Abstract
Because the quinolines inhibit heme crystallization within the malaria parasite much work has focused on mechanism of formation and inhibition of hemozoin. Here we review the recent evidence for heme crystallization within lipids in diverse parasites and the new implications of a lipid site of crystallization for drug targeting. Within leukocytes hemozoin can generate toxic radical lipid metabolites, which may alter immune function or reduce deformability of uninfected erythrocytes.
1. Introduction
Nature has evolved a colorful spectrum of heme and non-heme proteins for oxygen transport. Copper in the enormous 3. 5 million kDa protein hemocyanin imparts a blue color to the blood of certain invertebrates[1]. Clear, colorless hemerythrin found in leeches and certain methanotrophic marine bacteria contains a unique diiron site confined within a hydrophobic pocket and turns bright pink when bound by oxygen[2]. Green chlorocruorin of annelids shifts to faint red when bound by oxygen[3]. Hemoproteins are a biologically diverse super family of proteins that catalyze a wide variety of biochemical reactions by virtue of their use of heme or variant porphyrins as a prosthetic group[4]. Cytochrome-c, cytochrome p450 isoforms, catalase, nitric oxide synthase, and peroxidases are all representative examples of hemoproteins[5–7]. The much studied hemoprotein, hemoglobin, is observed across the evolutionary tree from bacteria to fungi and higher eukaryotes, although not all hemoglobins function in gas exchange[8].
Erythrocytes, sometimes described as membrane bound bags of hemoglobin, function primarily for gas exchange. The human erythrocyte occupies a volume of approximately 90–100 fL with a large surface area for gas transfer of 135 sq microns in the shape of a biconcave disc[9]. Hemoglobin comprises about 95% of the protein content of the mature erythrocyte and is present at a concentration of roughly 350 mg/ml or 5 mM. Interestingly hemoglobin is not the most abundant erythrocyte molecule. Water occupies almost 72% of erythrocyte volume[9] and is present at 721 mg/ml with approximately 115 mg/ml hydrated directly to hemoglobin[10]. This hydrated fraction represents a molar ratio of 1,260 moles H2O per mole of hemoglobin[9].
2. Toxic effects of free heme
While heme functions in useful enzymatic reactions, a number of deleterious effects are directly and indirectly attributable to free heme once separated from its protein component. Less than 10–20 μM heme can inhibit many Plasmodium enzymes in the digestive vacuole like plasmepsins and falcipains[11, 12] and also enzymes in cytosol like glycolytic glyceraldehyde-3-phosphate dehydrogenase or 6-phosphogluconate dehydrogenase [13, 14]. Integration of the lipophilic heme molecule into biological membranes diminishes erythrocytes deformability and induces hemolysis[15–18]. Membrane heme also weakens the lipid bilayer by making it more susceptible to H2O2 mediated lysis[16, 18]. Integration of heme or hemichromes also disrupts the RBC membrane’s normally dynamic interaction with its underlying cytoskeletal proteins[18, 19]. High heme concentrations cause increased oxidative stress and the presence of peroxidation products are associated with decreased RBC membrane fluidity which probably amplifies cellular rigidity in the parasitized red cell[20].
Free heme catalyzes the generation of toxic lipid peroxidation products by a number of biochemical mechanisms. Catabolism of hemoglobin, releasing reactive heme and iron, is associated with the generation of the redox reactive substance such as H2O2, superoxide radicals and the hydroxyl radical which is the direct mediator of lipid peroxidation[21, 22]. Interaction between heme, intracellular hydrogen peroxide and lipids can results in lipid peroxidation[23]. The Fenton reaction (iron-catalyzed Haber-Weiss reaction) is the primary mechanism by which the hydroxyl radical is formed in the digestive vacuole[24].
Fenton reaction rates are influenced by the availability of the reaction’s catalyst iron and also the abundance of the substrates O2− and H2O2[25–27]. Intact hemoglobin may also mediate formation of oxidants for interactions of peroxides with ferric hemoglobin to produce reactive ferryl iron (Fe IV) as well as protein-associated free radicals[28].
The Fitch hypothesis proposed that chloroquine or active quinolines when bound or complexed with heme enhanced toxicity by similar mechanisms as free heme. The chloroquine-heme complex can also lyse membranes[29], peroxidize lipids[30] or interfere with enzymes[11].
3. Heme disposition
Cells and blood feeding organisms have developed diverse means to metabolize toxic heme released after ingestion and degradation of hemoglobin. On a cellular level mammalian macrophages residing in the liver and spleen “professionally” ingest senescent erythrocytes, with protease degradation of hemoglobin occurring in the acidic lysosome[31]. Toxic free heme is rapidly transported to cytosolic microsomal compartments where heme oxygenase releases bioavailable iron and the fragmented porphyrin ring which becomes bilirubin[32]. Most mammalian cells have a capacity to degrade heme on a smaller scale. Mammalian heme transport across membranes in the intestine or out of organellar compartments has been an enigma. Recently a number of mammalian proteins including heme-carrier protein 1 (HCP1), ABC transporter ABCG2, and feline leukaemic virus receptor have been shown to be involved with heme transport[33–36].
On an organism level, the protozoan parasite, Entoambea histolytica, by pathogenic definition, ingests erythrocytes which they rapidly degrade, probably via a heme oxygenase activity similar to a macrophage[37]. Another protozoan parasite, Babesia, although residing in an erythrocyte ingests little hemoglobin, but relies on pinocytosis for nutrient uptake[38]. Malaria pigment or hemozoin has been associated with the Plasmodium parasite for centuries, recently reviewed by Hempelmann[39]. Additionally described eukaryotic parasites which biologically form heme crystals include Hemoproteus[40] and two trematodes- Schistosoma[41, 42] and recently Echinostoma trivolvis rediae in snails[43]. While most blood feeding insects like mosquitoes, lice and ticks do not make heme crystals probably because the pH of their digestive tracts is not acidic, the insect genus, Rhodnius, which transmit Chagas disease, do make heme crystals[44–46].
