bfr studied by Swartz et al. (2006) the B-site is occupied by magnesium ions octahedrally coordinated by water molecules. The exact function of the pore is so far unknown; however, as reported by Swartz et al. (2006) and Macedo et al. (2003), it is sufficiently large to accommodate an iron atom. Therefore, it may be able to play a role in iron transport from and to the core of Bfr. The pore in Ms-Bfr is lined by Asp66 from one subunit, Asn34 from the second, and Asp132, Glu135, Thr136, and Gln139 from the third subunit (Fig. 8). The channel is negatively charged like in other Bfrs, but in contrast to the three- and fourfold channels, it is closed from the inner side by the C-terminal residue Ala161. However, this does not preclude the function as a channel since the C-terminal part may, under certain conditions (such as pH), undergo minor conformational changes, thus opening up the channel and letting ions pass through it. With Gln139 from Ms-Bfr mutated to Glu in the Mtb protein, the B-site in M. tuberculosis Bfr is even more negatively charged than in Ms-Bfr, which may enhance iron transport through the pore. In difference electron density maps, peaks too strong to be just water molecules were found in all 12 B-site pores of Ms-Bfr. However, the anomalous difference Fourier maps did not exhibit any strong features at this site; therefore iron or zinc atoms can be ruled out. For the purification and crystallization the following metal ions were used: 10 mM Mg2+, 20 mM K+, Na+ (minor amounts as NaOH for adjusting of pH), plus all traces present in other chemicals, glassware, and whatever the protein could bind in the cells. Both magnesium and potassium were modeled into this pore, but the refined atomic temperature factor values were only reasonable when Mg2+ was assumed to be bound to this site, making the presence of Mg2+ the most likely scenario. The B-values for Mg2+ in the final model range from 40 to 60 Å2 (average 52 Å2). This metal is surrounded by the side chains of Asn34, Asp66, Asp132, and Gln139. The distances between Mg2+ and the surrounding ligands range from 3.2 to 4 Å. This may indicate that the first coordination sphere of the metal ion is water as in both structures of Av-Bfr studied by Swartz et al. (2006), but due to the relatively low resolution of the structure this additional coordination is not visible. The location of the B-site is closer to the ferroxidase center than any of the three- and fourfold channels. If the midpoint between both metal positions of the ferroxidase center is considered as the center of the di-metal-binding site, then the distance to the position of the magnesium ion at the B-site is ∼17 Å, whereas the distances to the center of the nearest three- and fourfold channels are about 21 and 32 Å, respectively. A possible path from the B-site to the ferroxidase center is lined with hydrophilic and mostly negatively charged amino acid residues. It starts with Asp132, Thr129 and then reaches Glu50 and Asp126, which are in the vicinity of the ferroxidase center. In addition, the four main-chain carbonyl groups of Ala125, Asp126, Glu128, Thr129 are lining the path. In contrast, a possible path from the threefold channel to the ferroxidase center is lined with both positively and negatively charged residues (Arg118, Gln122, and Asp126).
Another possible path for the iron ions to enter the ferroxidase center had been proposed by Macedo et al. (2003). A pore located just above the ferroxidase center, with the ions of the di-metal site located ∼6 Å beneath its presumed entrance, may also constitute a potential path for the iron ions. The dimensions of the pore and composition of the residues building it would allow ions like Fe or water molecules to enter or leave the pore. The pore of Dd-Bfr (Macedo et al. 2003) is formed mainly by hydrophilic residues arranged in three layers: a top layer consisting of Met22, His25, Gln98, and Thr102, a middle layer with Ala26, Tyr30, Thr102, and Tyr106, and the bottom layer consisting of the iron atoms Fe1 and Fe2, as well as four glutamates (Glu23, Glu56, Glu99, and Glu132). The composition of this pore in M. smegmatis Bfr does not preclude the possibility of transfer iron ions through it, although it is not as open as in the D. desulfuricans case and it is more hydrophobic. Apart from the residues corresponding to the layers in Dd-Bfr, Glu96 is located on the very top of the pore, making a salt bridge with Arg78 and thus constricting the entrance to the pore. The residues of Ms-Bfr corresponding to the top layer of Dd-Bfr are Ser17, Thr20, Ile93, and Val97, the middle layer includes Ala21, Tyr25, and Gln14, and the bottom layer consists of the metal atoms and glutamates Glu18, Glu51, Glu94, and Glu127. After oxidization in the di-metal site, iron may be translocated into the core of Bfr via a concerted movement of His54, Asp126, and probably additionally Glu50 located below the metal position. The corresponding residues in Dd-Bfr are His59 and Glu131 (Macedo et al. 2003).
