Abstract
The dorsal raphe nucleus (DRN) contains both serotonergic and non-serotonergic projection neurons. Retrograde tracing studies have demonstrated that components of the basal forebrain and extended amygdala are targeted heavily by input from nonserotonergic DRN neurons. The object of this investigation was to examine the terminal distribution of nonserotonergic DRN projections in the basal forebrain and extended amygdala, using a technique that allows selective anterograde tracing of nonserotonergic DRN projections. To trace nonserotonergic DRN projections, animals were pretreated with nomifensine, desipramine and the serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT), 7 days prior to placing an iontophoretic injection of biotinylated dextran amine (BDA) into the DRN. In animals treated with 5,7-DHT, numerous nonserotonergic BDA-labeled fibers ascended to the basal forebrain in the medial forebrain bundle system. Some of these labeled fibers crossed through the lateral hypothalamus, bed nucleus of the stria terminalis, and substantial innominata. These fibers entered the amygdala through the ansa peduncularis and ramified within the central and basolateral amygdaloid nuclei. Other fibers entered the diagonal band of Broca and formed a dense plexus of labeled fibers in the dorsal half of the intermediate portion of the lateral septal nucleus and the septohippocampal nucleus. These findings demonstrate that the basal forebrain and extended amygdala receive a dense projection from nonserotonergic DRN neurons. Given that the basal forebrain plays a critical role in processes such as motivation, affect, and behavioral control, these findings support the hypothesis that nonserotonergic DRN projections may exert substantial modulatory control over emotional and motivational functions.
Keywords: amygdala; bed nucleus; 5,7-dihydroxytryptamine; septal nuclei; biotinylated dextran amine; tyrosine hydroxylase
It is well known that the dorsal raphe nucleus contains more than half of all the serotonergic neurons in the brain (Leger and Wiklund, 1982) and is a source of projections that terminate extensively throughout the central nervous system (Vertes, 1991; Sim and Joseph, 1993; Vertes and Kocsis, 1994; Morin and Meyer-Bernstein, 1999). Serotonergic DRN neurons are clustered within dorsomedial (DRNdm), ventromedial (DRNvm), and lateral (DRNl) cell groups (Steinbusch et al., 1981). It has been proposed that the activity of serotonergic DRN neurons plays an important role in the coordination of sensory processing and motor output with the sleep—wake—arousal cycle (Jacobs and Azmitia, 1992; Jacobs and Fornal, 1993, 1999), and this system may play a role in a number of disease states, including depression, schizophrenia, anxiety, and obsessive-compulsive disorder (Lowry et al., 2005; Hensler, 2006).
Approximately 50–75% of DRN cells are nonserotonergic (Moore, 1981). Experiments combining retrograde tracing with serotonin (5-HT) immunostaining have established that many nonserotonergic DRN neurons project extrinsically (Beitz et al., 1986; Ma et al., 1991; Petrov et al., 1992, 1994; Van Bockstaele et al., 1993; Kirifides et al., 2001; Kim et al., 2004; Halberstadt and Balaban, 2006a). Nonserotonergic DRN projections are neurochemically heterogeneous, and may contain a number of transmitter substances, including glutamate (Schwarz and Schwarz, 1992; Kiss et al., 2002), dopamine (Pohle et al., 1984; Descarries et al., 1986; Yoshida et al., 1989; Stratford and Wirtshafter, 1990; Hasue and Shammah-Lagnado, 2002), GABA (Nanopoulos et al., 1982; Stamp and Semba, 1995; Ford et al., 1995; Jankowski and Sesack, 2002), nitric oxide (Xu and Hokfelt, 1997; Simpson et al., 2003), and neuropeptides such as vasoactive intestinal polypeptide (VIP) (Petit et al., 1995; Kozicz et al., 1998) and cholecystokinin (van der Kooy et al., 1981; Seroogy and Fallon, 1989).
Although it is likely that non-5-HT DRN projections influence processing in target regions (Weiss and Pellet, 1982; Hajés-Korcsok and Sharp, 2002), very little is known about the anatomical organization of this pathway. Anterograde tracing studies with Phaseolus vulgaris leucoagglutinin (PHA-L) or biotinylated dextran amine (BDA) have demonstrated that regions of the basal forebrain are heavily targeted by DRN projections (Vertes, 1991; Sim and Joseph, 1993; Morin and Meyer-Bernstein, 1999), and retrograde tracing has confirmed that this projection arises, in part, from nonserotonergic neurons (Descarries et al., 1986; Semba et al., 1988; Stratford and Wirtshafter, 1990; Li et al., 1990; Van Bockstaele et al., 1993; Petrov et al., 1994; Ford et al., 1995; Gasbarri et al., 1999; Hansue and Shammah-Lagnado, 2002; Halberstadt and Balaban, 2006a). A recent study combined BDA anterograde tracing with 5-HT-immunofluorescent staining to demonstrate that the vast majority of DRN fibers innervating the septum are nonserotonergic (Aznar et al., 2004). Unfortunately, there is substantial variation in the concentration of 5-HT present in serotonergic fibers, and it has not been conclusively established that the absence of 5-HT immunofluorescent labeling can be used to unequivocally identify fibers as being nonserotonergic (Nielsen et al., 2006). Therefore, it is important to develop other methodologies that can be used to reliably anterogradely trace nonserotonergic DRN projections.
Selective serotonergic neurotoxins, including 5,7-dihydroxytryptamine (5,7-DHT), have been shown to disrupt axonal transport in serotonergic neurons (Moore and Halaris, 1975; Halaris et al., 1976; Azmitia and Segal, 1978; Jacobs et al., 1978; Moore et al., 1978; Satoh, 1979; Araneda et al., 1980a,b; Zhou and Azmitia, 1983; Callahan et al., 2001). Our recent work in this laboratory (Halberstadt and Balaban, 2007) has verified that it is possible to selectively trace the projections of nonserotonergic DRN neurons with BDA after ablation of central serotonergic projections with 5,7-DHT. We used this approach to specifically examine the course and topography of nonserotonergic DRN projections to the basal forebrain and extended amygdala.
MATERIALS AND METHODS
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh, which certifies compliance with National Institutes of Health and United States Department of Agriculture standards for humane animal utilization. All efforts were made to minimize the number of animals used and their suffering. Pairs of animals were housed in suspended caging at 22 °C with a 12-h light/dark cycle and ad libitum access to food and water.
