Abstract
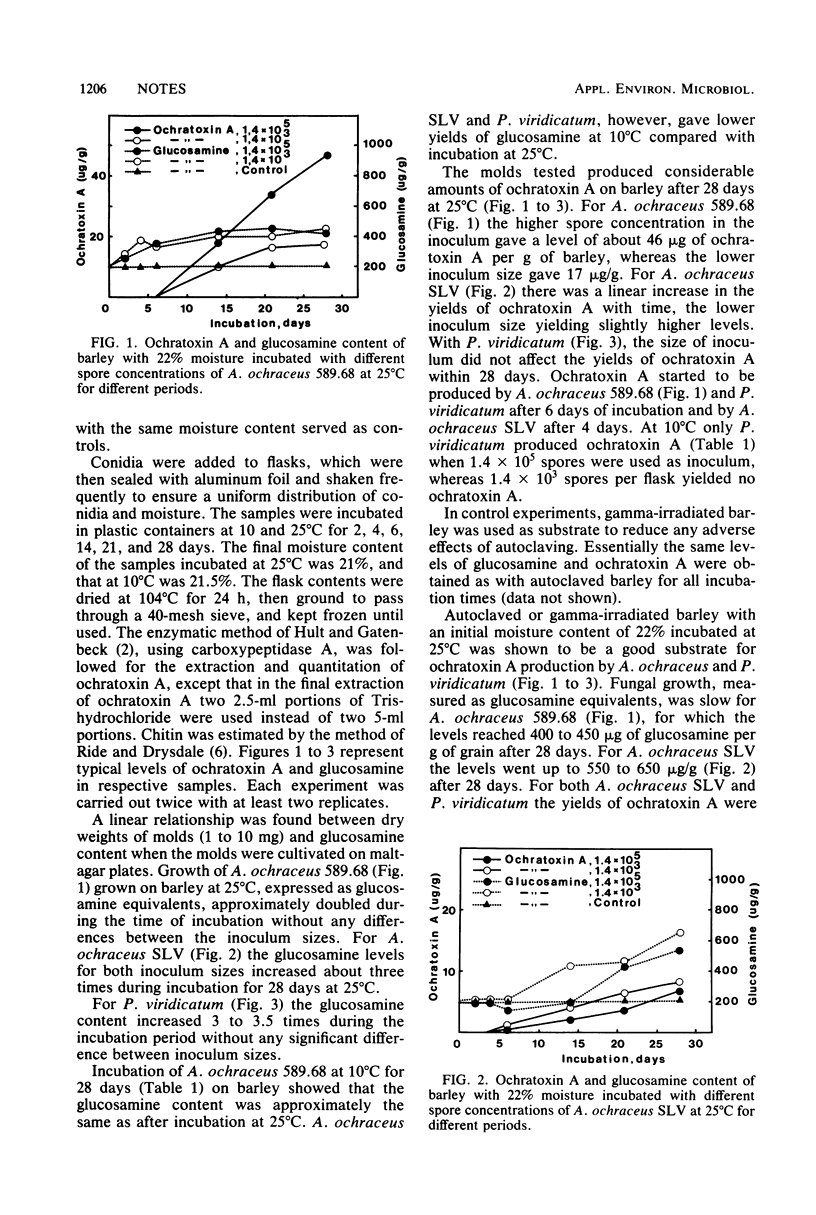
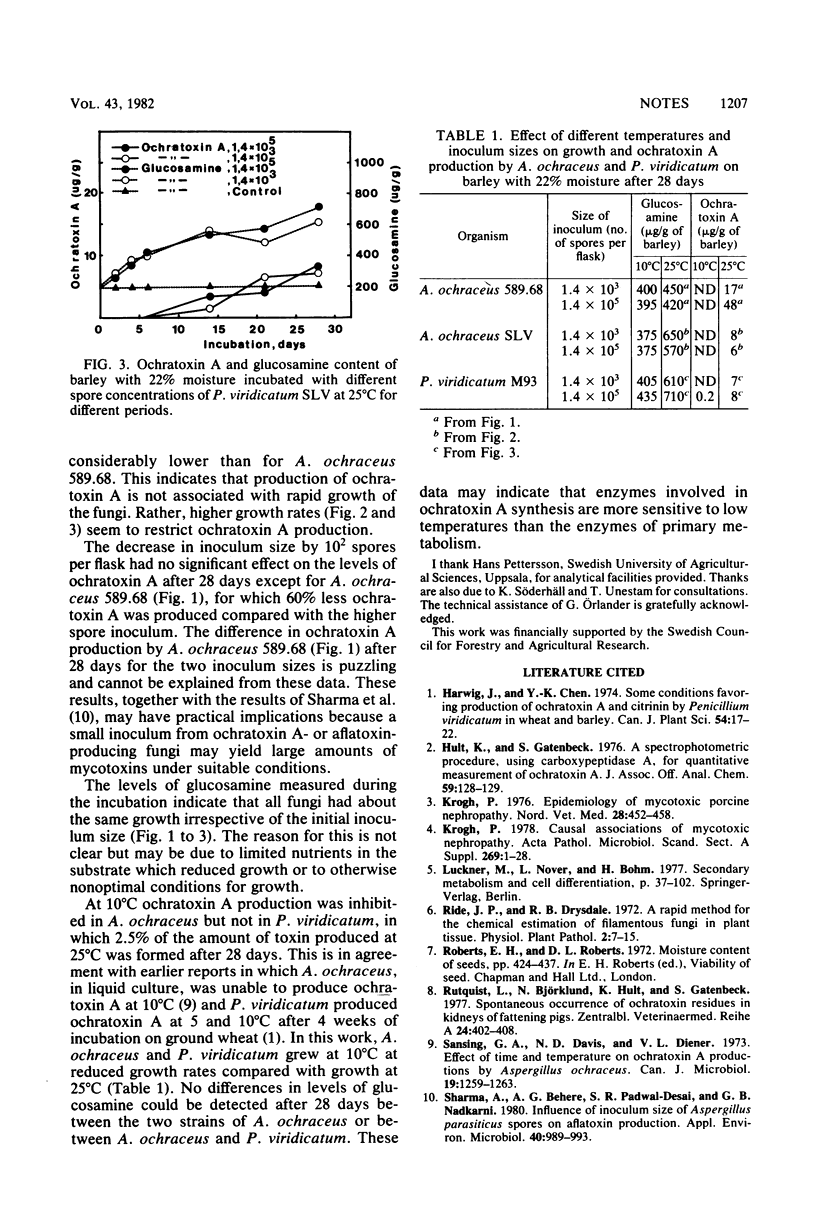
Moistened barley was inoculated with 1.4 x 10(3) and 1.4 x 10(5) spores, respectively, from ochratoxin A-producing strains of Aspergillus ochraceus and Penicillium varidicatum. To estimate fungal tissue in the barley, the amount of glucosamine was followed for 28 days at 10 and 25 degrees C. Ochratoxin A was also followed during the same period and under the same conditions. The data show that ochratoxin A could be detected 4 to 6 days after inoculation at 25 degrees C, and the maximal accumulation of ochratoxin A was observed 28 days after inoculation. After 28 days at 25 degrees C, the quantities of ochratoxin A were between 7 and 46 micrograms/g of grain. At 10 degrees C only P. viridicatum produced ochratoxin A. The results indicated that production of ochratoxin A is not associated with rapid increase of glucosamine in the barley.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hult K., Gatenbeck S. A spectrophotometric procedure, using carboxypeptidase A, for the quantitative measurement of ochratoxin A. J Assoc Off Anal Chem. 1976 Jan;59(1):128–129. [PubMed] [Google Scholar]