Conclusions
Bacterioferritin from M. smegmatis was isolated directly from its source organism, crystallized in a new space group, and its structure was solved by molecular replacement and refined to a resolution of 2.72 Å. It is the first Bfr structure from an organism closely related to an important human pathogen (M. tuberculosis). It occurs as a spherically shaped 24-mer and shows high structural similarity to other Bfrs and ferritins.
Unexpectedly, two zinc ions were identified in the di-metal-binding site of Ms-Bfr. This is a very surprising observation because the protein was isolated directly from its source M. smegmatis, it was not overexpressed, and no zinc had been added at any step of the isolation and preparation of the protein except during the growth of the bacteria in Middlebrook 7H9 medium, which contains both iron and zinc, albeit the latter one at a much lower concentration. The reason of the presence of Zn in the di-iron site and its function remain thus far unclear.
From the analysis of the channels connecting the inner part of the Bfr particle with the exterior it can be deduced that neither the three- nor fourfold channel is able to transfer iron ions in M. smegmatis. In Ms-Bfr the most likely route for iron ions in and out of the Bfr interior is via the so-called B-site.
Materials and Methods
Purification
Native bacterioferritin was extracted from its source organism, M. smegmatis, strain mc2155. To avoid contaminations during growth, the cells were transformed with an empty pMyNT vector (EMBL Hamburg) containing the hygromycin B resistance gene. Cells from an overnight preculture (50 mL per 1 L of culture) were grown in the medium consisting of 0.47% (w/v) Middlebrook 7H9 Medium powder (Difco), 0.2% (v/v) glycerol, 0.05% (v/v) Tween 80, 0.2% (w/v) glucose, and 50 μg mL−1 hygromycin B. The culture was incubated for ∼24 h at 310 K and 100 rpm and then harvested. The cells were frozen and stored at 253 K until further processing. Forty grams of cell pellet were dissolved in 40 mL of buffer A, composed of 10 mM HEPES pH 7.5, 10 mM MgCl2, 60 mM NH4Cl, 20 mM KAc, 1 μg/mL DNase, and two complete Mini, EDTA-free Protease Inhibitor Cocktail tablets (Roche), and lysed using a French press operated at 14,000 psi. The cell debris was pelleted by centrifugation for 45 min at 277 K and 22,000 rpm using a Beckman Ti45 rotor. This corresponds to a force of about 56,000g. The crude lysate was mixed (4:1) with a sucrose cushion (1.1 M sucrose in buffer A) and then spun down at 19,000 rpm for 3 h in a Ti45 rotor (≈42,000g) to pellet remaining cell debris and high molecular weight particles. Afterward, the supernatant was loaded above the layer of 1.1 M sucrose cushion in buffer A in a proportion 4:1, respectively, and spun down for 20 h at 40,000 rpm in a Ti45 rotor (≈186,000g) to harvest all other high molecular weight particles present in the solution. The pellet was dissolved in buffer A and loaded onto the sucrose gradient (10%–40% [w/v] sucrose in buffer A). After spinning down for 24 h at 25,000 rpm in a Beckman Ti15 rotor (≈59,000g), the fraction containing bacterioferritin was collected. The brownish color of the fraction provided visual evidence of the presence of bacterioferritin. The selected fraction was spun down overnight at 50,000 rpm using a Beckman Ti60 rotor (≈250,000g), the pellet dissolved in buffer A and left for crystallization. The yield of the purification was about 1 mg of the protein from each 2 g of the cells.