Surgical procedures
Twelve adult male Long-Evans rats (250–300 g; Charles River Laboratories, Wilmington, MA, USA) were anesthetized using a mixture of ketamine (50 mg/kg, i.m.), xylazine (6 mg/kg, i.m.), and acepromazine (0.5 mg/kg, i.m.). They were positioned in a stereotaxic apparatus using ear bars and a bite bar. The rats were pretreated with a combination of nomifensine and desipramine to prevent damage to dopaminergic and noradrenergic projections, respectively (Björklund et al., 1975; Baumgarten et al., 1982; Caillé et al., 2002). Thirty min after injection of nomifensine maleate (15 mg/kg, i.p.) and desipramine hydrochloride (15 mg/kg, i.p.), rats were administered 5,7-DHT creatinine sulfate (Fluka, Milwaukee, WI, USA) by the intracerebroventricular (i.c.v.) route. The injection was stereotaxically guided, and was made through a burr hole in the cranium. Using a 20 μl Hamilton syringe, 150 μg 5,7-DHT (dose calculated as the free base: 1 μg free base = 2.099 μg 5,7-DHT creatinine sulfate) in 15 μl 0.9% sterile saline containing 0.2% ascorbic acid was injected into the left lateral ventricle over 15 min. The burr hole was then closed with Gelfoam and the scalp incision sutured.
Seven days later, the animals were anesthetized using a mixture of ketamine (50 mg/kg, i.m.), xylazine (6 mg/kg, i.m.), and acepromazine (0.5 mg/kg, i.m.), and then positioned in a stereotaxic apparatus using ear bars and a bite bar. A burr hole was drilled in the cranium, and a solution of 7.5% BDA (10,000 MW; Molecular Probes, Eugene, OR, USA) in 10 mM phosphate-buffer containing 0.5 M NaCl, pH 7.0, was injected iontophoretically (4 μA positive pulsed square wave, 15 s duty cycle, 15 min) into DRN using glass micropipettes (~40 μm tip diameter). The micropipette was positioned laterally at an angle 26° to the vertical plane, and aimed at the following stereotaxic coordinates: 1.0 mm posterior to the interaural line, 0 mm lateral, and 6.5 mm ventral to the skull surface. The burr hole was then closed with Gelfoam and the scalp incision sutured.
Euthanasia, fixation and sectioning
After a 7 day survival period, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.), and perfused transcardially with 50 mM phosphate-buffered saline (PBS, pH 7.4) followed by paraformaldehyde-lysine-periodate fixative (McLean and Nakane, 1974). The brains were extracted from the skulls, post-fixed overnight at 4 °C, and cryoprotected for 48–72 h at 4 °C in 30% sucrose–PBS. Thirty-five μm sections were cut in the transverse plane on a freezing–sliding microtome, and sets of every sixth section were collected in PBS containing 30% sucrose and 30% ethylene glycol and stored indefinitely at 20 °C.
Tissue processing for SERT and BDA
Four of the six sets of sections from each animal were stained for BDA labeling. A onestep avidin–biotin–peroxidase procedure was used to visualize BDA labeling, as described previously (see Halberstadt and Balaban, 2006b). The remaining sets of sections were used to visualize immunoreactivity for the 5-HT transporter (SERT) or for tyrosine hydroxylase (TH). Immunoreactivity for SERT was visualized using previously established procedures (see Halberstadt and Balaban, 2003). The mouse monoclonal anti-SERT antibody (MAB1564; Chemicon International, Temecula, CA, USA; used at a concentration of 1:1000) was prepared against a rat neuronal SERT NH2-terminus/glutathione S-transferase fusion protein corresponding to the first 85 amino acids of rat SERT. This antiserum stains bands of 73 kD and 120 kD molecular weight on Western blot (Inazu et al., 2001). The distribution of serotonergic DRN neurons stained with this antiserum was consistent with that reported previously by studies using immunostaining for 5-HT (e.g. Steinbusch, 1981). Immunoreactivity for tyrosine hydroxylase (TH) was visualized using previously established procedures (Schuerger and Balaban, 1999). The mouse monoclonal anti-TH antibody (MAB318; Chemicon; used at a concentration of 1:1000), which was prepared against a 59–61 kDa protein on the outside of the regulatory N-terminus of TH purified from PC12 cells, does not cross react with other monoamine synthesis enzymes on Western blot.
Analysis
Digital images were prepared using a Nikon Eclipse E600N microscope equipped with a Spot RT Monochrome camera (Model 2.1.1, Diagnostic Instruments, Inc., Sterling Heights, MI, USA). The images were captured on a Pentium-based computer running MetaMorph software (Ver. 6.1r4, Universal Imaging Corporation, Downingtown, PA, USA). Adobe Photoshop 7.01 (Adobe Systems, San Jose, CA, USA) was used for brightness and contrast adjustments and cropping.
Camera lucida drawings were prepared using an Olympus BH-2 microscope (20× objective) equipped with a drawing tube (Olympus, Tokyo, Japan). The full-size drawings were reduced by 50%, traced in India ink, and scanned at 800 dpi in grayscale mode.
RESULTS
Anterograde transport of BDA, in combination with the serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT), was effective in demonstrating the distribution of the nonserotonergic DRN projection to the basal forebrain. These injection cases were available from a previous study which analyzed only the distribution of nonserotonergic DRN projections to the vestibular nuclei and adjacent brain stem nuclei (see: Halberstadt and Balaban, 2007); this communication presents anterograde transport data to the forebrain. In order to evaluate the effect of i.c.v. 5,7-DHT administration on serotonergic DRN neurons, sections from normal control rats (n = 4) and from 5,7-DHT-pretreated animals that had received an iontophoretic injection of BDA into the DRN (n = 4) were stained simultaneously for SERT-immunoreactivity. As described previously, i.c.v. administration of 5,7-DHT has profound effects on SERT expression within the DRN. Compared with untreated animals in which heavily SERT-immunopositive neuronal perikarya and fibers were found throughout the DRN, animals treated with 5,7-DHT showed a complete absence of SERT staining in the dorsomedial, ventromedial, and lateral subdivisions of the DRN (data not shown). In addition to being neurotoxic to serotonergic neurons, 5,7-DHT also induces degeneration of catecholamine-containing neurons (Baumgarten et al., 1982). In order to protect dopaminergic neurons in the DRN from the toxic action of 5,7-DHT, we pretreated animals with the dopamine uptake blocker nomifensine and the norepinephrine uptake blocker desipramine. To confirm that this pretreatment regimen is effective at preventing damage to dopaminergic DRN neurons, sections from 5,7-DHT-pretreated animals (n = 4) that had received an iontophoretic injection of BDA into the DRN were immunostained for TH. TH-positive cells bodies were detected in the ventromedial, dorsomedial, and lateral DRN subdivisions (Fig. 1). The TH-positive cell bodies in the medial DRN were distributed in a shell around the central portion, which had little staining. This shell region includes the dorsal margin of medial and lateral DRN. There was also a tendency for heavily stained neurons to be distributed around the periphery of lateral DRN. The density of labeled cells was greater in rostral DRN compared with intermediate and caudal DRN. Numerous TH-positive cells were also observed in locus coeruleus, substantia nigra pars compacta, and ventral tegmental area (data not shown). The TH immunostaining data confirms that 5,7-DHT has little effect on catecholaminergic neurons in animals pretreated with nomifensine and desipramine.
Figure 1.
