Abstract
A putative proton wire in potato soluble epoxide hydrolase 1, StEH1, was identified and investigated by means of site-directed mutagenesis, steady-state kinetic measurements, temperature inactivation studies, and X-ray crystallography. The chain of hydrogen bonds includes five water molecules coordinated through backbone carbonyl oxygens of Pro186, Leu266, His269, and the His153 imidazole. The hydroxyl of Tyr149 is also an integrated component of the chain, which leads to the hydroxyl of Tyr154. Available data suggest that Tyr154 functions as a final proton donor to the anionic alkylenzyme intermediate formed during catalysis. To investigate the role of the putative proton wire, mutants Y149F, H153F, and Y149F/H153F were constructed and purified. The structure of the Y149F mutant was solved by molecular replacement and refined to 2.0 Å resolution. Comparison with the structure of wild-type StEH1 revealed only subtle structural differences. The hydroxyl group lost as a result of the mutation was replaced by a water molecule, thus maintaining a functioning hydrogen bond network in the proton wire. All mutants showed decreased catalytic efficiencies with the R,R-enantiomer of trans-stilbene oxide, whereas with the S,S-enantiomer, k cat/K M was similar or slightly increased compared with the wild-type reactions. k cat for the Y149F mutant with either TSO enantiomer was increased; thus the lowered enzyme efficiencies were due to increases in K M. Thermal inactivation studies revealed that the mutated enzymes were more sensitive to elevated temperatures than the wild-type enzyme. Hence, structural alterations affecting the hydrogen bond chain caused increases in k cat but lowered thermostability.
Keywords: epoxide hydrolase, proton wire, thermostability, mutants, X-ray crystal structure
Soluble epoxide hydrolases (EC 3.3.2.10) are cofactor-independent enzymes belonging to the α/β-hydrolase fold superfamily (Ollis et al. 1992). The reaction catalyzed is the hydrolytic ring opening of an epoxide to form the corresponding vicinal diol (Fig. 1). Epoxide hydrolases show high catalytic activity as well as high enantioselectivity, which make them suitable for further development into specific and efficient biocatalysts (Archelas and Furstoss 2001; Koeller and Wong 2001; Steinreiber and Faber 2001). Such enzymes are widespread in nature; they are present in organisms from all kingdoms, with a variety of assigned functions. In some organisms, including humans, epoxide hydrolases function as detoxification enzymes, converting potentially harmful epoxides into less toxic and more easily excretable diol products. It has further been shown that human soluble epoxide hydrolase is a promising therapeutic target in ischemic stroke and is associated with hypertension-related kidney damage and systemic inflammation (Zhao et al. 2004; Imig 2005; Schmelzer et al. 2005; Zhang et al. 2007). Other known functions of epoxide hydrolases are in secondary catabolism and in regulation of signal molecules. In plants, epoxide hydrolases have been proposed to be involved in pathogen defense by participating in the synthesis of cutin (Kolattukudy 1981; Fauth et al. 1998; Arand et al. 2003; Morrisseau and Hammock 2005).
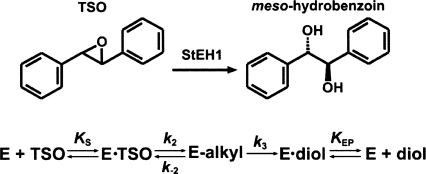
Figure 1.
Kinetic mechanism of StEH1 catalyzed hydrolysis of TSO.
The simplest view of the catalyzed hydrolysis encompasses four steps, illustrated for the model substrate trans-stilbene oxide (TSO) in Figure 1. Formation of the enzyme–substrate complex is followed by nucleophilic attack by an active-site aspartate carboxylate, which generates a covalent alkylenzyme intermediate. A general base-activated water reacts with the covalent intermediate, and the diol product is formed. Product release completes a cycle of catalytic turnover (for a review, see Armstrong and Cassidy 2000).