4. Heme crystal structure and morphology
Hemozoin has long interested malariologists and its microscopic identification provided the crucial link in the identification of the Plasmodium parasite responsible for malaria as well as the arthropod vector species[39]. Many investigations into the formation and inhibition of the heme crystals called hemozoin, revolve around solving the mechanism of action and resistance of the widely used quinolines like chloroquine. Hemozoin contains oxidized ferric iron and is a crystal substance capable of rotating the plane of polarized light and generating a small magnetic field, physical characteristics of use in microscopic diagnosis [47–50]. In 1991 the intermolecular bond of hemozoin heme was discovered through use of infrared and x-ray absorption spectroscopy which showed that the fundamental chemical bond in purified hemozoin is the iron III-carboxylate coordinate bond that exists between the central ferric iron of one heme monomer and the propionic carboxylate side chain of a second[51]. It was initially hypothesized that hemozoin was comprised of long chains of polymerized heme linked through hydrogen bonding to adjacent heme polymers[51, 52]. In 2000 Pagola and the Bohle group showed by x-ray powder diffraction analysis that hemozoin is instead comprised of unit head-to-tail heme dimers where each crystal dimer consists of two heme molecules covalently linked through reciprocal iron-carboxylate coordinate bonding[53]. These dimers are then integrated into the crystal lattice of hemozoin through hydrogen bonding. This crystalline form of dimeric heme is unique to crystallized heme and should not be confused with the aqueous μ-oxo dimer of molecular oxygen sandwiched by heme[54]. The unit cell of the heme crystal dimer is approximately an one nanometer cube[53]. Hemozoin crystals isolated from the human malaria parasite have a brick like morphology with approximately 100 nm × 100 nm × 500 nm dimensions. Murine Plasmodium crystals are slightly smaller[55]. Crystals from avian Plasmodium and Haemoproteus have a rounded globular morphology of approximately 200–500 nm in diameter which upon close inspection appear to be composed of many small 25–50 nm “bricks”[55, 56]. Similarly the hemozoin morphology of Schistosoma and Rhodnius have a rounded multicrystalline shape[44]. In vitro synthesis of β-hematin crystals sometimes produces a tapered needle like morphology that has been attributed to rapid growth at faces. Inhibitory concentration of the quinolines or xanthones also produce tapered needlelike crystals[57]. Figure 1 compares the sizes of heme, hemozoin, erythrocytes and a mosquito to water, glucose, antibodies, mammalian cells and ultimately a tennis ball on a log 10 scale. Antibodies and toll-like receptor 9 have both been proposed to interact directly with hemozoin[58–63]. Considering the relative size of the binding pocket of antibodies or TLR9, which are 10 to hundred times smaller than the smooth faces of heme crystals, these might be planar hydrogen bonding, possibly nonspecific low affinity interactions. Recent work suggests malaria DNA contamination in some experimental purifications of hemozoin used to measure TLR9 binding. This complicates the TLR9 hypothesis, because TLR9 normally binds DNA and may have bound DNA on hemozoin rather than to hemozoin itself[58].
Figure 1.
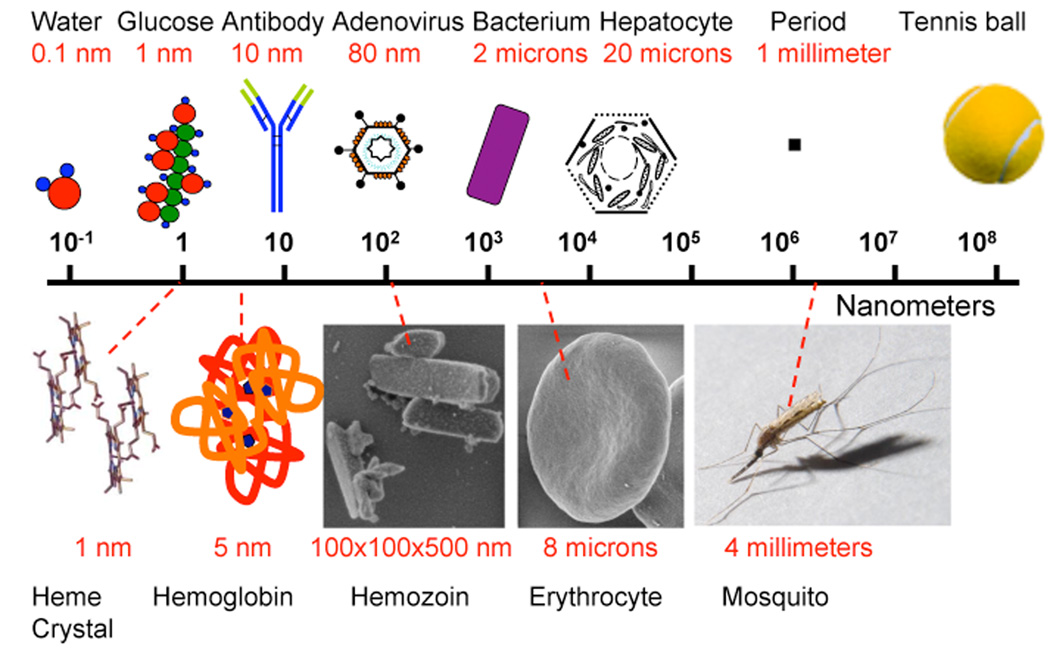
Nanometer scale bar illustrating the relative size of objects ranging from a 0. 1 nm with a water molecule (far left) to a 100 mm tennis ball (far right).