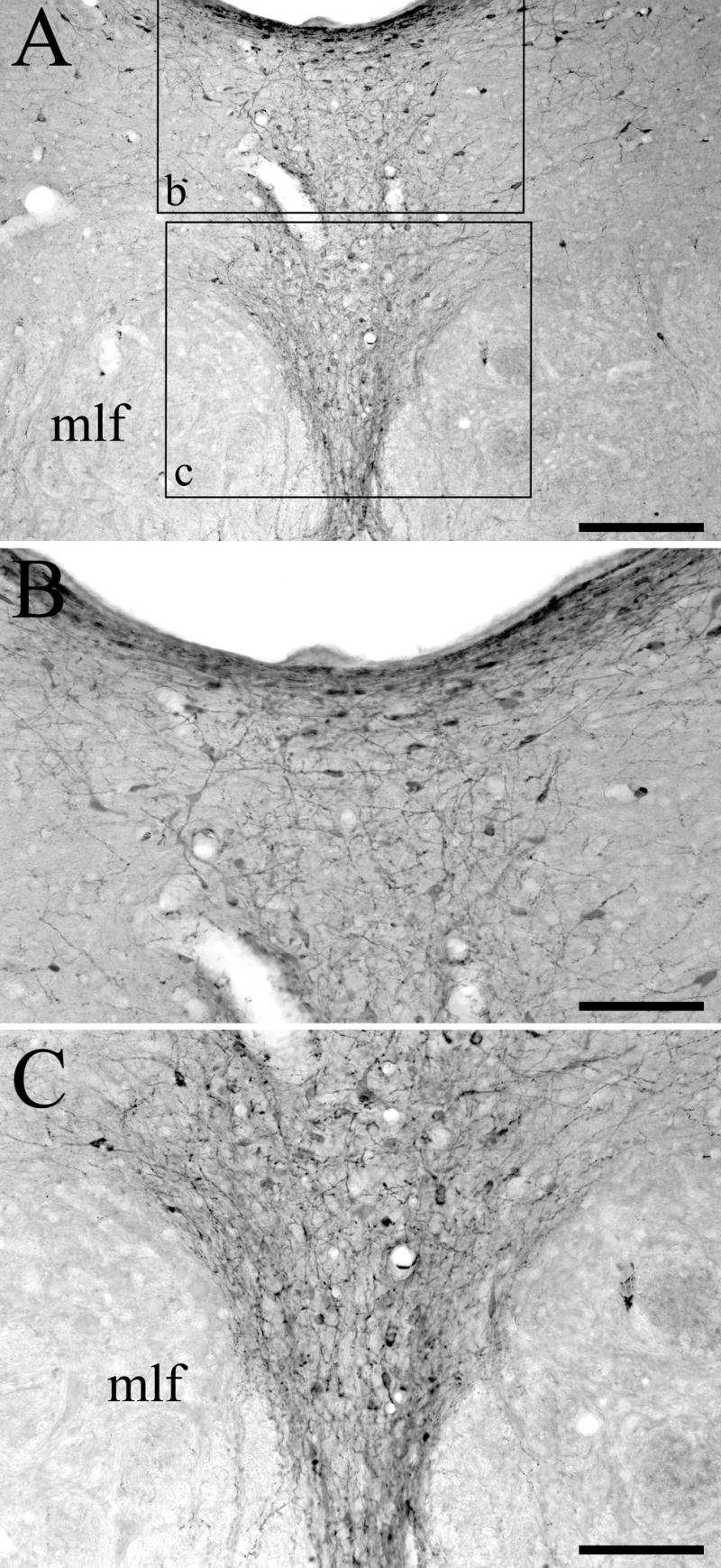
Tyrosine hydroxylase (TH) immunoreactivity in the DRN of animals treated with i.c.v. 5,7-DHT. (A) Numerous TH-positive cell bodies, dendrites, and fibers are located throughout the DRN. The boxes in panel A indicate the areas shown enlarged in panels B and C. (B) High-power photomicrograph showing TH immunostaining in the dorsomedial DRN subdivision. (C) High-power photomicrograph showing TH immunostaining in the ventromedial DRN subdivision. The medial longitudinal fasciculus (mlf) is also indicated. Scale bars = 200 μm in A and 100 μm in B and C.
BDA injections were made in twelve animals that had been pretreated with 150 μg 5,7-DHT i.c.v. seven days previously. The BDA injection site was centered within the DRN, with minimal spread to neighboring structures, in seven rats. The BDA injection site from one of these cases is illustrated in Figure 2. The BDA injection sites displayed a dense central core of labeled neuronal somata and neuropil surrounded by a peripheral halo region containing scattered neuronal perikarya with dendrites that extend into the core. These sites were generally ovoid and relatively small, with the diameter of the central core typically measuring 200 μm in the mediolateral direction and extending for a similar distance rostrocaudally across transverse sections. Scattered neuronal somata located on the periphery of the halo region were sometimes labeled via transport through dendrites that extend into the core of the injection site; these cells were regarded as part of the injection site halo. The injection sites in cases DHT-214A, DHT-222B, DHT-324B and DHT-331B involved both the caudal and intermediate levels of the DRN, and injection sites DHT-222C, DHT-324A, and DHT-709A were centered within the intermediate part of DRN. Although the BDA injection sites were centered within different subdivisions of the DRN, they typically spanned across multiple subdivisions. Injection cases DHT-222C and DHT-324B were relatively confined within lateral DRN (DRNl). Case DHT-324A was situated in DRNl with some spread into the mlf. The injection sites in cases DHT-214A and DHT-222B were located in DRNl with some involvement of medial DRN subdivisions. The injection site in case DHT-331B was primarily located within dorsomedial DRN (DRNdm), but also involved ventromedial DRN (DRNvm) and bilateral aspects of DRNl. Lastly, the tracer injection in case DHT-709A was made into DRNdm and the dorsal part of DRNvm.
Figure 2.
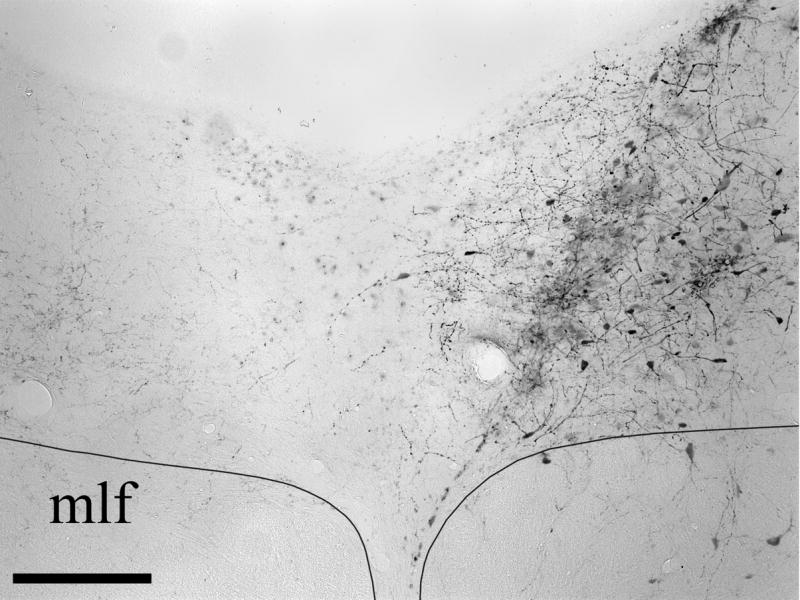
Photomicrographs of the BDA injection site in DRN in case DHT-324B. The medial longitudinal fasciculus (mlf) is also indicated. Scale bar = 200 μm.