Central structural features of the epoxide hydrolase fold are a core α/β domain and a mainly α-helical lid domain. A catalytic triad, consisting of the Asp nucleophile, His general base, and an Asp charge relay residue, is located on the α/β domain, while two key tyrosyl residues are placed on the lid. In StEH1, a soluble monomeric epoxide hydrolase from potato, these catalytic groups are contributed by Asp105 as nucleophile, His300 as general base, Asp265 as charge relay residue, and the Tyr154/Tyr235 pair (Elfström and Widersten 2005, 2006). From the X-ray crystal structure of StEH1 (Protein Data Bank [PDB] entry code 2CJP) (Mowbray et al. 2006), an additional catalytic residue, Glu35, was identified. This residue has been shown to be important, both in the activation of Asp105 and in the hydrolysis half-reaction, in the latter case by properly orienting the water molecule that attacks the alkylenzyme intermediate (Thomaeus et al. 2007). Strong conservation regarding both structure and chemical functionality is found between the potato enzyme and other plant isoenzymes. Conservation of the key catalytic residues extends also to the mammalian soluble epoxide hydrolases (Thomaeus et al. 2007). The combined data allow some general conclusions to be drawn regarding structure/function relationships in the entire enzyme family. For instance, the attack of the base-activated water molecule is proposed to take place in concert with protonation of the negatively charged alkylenzyme intermediate. The proton is suggested to be transferred from donor oxonium ions of the bulk solvent through a chain of water molecules that leads to the active-site Tyr154 (Elfström and Widersten 2006), originally identified in the StEH1 crystal structure (Mowbray et al. 2006). The waters in the protein tunnel are coordinated by the following residues of the enzyme (listed in consecutive order, proceeding from the active site in the interior to the protein surface): the hydroxyl group of Tyr154, the imidazole moiety of His153, the backbone carbonyl of Leu266, the hydroxyl of Tyr149, the backbone carbonyl of His269, and, finally, the backbone carbonyl of Pro186 (Fig. 2). The hydroxyl groups of tyrosines 149 and 154 can be viewed as integral components of the chain (Fig. 2). To assess the importance and role of this putative proton wire, three mutants of StEH1, Y149F, H153F, and Y149F/H153F, were constructed and expressed in Escherichia coli, allowing their functional properties to be compared with those of the wild-type enzyme. In addition, the X-ray crystal structure of mutant Y149F was determined to evaluate any structural effects caused by the mutation.
Figure 2.
Schematic overview of the putative proton wire in StEH1.
Results
Site-directed mutagenesis, protein expression, and purification
The single mutants of StEH1, Y149F and H153F, and the double-mutant Y149F/H153F, were constructed using plasmid pGTacStEH1-5H, coding for wild-type StEH1, as cDNA template, together with mutagenic primers in the polymerase chain reactions. The enzymes, both wild type and mutants, were expressed in E. coli XL1-Blue and purified to homogeneity according to established procedures (Elfström and Widersten 2005) with comparable yields (20 mg/L batch cultures). Purified proteins were stored at 4°C and retained unchanged enzyme activity during the time of experimental data collection.
Structure of mutant Y149F
The structure of the substrate-free form of the StEH1 Y149F mutant at pH 7.5 was solved by molecular replacement using the wild-type protein structure (PDB entry code 2CJP) as a search model, and subsequently refined to a resolution of 2 Å. Statistics concerning data collection and refinement are shown in Table 1. Electron density in the region of interest is shown in Figure 3A. Comparison with the wild-type structure indicated that the Y149F point mutation led only to subtle changes; the two molecules in each respective asymmetric unit had an RMSD of 0.2 Å when all Cα atoms were compared. When the site of mutation is inspected, it becomes clear that removing the hydroxyl group of the wild-type tyrosyl residue results in a space that is filled by a water molecule (Fig. 3B). The other water molecules of the putative proton wire coordinate to the protein in essentially the same way as in the wild-type enzyme. The same water coordination is observed in both protein molecules in the asymmetric units of wild-type and Y149F mutant structures.
Table 1.
Data collection and refinement statistics
Figure 3.
Active-site and hydrogen-bond chain. (A) Residues in the active site of the Y149F mutant are shown together with the chain of waters that compose the putative proton wire. Electron density from a SIGMAA-weighted |2Fobs − Fcalc| map (Read 1986) is shown contoured at a level of 1σ. Residues participating in catalysis (Tyr154, Tyr235, Asp105, and His153), as well as the mutated Phe149, are labeled. (B) The same residues and equivalent water molecules from the wild-type enzyme (shown in white) are superimposed, and the site of mutation indicated. Replacing Tyr149 with phenylalanine results in a change of the position in the closest water molecule.
Enzyme kinetics
The steady-state parameters for the hydrolysis of both enantiomers of TSO were collected at 30°C and pH 6.8, the previously determined pH optimum for the wild-type enzyme (Elfström and Widersten 2005). In addition, the rate constants for formation (k 2) and decomposition (k -2 and k 3) of the alkylenzyme intermediate were determined for the Y149F catalyzed hydrolysis and compared with data for the wild-type enzyme. The results are listed in Tables 2 and 3 and commented on below. Data for the wild-type enzyme were collected under identical conditions to those for the mutants and were reproducible between enzyme batches.
Table 2.
Steady-state kinetic parameters of TSO hydrolysis catalyzed by wild-type StEH1, and mutants Y149F, H153F, and Y149F/H153F at pH 6.8
Table 3.