Ferrous-protoporphyrin IX released from hemoglobin is rapidly oxidized to ferric-protoporphyrin IX in an aqueous acidic oxygen rich environment of the digestive vacuole. Abundant water molecules also quickly complex with axial heme iron. Disagreement exists on the nature of aqueous heme inside the digestive vacuole whether as the μ-oxo dimer with the iron ligands directed inward to oxygen[54, 64] and or as a non crystalline planar porphyrin dimer with outward iron coordinated with H2O or –OH (hematin)[65]. Roepe and colleagues have reviewed the longstanding evidence for the μ-oxo dimer or alternative heme dimers or aggregates at an acid pH[66]. Recent evidence by Egan and colleagues suggest another non crystalline dimer suggested to be in the digestive vacuole[65]. The transition of either aqueous species to heme crystal dimmers would be quite different. The μ-oxo dimer requires removal of axial oxygen with lateral protoporphyrin IX shift to approximate the iron propionate linkage and the Egan heme dimer requires removal of outward axial hydroxides and iron to transition through the plane of the protoporphyrin ring.
5. Lipid formation of heme crystals
How exactly aqueous heme is removed from the acidic digestive vacuolar solution and converted into harmless hemozoin in order to avert membrane lysis, lipid peroxidation and inevitable parasite destruction has been the subject of much debate and previous reviews[56, 67–70]. While β-hematin heme crystals can be synthesized as a chemical process taking place more rapidly with high temperatures or high concentrations of protons or heme[71], the catalyst in a biologic protein/lipid aqueous solution has not been fully resolved. The weight of evidence has recently shifted away from histidine-rich protein II or III[30, 72–74]to lipid[75–78] initiation. Also diminishing the current evidence for protein initiation is the fact that a P. falciparum laboratory clone that lacks both histidine-rich protein II and III, still makes hemozoin. P. vivax and the murine Plasmodium species also lack orthologues to histidine-rich protein II or III while they still make hemozoin[56]. Bendrat and Cerami[79] initially proposed possible polar lipid initiation, substantiated by Dorn and Ridley[80, 81]. Fitch also proposed a role for neutral lipids, but was not able to identify digestive vacuolar lipids[76]. Tripathi and others suggested a combination of proteins and lipids[75, 82]. Recent work in Plasmodium associated neutral lipid bodies both on the interior and the exterior of digestive vacuoles[83–85]. Based on the published electron micrographic evidence of intra vacuolar lipid bodies preserved by malachite green, Pisciotta identified by mass spectrometric analysis neutral lipids closely associated with hemozoin[86]. Many experiments however show that erythrocyte membrane ghosts alone do not efficiently initiate heme crystals[76, 86, 87]. Alcohols and even detergents titrated to particular concentrations also efficiently promote crystallization[88]. In the intestines of Schistosoma and Rhodnius heme crystals are visualized at surface of lipid droplets[77, 87]. Formalin pigment forms in lipid-rich blood tissues that have been fixed in formaldehyde which gradually turns into formic acid[89]. Subcellular fractionation of Plasmodium hemozoin has identified both neutral lipids and polar lipids in these digestive vacuole lipid bodies suggesting some polar lipids may be at surface with interior composed of neutral lipids[86]. Recent images in Schistosoma also demonstrated bilaminar membranes encircling lipid bodies[77]. Yet to be proven is existence of a lipase or phospholipase in situ at place of heme crystallization, which would produce a neutral lipid body from polar membranes like the parasitophorus vacuole membrane as proposed by Hempelmann[90]. Cleavage of the polar head group by phospholipase C would produce diacylglycerols rather than cleavage of a fatty acid by phosplipase A (snake venoms) which produces the detergent-like lysolecithins that induce pores in membranes.
Crystallographers set up thousands of conditions to carefully remove surface water from hydrated proteins to promote crystal growth. Aqueous heme likewise is hydrated by axial iron hydroxide in equilibrium with abundant digestive vacuole water at an acid pH. The oily lipid-water interface is a means to transiently remove axial iron bound water so that the central iron of one heme is free to coordinate to a propionate carboxylate –OH of an adjacent heme. Egan expands on the chemistry and equilibrium dynamics of axial water removal at the lipid interface in a formal molecular dynamic calculation[78]. Formation of the head to tail heme crystal dimer is just the first step. The unit heme crystal dimer may still be able to enter membranes to cause hemolysis or bind to active sites on proteins or enzymes. The subsequent hemozoin construction step is efficient assembly of individual heme crystal dimers into a large 100 nm × 100 nm × 500 nm crystal potentially containing 10,000,000 hemes. The neutral lipid environment may also protect from interference of peroxide degradation[40, 55, 86], small oligopeptides or other molecule crystal disrupters. Imaged heme crystals have no “gaps” in the lattice[91]. Hemozoin inhibitors can act at either the formation of the unit head to tail heme crystal dimer by drug-heme complex competition or assembly into large crystal by drug-heme complex binding to growing face to prevent addition of more heme crystal dimers. The reversible inhibition characterized by Egan[92] and Chong[93] suggest that binding to growing face can change over time. This also suggests that different quinoline-heme complexes may have different affinities at growing face with different “on” or “off” binding rates. Evidence of drug binding to heme crystal dimer has not been investigated in contrast to much reported drug monomeric or dimeric heme binding.
6. Drug Inhibition
The extensive chemistry of heme-drug interactions and inhibition of heme crystal formation has been eloquently reviewed[67, 69, 94–96]. The site of lipid heme crystallization has important implications for drug inhibition. These new findings predict that drug inhibition is nonaqueous chemistry in an oily hydrophobic environment. The weak bases like the quinolines accumulate to high concentrations in the acidic digestive vacuole compartment. Warhurst and colleagues recently have shown a high aqueous vacuolar accumulation ratio for chloroquine as a free drug (meaning not complexed to heme). The lipid accumulation ratio of free drug was much smaller, almost 1,000 fold, and constant despite changes in pH which affected aqueous vacuolar accumulation ratio[97]. It would be interesting to model a drug heme complex which would be predicted to have a higher lipid accumulation ratio. Bray and Ward also noted that higher lipophilicity constants amongst a diverse set of quinolines was correlated with lower inhibitory concentrations against chloroquine-resistant parasites[98]. A long standing piece of the puzzle regarding P. falciparum quinoline resistance has been lack of correlation of resistance amongst the active quinolines in contrast to multidrug resistant cancer cells which are resistant to diverse compounds. Mefloquine which is very lipophilic often has inverse drug sensitivity profiles to chloroquine which has low lipophilicity[99]. Mefloquine is effective despite lower total vacuolar accumulation because more may concentrate in the lipid target site[99].
