Abstract
RNAi is widely applied to inhibit expression of specific genes, but it is limited by variable efficiency and specificity of empirically designed siRNA or shRNA constructs. This complicates studies targeting individual genes and significantly impairs large-scale screens using genome-wide knockdown libraries. Here, we show that ectopic expression of the RISC slicer Argonaute-2 (Ago2, eIF2C2) dramatically enhances RNAi specifically for mRNA targets with perfectly matched binding sites. This effect depends on its endonuclease activity and is uncoupled from its regulation of microRNA expression. To model the application of Ago2 coexpression with shRNA knockdown, we targeted the EGF receptor (EGFR) in lung cancer cells exhibiting oncogene addiction to EGFR. Whereas multiple empirically designed shRNA constructs exhibited highly divergent efficiencies in mediating EGFR knockdown and cell killing, coexpression of Ago2 resulted in uniform and highly specific target gene suppression and apoptosis in EGFR-dependent cells. Codelivery of Ago2 with shRNA constructs or siRNA duplexes thus provides a strategy to enhance the efficacy and the specificity of RNAi in experimental and potentially therapeutic settings.
Keywords: microRNA, RNAi screening, shRNA, siRNA, Ago2
Short RNA molecules can inhibit gene expression of specific target mRNA transcripts by reducing mRNA stability or inhibiting translation. This process, called RNAi, is mediated by either ectopic short RNA molecules (siRNA, shRNA) or by the endogenous microRNA (miRNA) class derived from longer precursors. Beyond the knockdown of single genes of interest, genome-wide siRNA or shRNA libraries have been created for different species that allow broad and unbiased screening for genetic modifiers of various cellular phenotypes (1–8). However, the application of RNAi is limited by the variable efficiency of empirically designed targeting sequences, with only a small fraction of constructs resulting in effective and specific knockdown of their targeted transcript. For experimental strategies aimed at targeting individual genes, this requires testing and validation of multiple constructs. When applied in genome-wide screens using shRNA or siRNA libraries to uncover novel regulators of cellular phenotypes, the variable effectiveness of empiric knockdown constructs requires the use of at least five constructs per gene to ensure adequate coverage. In addition to their inconsistent efficacy in mediating knockdown of target gene expression, some constructs confound this effect by off-target effects, including targeting of nonperfectly matched binding sites (9) and nonspecific competition with endogenous miRNA pathways at high siRNA concentrations (10). Thus, approaches capable of improving RNAi efficiency, while preserving specificity, are essential for its broad application in screening approaches. A number of protein factors capable of influencing mature miRNA or shRNA expression have been identified, including members of the Argonaute family and Exportin-5 (Exp-5, XPO5) (11–14). However, increased expression of mature miRNAs alone does not ensure the required specificity of targeting and could enhance off-target effects and the desired knockdown.
We recently demonstrated that Argonaute-2 (Ago2), the slicer in the RNA Induced Silencing Complex (RISC), also functions in endogenous miRNA biogenesis (11), in addition to its effector role in target mRNA cleavage. Here, we show that ectopic coexpression of Ago2 has a profound effect on RNAi mediated by siRNA, shRNA, or miRNA constructs, dramatically and selectively enhancing cleavage of perfectly matched RNA targets. This observation has immediate applications for the optimal design of RNAi strategies.