Kinetic parameters of TSO hydrolysis catalyzed by wild-type StEH1 and mutant Y149F at pH 6.8
Y149F mutant
For the reaction with R,R-TSO, both the k cat and K M parameters were elevated 1.9- to 3.6-fold, respectively, compared with the wild-type catalyzed reaction. The resulting catalytic efficiency was half that exhibited by the wild-type enzyme. For the reaction with S,S-TSO, k cat and K M were only slightly increased and the k cat/K M parameter was not significantly changed. The observed rates (k obs) for transient buildup of the alkylenzyme intermediate, reflected by quenching of intrinsic tryptophan fluorescence (Elfström and Widersten 2006), displayed substrate concentration dependencies that allowed for determination of k 2, k −2, and k 3 and the equilibrium dissociation constant of the Michaelis complexes, K S, as described in the Materials and Methods section (Fig. 4). In the reaction with R,R-TSO, however, enzyme saturation was not reached within the solubility range of the substrate, and so values for K S, k 2, k −2, and k 3, could not be determined with high accuracies.
Figure 4.
Substrate concentration dependence of observed rates (k obs) of alkylenzyme formation. Filled squares indicate R,R-TSO; filled circles, S,S-TSO. Solid lines represent the fits of Equation 3 to the observed rates.
However, it is clear from the results that the increases in k cat displayed by the Y149F mutant with either TSO enantiomer can be linked to higher hydrolysis rates (k 3) underlining this step as primarily rate limiting. In the S,S-TSO reaction, the alkylenzyme formation rate was increased twofold while k -2 and K S were essentially unchanged by the mutation.
H153F mutant
With R,R-TSO as substrate, the k cat parameter was essentially unchanged compared with the wild-type reaction, while K M was ninefold higher, resulting in a 15-fold drop in catalytic efficiency. With the S,S-enantiomer, k cat and K M were both increased two- to threefold in a compensatory manner, resulting in a virtually unchanged k cat/K M.
Y149F/H153F mutant
With the double mutant, the hydrolysis of R,R-TSO presented experimental difficulties due to the inability to reach enzyme saturation within the solubility range of the substrate (≤80 μM). Hence, the k cat and K M parameters could not be determined individually. However, the turnover rate was not severely affected, as judged by the observed rate at the highest substrate concentration (>10 s−1). The catalytic efficiency could be accurately determined and showed a more than 10-fold decrease compared with the corresponding wild-type value. k cat S,S -TSO was increased while K M S,S -TSO was virtually unaffected, thus resulting in a twofold elevated catalytic efficiency; k cat/K M S,S -TSO was 11-fold higher than the corresponding value for R,R-TSO. For the wild-type enzyme, R,R-TSO was the slightly preferred substrate, while the double mutant had a strong preference for the S,S-enantiomer; the difference in enantiospecificity between the wild-type enzyme and the Y149F/H153F mutant was thus 33-fold.
pH Dependence of catalysis
The pH dependence of k cat for the Y149F mutant was determined with the procedure previously used for the wild-type StEH1 (Elfström and Widersten 2005); the results are shown in Figure 5. The pH dependence of k cat reflects the titration of ionizable groups from the enzyme–substrate complex through the alkylenzyme intermediate leading to product and titrates with a bell-shaped profile in the wild-type enzyme. The observed titration profiles of k cat of the Y149F mutant suggested similar pH dependencies to the wild-type StEH1.
Figure 5.
pH Dependence of k cat. Effect of pH on the catalytic turnover, k cat, for wild-type StEH1 catalyzed (unfilled square), mutant Y149F catalyzed (filled squares) reactions with R,R-TSO and the effect of pH on k cat for wild-type StEH1 catalyzed (unfilled circles), and mutant Y149F catalyzed (filled circles) reactions with S,S-TSO. Lines represent the fits of Equation 1 to the experimental data. Error bars, the standard error of k cat obs. Data for k cat R,R -TSO and k cat S,S -TSO with wild-type StEH1 were adapted from Elfström and Widersten (2005).
Thermostability of activity
The catalytic activities of the reactions with R,R-TSO in the presence of wild-type StEH1, Y149F, H153F, or Y149F/H153F, following incubation of the enzymes at 55°C, were determined. The wild-type activity was the most stable at this temperature, with an estimated half-life of 2 h and 15 min (Fig. 6A). The activities of the single mutants showed intermediate half-lives, 1 h at 55°C, whereas the double mutant exhibited a dramatic drop in enzyme activity, with an interpolated half-life of only 20 min. The effects on thermal inactivation appear to be additive; the sum of the inactivation times for each single mutant gives a good approximation of the sensitivity exhibited by the double mutant. The observed temperature inactivation was not reversible. Wild-type StEH1 was also more resistant to thermal inactivation than the Y149F mutant in a temperature range between 55°C and 64°C. The wild type retained ∼65% activity after 5-min incubation at 60°C while, under the same conditions, the Y149F mutant had lost 70% of its initial activity at 30°C (Fig. 6B). Hence, the results of the thermal inactivation of wild type and Y149F mutant after fixed-time incubations at varying temperatures are in agreement with the results from the study of time-dependent temperature inactivation following incubations at 55°C.
Figure 6.