After iontophoretic injection of BDA into the DRN of animals pretreated with 5,7-DHT, anterogradely-labeled fibers and varicose arborizations were distributed in moderate to heavy density within medial portions of the basal forebrain and extended amygdala. The distribution of BDA-labeled fibers and terminals within the basal forebrain and extended amygdala of injection case DHT-331B is illustrated in Fig. 3 as a series of camera lucida drawings. Transport ipsilateral to the injection site is illustrated to the right side of the figure. The terminal innervation was located primarily on the side ipsilateral to the injection site when the injection site was confined unilaterally, whereas for injection cases involving bilateral aspects of DRN (i.e., DHT-331B and DHT-709A), the projections were more symmetric. Although the exact location of the BDA injection site varied from case to case, the distribution of terminal regions was consistent across cases.
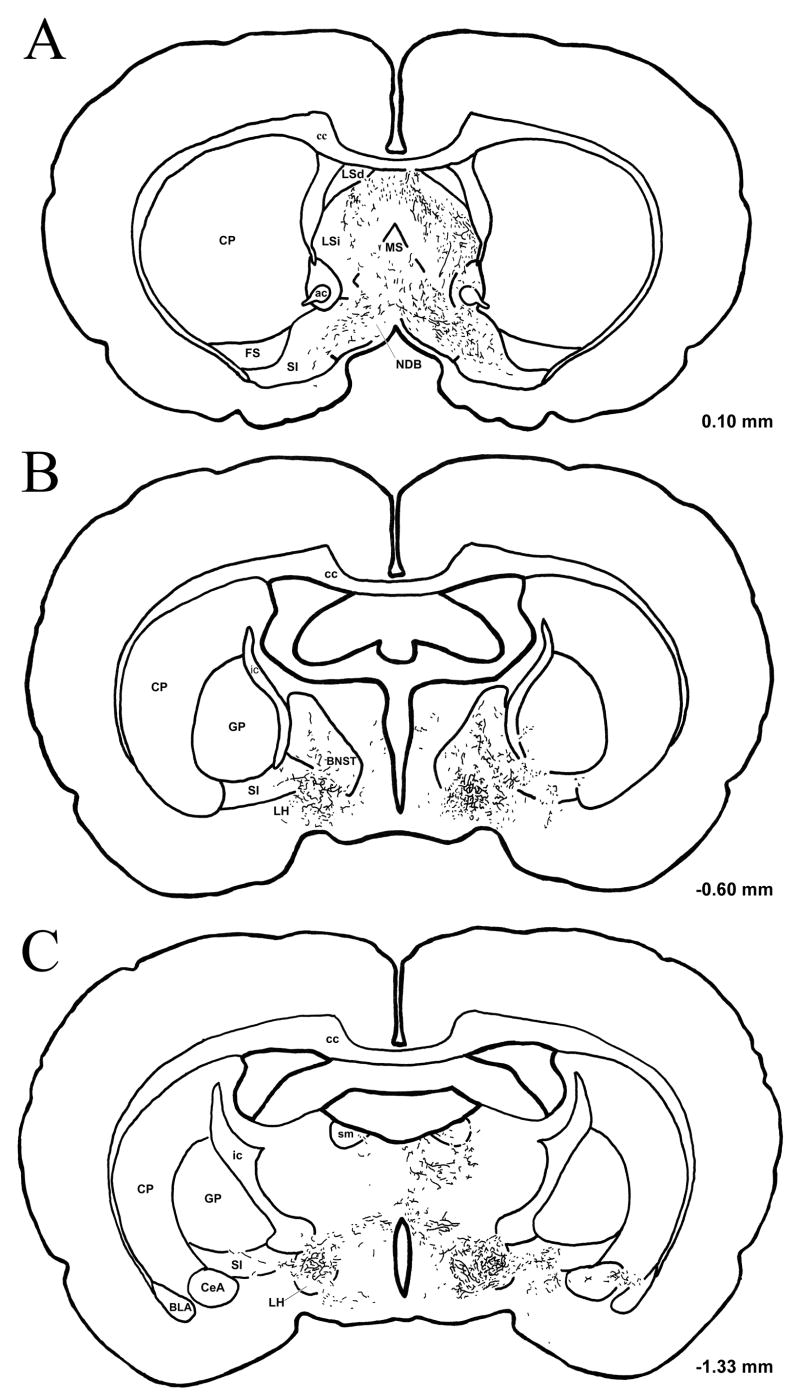
Figure 3.
Distribution of anterogradely labeled fibers in a series of transverse sections spanning the basal forebrain from case DHT-331B. BDA-labeled fibers are charted in camera lucida drawings of three progressively caudal sections (A–C) located at the indicated AP level (relative to bregma). Transport ipsilateral to the injection site is illustrated on the right side of the figure. (Abbreviations: ac, anterior commissure; BLA, basolateral amygdaloid nucleus; BNST, bed nucleus of the stria terminalis; cc, corpus callosum; CeA, central amygdaloid nucleus; CP, caudate putamen; FS, fundus striati; GP, globus pallidus; ic, internal capsule; LH, lateral hypothalamic area; LSd, dorsal lateral septal nucleus; LSi, intermediate lateral septal nucleus; MS, medial septal nucleus; NDB, nucleus of the diagonal band of Broca; SI, substantia immominata; sm, stria medullaris).
The ascending fibers from DRN injection sites followed the same course described by Vertes (1991) for PHA-L transport in rats with intact serotonergic neurons. The main group of fibers traveled ventrally toward the median raphe nucleus, then turned rostrally; a second group traveled laterally into the pontine and midbrain tegmentum. The medial group of fibers ascended to the caudal pole of the diencephalon, where one bundle of fibers passed dorsally to innervate the thalamus and second bundle turned laterally, and ascended within the medial forebrain bundle to the basal forebrain Some labeled fibers terminated locally within the lateral hypothalamic area (LH). Labeled fibers crossed through the medial part of the substantia immominata (SI) before entering the amygdala through the ansa peduncularis; these fibers ramified primarily within the central amygdaloid nucleus (CeA)—especially the lateral aspect—and less densely within the basolateral nucleus. The medial and basomedial amygdaloid nuclei were essentially devoid of labeling. Examples of BDA-labeled fibers and terminals from the CeA are illustrated in Fig. 4A. Labeled fibers were also heavily distributed in the bed nucleus of the stria terminalis (BNST), especially in the medial and lateral subdivisions (see Fig. 4B).