Roepe and colleagues have used realtime spinning disk confocal microscopy to obtain precise measurements of hemozoin growth in live parasites[100]. There were only minor differences in sigmoidal growth curves between chloroquine-resistant and chloroquine-sensitive parasites with a peak hemozoin growth at the ring-trophozoite stage approximately 15–30 hours. Interestingly chloroquine did not inhibit initial growth up in the 15 to 22 hour window, where more than half of hemozoin was made. There were decreases in amount made after this time point, which when graphed indicated a linear relationship between inhibition of hemozoin and growth. As stated in discussion the effect on late hemozoin growth was suggestive of effect on not initial nucleation but subsequent large crystal formation such as hypothesized to block at growing crystal face. They had the intriguing note that chloroquine did not effect parasite morphology during the cell cycle administered, but had marked effect on subsequent cycle which may be followed up in further studies.
7. Lipid toxicity products
Lipid peroxidation products are elevated in the parasitized RBC. The major lipid peroxidation products observed to date are hydroxyeicosatetraenoic acids (HETE) and hydroperoxyoctadecadienoic acids (HODE) which also are directly associated with hemozoin[101]. HETE arises from peroxidation of arachadonic acid[102]. Peroxidation linoleic acid causes HODE formation[101]. These lipids are present in high concentration in parasitized cells versus uninfected cells and have cytotoxic properties[101, 103].
In the mid 1990’s a dramatic increase in the intracellular monocytic concentration of a very highly reactive, electrophilic lipid peroxidation product called 4-hydroxynonenal (HNE) was observed[104]. The difference was between resting monocytes with 5 nmoles HNE per 1010 cells and monocytes that had phagocytosed hemozoin with 230 nmoles HNE per 1010 cells[104]. Hemozoin, known to persist in white blood cells (WBCs) since before the time of Lavaran, induces a protracted, though ineffective WBC oxidative burst with subsequent anergy after hemozoin ingestion[105]. Since the concentration of HNE within hemozoin treated WBCs increased over the course of the oxidative burst is stood to reason that this normally potent antimicrobial WBC response had backfired in the form of runaway heme mediated lipoperoxidation of host cell lipids[70, 104]. Since the acidic pH of the phagolysosome is comparable to the parasites digestive vacuole which is required for hemozoin formation a comparable mechanism may be at work in the parasites. Synthetic hemozoin (beta-hematin) is now known to generate HNE when exposed to polyunsaturated lipids in vitro[106]. HNE has not yet been identified in parasitized RBCs but HNE-protein adducts have been reported to decrease erythrocyte membrane deformability[107]. This illustrates that downstream protein oxidation events can be caused indirectly by heme. Potential mechanisms may include HNE’s electrophilic attack of sulfydral groups, thioester to hemiacytal formation, electrophilic attack of nucleophilic proteins and stable Michael adducts. Such alterations can result in disrupted enzymatic and structural function of the cell and HNE is recognized as a toxin capable of inducing apoptosis in some cell types[108, 109]. Gowda has described a role for GPI anchors as malaria toxins[110]. Lipid peroxidation products such as HNE appear to fit the description of an additional non specific malaria toxin.
8. Summary and questions with uncrystallized answers
Lipid droplets catalyze the biomineralization of heme to hemozoin by concentrating lipophilic heme from the aqueous environment in which hemoglobin digestion transpires. The exclusion of water facilitates heme’s transition from the aqueous dimer to the iron (III)--carboxylate heme crystal dimer. Inside the non-polar, hydrophobic microenvironment of the lipid droplet individual heme crystal dimers then integrate via hydrogen bonding into the growing face of the hemozoin crystal. Questions with uncrystallized answers include: Do quinoline antimalarials block formation of the heme crystal dimer and/or its integration into the growing crystal? How toxic are the individual heme crystal dimers? Is the drug heme complex the toxin that kills the parasite or heme crystal dimers that do not form into the larger crystal? Once made do they return to an aqueous environment to bind to enzymes? What is the state of the aqueous heme before crystallization? What role do proteins function in the process of chaperoning heme to lipid interfaces? How does the parasite synthesize and traffic to the digestive vacuole the lipids involved in hemozoin formation? What are the lipid peroxidation products generated by hemozoin within the infected erythrocyte and do they have an effect on the parasite? Could the lipid droplet serve as a sink for both toxic lipids and uncrystallised heme? Can drug heme complexes still make heme crystal dimers? Finally, why are snowflake crystals each different, but hemozoin regular?
Figure 2.

Hemoglobin ingested from the host erythrocyte cytosol (RBC) via the cytostome and transport vescicles (TV) is hydrolytically degraded by parasite proteases inside the acidic digestive vacuole (DV). Hydrophobic free heme released during hemoglobin catabolism is believed to accumulate within neutral lipid nanospheres present within the DV. The entry of hydrophobic heme into the NLN excludes water and favors the formation of head to tail heme dimers at the NLN interface. Concentrated heme dimers then assimilate into the growing face of the hemozoin crystal lattice. Heme chloroquine complexes are required for delivery of quinolines into the NLN interior where they may disrupt either dimer formation and/or dimer integration into larger crystal. Nucleus (N), mitochondria (M), apicoplast (A), plasma membrane (PM) and parasitophorous vacuole membrane (PVM). Cartoon is not to scale.
