Abstract
Polyethylene glycol-treated protoplasts prepared from Streptococcus lactis LM3302, a lactose-negative (Lac−) derivative of S. lactis ML3, were transformed to lactose-fermenting ability by a transductionally shortened plasmid (pLM2103) coding for lactose utilization.
Full text
PDF
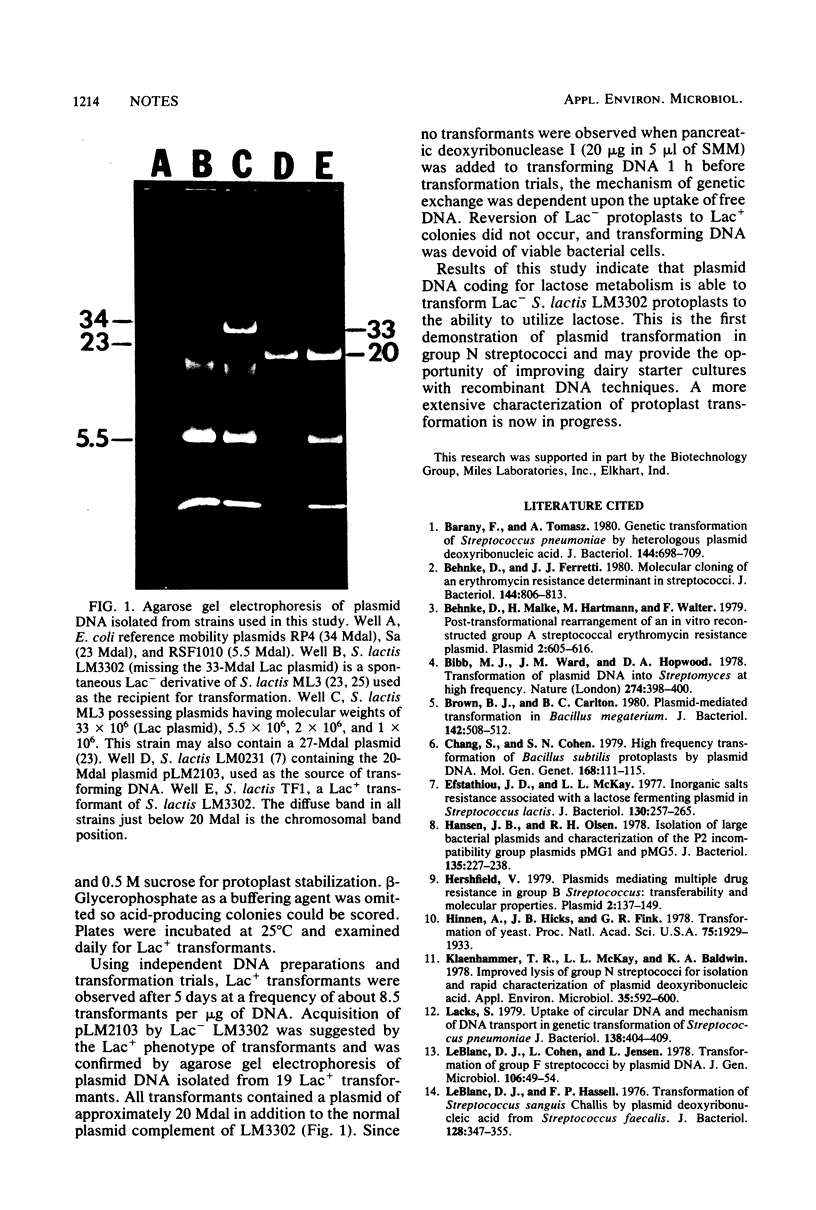

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F., Tomasz A. Genetic transformation of Streptococcus pneumoniae by heterologous plasmid deoxyribonucleic acid. J Bacteriol. 1980 Nov;144(2):698–709. doi: 10.1128/jb.144.2.698-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Molecular cloning of an erythromycin resistance determinant in streptococci. J Bacteriol. 1980 Nov;144(2):806–813. doi: 10.1128/jb.144.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Malke H., Hartmann M., Walter F. Post-transformational rearrangement of an in vitro reconstructed group-A streptococcal erythromycin resistance plasmid. Plasmid. 1979 Oct;2(4):605–616. doi: 10.1016/0147-619x(79)90058-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Brown B. J., Carlton B. C. Plasmid-mediated transformation in Bacillus megaterium. J Bacteriol. 1980 May;142(2):508–512. doi: 10.1128/jb.142.2.508-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Cohen L., Jensen L. Transformation of group F streptococci by plasmid DNA. J Gen Microbiol. 1978 May;106(1):49–54. doi: 10.1099/00221287-106-1-49. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980 Jun;28(3):692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Reichardt W., Hartmann M., Walter F. Genetic study of plasmid-associated zonal resistance to lincomycin in Streptococcus pyogenes. Antimicrob Agents Chemother. 1981 Jan;19(1):91–100. doi: 10.1128/aac.19.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Lohr J. R., Dean D. H. Transformation of Bacillus thuringiensis protoplasts by plasmid deoxyribonucleic acid. J Bacteriol. 1981 Feb;145(2):980–983. doi: 10.1128/jb.145.2.980-983.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Phillips S., Edelman I., Novick R. P. Tn554: isolation and characterization of plasmid insertions. Plasmid. 1981 May;5(3):292–305. doi: 10.1016/0147-619x(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Monomer plasmid DNA transforms Streptococcus pneumoniae. Mol Gen Genet. 1981;181(1):57–62. doi: 10.1007/BF00339005. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Properties and transforming activities of two plasmids in Streptococcus pneumoniae. Mol Gen Genet. 1980;180(3):573–578. doi: 10.1007/BF00268062. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R. J., McKay L. L., Ahlstrand G. G. Transduction of Lactose Metabolism by Streptococcus cremoris C3 Temperate Phage. Appl Environ Microbiol. 1981 Nov;42(5):897–903. doi: 10.1128/aem.42.5.897-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]