Figure 4.
Photomicrographs of BDA-labeled fibers and terminals within regions of the basal forebrain and extended amygdala. (A) Anterogradely labeled fibers and terminals in central amygdaloid nucleus. (B) Anterograde labeling in the bed nucleus of the stria terminalis. (C) Low-power photomicrograph showing the distribution of anterograde labeling in the intermediate part of the lateral septal nucleus. Note the presence of a dense diagonal band of labeling. (D) High-power photomicrograph of the area indicated by the rectangle in panel C, showing labeled terminals. Scale bars = 50 μm in A, B and D, and 100 μm in C.
Further rostrally, anterogradely-labeled fibers entered the nucleus of the diagonal band of Broca (NDB) and the medial septal nucleus (MS). A large number of fibers were also found to ramify within the dorsal half of the intermediate portion of the lateral septal nucleus (LSi), forming a dense diagonal plexus which was located ventromedial to the border of the dorsal lateral septal nucleus. This terminal region spanned the medial wall of the lateral ventricle and the septohippocampal nucleus (Fig. 4C,D).
The innervation of lateral regions of the forebrain was substantially lighter than was observed for nuclei situated medially. For example, very few, if any BDA-labeled fibers were identified in the islands of Calleja, hippocampus, nucleus accumbens, caudate-putamen (CPu), globus pallidus, claustrum, or the neocortex.
DISCUSSION
This study confirms that it is possible to trace the projections of nonserotonergic DRN neurons anterogradely (and selectively) by using BDA in combination with the serotonergic neurotoxin 5,7-DHT. The rationale for using such a combination is that pretreatment with 5,7-DHT eliminates the transport of BDA by serotonergic DRN projections while sparing transport of BDA by nonserotonergic DRN efferents. A substantial body of experimental evidence demonstrates that selective 5-HT neurotoxins prevent anterograde (Moore and Halaris, 1975; Halaris et al., 1976; Moore et al., 1978; Azmitia and Segal, 1978; Callahan et al., 2001) and retrograde (Jacobs et al., 1978; Satoh, 1979; Araneda et al., 1980a,b; Zhou and Azmitia, 1983) axonal transport in serotonergic cells but not in nonserotonergic cells. Indeed, studies were able to demonstrate the presence of nonserotonergic neurons within the DRN by injection of the retrograde tracer horseradish peroxidase into the striatum (Jacobs et al., 1978) or forebrain (Baumgarten et al., 1974) of rats pretreated with 5,7-DHT. Importantly, electrophysiological findings indicate that putative nonserotonergic DRN cells are not damaged by 5,7-DHT (Aghajanian et al., 1978).
One potential concern with i.c.v. 5,7-DHT administration is that serotonergic DRN fibers and neurons located adjacent to the cerebral aqueduct were exposed to higher concentrations of 5,7-DHT than were fibers and neurons located deeper in the DRN. However, the fact that SERT immunostaining was eliminated throughout the DRN, and not just in regions adjacent to the ventricular system, strongly indicates that serotonergic neurons throughout the DRN were exposed to toxic concentrations of 5,7-DHT after i.c.v. administration. Indeed, our anti-SERT immunohistochemical findings are consistent with those of Serrats et al. (2005) who found that i.c.v. administration of 5,7-DHT causes a complete loss of SERT mRNA expression throughout the DRN.
The possibility of fiber-of-passage uptake is always a concern in the interpretation of anterograde neuronal tracing data. We do not believe that this is likely to be a major confounding factor in our results, given that there is evidence that BDA uptake into fibers of passage is minimal from iontophoretic application sites (Alipour et al., 1997; Power and Mitrofanis, 2002; van Dongen et al., 2005). Nonetheless, it appears that the uptake of BDA by fibers of passage can occur in axons damaged by the pipet or microsyringe used to make the injection (Reiner et al., 2000), which will occur for any injection method. Thus, one cannot preclude the possibility that a minor component of fiber of passage uptake contributed to the anterograde labeling observed in this study.
The combination of centrally administered 5,7-DHT and intra-DRN BDA injections produced robust anterograde labeling of nonserotonergic DRN projections to the basal forebrain. Although it is well established in the literature that the DRN projection system contains a nonserotonergic component, very little is actually known about the organization of this subset of DRN efferents. The present report demonstrates that several regions of the basal forebrain and extended amygdala are innervated moderately-to-heavily by projections from nonserotonergic DRN neurons. In particular, appreciable anterograde labeling was present in the LH, BNST, SI, CeA, and septal nuclei after injection of BDA into the DRN of 5,7-DHT-pretreated rats. It is noteworthy that the terminal innervation of the basal forebrain and extended amygdala was located primarily on the side ipsilateral to the injection site. Retrograde tracing studies have demonstrated that the majority of serotonergic DRN neurons projecting to caudate-putamen (Van der Kooy, 1979), neocortex (O’Hearn and Molliver, 1984), prefrontal cortex and nucleus accumbens (Van Bockstaele et al., 1993), and components of the trigeminal somatosensory pathway (Kirifides et al., 2001) are located ipsilateral to the tracer injection site. The present anterograde tracing data indicate that the nonserotonergic projection from the DRN to the basal forebrain and the extended amygdala also originates primarily from the ipsilateral DRN.
A number of studies have used PHA-L to visualize the ascending projections of the DRN (e.g., Kosofsky and Molliver, 1987; Vertes, 1991; Sim and Joseph, 1993; Morin and Meyer-Bernstein, 1999). Compared with the reported regional distribution of DRN fibers in normal animals, animals treated with 5,7-DHT display notable differences in the distribution of anterograde labeling. Specifically, after treatment with 5,7-DHT, there was a loss of anterograde labeling within several forebrain regions previously reported to be heavily targeted by DRN projections. For example, although it was previously reported that the frontal cortex and CPu are normally moderately to heavily innervated by DRN fibers (Kosofsky and Molliver, 1987; Vertes, 1991; Morin and Meyer-Bernstein, 1999), we observed at most only sparse BDA labeling in those two regions after administration of 5,7-DHT. By contrast, it appears that the DRN projections to certain forebrain regions—most notably the septal nuclei—are spared by 5,7-DHT. The fact that treatment with 5,7-DHT produced a loss of anterograde labeling in some but not all DRN target regions, as opposed to just reducing the density of labeled fibers in a regionally-nonspecific manner, argues strongly that the relative density of serotonergic and nonserotonergic DRN projections varies across target regions. Although the present results indicate that there is an appreciable nonserotonergic component to the DRN projection to septum, the complete absence of neocortical labeling argues that the DRN projection to neocortex originates predominantly from serotonergic neurons. Indeed, studies combining anterograde tracing with immunofluorescent staining for 5-HT have concluded that the DRN projection to the septal nuclei is primarily nonserotonergic (Aznar et al., 2004), whereas the DRN projection to neocortex originates primarily from serotonergic neurons (Kosofsky and Molliver, 1987).