Results
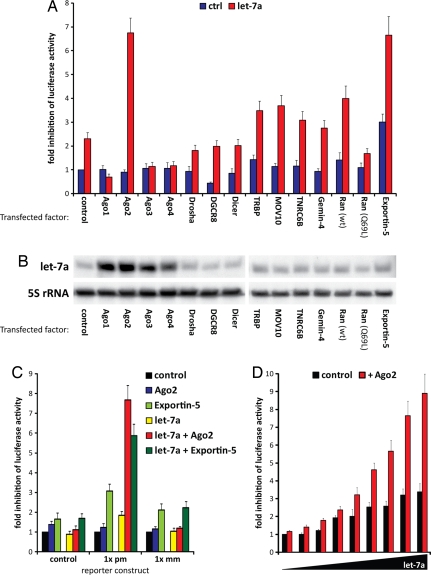
To improve RNAi mediated by small RNAs, we screened human factors involved in miRNA processing and in the effector phase of RNAi for their effect on target knockdown, using a reporter construct encoding luciferase fused to a 3′ untranslated region (3′-UTR) with perfectly matched binding sites complementary to the miRNA let-7a. Transfection of 293 cells with a construct encoding the let-7a primary miRNA (pri-miRNA) inhibited luciferase activity 2.3-fold. Of 14 candidate RNAi factors tested, coexpression of either Ago2 or Exportin-5 (Exp-5) led to >6-fold suppression of luciferase activity (Fig. 1A). In contrast to previous findings in Drosophila melanogaster where expression of Dicer-2 increased RNAi potency (2), human Dicer did not have any impact on RNAi in mammalian cells. We have shown that all Ago proteins enhance mature miRNA expression (11), which was also evident with let-7a (Fig. 1B). However, neither Ago1 nor Ago3 or Ago4 had any effect on expression of the luciferase reporter indicating that increased miRNA expression alone was insufficient to mediate the strong enhancement in RNAi. Along with Ago2, only the miRNA processing factor Exp-5, which mediates nuclear export of miRNA and shRNA precursors (15, 16), enhanced miRNA efficacy (12). To determine the most specific enhancer of RNAi, we compared the efficiency of Ago2 and Exp-5 toward different luciferase constructs (Fig. 1C). Exp-5 enhanced inhibition of the reporter by endogenous miRNAs even in the absence of ectopic let-7a, and its effect was also evident with nonperfectly matched binding sites. In marked contrast, enhancement of miRNA-mediated reporter knockdown by coexpression of Ago2 was restricted to perfectly matched binding sites and did not affect endogenous RNAi activity. Because off-target effects of RNAi have been linked to saturation of endogenous miRNA pathways by the ectopic short RNAs (10), we determined whether coexpression of Ago2 also allowed a reduction of the ectopic small RNA concentration by cotransfecting variable amounts of let-7a expression plasmids with or without Ago2. Remarkably, the presence of Ago2 resulted in a maximal luciferase inhibition by let-7a at a 16-fold lower miRNA concentration than achieved with the miRNA alone (Fig. 1D).
Fig. 1.
Identification of enhancers of RNAi. (A) 293 cells were transfected with a firefly luciferase construct containing multiple perfectly matched let-7a-binding sites in its 3′UTR, let-7a, and miRNA processing/RNAi factors, as indicated. Renilla luciferase was cotransfected for normalization. Expression levels of the transfected proteins have been documented (11). Depicted is the mean fold inhibition (+SEM) of firefly luciferase activity compared with control (luciferase alone). Although let-7a alone weakly inhibited luciferase activity, Argonaute-2 (Ago2) and Exportin-5 (Exp-5) greatly enhanced its inhibitory function. Statistical analyses for all experiments are listed in Dataset S2. (B) 293 cells were cotransfected with pri-let-7a and either a control plasmid or expression constructs for different processing/RNAi factors. Northern blot analysis showed that expression of all Argonaute proteins led to increased mature let-7a expression. (C) Comparison of Ago2 and Exp-5 in 293 cells transfected with luciferase constructs containing different binding sites in their 3′UTR (pm, perfect match; mm, two mismatches in middle of miRNA-binding site) revealed higher specificity for Ago2 in let-7a-mediated repression. (D) 293 cells were transfected with increasing amounts of let-7a expression constructs in 2-fold increments. Luciferase assays with a reporter containing multiple let-7a binding sites revealed that the maximum knockdown level of let-7a alone was reached at 16-fold lower miRNA transfection levels when cotransfected with Ago2.