Crystallization of bacterioferritin
Native bacterioferritin from M. smegmatis was crystallized using the sitting-drop vapor-diffusion method at 292 K. The initial crystallization screening for the protein was performed using sparse-matrix screens (Jancarik and Kim 1991) from Hampton Research and Jena Bioscience. The protein crystallizes from a wide range of conditions, but the best crystals were obtained from condition no. 41 of the Crystal Screen composed of 100 mM HEPES pH 7.5, 10% (v/v) 2-propanol, and 20% (w/v) PEG 4000. To improve the quality of the crystals, optimization was performed that resulted in the growth of crystals suitable for diffraction experiments from 100 mM HEPES pH 7.5, 10% (v/v) 2-propanol, and 14% (w/v) PEG 4000. The colored crystals appeared after 3–7 d (Fig. 9) and grew to a maximum size of 40 × 15 × 15 μm3.
Figure 9.
A monoclinic crystal of bacterioferritin isolated from Mycobacterium smegmatis.
Data collection and processing
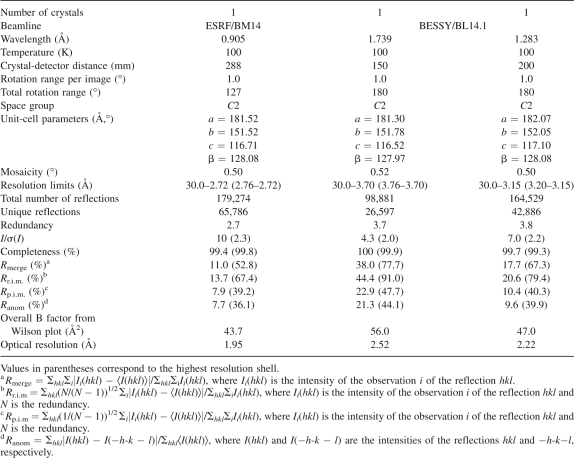
A crystal with the dimensions 40 × 15 × 15 μm3 was mounted in a nylon-fiber loop and flash-cooled to 100 K in a nitrogen-gas stream. Cryoprotection was performed for ∼5 sec in the reservoir solution enriched with 20% (v/v) PEG 400. Diffraction data were collected on beamline BM14 (ESRF Grenoble, France) using a MAR-Mosaic CCD detector (225 mm) and a wavelength of 0.905 Å. One hundred twenty-seven images were collected to 2.7 Å resolution with an oscillation range of 1° per image and a crystal-to-detector distance of 288 mm. To identify the metals present in the crystals of Ms-Bfr, fluorescence scans at the K-absorption edges of Mn, Fe, Co, Ni, Cu, and Zn were performed at the beamline 14.1 at BESSY, Berlin. The fluorescence scans were then analyzed with the program CHOOCH (Evans and Pettifer 2001) in order to determine the anomalous scattering lengths Δf′ and Δf″. In addition, two data sets were collected on beamline 14.1 at BESSY, Berlin, using a MAR-Mosaic CCD detector (225 mm). The first data set was collected to 3.7 Å resolution at the peak of the iron absorption edge, at a wavelength of 1.739 Å and a crystal-to-detector distance of 150 mm. The second data set was collected to 3.15 Å resolution at the peak of the zinc absorption edge, at a wavelength of 1.283 Å and a crystal-to-detector distance of 200 mm. In both cases, 360 images were collected with an oscillation range of 1° per image, but because of radiation damage, only the first 180 images turned out to be useful for inclusion into the scaling and merging of the diffraction data. The data were indexed and integrated using DENZO (Otwinowski and Minor 1997) and scaled using SCALEPACK (Otwinowski and Minor 1997). The redundancy-independent merging R-factor R r.i.m. as well as the precision-indicating merging R-factor R p.i.m. (Weiss 2001) were calculated using the program RMERGE (available from http://www.embl-hamburg.de/∼msweiss/projects/msw_qual.html or from M.S.W. upon request). Intensities were converted to structure factor amplitudes using the program TRUNCATE (French and Wilson 1978; Collaborative Computational Project, Number 4 1994). The optical resolution was calculated using the program SFCHECK (Vaguine et al. 1999; Collaborative Computation Project, Number 4 1994). Table 3 summarizes the data collection and processing statistics.
Table 3.