Acknowledgements
D. S. would like to acknowledge the support of the NIH RO1 AI045774 and Abhai Tripathi for a review of manuscript. Rebecca Kent photographed the Anopheles arabiensis mosquito in Macha, Zambia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin AG, Depoix F, Stohr M, Meissner U, Hagner-Holler S, Hammouti K, et al. Limulus polyphemus hemocyanin: 10 A cryo-EM structure, sequence analysis, molecular modelling and rigid-body fitting reveal the interfaces between the eight hexamers. J.Mol.Biol. 2007;366:1332. doi: 10.1016/j.jmb.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 2.Karlsen OA, Ramsevik L, Bruseth LJ, Larsen O, Brenner A, Berven FS, et al. Characterization of a prokaryotic haemerythrin from the methanotrophic bacterium Methylococcus capsulatus (Bath) FEBS J. 2005;272:2428. doi: 10.1111/j.1742-4658.2005.04663.x. [DOI] [PubMed] [Google Scholar]
- 3.Imai K, Sharma PK, Vinogradov SN. Oxygen binding properties of Eudistylia vancouverii chlorocruorin and its dodecamer subunit. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:613–618. doi: 10.1016/0305-0491(96)83567-x. [DOI] [PubMed] [Google Scholar]
- 4.Mense SM, Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16:681. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- 5.Horie S, Hasumi H, Takizawa N. Heme-linked spectral changes of the protein moiety of hemoproteins in the near ultraviolet region. J Biochem (Tokyo) 1985;97:281–293. doi: 10.1093/oxfordjournals.jbchem.a135052. [DOI] [PubMed] [Google Scholar]
- 6.Beri R, Chandra R. Chemistry and biology of heme. Effect of metal salts, organometals, and metalloporphyrins on heme synthesis and catabolism, with special reference to clinical implications and interactions with cytochrome P-450. Drug Metab Rev. 1993;25:49–152. doi: 10.3109/03602539308993973. [DOI] [PubMed] [Google Scholar]
- 7.Rydberg P, Sigfridsson E, Ryde U. On the role of the axial ligand in heme proteins: a theoretical study. J Biol Inorg Chem. 2004;9:203–223. doi: 10.1007/s00775-003-0515-y. [DOI] [PubMed] [Google Scholar]
- 8.Frey AD, Farres J, Bollinger CJ, Kallio PT. Bacterial hemoglobins and flavohemoglobins for alleviation of nitrosative stress in Escherichia coli. Appl.Environ.Microbiol. 2002;68:4835. doi: 10.1128/AEM.68.10.4835-4840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin RL, Cravalho EG, Huggins CE. Effect of hydration on the water content of human erythrocytes. Biophys J. 1976;16:1411–1426. doi: 10.1016/S0006-3495(76)85784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drabkin DL. Spectrophotometric studies. XV. Hydration of macro sized crystals of human hemoglobin, and osmotic concentrations in red cells. J Biol Chem. 1950;185:231–245. [PubMed] [Google Scholar]
- 11.Gluzman IY, Francis SE, Oksman A, Smith CE, Duffin KL, Goldberg DE. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J Clin Invest. 1994;93:1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Jagt DL, Hunsaker LA, Campos NM. Comparison of proteases from chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Biochem Pharmacol. 1987;36:3285–3291. doi: 10.1016/0006-2952(87)90646-0. [DOI] [PubMed] [Google Scholar]
- 13.Campanale N, Nickel C, Daubenberger CA, Wehlan DA, Gorman JJ, Klonis N, et al. Identification and characterization of heme-interacting proteins in the malaria parasite, Plasmodium falciparum. J Biol Chem. 2003;278:27354–27361. doi: 10.1074/jbc.M303634200. [DOI] [PubMed] [Google Scholar]
- 14.Famin O, Ginsburg H. The treatment of Plasmodium falciparum-infected erythrocytes with chloroquine leads to accumulation of ferriprotoporphyrin IX bound to particular parasite proteins and to the inhibition of the parasite's 6-phosphogluconate dehydrogenase. Parasite. 2003;10:39–50. doi: 10.1051/parasite/2003101p39. [DOI] [PubMed] [Google Scholar]
- 15.Light WR, 3rd, Olson JS. Transmembrane movement of heme. J Biol Chem. 1990;265:15623–15631. [PubMed] [Google Scholar]
- 16.Chou AC, Fitch CD. Mechanism of hemolysis induced by ferriprotoporphyrin IX. J.Clin.Invest. 1981;68:672. doi: 10.1172/JCI110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitch CD, Chevli R, Kanjananggulpan P, Dutta P, Chevli K, Chou AC. Intracellular ferriprotoporphyrin IX is a lytic agent. Blood. 1983;62:1165. [PubMed] [Google Scholar]
- 18.Omodeo-Sale F, Motti A, Dondorp A, White NJ, Taramelli D. Destabilisation and subsequent lysis of human erythrocytes induced by Plasmodium falciparum haem products. Eur J Haematol. 2005;74:324–332. doi: 10.1111/j.1600-0609.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 19.Meera S, Rao AV, D'Souza V, Rao SB. In vitro studies on peroxidative changes leading to hemolysis of erythrocytes infested with malarial parasite Plasmodium vivax. Indian J.Exp.Biol. 1999;37:729. [PubMed] [Google Scholar]
- 20.Levin G, Cogan U, Levy Y, Mokady S. Riboflavin deficiency and the function and fluidity of rat erythrocyte membranes. J.Nutr. 1990;120:857. doi: 10.1093/jn/120.8.857. [DOI] [PubMed] [Google Scholar]
- 21.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int.J.Parasitol. 2004;34:163. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Har-El R, Marva E, Chevion M, Golenser J. Is hemin responsible for the susceptibility of Plasmodia to oxidant stress? Free Radic Res Commun. 1993;18:279–290. doi: 10.3109/10715769309147495. [DOI] [PubMed] [Google Scholar]
- 23.Klouche K, Morena M, Canaud B, Descomps B, Beraud JJ, Cristol JP. Mechanism of in vitro heme-induced LDL oxidation: effects of antioxidants. Eur.J.Clin.Invest. 2004;34:619. doi: 10.1111/j.1365-2362.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 24.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 25.Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia. Semin.Hematol. 1990;27:70. [PubMed] [Google Scholar]