To further test the sequence specificity of Ago2-enhanced knockdown, we generated a series of reporters with variations in the miRNA-binding sequence (Fig. 2A). As expected, ectopic let-7a, Ago2, or coexpression of the two constructs had no inhibitory effect on a control 3′UTR. A reporter with perfectly matched binding sites (pm) was unaffected by expression of Ago2 alone, weakly suppressed by exogenous let-7a but strongly suppressed by coexpression of the two constructs. Of note, coexpression of Ago2 improved let-7a-mediated knockdown of a reporter with a single binding site (1X) to a higher level than observed with the miRNA alone targeting a reporter with four multimerized binding sites (4X). Addition of Ago2 to the 4X reporter enhanced let-7a-mediated knockdown even further. A 2-nt mismatch in the middle of the binding site (mm) abrogated the effect of let-7a alone and displayed no enhancement after Ago2 expression, despite the presence of 10 perfectly matched base pairs (including the seed sequence) on either side of the mismatch. A 2-nt mismatch in the seed sequence similarly inhibited the effect of Ago2 on let-7a-mediated knockdown (seed mm). Because Ago2 is known to cleave perfectly matched duplexes, we tested whether its intrinsic RNase activity was required for enhancement of miRNA knockdown. Indeed, RNase-deficient Ago2 mutants D597A and D669A (17) failed to enhance miRNA-mediated inhibition (Fig. 2B). Notably, Ago1, Ago3, and Ago4 lack intrinsic RNase activity, and expression of these family members [supporting information (SI) Fig. S1A] or RNase-inactive Ago2 (Fig. 2B) inhibited miRNA-mediated knockdown, raising the possibility of competition with endogenous Ago2. Ectopic expression of the let-7a pri-miRNA is analogous to the experimental transfection of shRNA constructs, which are also processed through the miRNA biogenesis pathway, whereas siRNA duplexes are loaded into the RISC largely independent of the biosynthetic processing machinery. To test whether enhancement of RNAi by Ago2 observed for miRNA/shRNA constructs was also evident with siRNA-mediated knockdown, we transfected 293 cells with the luciferase reporter along with double-stranded let-7a RNA oligonucleotides (Fig. 2C). Inhibition of the reporter by the siRNA duplex was enhanced by Ago2 to an extent comparable to that evident with miRNA precursors. We also confirmed Ago2-mediated enhancement of RNAi using a second well studied miRNA, miR-143, coexpressed with a luciferase reporter containing matched binding sites in its 3′UTR (Fig. 2D).
Fig. 2.
Ago2 RNase activity is required for RNAi enhancement at perfectly matched targets. (A) 293 cells were transfected with a firefly luciferase construct containing different let-7a binding sites in its 3′ UTR (pm, perfect match; mm, two mismatches in middle of miRNA-binding site; seed mm, two mismatches in seed region of miRNA–3′UTR interaction site; 1x, one binding site; 4x, four tandem binding sites), renilla luciferase and expression plasmids for let-7a or/and Ago2. Depicted is the mean fold inhibition (+SEM) of firefly luciferase activity compared with a transfection neither containing the ectopic miRNA nor Ago2 (ctrl). Let-7a only weakly inhibited luciferase activity, whereas Ago2 enhanced it. RNAi specificity was conserved because neither mismatch nor seed mutant controls were affected at all by Ago2 expression. (B) RNase deficient Ago2 mutants (D597A, D669A) failed to enhance inhibition of luciferase activity by let-7a in 293 cells. (C) Ago2 cotransfection into 293 cells also enhanced the inhibition of let-7a siRNA transfected as double-stranded siRNA oligonucleotide. (D) Efficiency of miR-143 toward a luciferase construct containing perfectly matched miR-143 binding sites in its 3′UTR was also enhanced by Ago2 cotransfection into 293 cells.