Data collection and processing statistics
Structure determination and refinement
The calculation of the Matthews parameter (Matthews 1968) combined with the investigation of the self-rotation function obtained from MOLREP (Collaborative Computational Project, Number 4 1994; Vagin and Teplyakov 1997) led to the conclusion that the most likely content of the asymmetric unit of the monoclinic crystals is 12 protein chains. This corresponds to a Matthews parameter of 2.85 Å3 Da−1 and a solvent content of 57% (when the inside of the Bfr sphere is counted as solvent). The structure of M. smegmatis bacterioferritin was then solved by means of molecular replacement using the program MOLREP (Collaborative Computational Project, Number 4 1994) to a resolution of 3.1 Å based on the data set collected at a wavelength of 0.905 Å. As a search model the dimer AB of A. vinelandii bacterioferritin was used (PDB ID 1sof) (Liu et al. 2004), because the amino acid sequence of this protein exhibits 46% identity and 65% similarity to M. smegmatis bacterioferritin sequence for a 155 amino acid overlap. Five dimers could be located. After displaying the solution using the graphic program Coot (Emsley and Cowtan 2004), two additional monomers were placed manually in their expected positions. Each of them forms a dimer via the crystallographic twofold axis.
The structure was then refined to 2.72 Å resolution using the program REFMAC5 (Murshudov et al. 1997) using the maximum-likelihood target function and including TLS parameters (Winn et al. 2001) and NCS restraints. After manual rebuilding in Coot (Emsley and Cowtan 2004), the final refined model was characterized by R and R free factors of 18.0% and 22.8%, respectively. The good quality of the electron density map allowed the inclusion of waters, several ions, and HEPES molecules. The sigmaA-weighted (2Fobs-Fcalc,αcalc)-electron density map (contoured at 1.2σ) is well defined for all 161 residues of the 12 independent molecules. For metal identification, anomalous difference Fourier electron density maps were computed based on anomalous differences obtained from diffraction data sets collected at the K-edges of Zn and Fe, respectively. For both maps, the final refined model phases were used.
Stereochemical analysis of the final model was carried out using the program PROCHECK (Laskowski et al. 1993). It indicates that there are no residues with generously allowed or unfavorable backbone dihedral angles and that 97% of all residues are in the core region of the Ramachandran plot. The results of the refinement are shown in Table 4. The refined structure and the structure factor amplitudes were deposited with the PDB under the accession code 3bkn.
Table 4.
Refinement statistics
Table 5.
Zn-ligand distances in the structures of Ms-Bfr and of the aminopeptidase from S. griseus (PDB ID 1qq9) (Gilboa et al. 2000)
Three-dimensional structural alignment was performed using secondary structure matching (SSM) as implemented in the SSM server (http://www.ebi.ac.uk/msd) (Krissinel and Henrick 2004) using the subunit A of Ms-Bfr as the search chain.
Acknowledgments
We would like to acknowledge German Israeli Foundation grant no. G-853-284.9/2004; Professor Ada Yonath for giving us the access to the equipment and valuable discussion, and Dr. Anat Bashan and Dina Levy for help in the laboratory. We also acknowledge the access to beam time at the ESRF (Grenoble, France), the BESSY (Berlin-Adlershof, Germany) synchrotrons, and the help of the synchrotron MX staff during data collection.
Footnotes
Reprint requests to: Manfred S. Weiss, EMBL Hamburg Outstation, c/o DESY, Notkestasse 85, D-22603 Hamburg, Germany; e-mail: msweiss@embl-hamburg.de; fax: 49-40-89902-149.
Abbreviations: Av, Azotobacter vinelandii; Dd, Desulfovibrio desulfuricans; Ec, Escherichia coli; Rc, Rhodobacter capsulatus; Mtb, Mycobacterium tuberculosis; Ms, Mycobacterium smegmatis; Bfr, bacterioferritin.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.034819.108.