Thermal inactivation of enzyme activity. Effect of incubation at 55°C over time on the activity with 50 μM R,R-TSO with wild-type StEH1 (square), mutant Y149F (circle), mutant H153F (diamond), and mutant Y149F/H153F (triangle). Activities prior to incubation at 55°C were normalized to 100%. (B) Remaining enzyme activities with R,R-TSO (50 μM) after 5-min incubations at temperatures between 50°C and 70°C. Wild-type StEH1 (square) and mutant Y149F (circle). Activities prior to incubations at elevated temperatures were normalized to 100%.
Discussion
Proton transport can be accomplished by a continuous chain of hydrogen bonds, which may consist of protein functional groups or a combination of both protein and water (Nagle and Morowitz 1978). Proton wires made up only of water molecules occur in various types of proteins, ranging from transmembrane pores and thiamine-dependent enzymes to photosynthetic reaction centers (Pomès and Roux 1998; Frank et al. 2004). The process of proton hopping from one water molecule to the next, including the reorientation of the waters needed for proton transfer, is referred to as the Grotthuss mechanism. This hop-and-turn process provides a more rapid translocation of a proton than when transfer occurs solely by molecular diffusion. The reorganization of the hydrogen bonds between the water molecules after the translocation of the proton is the rate-limiting step. The proton transfer rates depend on the pKa values of engaged acid/base pairs and reach higher values when the pKas are matched (Pomès and Roux 1998). For both reasons, the transfer is slower if the proton transfer chain also contains hydrogen bond donors/acceptors from the protein itself (Pomès and Roux 1996, 1998; Decornez et al. 1999; Decoursey 2003).
In potato epoxide hydrolase StEH1, a proton wire-like structure has been identified, which could provide a route for the entry of the proton that is added to the anionic alkylenzyme intermediate during the catalytic cycle (Elfström and Widersten 2006). The protein-provided groups that coordinate the five water molecules of the hydrogen-bonding chain include backbone carbonyl oxygens and a histidyl imidazole (Fig. 2). In addition, Tyr149 and Tyr154 participate through their phenolic hydroxyls. The final member in the chain, Tyr154, acts together with Tyr235 to provide the hydrogen bond donors needed to position the oxirane oxygen of the epoxide substrate. Such positioning allows efficient nucleophilic attack by Asp105, which in turn leads to the formation of a covalent alkylenzyme intermediate. Mutation of either Tyr154 or Tyr235 to phenylalanine results in loss of activity (Elfström and Widersten 2005, 2006). A corresponding mutation of Tyr149, by contrast, allowed for an additional water molecule to fill the available space and participate in the chain (Fig. 3), thereby maintaining efficient proton transfer and a catalytically competent enzyme. The observed increases in k cat, however (Table 2), are unlikely to primarily originate from a facilitated proton transport from solvent to Tyr154 for two reasons: (1) The intrinsic rates of proton transfer between electronegative atoms are expected to be much faster (>1010 s−1) than the catalytic turnover numbers (<300 s−1), and (2) the rate-limiting step in StEH1-catalyzed TSO hydrolysis is not protonation of the alkylenzyme, but rather its hydrolysis (Thomaeus et al. 2007). Hence, k cat is mainly dictated by the energy barrier for the formation of the transition state of the hydrolytic half-reaction. If, however, protonation of the alkylenzyme facilitates the formation of this transition state, an increased value of k cat for Y149F-catalyzed TSO hydrolysis would be expected. This could be due, for instance, to a reduction in the electrostatic repulsion that would otherwise exist in a doubly negatively charged high-energy species. In essence, the determined rates of formation and hydrolysis of the alkylenzymes in the Y149F mutant support these arguments. The increased values of k 2 and k 3 indicate that both proton transfer and alkylenzyme hydrolysis have been facilitated by the mutation.
The reactions with the two TSO enantiomers are affected differently by the mutations; the hydrolysis efficiency with the S,S-enantiomer is weakly enhanced by removal of the protein–water hydrogen bonds in the proton wire. These differences can be attributed to distinct characteristics of the hydrolysis of the two enantiomers. S,S-TSO is bound to the enzyme with higher affinity than is R,R-TSO (Elfström and Widersten 2005), so accumulation of the Michaelis complex contributes to K M to a larger degree. In the R,R-TSO reaction, formation of the alkylenzyme is more efficient and exceeds hydrolysis rates by an order of magnitude, resulting in accumulation of the alkylenzyme. Hence, a facilitated alkylenzyme hydrolysis will, under such circumstances, also result in higher values of K M. The observed changes in enantiospecificity are in any case noteworthy: Mutations distant from the central catalytic residues that resulted in the loss of three protein–water hydrogen bonds caused a shift in enantiopreference exceeding 30-fold.
For the Y149F mutant, the catalytic turnover numbers are increased for the respective enantiomers of TSO over the pH range from 5.1 to 7.9 (Fig. 5). This increase may reflect facilitation of product formation by lowering the activation energy for formation of the tetrahedral intermediate. We have previously reported that the pH dependence of k cat in StEH1 can be explained by different protonation states of His300 with altering acidity through the catalytic cycle, presumably as an effect of the changing ionic states of the active-site micro-environment (Thomaeus et al. 2007). The curvatures of the respective pH profiles for the R,R- and S,S-TSO reactions, however, do not indicate that major changes in the acidity of the catalytically important His300 have occurred as a result of the Y149F mutation (Fig. 5). Hence, the observed increases in k cat and K M at pH 6.8 are probably not an effect of a change in rate-limiting steps dependent on His300 protonation states.