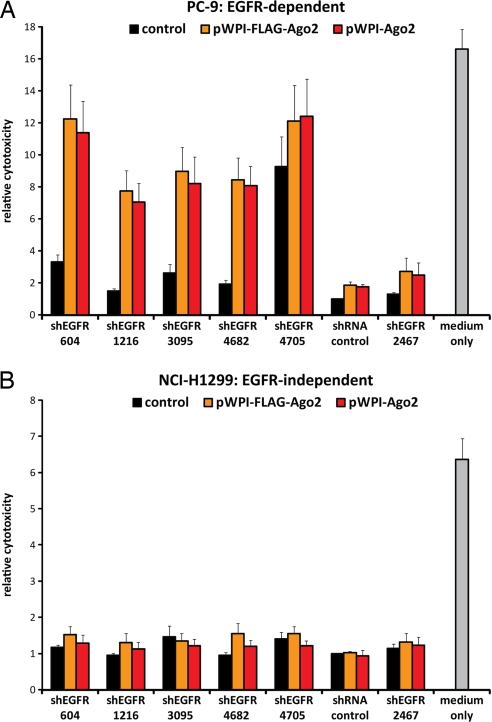
Given the effectiveness of Ago2 in enhancing RNAi targeting synthetic reporter constructs, we tested its impact on endogenous transcripts. As noted above, the need for effective “on-target” knockdown is typically met by testing different empirically designed shRNAs, whereas false-positive “off-target” effects are mitigated by testing multiple effective constructs. Such analyses are feasible for individual genes of interest, but they present a serious challenge for genome-wide screens, where false-positive and -negative results are common. To screen for cell cycle arrest phenotypes using the RNAi Consortium (TRC) library, five shRNA clones were tested against each target to ensure that one resulted in effective knockdown (4). To test whether coexpression of Ago2 with shRNA constructs provided a solution to this problem, we obtained the six TRC library, empirically designed lentiviral shRNA constructs targeting EGFR, a target gene whose knockdown has a dramatic effect in a subset of non-small cell lung cancers (NSCLC) (18). For these experiments, we made use of PC-9 NSCLC cells, endogenously harboring an amplified mutant (delE746-A750) EGFR gene. Flow cytometry 48 h after infection with each of these shRNAs revealed that, of six constructs tested, only one shRNA (shEGFR-4705) was capable of a >50% reduction in EGFR-expressing cells (Fig. 3 A and B, Figs. S2 and S3). However, coinfection with Ago2 resulted in a comparable reduction in EGFR-expressing cells using four previously ineffective shEGFR constructs, and it improved the effect of shEGFR-4705 to 80%. Of interest, the target site of the sixth shRNA construct (shEGFR-2467) is partially disrupted by the specific endogenous EGFR gene mutation in PC-9 cells. Ago2 did not enhance EGFR knockdown by shEGFR-2467 in PC-9 cells, further demonstrating the remarkable specificity of this effect for perfectly matched binding sites. PC-9 cells are exquisitely sensitive to loss of mutant EGFR signaling, illustrating the phenomenon of “oncogene addiction,” which underlies the dramatic response of a subset of NSCLC to EGFR kinase inhibitors such as gefitinib and erlotinib (18). Indeed, apoptosis was observed in these cells 96 h after infection with shRNA constructs targeting EGFR. Only one of the shRNA constructs obtained from the TRC library induced cell death, grossly measurable by either Syto60 staining or MTT assays (Fig. 4; Fig. S4). Coinfection with lentiviral constructs expressing either tagged or untagged Ago2 vastly increased cell death after expression of all five perfectly matched shEGFR constructs. Ago2 alone had no cytotoxic effects on PC-9 cells, and coinfection of Ago2 together with shEGFR constructs did not cause toxicity in NSCLC cells with wild-type EGFR (NCI-H1299), which do not depend on this pathway for cell survival (Fig. 4B). Coinfection of Ago2 with shEGFR-2467, which targets wild-type EGFR but not the mutant allele in PC-9 cells, did not induce cell death, supporting the biological significance of knocking down the “oncogene addicting” mutant EGFR allele, rather than the wild-type allele in these cells.
Fig. 3.
Ago2 enhances shRNA-mediated knockdown of endogenous EGFR. (A and B) PC-9 cells were infected with different shRNA constructs targeting EGFR and pWPI-Ago2 containing an IRES-EGFP cassette to distinguish Ago2-expressing (green) from nonexpressing cells. 48 h after infection, EGFR expression was analyzed by flow cytometry. shEGFR-2467 targets a region within EGFR that is deleted in PC-9 cells and thus served as additional control next to shEGFP, shMET or no shRNA showing specificity and viability of the Ago2 infection. (A) Histogram plots and dot plots of representative shRNAs illustrate that the EGFR expression was lower in Ago2-expressing, EGFP-positive cells than in cells not expressing Ago2. (B) The bar graph shows the mean (+SEM) percentage of EGFR-negative cells from three independent experiments. Focusing first on EGFP-negative and thus non-Ago2-expressing cells (red bars), only shEGFR-4705 was able to induce >50% EGFR-negative cells, whereas all other shRNA constructs failed to reach this level of knockdown by far. For EGFP-positive and thus Ago2-expressing cells (green bars), all shEGFR constructs increased the fraction of EGFR-negative cells to 40–80%.