References
- Andrews, S.C. Iron storage in bacteria. Adv. Microb. Physiol. 1998;40:281–351. doi: 10.1016/s0065-2911(08)60134-4. [DOI] [PubMed] [Google Scholar]
- Andrews, S.C., Le Brun, N.E., Barynin, V., Thomson, A.J., Moore, G.R., Guest, J.R., Harrison, P.M. Site-directed replacement of the coaxial heme ligands of bacterioferritin generates heme-free variants. J. Biol. Chem. 1995;270:23268–23274. doi: 10.1074/jbc.270.40.23268. [DOI] [PubMed] [Google Scholar]
- Bou-Abdallah, F., Lewin, A.C., Le Brun, N.E., Moore, G.R., Chasteen, N.D. Iron detoxification properties of Escherichia coli bacterioferritin. Attenuation of oxyradical chemistry. J. Biol. Chem. 2002;277:37064–37069. doi: 10.1074/jbc.M205712200. [DOI] [PubMed] [Google Scholar]
- Brooks, B.W., Robertson, R.H., Corner, A.H., Samagh, B.S., Garcia, M.M., Turcotte, C., Duncan, J.R. Evaluation of the serological response of sheep in one flock to Mycobacterium paratuberculosis by crossed immunoelectrophoresis. Can. J. Vet. Res. 1988;52:199–204. [PMC free article] [PubMed] [Google Scholar]
- Chasteen, N.D. Ferritin. Uptake, storage, and release of iron. Met. Ions Biol. Syst. 1998;35:479–514. [PubMed] [Google Scholar]
- Chasteen, N.D., Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999;126:182–194. doi: 10.1006/jsbi.1999.4118. [DOI] [PubMed] [Google Scholar]
- Cobessi, D., Huang, L.S., Ban, M., Pon, N.G., Daldal, F., Berry, E.A. The 2.6 Å resolution structure of Rhodobacter capsulatus bacterioferritin with metal-free dinuclear site and heme iron in a crystallographic “special position.”. Acta Crystallogr. D Biol. Crystallogr. 2002;58:29–38. doi: 10.1107/s0907444901017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Dautant, A., Meyer, J.B., Yariv, J., Précigoux, G., Sweet, R.M., Kalb, A.J., Frolow, F. Structure of a monoclinic crystal from cyctochrome b1 (Bacterioferritin) from E. coli . Acta Crystallogr. D Biol. Crystallogr. 1998;54:16–24. doi: 10.1107/s0907444997006811. [DOI] [PubMed] [Google Scholar]
- Desmarais, W.T., Bienvenue, D.L., Bzymek, K.P., Holz, R.C., Petsko, G.A., Ringe, D. The 1.20 Å resolution crystal structure of the aminopeptidase from Aeromonas proteolytica complexed with Tris: A tale of buffer inhibition. Structure. 2002;10:1063–1072. doi: 10.1016/s0969-2126(02)00810-9. [DOI] [PubMed] [Google Scholar]
- Douglas, T., Ripoll, D.R. Calculated electrostatic gradients in recombinant human H-chain ferritin. Protein Sci. 1998;7:1083–1091. doi: 10.1002/pro.5560070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley, P., Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans, G., Pettifer, R.F. CHOOCH: A program for deriving anomalous-scattering factors from X-ray fluorescence spectra. J. Appl. Crystallogr. 2001;34:82–86. [Google Scholar]
- Ford, G.C., Harrison, P.M., Rice, D.W., Smith, J.M.A., Treffry, A., White, J.L., Yariv, J. Ferritin design and formation of an iron storage molecule. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984;304:551–566. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- French, G.S., Wilson, K.S. On the treatment of negative intensity observations. Acta Crystallogr. A. 1978;34:517–525. [Google Scholar]
- Frolow, F., Kalb, A.J., Yariv, J. Structure of a unique twofold symmetric haem-binding site. Nat. Struct. Biol. 1994;1:453–460. doi: 10.1038/nsb0794-453. [DOI] [PubMed] [Google Scholar]
- Gilboa, R., Greenblatt, H.M., Perach, M., Spungin-Bialik, A., Lessel, U., Wohlfahrt, G., Schomburg, D., Blumberg, S., Shoham, G. Interactions of Streptomyces griseus aminopeptidase with a methionine product analogue: A structural study at 1.53 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 2000;56:551–558. doi: 10.1107/s0907444900002420. [DOI] [PubMed] [Google Scholar]