It is noteworthy that we failed to detect BDA-labeled fibers in neocortical areas, given that Kosofsky and Molliver (1987) have reported that approximately one-fifth of the DRN fibers in neocortex are nonserotonergic. This discrepancy may be explained by the fact that our injections sites were relatively small, compared with those made by Kosofsky and Molliver (1987), and thus are likely to have labeled of only a small portion of the entire DRN projection. It is entirely possible that larger BDA injections would have produced labeling in the neocortex, as well as in other brain regions in which little or no anterograde transport was detected. This conclusion seems likely, given that there is both neurochemical (Jankowski and Sesack, 2002) and electrophysiological (Puig et al., 2005) evidence that the DRN sends a GABAergic projection to medial prefrontal cortex.
After injection of BDA into the DRN of animals pretreated with 5,7-DHT, anterograde labeling in the lateral septum (LS) was found primarily within a distinctive band that followed the border of the dorsal and intermediate subdivisions of the nucleus (see Figs. 3 and 4). This labeling overlaps completely with the pattern of anterograde labeling that was observed in the LS in previous studies in which PHA-L was injected into the DRN of normal rats and hamsters (Vertes, 1991; Morin and Meyer-Bernstain, 1999). The fact that pretreatment with 5,7-DHT has little apparent effect on the distribution of anterogradely-labeled DRN projections within the LS indicates that the projection from DRN to LS contains a substantial nonserotonergic component. Indeed, this conclusion is consistent with the findings of Aznar and colleagues (Aznar et al., 2004) who observed that after injection of BDA into the DRN only 14% of the anterogradely-labeled fibers in the LS were also immunopositive for 5-HT.
The anterograde transport results also demonstrate that the NDB and MS are targeted by an appreciable nonserotonergic projection from DRN, which confirm the inference that the vast majority of DRN fibers within the NDB and MS are nonserotonergic (Aznar et al., 2004). It is well established that the rhythmic firing of GABAergic cells in the NDB/MS complex is responsible for the generation of hippocampal theta rhythm (Freund and Antal, 1988). There is electrophysiological evidence that the firing of a subpopulation of nonserotonergic DRN neurons is synchronized with hippocampal theta rhythm (Kocsis and Vertes, 1992, 1996). Given that nonserotonergic DRN fibers have been shown to make contact with parvalbumin- and calbindin-positive NDB/MS cells (Aznar et al., 2004), it seems likely that the nonserotonergic DRN projection to the NDB/MS complex is involved in the modulatory control of hippocampal rhythmic activity.
Several components of the extended amygdala are targeted moderately to heavily by nonserotonergic DRN projections. Previous anterograde tracing studies have demonstrated that the CeA is moderately innervated by DRN projections (Vertes, 1991; Sim and Joseph, 1993; Morin and Meyer-Bernstein, 1999; Commons et al., 2003). Retrograde tracing studies have shown that that this projection is partially derived from nonserotonergic DRN cells (Li et al., 1991; Petrov et al., 1994; Halberstadt and Balaban, 2006a), and the present findings provide additional confirmation that the DRN-to-CeA projection contains a nonserotonergic component. Although the neurochemical identity of the nonserotonergic DRN terminals located within the CeA is uncertain, they may in large part be dopaminergic given that approximately half of the DRN cells projecting to the CeA are immunoreactive for TH (Hasue and Shammah-Lagnado, 2002). Because 5,7-DHT (without pretreatment) is known to be toxic to dopaminergic neurons (Baumgarten et al., 1982), immunostaining was used to confirm that TH-positive DRN neurons are preserved after 5,7-DHT treatment in animals pretreated with nomifensine and desipramine. Another component of the extended amygdala, the BNST, has previously been shown to be densely innervated by DRN projections (Vertes, 1991; Sim and Joseph, 1993; Commons et al., 2003), and our findings demonstrate that this projection is partially nonserotonergic. It is known that DRN neurons containing VIP immunoreactivity send projections to the BNST (Petit et al., 1995; Kozicz et al., 1998), but it is not known whether those VIP-positive neurons colocalize 5-HT. As with the CeA, there is evidence that dopaminergic DRN neurons project to BNST (Hansue and Shammah-Lagnado, 2002). Our data also indicate that the SI also receives a prominent nonserotonergic DRN projection, a finding supported by the results of a retrograde tracing experiment which demonstrated that only 10–15% of the DRN cells projecting to SI are tryptophan hydroxylase-immunopositive (Gasbarri et al., 1999).
This study has demonstrated that regions of the basal forebrain are targeted heavily by projections from nonserotonergic DRN neurons. These results also confirm that the relative density of innervation by serotonergic and nonserotonergic DRN projections varies across target regions. Based on these findings, it is likely that the influence of DRN activity on neuronal activity within a given region of the basal forebrain depends not only on the overall density of DRN innervation but also on the exact proportion of serotonergic to nonserotonergic DRN input. Importantly, there appear to be differences in the activity and firing properties of putative serotonergic and nonserotonergic DRN units. The firing activity of putative serotonergic DRN neurons in behaving cats increases across the sleep–wake–arousal cycle (Trulson and Jacobs, 1979; Sakai and Crochet, 2001) and reflects the level of ongoing motor activity (Trulson et al., 1981; Steinfels et al., 1983). Further, at least certain subpopulations of putative serotonergic DRN neurons are excited by the presentation of auditory and visual stimuli (Heym et al., 1982; Shima et al., 1986), and during rhythmic oral-buccal movements and in response to tactile stimulation (Fornal et al., 1996). Although extensive electrophysiological work has been devoted to examining the firing properties of putative serotonergic DRN neurons in behaving animals (Trulson and Jacobs, 1979; Trulson et al., 1981; Heym et al., 1982; Steinfels et al., 1983; Fornal et al., 1996; Veasey et al., 1997; Guzman-Marin et al., 2000; Sakai and Crochet, 2001), much less is known about the response properties of nonserotonergic DRN neurons. Experiments in chloral hydrate-anesthetized rats have demonstrated that stimulation of the sciatic nerve suppresses the firing of putative serotonergic DRN cells but increases the firing of putative nonserotonergic cells (Aghajanian et al., 1978). Recording from the DRN of behaving rats, Waterhouse and colleagues (Waterhouse et al., 2004) reported that although the firing activity of both serotonergic and nonserotonergic neurons was tied to the sleep–wake cycle, serotonergic DRN cells were generally insensitive to sensorimotor stimulation whereas nonserotonergic neurons displayed responses to specific sensory and motor events. Given these significant differences in the response properties of serotonergic and nonserotonergic DRN neurons, the diversity in the relative patterns of serotonergic and nonserotonergic innervation may indicate profound differences in the influence of DRN on information processing in different basal forebrain regions. Indeed, it would appear that the septal nuclei are primarily targeted by nonserotonergic DRN projections, whereas cortical regions are targeted primarily by serotonergic DRN projections. However, it will not be possible to determine how input from nonserotonergic DRN cells influences processing within the basal forebrain prior to identifying (1) the sources of afferent input to nonserotonergic DRN cells, and (2) the transmitter substance(s) that are synthesized and released by nonserotonergic DRN projections in each region.