Fig. 4.
Ago2 improves shRNA-mediated cell death specifically in sensitive cells. (A and B) PC-9 cells contain a small deletion in EGFR leading to constitutionally active EGFR and making PC-9 cells exquisitely sensitive to loss of EGFR signaling (18), whereas NCI-H1299 cells are not sensitive to EGFR knockdown. Both cell lines were lentivirally infected with different shEGFR constructs containing a Puromycin resistance cassette combined with an empty pWPI lentivirus or expression constructs for tagged or untagged Ago2, selected by Puromycin and analyzed for cell density by Syto60 staining. Depicted is the mean growth inhibition (+SEM) as compared to cells infected with shEGFP and empty pWPI. Without Ago2 expression, only shEGFR-4705 induced cell death in these cells mirroring the results from protein knockdown. With ectopic Ago2, all shEGFR constructs targeting EGFR in PC-9 cells were capable of inducing cell death. As controls, shEGFP or shEGFR-2467 (located within deletion) infected cells were viable whereas noninfected cells died in Puromycin selection. Ago2 expression had very little effects on controls. NCI-H1299 cells did not show any sensitivity to the shRNAs, neither without nor with additional Ago2.
Discussion
We have shown that coexpression of Ago2 enhances RNAi mediated by ectopic miRNA, siRNA and shRNA, in a manner that is specific to perfectly complementary sequences and does not affect endogenous miRNA pathways. In our screen for factors improving RNAi, we identified Ago2 and Exp-5 as the strongest RNAi enhancers. Our data on Exp-5 are consistent with previous findings by Cullen and coworkers (12), although we find that Exp-5 also enhances endogenous miRNA activity and partially affects mismatched sequences, making it more prone to off-target effects. In contrast, Ago2 is a potent enhancer of RNAi that possesses a higher specificity toward perfectly matched binding sites making it uniquely valuable for RNAi screens.
The mechanism of action of Ago2 most likely reflects its unique RNase activity, which is required for target mRNA cleavage (17, 19). However, Ago2 additionally plays an active role in miRNA biogenesis: It cleaves the precursor miRNA hairpin in the passenger strand arm resulting in the ac-pre-miRNA intermediate (11), and it also cuts the nonproductive strand of the siRNA duplex within the RISC complex and thus facilitates strand dissociation (20–23). Expression of all Ago proteins increases the abundance of mature miRNAs in vivo and in vitro (11, 13, 14), but only Ago2 enhances RNAi efficiency. Thus, expression levels of the mature miRNA seem not to be the major determinant for RNAi efficiency, but properties intrinsic to Ago2 appear critical, emphasizing the importance of the Ago2-specific endonuclease. In this context, the inhibitory effects of other Ago proteins on RNAi efficiency even suggest dominant-negative effects, possibly through competition with endogenous Ago2. Of note, Ago3 and Ago4 have been shown to inhibit translation when artificially tethered to the 3′UTR of target mRNAs and are hence effectors of RNAi (24). Nonetheless, they do not enhance RNAi by let-7a toward perfectly matched binding sites in our assays. We therefore conclude that the ability of Ago2 to increase the degradation of the targeted mRNA is essential to its potent effect on enhancing RNAi. The requirement of Ago2 RNase activity for a perfectly complementary duplex likely underlies the strict preservation of RNAi target specificity mediated by Ago2 and minimizes the risk of enhancing off-target effects.