- Harrison, P.M., Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Harrison, P.M., Ford, G.C., Rice, D.W., Smith, J.M.A., Treffry, A., White, J.L., Xavier, A.V. The three-dimensional structure of apoferritin: A framework controlling ferritin's iron storage and release. Front. Bioinorg. Chem. 1986;2:268–277. [Google Scholar]
- Hempstead, P.D., Yewdall, S.J., Fernie, A.R., Lawson, D.M., Artymiuk, P.J., Rice, D.W., Ford, G.C., Harrison, P.M. Comparison of the three-dimensional structures of recombinant human H and horse L ferritins at high resolution. J. Mol. Biol. 1997;268:424–448. doi: 10.1006/jmbi.1997.0970. [DOI] [PubMed] [Google Scholar]
- Hunter, S.W., Rivoire, B., Mehra, V., Bloom, B.R., Brennan, P.J. The major native proteins of the leprosy bacillus. J. Biol. Chem. 1990;265:14065–14068. [PubMed] [Google Scholar]
- Ilari, A., Stefanini, S., Chiancone, E., Tsernoglou, D. The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site. Nat. Struct. Biol. 2000;7:38–43. doi: 10.1038/71236. [DOI] [PubMed] [Google Scholar]
- Jancarik, J., Kim, S.-H. Sparse matrix sampling: A screening method for crystallization of proteins. J. Appl. Crystallogr. 1991;24:409–411. [Google Scholar]
- Jozic, D., Bourenkow, G., Bartunik, H., Scholze, H., Dive, V., Henrich, B., Huber, R., Bode, W., Maskos, K. Crystal structure of the di-nuclear zinc aminopeptidase PepV from Lactobacillus delbrueckii unravels its preference for dipeptides. Structure. 2002;10:1097–1106. doi: 10.1016/s0969-2126(02)00805-5. [DOI] [PubMed] [Google Scholar]
- Krissinel, E., Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., MacArthur, M.W., Moss, D.S., Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- Levi, S., Santambrogio, P., Corsi, B., Cozzi, A., Arosio, P. Evidence that residues exposed on the three-fold channels have active roles in the mechanism of ferritin iron incorporation. Biochem. J. 1996;317:467–473. doi: 10.1042/bj3170467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.L., Zhou, H.N., Xing, W.M., Zhao, J.F., Li, S.X., Huang, J.F., Bi, R.C. 2.6 Å resolution crystal structure of the bacterioferritin from Azotobacter vinelandii . FEBS Lett. 2004;573:93–98. doi: 10.1016/j.febslet.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Macedo, S., Romao, C.V., Mitchell, E., Matias, P.M., Liu, M.Y., Xavier, A.V., LeGall, J., Teixeira, M., Lindley, P., Carrondo, M.A. The nature of the di-iron site in the bacterioferritin from Desulfovibrio desulfuricans . Nat. Struct. Biol. 2003;10:285–290. doi: 10.1038/nsb909. [DOI] [PubMed] [Google Scholar]
- Maeda, Y., Mukai, T., Spencer, J., Makino, M. Identification of an immunomodulating agent from Mycobacterium leprae . Infect. Immun. 2005;73:2744–2750. doi: 10.1128/IAI.73.5.2744-2750.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, B.W. The solvent content of protein crystals. J. Mol. Biol. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Murshudov, G.N., Vagin, A., Dodson, E. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Nordlund, P., Eklund, H. Di-iron-carboxylate proteins. Curr. Opin. Struct. Biol. 1995;5:758–766. doi: 10.1016/0959-440x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z., Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pessolani, M.C., Smith, D.R., Rivoire, B., McCormick, J., Hefta, S.A., Cole, S.T., Brennan, P.J. Purification, characterization, gene sequence, and significance of a bacterioferritin from Mycobacterium leprae . J. Exp. Med. 1994;180:319–327. doi: 10.1084/jem.180.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Audigier, C., Jouanguy, E., Lamhamedi, S., Altare, F., Rauzier, J., Vincent, V., Canioni, D., Emile, J.F., Fischer, A., Blanche, S., et al. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon γ receptor deficiency. Clin. Infect. Dis. 1997;24:982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- Pryor, W.A., Houk, K.N., Foote, C.S., Fukuto, J.M., Ignarro, L.J., Squadrito, G.L., Davies, K.J. Free radical biology and medicine: It's a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- Richards, T.D., Pitts, K.R., Watt, G.D. A kinetic study of iron release from Azotobacter vinelandii bacterial ferritin. J. Inorg. Biochem. 