Hydrogen bonds between protein and water molecules may contribute substantial stabilization to protein structure. In StEH1, the H153F and Y149F mutants lose one and two protein–water hydrogen bonding interactions, respectively, which appears to destabilize the structure. Removing three interactions in mutant Y149F/H153F (Figs. 2, 6) is linked to a lowering of the half-life at 55°C from 2.25 h to 20 min. It should be noted, however, that the measured feature here is loss in catalytic activity rather than a direct analysis of structural unfolding.
A structure-based sequence alignment was used to compare the potato epoxide hydrolase and nine other plant epoxide hydrolases and shows strong conservation of a tyrosyl residue corresponding to Tyr149 in StEH1 (Fig. 7). The residue is conserved in eight out of the 10 plant epoxide hydrolases, a higher degree of conservation than seems to be required by the structural setting. The conservation thus supports the notion that a tyrosyl residue in this position may contribute its hydroxyl to the hydrogen-bond chain of water molecules in many other plant species. By contrast, the histidyl residue at position 153 (in StEH1) is only conserved in the rice epoxide hydrolase; most other plant isoenzymes contain a tyrosyl residue in the corresponding position. Modeling of this mutation in the StEH1 structure indicates that the hydroxyl group of a tyrosine could replace the histidine imidazole moiety in the hydrogen-bonding chain. Hydrogen bonding capacity is thus a conserved feature at this position. The residues corresponding to Pro186, Leu266, and His269 in StEH1 are reasonably well conserved although the contributions of these residues to the putative wire are via backbone oxygen interactions with the water molecules. The conservation seems to be based on the need to preserve the correct local structure, rather than a more direct role. The observed patterns of residue conservation imply similar functions in other plant epoxide hydrolases, i.e., contributing to coordination of a water–hydrogen-bond chain.
Figure 7.
Sequence alignment of soluble plant epoxide hydrolases. Regions of interest for the putative proton wire are shown; residues directly participating are indicated by white letters on black background. StEH1 (Swiss-Prot entry Q41415_SOLTU), soybean (Q39856_SOYBN), rough lemon (Q76E11_9ROSI), rape (Q8L5G6_BRANA), rice (Q9S7P1_ORYSA), pineapple (Q8H289_ANACO), E. lagascae (Q84ZZ3_EUPLA), barrel medic (A2Q320_MEDTR), chickpea (Q8LPE6_CICAR), and mouse-ear cress (Q42566_ARATH).
The water channel in StEH1 seems to serve two functions: to act as a proton wire for efficient protonation of the alkylenzyme intermediate during catalysis, and to stabilize the enzyme structure through hydrogen bonding with protein side chains. Furthermore, the combination of the results from the present study with a previous report describing a plausible proton escape route out of the active site (Thomaeus et al. 2007) suggests that plant epoxide hydrolases may have evolved to efficiently supply and remove protons to the catalyzed reaction. While the exit route for protons appears to be structurally conserved also in mammalian epoxide hydrolases (Thomaeus et al. 2007), we have not been able to identify structural features in epoxide hydrolase crystal structures of mammalian (1VJ5, 1CQZ), bacterial (1EHY), or fungal (1Q07) origin that corresponds to the water entry channel found in the plant enzyme.
The His300 imidazole must exist in an unprotonated state in order to function as a catalytic base. This relies on efficient proton transfer from the corresponding imidazolium, formed during the initial stages of the catalytic cycle, out of the active site. On the other hand, the anionic alkylenzyme intermediate requires protonation prior to its hydrolysis to form the diol product. Our analysis of the enzyme structure suggests that in plant enzymes both entrance and exit pathways for protons are integrated into the protein structure, forming a continuous path that runs from the surface of the protein to the buried active site then back to solvent (Fig. 8).
Figure 8.
Possible lateral proton translocation in StEH1. The gray arrow indicates the possible entrance and exit pathways for protons in and out of the active site. The side chains of residues Tyr149 and His153 and water molecules facilitate proton transfer into the active site (highlighted by a dotted circle) and the catalytic residue Tyr154. From the active site, a chain of hydrogen bonds, consisting of a water molecule, and the side-chain groups of Glu35, Ser39, Tyr219, Arg41, and Glu215 links the active site with bulk solvent and allows for the liberation of a proton at the other face of the enzyme.