- Krogh P. Casual associations of mycotoxic nephropathy. Acta Pathol Microbiol Scand Suppl. 1978;(269):1–28. [PubMed] [Google Scholar]
- Krogh P. Epidemiology of mycotoxic porcine nephropathy. Nord Vet Med. 1976 Sep;28(9):452–458. [PubMed] [Google Scholar]
- Luckner M., Nover L. Expression of secondary metabolism. An aspect of cell specialization of microorganisms, higher plants, and animals. Mol Biol Biochem Biophys. 1977;23:3–102. [PubMed] [Google Scholar]
- Rutqvist L., Björklund N. E., Hult K., Gatenbeck S. Spontaneous occurrence of ochratoxin residues in kidneys of fattening pigs. Zentralbl Veterinarmed A. 1977 May;24(5):402–408. doi: 10.1111/j.1439-0442.1977.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Sansing G. A., Davis N. D., Diener U. L. Effect of time and temperature on ochratoxin A production by Aspergillus ochraceus. Can J Microbiol. 1973 Oct;19(10):1259–1263. doi: 10.1139/m73-203. [DOI] [PubMed] [Google Scholar]
- Sharma A., Behere A. G., Padwal-Desai S. R., Nadkarni G. B. Influence of inoculum size of Aspergillus parasiticus spores on aflatoxin production. Appl Environ Microbiol. 1980 Dec;40(6):989–993. doi: 10.1128/aem.40.6.989-993.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