1996;61:1–13. doi: 10.1016/0162-0134(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Rohrer, J.S., Islam, Q.T., Watt, G.D., Sayers, D.E., Theil, E.C. Iron environment in ferritin with large amounts of phosphate, from Azotobacter vinelandii and horse spleen, analyzed using extended X-ray absorption fine structure EXAFS. Biochemistry. 1990;29:259–264. doi: 10.1021/bi00453a035. [DOI] [PubMed] [Google Scholar]
- Stillman, T.J., Hempstead, P.D., Artymiuk, P.J., Andrews, S.C., Hudson, A.J., Treffry, A., Guest, J.R., Harrison, P.M. The high-resolution X-ray crystallographic structure of the ferritin EcFtnA of Escherichia coli; comparison with human H ferritin HuHF and the structures of the Fe3+ and Zn2+ derivatives. J. Mol. Biol. 2001;307:587–603. doi: 10.1006/jmbi.2001.4475. [DOI] [PubMed] [Google Scholar]
- Sugden, E.A., Brooks, B.W., Young, N.M., Watson, D.C., Nielsen, K.H., Corner, A.H., Turcotte, C., Michaelides, A., Stewart, R.B. Chromatographic purification and characterization of antigens A and D from Mycobacterium paratuberculosis and their use in enzyme-linked immunosorbent assays for diagnosis of paratuberculosis in sheep. J. Clin. Microbiol. 1991;29:1659–1664. doi: 10.1128/jcm.29.8.1659-1664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, L., Kuchinskas, M., Li, H., Poulos, T.L., Lanzilotta, W.N. Redox-dependent structural changes in the Azotobacter vinelandii bacterioferritin: New insights into the ferroxidase and iron transport mechanism. Biochemistry. 2006;45:4421–4428. doi: 10.1021/bi060146w. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., Kuyucak, S. Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophys. J. 2003;84:2256–2263. doi: 10.1016/S0006-3495(03)75031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil, E.C. Ferritin. In: Messerschmidt A., et al., editors. Handbook of metalloproteins. Vol. 2. Wiley, Chichester; UK: 2001. pp. 771–781. [Google Scholar]
- Treffry, A., Bauminger, E.R., Hechel, D., Hodson, N.W., Nowik, I., Yewdall, S.J., Harrison, P.M. Defining the roles of the threefold channels in iron uptake, iron oxidation and iron-core formation in ferritin: A study aided by site-directed mutagenesis. Biochem. J. 1993;296:721–728. doi: 10.1042/bj2960721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin, A.A., Teplyakov, A. MOLREP: An automated program for molecular replacement. J. Appl. Crystallogr. 1997;30:1022–1025. [Google Scholar]
- Vaguine, A.A., Richelle, J., Wodak, S.J. SFCHECK: A unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. D Biol. Crystallogr. 1999;55:191–205. doi: 10.1107/S0907444998006684. [DOI] [PubMed] [Google Scholar]
- van Eerde, A., Wolterink-van Loo, S., van der Oost, J., Dijkstra, B.W. Fortuitous structure determination of “as-isolated” Escherichia coli bacterioferritin in a novel crystal form. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;62:1061–1066. doi: 10.1107/S1744309106039583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, V.J., Treffry, A., Laulhère, J.P., Bauminger, E.R., Cleton, M.I., Mann, S., Briat, J.F., Harrison, P.M. Structure and composition of ferritin cores from pea seed Pisum sativum . Biochim. Biophys. Acta. 1993;1161:91–96. doi: 10.1016/0167-4838(93)90201-2. [DOI] [PubMed] [Google Scholar]
- Wallace R.J., Jr, Nash, D.R., Tsukamura, M., Blacklock, Z.M., Silcox, V.A. Human disease due to Mycobacterium smegmatis . J. Infect. Dis. 1988;158:52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- Weiss, M.S. Global indicators of X-ray data quality. J. Appl. Crystallogr. 2001;34:130–135. [Google Scholar]
- Winn, M.D., Isupov, M.N., Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]