Materials and Methods
Site-directed mutagenesis
Mutants were constructed using polymerase chain reactions including mutagenic primers (Thermo Scientific) and plasmid pGTacStEH1-5H (Elfström and Widersten, 2005) coding for wild-type StEH1 as template. Mutant Y149F was constructed by substituting a TAT codon with a TTT at position 149 in the amplified cDNA. The H153F mutant was constructed by changing the codon CAT at position 153 in the cDNA for a TTT codon. The double mutant Y149F/H153F was constructed by introducing both codon substitutions at position 149 and 153 in the cDNA. The mutated cDNA fragments were subcloned between the MunI and XhoI sites of pGTacStEH1-5H, and the plasmid constructs were sequenced in full to confirm the mutations and ensure that no further alterations in the cDNAs had occurred.
Structure alignment
Sequences similar to StEH1 were identified using BLAST (Altschul et al. 1997). Structure-based sequence alignments were carried out using Indonesia (D. Madsen, P. Johansson, and G.J. Kleywegt, in prep.) (http://xray.bmc.uu.se/%7edennis/).
Protein expression and purification
Expression plasmids encoding wild-type or mutant StEH1 were transformed into E. coli XL1-Blue bacteria by electroporation. Protein expression and purification of the His-tagged wild-type and mutant enzymes were performed as described earlier (Elfström and Widersten 2005). Protein concentrations of collected fractions were determined from the absorbance values at 280 nm. The molar absorbance coefficients used, calculated from the respective amino acid compositions, were 59,030 M−1cm−1 for wild-type and mutant H153F and were 57,630 M−1cm−1 for mutant Y149F and double-mutant Y149F/H153F.
Crystallization of mutant Y149F
The Y149F mutant protein was crystallized by vapor diffusion in hanging drops of 2 μL protein and 2 μL reservoir solution (25% PEG 10,000, 0.1 M HEPES, pH 7.5) at room temperature. Crystals (∼0.1 × 0.05 × 1 mm3) appeared within 3 d. Before being flash-cooled in liquid nitrogen, crystals were transferred to cryoprotectant composed of 20% glycerol, 25% PEG 10,000, and 0.1 M HEPES, pH 7.5. X-ray data were collected at beamline ID23 at the European Synchrotron Radiation Facility (ESFR), Grenoble. A data set of 99.6% completeness to 2 Å resolution was obtained. Diffraction data were indexed using MOSFLM (Leslie 1999) and processed with SCALA (Evans 1993) as implemented in the CCP4 interface (Potterton et al. 2003). The crystals possessed the symmetry of the space group P212121. Statistics for the data set are shown in Table 1 (Engh and Huber 1991; Kleywegt and Jones 1996).
Structure determination and refinement of mutant Y149F
The structure of the mutant Y149F was solved by molecular replacement with MOLREP (Vagin and Teplyakov 1997). The search model included all protein atoms from the earlier 2 Å resolution wild-type structure (PDB [Berman et al. 2002] entry code 2CJP [Mowbray et al. 2006]). Two molecules were found in the asymmetric unit. The structure was refined by alternating cycles of refinement in REFMAC (Murshudov et al. 1997) and model rebuilding in O (Jones et al. 1991). Progressive inclusion of water molecules and a fragment of PEG, modeled as tetraethylene glycol, dropped the R-factor to 17.0% (Rfree = 20.3%). Final refinement statistics are given in Table 1. The final model and experimental diffraction data are deposited at the PDB with entry code 3cxu.
Steady-state kinetics and pH dependencies
The enzyme-catalyzed initial rates of TSO hydrolysis were determined in 0.1 M sodium phosphate (pH 6.8) at 30°C. TSO was dissolved in acetonitrile and added to the reaction mixture, resulting in a final concentration of 1% (v/v) acetonitrile. The conversion from epoxide to diol was detected spectrophotometrically by measuring the decline in absorbance over time at 229 nm (Δε = −15 mM−1cm−1) (Wixtrom and Hammock 1988). Reactions were recorded for 20–60 s. Enzyme concentrations used in reactions were 25 nM, 26 nM, and 50 nM, for mutants H153F, Y149F/H153F, and Y149F, respectively. The concentrations of R,R-TSO or S,S-TSO were varied between 1.6 and 70 μM. At least three measurements at every substrate concentration were made. k cat, K M, and k cat/K M were determined by fitting the experimental data to the Michaelis-Menten equation in the MMFIT or RFFIT programs in SIMFIT (http://www.simfit.man.ac.uk). The pH dependence of k cat for the Y149F mutant was determined in 0.1 M sodium phosphate in the pH range of 5.1–7.9 using the same substrate concentration range as well as enzyme concentration. The determined k cat values were fitted to Equation 1 with RRFIT:
 |
In Equation 1, describing a doubly ionizing system, L H is the pH-dependent kinetic parameter k cat. LH2A, LHA-, and LA2- are the respective amounts of the protonated and deprotonated states of the enzyme-substrate complexes, and Ka1 and Ka2, the respective apparent acid constants.