Acknowledgments
This work was supported in part by NIDCD Grants R01 DC00739 and F31 DC006772. We would like to thank Gloria Limetti and Jean Betsch for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. doi: 10.1016/0006-8993(78)91140-x. [DOI] [PubMed] [Google Scholar]
- Alipour M, Chen Y, Jurgens U. Anterograde projections of the cortical tongue area of the tree shrew (Tupaia belangeri) J Hirnforsch. 1997;38:405–423. [PubMed] [Google Scholar]
- Araneda S, Bobillier P, Buda M, Pujol JF. Retrograde axonal transport following injection of [3H]serotonin in the olfactory bulb. I. Biochemical study. Brain Res. 1980a;196:405–415. doi: 10.1016/0006-8993(80)90404-7. [DOI] [PubMed] [Google Scholar]
- Araneda S, Gamrani H, Font C, Calas A, Pujol JF, Bobillier P. Retrograde axonal transport following injection of [3H]serotonin into the olfactory bulb. II. Radioautoradiographic study. Brain Res. 1980b;196:417–427. doi: 10.1016/0006-8993(80)90405-9. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian ZX, Knudsen GM. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience. 2004;124:573–581. doi: 10.1016/j.neuroscience.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Björklund A, Lachenmayer L, Nobin A. Evaluation of the effects of 5,7-dihydroxytryptamine on serotonin and catecholamine neurons in the rat CNS. Acta Physiol Scand Suppl. 1974;391:1–19. [PubMed] [Google Scholar]
- Baumgarten HG, Klemm HP, Sievers J, Schlossberger HG. Dihydroxytryptamines as tools to study the neurobiology of serotonin. Brain Res Bull. 1982;9:131–150. doi: 10.1016/0361-9230(82)90128-9. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Clements JR, Mullett MA, Ecklund LJ. Differential origin of brainstem serotonergic projections to the midbrain periaqueductal gray and superior colliculus of the rat. J Comp Neurol. 1986;250:498–509. doi: 10.1002/cne.902500408. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Servadio A, Forloni G, Angeretti N, Samanin R. Increased tryptophan hydroxylase mRNA in raphe serotonergic neurons spared by 5,7-dihydroxytryptamine. Mol Brain Res. 1990;8:343–348. doi: 10.1016/0169-328x(90)90048-i. [DOI] [PubMed] [Google Scholar]
- Björklund A, Baumgarten HG, Rensch A. 5,7-Dihydroxytryptamine: improvement of its selectivity for serotonin neurons in the CNS by pretreatment with desipramine. J Neurochem. 1975;24:833–835. [PubMed] [Google Scholar]
- Caillé S, Espejo EF, Koob GF, Stinus L. Dorsal and median raphe serotonergic system lesion does not alter the opiate withdrawal syndrome. Pharmacol Biochem Behav. 2002;72:979–986. doi: 10.1016/s0091-3057(02)00810-9. [DOI] [PubMed] [Google Scholar]
- Callahan BT, Cord BJ, Ricaurte GA. Long-term impairment of anterograde axonal transport along fiber projections originating in the rostral raphe nuclei after treatment with fenfluramine or methylenedioxyamphetamine. Synapse. 2001;40:113–121. doi: 10.1002/syn.1032. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role of affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Datiche F, Luppi PH, Cattarelli M. Serotonergic and non-serotonergic projections from the raphe nuclei to the piriform cortex in the rat: a cholera toxin B subunit (CTb) and 5-HT immunohistochemical study. Brain Res. 1995;671:27–37. doi: 10.1016/0006-8993(94)01293-q. [DOI] [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, Beaudet A. Dopaminergic projections from nucleus raphe dorsalis to neostriatum in rat. J Comp Neurol. 1986;249:511–520. doi: 10.1002/cne.902490407. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Marrosu F, Ribiero-do-Valle LE, Jacobs BL. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 1996;716:123–133. doi: 10.1016/0006-8993(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Pacitti C, McGaugh JL. Serotonergic input to cholinergic neurons in the substantia nigra and nucleus basalis magnocellularis in the rat. Neuroscience. 1999;91:1129–1142. doi: 10.1016/s0306-4522(98)00672-1. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, Gong H, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Res. 2000;875:23–34. doi: 10.1016/s0006-8993(00)02561-0. [DOI] [PubMed] [Google Scholar]
- Halaris AE, Jones BE, Moore RY. Axonal transport in serotonin neurons of the midbrain raphe. Brain Res. 1976;107:555–574. doi: 10.1016/0006-8993(76)90144-x. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Organization of projections from the raphe nuclei to the vestibular nuclei in rats. Neuroscience. 2003;120:573–594. doi: 10.1016/s0306-4522(02)00952-1. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006a;140:1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Anterograde tracing of projections from the dorsal raphe nucleus to the vestibular nuclei. Neuroscience. 2006b;143:641–54. doi: 10.1016/j.neuroscience.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Selective anterograde tracing of the individual serotonergic and nonserotonergic components of the dorsal raphe nucleus projection to the vestibular nuclei. Neuroscience. 2007;147:207–223. doi: 10.1016/j.neuroscience.2007.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajés-Korcsok É, Sharp T. Electrical stimulation of the dorsal and median raphe nuclei increases extracellular noradrenaline in rat hippocampus: evidence for a 5-HT-independent mechanism. Pharmacol Biochem Behav. 2002;71:807–813. doi: 10.1016/s0091-3057(01)00718-3. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ferry RC, Labow DM, Kovachich GB, Frazer A. Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus. Synapse. 1994;17:1–15. doi: 10.1002/syn.890170102. [DOI] [PubMed] [Google Scholar]
- Heym J, Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: effects of phasic auditory and visual stimuli. Brain Res. 1982;232:29–39. doi: 10.1016/0006-8993(82)90608-4. [DOI] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Ikoshi H, Sugisawa M, Uchida Y, Matsumiya T. Pharmacological characterization and visualization of the glial serotonin transporter. Neurochem Int. 2001;39:39–49. doi: 10.1016/s0197-0186(01)00010-9. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Foote SL, Bloom FE. Differential projections of neurons within the dorsal raphe nucleus of the rat: a horseradish peroxidase (HRP) study. Brain Res. 1978;147:149–153. doi: 10.1016/0006-8993(78)90779-5. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2 Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Sesack SR. Electron microscopic analysis of the GABA projection from the dorsal raphe nucleus to the prefrontal cortex in the rat. Soc Neurosci Abstr. 2002;28:587–588. [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RCS, Waterhouse BD. Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. J Comp Neurol. 2001;435:325–340. doi: 10.1002/cne.1033. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csáki Á, Bokor H, Kocsis K, Kocsis B. Possible glutamatergic/aspartatergic projections to the supramammillary nucleus and their origins in the rat studied by selective [3H]D-aspartate labeling and immunocytochemistry. Neuroscience. 2002;111:671–691. doi: 10.1016/s0306-4522(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Dorsal raphe nucleus: synchronous discharge with theta rhythm of the hippocampus in the freely behaving rat. J Neurophysiol. 1992;68:1463–1467. doi: 10.1152/jn.1992.68.4.1463. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Midbrain raphe cell firing and hippocampal theta rhythm in urethane-anaesthetized rats. Neuroreport. 1996;7:2867–2872. doi: 10.1097/00001756-199611250-00012. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotonergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. The source of origin of PACAP- and VIP-immunoreactive fibers in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Res. 1998;810:211–219. doi: 10.1016/s0006-8993(98)00692-1. [DOI] [PubMed] [Google Scholar]