- 26.Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat.Res. 1996;145:523. [PubMed] [Google Scholar]
- 27.Natella F, Nardini M, Ursini F, Scaccini C. Oxidative modification of human low-density lipoprotein by horseradish peroxidase in the absence of hydrogen peroxide. Free Radic.Res. 1998;29:427. doi: 10.1080/10715769800300471. [DOI] [PubMed] [Google Scholar]
- 28.Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, Wilson MT, et al. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem.J. 2006;399:513. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitch CD. Mode of action of antimalarial drugs. Ciba Found Symp. 1983;94:222–232. [PubMed] [Google Scholar]
- 30.Loria P, Miller S, Foley M, Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem J. 1999;339(Pt 2):363–370. [PMC free article] [PubMed] [Google Scholar]
- 31.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 32.Zuwala-Jagiello J. Haemoglobin scavenger receptor: function in relation to disease. Acta Biochim Pol. 2006;53:257–268. [PubMed] [Google Scholar]
- 33.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Rouault TA. The intestinal heme transporter revealed. Cell. 2005;122:649. doi: 10.1016/j.cell.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Latunde-Dada GO, Simpson RJ, McKie AT. Recent advances in mammalian haem transport. Trends Biochem.Sci. 2006;31:182. doi: 10.1016/j.tibs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Hamza I. Intracellular trafficking of porphyrins. ACS Chem.Biol. 2006;1:627. doi: 10.1021/cb600442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boettner DR, Huston CD, Sullivan JA, Petri WA., Jr Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect Immun. 2005;73:3422–3430. doi: 10.1128/IAI.73.6.3422-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langreth SG. Feeding mechanisms in extracellular Babesia microti and Plasmodium lophurae. J Protozool. 1976;23:215–223. doi: 10.1111/j.1550-7408.1976.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 39.Hempelmann E. Hemozoin Biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Res. 2007;100:671–676. doi: 10.1007/s00436-006-0313-x. [DOI] [PubMed] [Google Scholar]
- 40.Chen MM, Shi L, Sullivan DJ., Jr Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and beta-hematin. Mol Biochem Parasitol. 2001;113:1–8. doi: 10.1016/s0166-6851(00)00365-0. [DOI] [PubMed] [Google Scholar]
- 41.Moore GA, Homewood CA, Gilles HM. A comparison of pigment from Schistosoma mansoni and Plasmodium berghei. Ann Trop Med Parasitol. 1975;69:373–374. doi: 10.1080/00034983.1975.11687021. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira MF, d'Avila JC, Torres CR, Oliveira PL, Tempone AJ, Rumjanek FD, et al. Haemozoin in Schistosoma mansoni. Mol Biochem Parasitol. 2000;111:217–221. doi: 10.1016/s0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- 43.Pisciotta JM, Ponder EL, Fried B, Sullivan D. Hemozoin formation in Echinostoma trivolvis rediae. Int J Parasitol. 2005;35:1037–1042. doi: 10.1016/j.ijpara.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, Hempelmann E, et al. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 2005;579:6010–6016. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira MF, Silva JR, Dansa-Petretski M, de Souza W, Braga CM, Masuda H, et al. Haemozoin formation in the midgut of the blood-sucking insect Rhodnius prolixus. FEBS Lett. 2000;477:95–98. doi: 10.1016/s0014-5793(00)01786-5. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira MF, Silva JR, Dansa-Petretski M, de Souza W, Lins U, Braga CM, et al. Haem detoxification by an insect. Nature. 1999;400:517–518. doi: 10.1038/22910. [DOI] [PubMed] [Google Scholar]
- 47.Homewood CA, Moore GA, Wwarhurst DC, Atkinson EM. Purification and some properties of malarial pigment. Ann.Trop.Med.Parasitol. 1975;69:283. doi: 10.1080/00034983.1975.11687012. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence C, Olson JA. Birefringent hemozoin identifies malaria. Am J Clin Pathol. 1986;86:360–363. doi: 10.1093/ajcp/86.3.360. [DOI] [PubMed] [Google Scholar]
- 49.Trang DT, Huy NT, Kariu T, Tajima K, Kamei K. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar J. 2004;3:7. doi: 10.1186/1475-2875-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairlamb AH, Paul F, Warhurst DC. A simple magnetic method for the purification of malarial pigment. Mol Biochem Parasitol. 1984;12:307–312. doi: 10.1016/0166-6851(84)90087-2. [DOI] [PubMed] [Google Scholar]
- 51.Slater AF, Swiggard WJ, Orton BR, Flitter WD, Goldberg DE, Cerami A, et al. An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci U S A. 1991;88:325–329. doi: 10.1073/pnas.88.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slater AF, Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992;355:167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- 53.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 54.Leed A, DuBay K, Ursos LM, Sears D, De Dios AC, Roepe PD. Solution structures of antimalarial drug-heme complexes. Biochemistry. 2002;41:10245–10255. doi: 10.1021/bi020195i. [DOI] [PubMed] [Google Scholar]
- 55.Noland GS, Briones N, Sullivan DJ., Jr The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol Biochem Parasitol. 2003;130:91–99. doi: 10.1016/s0166-6851(03)00163-4. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan DJ. Theories on malarial pigment formation and quinoline action. Int J Parasitol. 2002;32:1645–1653. doi: 10.1016/s0020-7519(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 57.Solomonov I, Osipova M, Feldman Y, Baehtz C, Kjaer K, Robinson IK, et al. Crystal nucleation, growth, and morphology of the synthetic malaria pigment beta-hematin and the effect thereon by quinoline additives: the malaria pigment as a target of various antimalarial drugs. J Am Chem Soc. 2007;129:2615–2627. doi: 10.1021/ja0674183. [DOI] [PubMed] [Google Scholar]