Pre-steady-state kinetics
Microscopic rate constants and the equilibrium dissociation constant of ES (K S) were determined by following the transient pre-steady-state reaction phases in a SX20 stopped-flow spectrometer (Applied Photophysics, Ltd.). The reaction progresses were followed by recording the change in intrinsic tryptophan fluorescence of wild-type StEH1 and mutant Y149F during the reaction with either enantiomer of TSO. An excitation wavelength of 290 nm was used, and the emitted fluorescence was detected after passing a 320-nm cut-off filter. The apparent rate constants were extracted by fitting a single exponential with floating end point to the detected fluorescence trace,
where A is the amplitude of the fluorescence change, k obs the observed rate, and C the floating end point of the progression curve. Each k obs used for parameter extraction was determined from the average of eight to 12 reaction traces. The pre-steady-state parameters, K S, k 2, k −2, and k 3 were determined at enzyme concentrations of 0.5–4 μM with varying substrate concentrations in 0.1 M sodium-phosphate, pH 6.8, at 30°C. The substrate-dependence of k obs was plotted and fitted to Equation 3 with QNFIT in SIMFIT, allowing for determination of K S, k 2, and (k −2 + k 3). The individual values of k −2 + k 3 were determined from the expression of k cat (Equation 4) inserting the values of k 2 and k cat:
 |
 |
Thermostability
The remaining enzyme activities with 50 μM R,R-TSO in 0.1 M sodium phosphate, pH 6.8, at 30°C were determined for the wild-type and various mutants after preincubation at 55°C. The enzyme concentrations used were 50 nM for wild type, 51 nM for the Y149F mutant, 25 nM for the H153F mutant, and 26 nM for the double mutant. Substrates were added to the reaction mixtures at a volume resulting in a final concentration of 1% (v/v) acetonitrile. Aliquots were withdrawn every half hour for activity measurements. The activity prior to incubation at 55°C was set to 100% relative activity. To test for possible reversibility in inactivation, measurements on the Y149F mutant and the wild-type enzymes were performed every hour following a 3-h incubation at 55°C, followed by incubation at room temperature. At least three measurements for every incubation time point were made, and three different enzyme samples were used for all variants. In addition, the thermostabilities of wild-type and Y149F mutant enzymes (50 nM concentrations) were analyzed after incubation at varying temperatures, 30°C–85°C, for a defined period of time, 5 min. The remaining enzyme activities in the presence of 50 μM R,R-TSO in 0.1 M sodium phosphate, pH 6.8, were determined at 30°C. Retained activity after incubations was expressed as percent of enzyme activity determined prior to incubation.
Acknowledgments
We thank Mattias Engman for assisting in purification of the TSO enantiomers. This work was supported by the Ingegerd Bergh and Carl Trygger Foundations (M.W.) and by the Swedish Research Council (M.W. and S.L.M.). A.T. is a recipient of a stipend from the Lawski Foundation.
Footnotes
Reprint requests to: Mikael Widersten, Department of Biochemistry and Organic Chemistry, Uppsala University, Box 576, Biomedical Center, SE-751 23 Uppsala, Sweden; e-mail: mikael.widersten@biorg.uu.se; fax: 46 (0)18-55-8431.
Abbreviations: StEH1, epoxide hydrolase 1 from Solanum tuberosum; TSO, trans-stilbene oxide; PEG, polyethylene glycol; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.034173.107.
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arand, M., Cronin, A., Oesch, F., Mowbray, S.L., Jones, T.A. The telltale of epoxide hydrolases. Drug Metab. Rev. 2003;35:365–383. doi: 10.1081/dmr-120026498. [DOI] [PubMed] [Google Scholar]
- Archelas, A., Furstoss, R. Synthetic applications of epoxide hydrolases. Curr. Opin. Chem. Biol. 2001;5:112–119. doi: 10.1016/s1367-5931(00)00179-4. [DOI] [PubMed] [Google Scholar]
- Armstrong, R.N., Cassidy, C.S. New structural and chemical insights into the catalytic mechanism of epoxide hydrolases. Drug Metab. Rev. 2000;32:327–338. doi: 10.1081/dmr-100102337. [DOI] [PubMed] [Google Scholar]
- Berman, H.M., Battistuz, T., Bhat, T.N., Bluhm, W.F., Bourne, P.E., Burkhardt, K., Feng, Z., Gilliland, G.L., Iype, L., Jain, S., et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Decornez, H., Drukker, K., Hammes-Schiffer, S. Solvation and hydrogen-bonding effects on proton wires. J. Phys. Chem. A. 1999;103:2891–2898. [Google Scholar]
- Decoursey, T.E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- Elfström, L.T., Widersten, M. Catalysis of potato epoxide hydrolase, StEH1. Biochem. J. 2005;390:633–640. doi: 10.1042/BJ20050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfström, L.T., Widersten, M. Implications for an ionized alkyl-enzyme intermediate during StEH1-catalyzed trans-stilbene oxide hydrolysis. Biochemistry. 2006;45:205–212. doi: 10.1021/bi051893g. [DOI] [PubMed] [Google Scholar]