- Leger L, Wiklund L. Distribution and numbers of indoleamine cell bodies in the cat brainstem determined with Falck-Hillarp fluorescence histochemistry. Brain Res Bull. 1982;9:245–251. doi: 10.1016/0361-9230(82)90137-x. [DOI] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Li YQ, Kaneko T, Mizuno N. Serotonergic innervation of mesencephalic trigeminal nucleus neurons: a light and electron microscopic study in the rat. Neurosci Res. 2000;37:127–140. doi: 10.1016/s0168-0102(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Li YQ, Jia HG, Rao ZR, Shi JW. Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett. 1990;120:124–127. doi: 10.1016/0304-3940(90)90184-b. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett. 1991;134:21–24. doi: 10.1016/0304-3940(91)90499-j. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Moore RY. The anatomy of central serotonin systems in the rat brain. In: Jacobs BL, Gelperin A, editors. Serotonin Neurotransmission and Behavior. MIT Press; Cambridge, MA: 1981. pp. 35–71. [Google Scholar]
- Moore RY, Halaris AE. Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol. 1975;164:171–184. doi: 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/s0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse. 2006;59:270–276. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nerve nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- Pohle W, Ott T, Muller-Welde P. Identification of neurons of origin providing the dopaminergic innervation of the hippocampus. J Hirnforsch. 1984;25:1–10. [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Ultrastructure of afferents from the zona incerta to the posterior and parafascicular thalamic nuclei of rats. J Comp Neurol. 2002;451:33–44. doi: 10.1002/cne.10332. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Satoh K. The origin of reticulospinal fibers in the rat: a HRP study. J Hirnforsch. 1979;20:313–322. [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD. Organization of the coeruleo-vestibular pathway in rats, rabbits, and monkeys. Brain Res Rev. 1999;30:189–217. doi: 10.1016/s0165-0173(99)00015-6. [DOI] [PubMed] [Google Scholar]
- Schwarz DW, Schwarz IE. The distribution of neurons labelled retrogradely with [3H]-D-aspartate injected into the colliculus inferior of the cat. J Otolaryngol. 1992;21:339–342. [PubMed] [Google Scholar]
- Semba K, Reiner PB, McGeer EG, Fibiger HC. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol. 1988;267:433–53. doi: 10.1002/cne.902670311. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Fallon JH. Forebrain projections from cholecystokininlike-immunoreactive neurons in the rat midbrain. J Comp Neurol. 1989;279:415–435. doi: 10.1002/cne.902790307. [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortés R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Shima K, Nakahama H, Yamamoto M. Firing properties of two types of nucleus raphe dorsalis neurons during the sleep-waking cycle and their responses to sensory stimuli. Brain Res. 1986;399:317–326. doi: 10.1016/0006-8993(86)91522-2. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Dorsal raphe nucleus efferents: termination in peptidergic fields. Peptides. 1993;14:75–83. doi: 10.1016/0196-9781(93)90013-7. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Waterhouse BD, Lin RCS. Differential expression of nitric oxide in serotonergic projection neurons: neurochemical identification of dorsal raphe inputs to rodent trigeminal somatosensory targets. J Comp Neurol. 2003;466:495–512. doi: 10.1002/cne.10912. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol (Paris) 1981;77:157–174. [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Raphe unit activity in freely moving cats is altered by manipulations of central but not peripheral motor systems. Brain Res. 1983;279:77–84. doi: 10.1016/0006-8993(83)90164-6. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 1990;511:173–176. doi: 10.1016/0006-8993(90)90239-8. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Moore KR, Thompson GC. Distribution and origin of serotoninergic afferents to guinea pig cochlear nucleus. J Comp Neurol. 1995;351:104–16. doi: 10.1002/cne.903510110. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL, Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 1981;226:75–91. doi: 10.1016/0006-8993(81)91084-2. [DOI] [PubMed] [Google Scholar]
- Van Boeckstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- van der Kooy D. The organization of the thalamic, nigral and raphe cells projecting to the medial vs lateral caudate-putamen in rat. A fluorescent retrograde double labeling study. Brain Res. 1979;169:381–387. doi: 10.1016/0006-8993(79)91038-2. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Hunt SP, Steinbusch HW, Verhofstad AA. Separate populations of cholecystokinin and 5-hydroxytryptamine-containing neuronal cells in the rat dorsal raphe, and their contribution to the ascending raphe projections. Neurosci Lett. 1981;26:25–30. doi: 10.1016/0304-3940(81)90420-1. [DOI] [PubMed] [Google Scholar]
- van Dongen YC, Deniau JM, Pennartz CM, Galis-de Graaf Y, Voorn P, Thierry AM, Groenewegen HJ. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience. 2005;136:1049–1071. doi: 10.1016/j.neuroscience.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Devilbiss D, Seiple S, Markowitz R. Sensorimotor-related discharge of simultaneously recorded, single neurons in the dorsal raphe nucleus of the awake, unrestrained rat. Brain Res. 2004;1000:183–191. doi: 10.1016/j.brainres.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Weiss M, Pellet J. Raphe-cerebellum interactions. I. Effects of cerebellar stimulation and harmaline administration on single unit activity of midbrain raphe neurons in the rat. Exp Brain Res. 1982;48:163–170. doi: 10.1007/BF00237211. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Hokfelt T. Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat. 1997;13:169–187. doi: 10.1016/s0891-0618(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Shirouzu M, Tanaka M, Semba K, Fibiger HC. Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res. 1989;496:373–376. doi: 10.1016/0006-8993(89)91091-3. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Azmitia EC. Effects of 5,7-dihydroxytryptamine on HRP retrograde transport from hippocampus to midbrain raphe nuclei in the rat. Brain Res Bull. 1983;10:445–451. doi: 10.1016/0361-9230(83)90142-9. [DOI] [PubMed] [Google Scholar]