- 58.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coban C, Ishii KJ, Uematsu S, Arisue N, Sato S, Yamamoto M, et al. Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol. 2007;19:67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 60.Ishii KJ, Uematsu S, Akira S. 'Toll' gates for future immunotherapy. Curr Pharm Des. 2006;12:4135–4142. doi: 10.2174/138161206778743484. [DOI] [PubMed] [Google Scholar]
- 61.Engwerda CR, Good MF. Interactions between malaria parasites and the host immune system. Curr Opin Immunol. 2005;17:381–387. doi: 10.1016/j.coi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Togbe D, Schofield L, Grau GE, Schnyder B, Boissay V, Charron S, et al. Murine cerebral malaria development is independent of toll-like receptor signaling. Am J Pathol. 2007;170:1640–1648. doi: 10.2353/ajpath.2007.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egan TJ. Interactions of quinoline antimalarials with hematin in solution. J Inorg Biochem. 2006;100:916–926. doi: 10.1016/j.jinorgbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 65.de Villiers KA, Kaschula CH, Egan TJ, Marques HM. Speciation and structure of ferriprotoporphyrin IX in aqueous solution: spectroscopic and diffusion measurements demonstrate dimerization, but not mu-oxo dimer formation. J Biol Inorg Chem. 2007;12:101–117. doi: 10.1007/s00775-006-0170-1. [DOI] [PubMed] [Google Scholar]
- 66.Ursos LM, Roepe PD. Chloroquine resistance in the malarial parasite, Plasmodium falciparum. Med Res Rev. 2002;22:465–491. doi: 10.1002/med.10016. [DOI] [PubMed] [Google Scholar]
- 67.Egan TJ. Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. J Inorg Biochem. 2002;91:19–26. doi: 10.1016/s0162-0134(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 68.O'Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines--past, present, and future: a chemical perspective. Pharmacol Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 69.Slater AF. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol.Ther. 1993;57:203. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]
- 70.Arese P, Schwarzer E. Malarial pigment (haemozoin): a very active 'inert' substance. Ann Trop Med Parasitol. 1997;91:501–516. doi: 10.1080/00034989760879. [DOI] [PubMed] [Google Scholar]
- 71.Egan TJ, Ross DC, Adams PA. Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment) FEBS Lett. 1994;352:54–57. doi: 10.1016/0014-5793(94)00921-x. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan DJ, Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996;271:219–222. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 73.Choi CY, Cerda JF, Chu HA, Babcock GT, Marletta MA. Spectroscopic characterization of the heme-binding sites in Plasmodium falciparum histidine-rich protein 2. Biochemistry. 1999;38:16916–16924. doi: 10.1021/bi991665k. [DOI] [PubMed] [Google Scholar]
- 74.Schneider EL, Marletta MA. Heme binding to the histidine-rich protein II from Plasmodium falciparum. Biochemistry. 2005;44:979–986. doi: 10.1021/bi048570p. [DOI] [PubMed] [Google Scholar]
- 75.Tripathi AK, Garg SK, Tekwani BL. A physiochemical mechanism of hemozoin (beta-hematin) synthesis by malaria parasite. Biochem Biophys Res Commun. 2002;290:595–601. doi: 10.1006/bbrc.2001.6231. [DOI] [PubMed] [Google Scholar]
- 76.Fitch CD, Cai GZ, Chen YF, Shoemaker JD. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim Biophys Acta. 1999;1454:31–37. doi: 10.1016/s0925-4439(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 77.Correa Soares JB, Maya-Monteiro CM, Bittencourt-Cunha PR, Atella GC, Lara FA, d'Avila JC, et al. Extracellular lipid droplets promote hemozoin crystallization in the gut of the blood fluke Schistosoma mansoni. FEBS Lett. 2007;581:1742–1750. doi: 10.1016/j.febslet.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 78.Egan TJ, Chen JY, de Villiers KA, Mabotha TE, Naidoo KJ, Ncokazi KK, et al. Haemozoin (beta-haematin) biomineralization occurs by self-assembly near the lipid/water interface. FEBS Lett. 2006;580:5105–5110. doi: 10.1016/j.febslet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 79.Bendrat K, Berger BJ, Cerami A. Haem polymerization in malaria. Nature. 1995;378:138–139. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- 80.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 81.Dorn A, Vippagunta SR, Matile H, Bubendorf A, Vennerstrom JL, Ridley RG. A comparison and analysis of several ways to promote haematin (haem) polymerisation and an assessment of its initiation in vitro. Biochem Pharmacol. 1998;55:737–747. doi: 10.1016/s0006-2952(97)00509-1. [DOI] [PubMed] [Google Scholar]
- 82.Pandey AV, Babbarwal VK, Okoyeh JN, Joshi RM, Puri SK, Singh RL, et al. Hemozoin formation in malaria: a two-step process involving histidine-rich proteins and lipids. Biochem Biophys Res Commun. 2003;308:736–743. doi: 10.1016/s0006-291x(03)01465-7. [DOI] [PubMed] [Google Scholar]
- 83.Vielemeyer O, McIntosh MT, Joiner KA, Coppens I. Neutral lipid synthesis and storage in the intraerythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol. 2004;135:197–209. doi: 10.1016/j.molbiopara.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 84.Jackson KE, Klonis N, Ferguson DJ, Adisa A, Dogovski C, Tilley L. Food vacuole-associated lipid bodies and heterogeneous lipid environments in the malaria parasite, Plasmodium falciparum. Mol Microbiol. 2004;54:109–122. doi: 10.1111/j.1365-2958.2004.04284.x. [DOI] [PubMed] [Google Scholar]