- Engh, R.A., Huber, R. Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr. 1991;A47:392–400. [Google Scholar]
- Evans, P.R. Warrington; England: 1993. Proceedings of CCP4 study weekend on data collection and processing. [Google Scholar]
- Fauth, M., Schweizer, O., Buchala, A., Markstädter, C., Riederer, M., Kato, T., Kauss, H. Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol. 1998;117:1373–1380. doi: 10.1104/pp.117.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, R.A.W., Titman, C.M., Pratap, J.V., Luisi, B.F., Preham, R.N. A molecular switch and proton wire synchronize the active sites in thiamine enzymes. Science. 2004;306:872–876. doi: 10.1126/science.1101030. [DOI] [PubMed] [Google Scholar]
- Imig, J.D. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am. J. Physiol. Renal Physiol. 2005;289:496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.-Y., Cowan, S.W., Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystalllogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kleywegt, G.J., Jones, T.A. Phi/Psi-cology: Ramachandran revisited. Structure. 1996;4:1395–1400. doi: 10.1016/s0969-2126(96)00147-5. [DOI] [PubMed] [Google Scholar]
- Koeller, K.M., Wong, C.-H. Enzymes for chemical synthesis. Nature. 2001;409:232–240. doi: 10.1038/35051706. [DOI] [PubMed] [Google Scholar]
- Kolattukudy, P.E. Structure, biosynthesis, and biodegradation of cutin and suberin. Annu. Rev. Plant Physiol. 1981;32:539–567. [Google Scholar]
- Leslie, A.G. Integration of macromolecular diffraction data. Acta Crystallogr. 1999;D55:1696–1702. doi: 10.1107/s090744499900846x. [DOI] [PubMed] [Google Scholar]
- Morrisseau, C., Hammock, B.D. Epoxide hydrolases: Mechanisms, inhibitor designs and biological roles. Annu. Rev. Pharmacol. Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- Mowbray, S.L., Elfström, L.T., Ahlgren, K.M., Andersson, C.E., Widersten, M. X-Ray structure of potato epoxide hydrolase sheds light on substrate specificity in plant enzymes. Protein Sci. 2006;15:1628–1637. doi: 10.1110/ps.051792106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov, G.N., Vagin, A.A., Dodson, E.J. Refinement of macromolecular structures by the maximum-likehood method. Acta Crystallogr. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Nagle, J.F., Morowitz, H.J. Molecular mechanisms for proton transport in membranes. Proc. Natl. Acad. Sci. 1978;75:298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis, D.L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S.M., Harel, M., Remington, S.J., Silman, I., Schrag, J., et al. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Pomès, R., Roux, B. Structure and dynamics of a proton wire: A theoretical study of H+ translocation along the single-file water chain in the Gramicidin A channel. Biophys. J. 1996;71:19–39. doi: 10.1016/S0006-3495(96)79211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomès, R., Roux, B. Free energy profiles for H+ conduction along hydrogen-bonded chains of water molecules. Biophys. J. 1998;75:33–40. doi: 10.1016/S0006-3495(98)77492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterton, E., Briggs, P., Turkenburg, M., Dodson, E. A graphical user interface to the CCP4 program suite. Acta Crystallogr. 2003;D59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. 1986;A42:140–149. [Google Scholar]
- Schmelzer, K.R., Kubala, L., Newman, J.W., Kim, I.-H., Eiserich, J.P., Hammock, B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinreiber, A., Faber, K. Microbial epoxide hydrolases for preparative biotransformations. Curr. Opin. Biotechnol. 2001;12:552–558. doi: 10.1016/s0958-1669(01)00262-2. [DOI] [PubMed] [Google Scholar]
- Thomaeus, A., Carlsson, J., Åqvist, J., Widersten, M. Active site of epoxide hydrolases revisited: A noncanonical residue in potato StEH1 promotes both formation and breakdown of the alkylenzyme intermediate. Biochemistry. 2007;46:2466–2479. doi: 10.1021/bi062052s. [DOI] [PubMed] [Google Scholar]
- Vagin, A., Teplyakov, A. MOLREP: An automated program for molecular replacement. J. Appl. Crystallogr. 1997;30:1022–1025. [Google Scholar]
- Wixtrom, R.N., Hammock, B.D. Continuous spectrophotometric assays for cytosolic epoxide hydrolase. Anal. Biochem. 1988;174:291–299. doi: 10.1016/0003-2697(88)90548-9. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Koerner, I.P., Noppens, R., Grafe, M., Tsai, H.-J., Morisseau, C., Luria, A., Hammock, B.D., Falck, J.R., Alkayed, N.J. Soluble epoxide hydrolase: A novel therapeutic target in stroke. J. Cereb. Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Yamamoto, T., Newman, J.W., Kim, I.-H., Watanabe, T., Hammock, B.D., Stewart, J., Pollock, J.S., Pollock, D.M., Imig, J.D. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J. Am. Soc. Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]