- 85.Palacpac NM, Hiramine Y, Seto S, Hiramatsu R, Horii T, Mitamura T. Evidence that Plasmodium falciparum diacylglycerol acyltransferase is essential for intraerythrocytic proliferation. Biochem Biophys Res Commun. 2004;321:1062–1068. doi: 10.1016/j.bbrc.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 86.Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, Bajad S, et al. The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J. 2007;402:197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silva JR, Mury FB, Oliveira MF, Oliveira PL, Silva CP, Dansa-Petretski M. Perimicrovillar membranes promote hemozoin formation into Rhodnius prolixus midgut. Insect Biochem Mol Biol. 2007;37:523–531. doi: 10.1016/j.ibmb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Huy NT, Maeda A, Uyen DT, Trang DT, Sasai M, Shiono T, et al. Alcohols induce beta-hematin formation via the dissociation of aggregated heme and reduction in interfacial tension of the solution. Acta Trop. 2007;101:130–138. doi: 10.1016/j.actatropica.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Pizzolato P. Formalin pigment (acid hematin) and related pigments. Am J Med Technol. 1976;42:436–440. [PubMed] [Google Scholar]
- 90.Hempelmann E, Motta C, Hughes R, Ward SA, Bray PG. Plasmodium falciparum: sacrificing membrane to grow crystals? Trends Parasitol. 2003;19:23–26. doi: 10.1016/s1471-4922(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 91.Moore GA, Boothroyd B. Direct resolution of the lattice planes of malarial pigment. Ann Trop Med Parasitol. 1974;68:489. doi: 10.1080/00034983.1974.11686977. [DOI] [PubMed] [Google Scholar]
- 92.Egan TJ, Ncokazi KK. Quinoline antimalarials decrease the rate of beta-hematin formation. J Inorg Biochem. 2005;99:1532–1539. doi: 10.1016/j.jinorgbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 93.Chong CR, Sullivan DJ., Jr Inhibition of heme crystal growth by antimalarials and other compounds: implications for drug discovery. Biochem Pharmacol. 2003;66:2201–2212. doi: 10.1016/j.bcp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Bray PG, Ward SA, O'Neill PM. Quinolines and artemisinin: chemistry, biology and history. Curr Top Microbiol Immunol. 2005;295:3–38. doi: 10.1007/3-540-29088-5_1. [DOI] [PubMed] [Google Scholar]
- 95.O'Niell PM, Bray PG, Hawley SR, Ward SA, Park KB. 4-Aminoquinolines-Past, Present and Future: a Chemical Perspective. Pharmocol. Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 96.de Dios AC, Casabianca LB, Kosar A, Roepe PD. Structure of the amodiaquine-FPIX mu oxo dimer solution complex at atomic resolution. Inorg Chem. 2004;43:8078–8084. doi: 10.1021/ic0489948. [DOI] [PubMed] [Google Scholar]
- 97.Warhurst DC, Craig JC, Adagu IS, Guy RK, Madrid PB, Fivelman QL. Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and resistant blood-stages of Plasmodium falciparum. Role of beta-haematin inhibition and drug concentration in vacuolar water- and lipid-phases. Biochem Pharmacol. 2007;73:1910–1926. doi: 10.1016/j.bcp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 98.Bray PG, Hawley SR, Mungthin M, Ward SA. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol Pharmacol. 1996;50:1559–1566. [PubMed] [Google Scholar]
- 99.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79:55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 100.Gligorijevic B, McAllister R, Urbach JS, Roepe PD. Spinning disk confocal microscopy of live, intraerythrocytic malarial parasites. 1. Quantification of hemozoin development for drug sensitive versus resistant malaria. Biochemistry. 2006;45:12400–12410. doi: 10.1021/bi061033f. [DOI] [PubMed] [Google Scholar]
- 101.Schwarzer E, Kuhn H, Valente E, Arese P. Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood. 2003;101:722–728. doi: 10.1182/blood-2002-03-0979. [DOI] [PubMed] [Google Scholar]
- 102.Green MD, Xiao V, Lal AA. Formation of hydroxyeicosatetraenoic acids from hemozoin-catalyzed oxidation of arachidonic acid. Mol Biochem Parasitol. 1996;83:183–188. doi: 10.1016/s0166-6851(96)02769-7. [DOI] [PubMed] [Google Scholar]
- 103.Schwarzer E, Ludwig P, Valente E, Arese P. 15(S)-hydroxyeicosatetraenoic acid (15-HETE), a product of arachidonic acid peroxidation, is an active component of hemozoin toxicity to monocytes. Parassitologia. 1999;41:199–202. [PubMed] [Google Scholar]
- 104.Schwarzer E, Muller O, Arese P, Siems WG, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- 105.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller CM, Carney CK, Schrimpe AC, Wright DW. beta-Hematin (hemozoin) mediated decompostion of polyunsaturated fatty acids to 4-hydroxy-2-nonenal. Inorg Chem. 2005;44:2134–2136. doi: 10.1021/ic048821i. [DOI] [PubMed] [Google Scholar]
- 107.Skorokhod A, Schwarzer E, Gremo G, Arese P. HNE produced by the malaria parasite Plasmodium falciparum generates HNE-protein adducts and decreases erythrocyte deformability. Redox Rep. 2007;12:73–75. doi: 10.1179/135100007X162284. [DOI] [PubMed] [Google Scholar]
- 108.Eckl PM. Genotoxicity of HNE. Mol.Aspects Med. 2003;24:161. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 109.West JD, Ji C, Duncan ST, Amarnath V, Schneider C, Rizzo CJ, et al. Induction of apoptosis in colorectal carcinoma cells treated with 4-hydroxy-2-nonenal and structurally related aldehydic products of lipid peroxidation. Chem.Res.Toxicol. 2004;17:453. doi: 10.1021/tx034248o. [DOI] [PubMed] [Google Scholar]
- 110.Perraut R, Diatta B, Marrama L, Garraud O, Jambou R, Longacre S, et al. Differential antibody responses to Plasmodium falciparum glycosylphosphatidylinositol anchors in patients with cerebral and mild malaria. Microbes Infect. 2005;7:682–687. doi: 10.1016/j.micinf.2005.01.002. [DOI] [PubMed] [Google Scholar]