In addition to providing insight into different properties of RNAi effectors, our observations have immediate applications for the design of RNAi in experimental settings. Ectopic Ago2 not only optimizes targeted RNAi but also may help minimize nonspecific toxicity attributed to oversaturation of miRNA pathways by high siRNA loads (10): Ago2 coexpression allows a reduction in the concentration of targeting construct required because of its increased potency and provides an additional amount of a rate-limiting component in the miRNA pathway. Thus, coexpression of Ago2 may well prove to be universally effective in RNAi experiments. Its use may be particularly critical in high-throughput screening approaches using siRNA or shRNA libraries. Currently, such genome-wide RNAi screens are greatly limited by evident false-positive and presumed false-negative results (25), and coexpression of Ago2 may provide the increased efficacy and specificity required to identify the full complement of genes involved in a phenotype under investigation. Finally, because the therapeutic utility of RNAi is explored in a number of different clinical contexts (26), its enhancement by coexpression of Ago2 may increase the potency and broaden the range of potential applications.
Materials and Methods
Cloning of Expression Plasmids and Lentiviruses.
Expression plasmids for RNAi factors have been described in ref. 11. Expression plasmids for let-7a-3 and miR-143 have also been described in ref. 27. The pWPI vector was generously provided by Didier Trono. For luciferase assays, the Firefly luciferase coding sequence was cloned into the pcDNA3.1D plasmid using directional TOPO cloning. In a second cloning step, the desired binding sites were cloned as double-stranded DNA oligonucleotides into the 3′ UTR using the XhoI and XbaI binding sites. pLKO1-shRNA lentiviruses targeting EGFR were obtained from the Broad collection (4). Ago2 was cloned into a lentiviral pWPI vector using GATEWAY technology from the pENTR3C entry vector. All clones were verified by DNA sequencing. Oligonucleotide sequences are listed in Dataset S1.
Transfection and Luciferase Assay.
For luciferase assays, 293 cells were seeded to reach 80% confluence and transfected with 1.6 μg of DNA plasmid and 4.0 μl of Lipofectamine2000 in a 12-well plate according to the manufacturer's recommendations (Invitrogen). Each transfection contained 50 ng of pRL-SV40 Renilla expression construct (Promega), 150 ng of pcDNA3.1D-Firefly expression construct including the 3′ UTR of interest, 700 ng of expression plasmid for the miRNA of interest and 700 ng of expression plasmid for the protein factor to be analyzed. For siRNA transfection, 10 pmol double-stranded let-7a siRNA were transfected instead of the miRNA expression plasmid. For luciferase assays, cells were washed with 500 μl of PBS and lysed in 150 μl of Passive Lysis Buffer (Promega). Ten microliters of lysate was subsequently analyzed in a Centro LB960 Luminometer (Berthold Technologies) using 25 μl of LAR II and 25 μl of Stop&Glow to determine Firefly and Renilla luciferase activity (Dual Luciferase Reporter Assay System; Promega). Each sample was analyzed in quadruplicate, and each transfection was carried out at least three times. Firefly activity was normalized to Renilla activity, and mean values plus standard error of mean are depicted.
Lentiviral Transduction.
Lentiviral infection was carried out as described in ref. 4. Updated protocols are available at www.broad.mit.edu/genome_bio/trc/publicProtocols.html.
Lentiviral supernatants were titrated by using A549 cells in a 96-well format (2,500 cells per well). pLKO1-shRNA lentiviral constructs contained a Puromycin resistance marker. Therefore, transduced A549 cells were selected in 1 μg/ml Puromycin for 48 h and then analyzed by using Syto60 staining to assess cell density. A transduction efficiency of 100% was defined as the lowest concentration at which no difference between the cell density of Puromycin-treated and nontreated cells was observed. This amount of shRNA virus supernatant was used for all experiments. Ago2-containing lentiviruses did not contain a resistance marker, but an IRES-EGFP cassette. Ago-2 encoding lentiviruses were titered by determining the fraction of EGFP-positive cells by fluorescence microscopy or flow cytometry. For flow cytometric determination of EGFR knockdown in the absence or presence of Ago2, a transduction efficiency of 50% was used to generate Ago2-expressing and nonexpressing cells in the same sample. The experiments for cell viability were carried out comparing either the same amount of transducing viruses or the same volume of pWPI control virus or pWPI-FLAG-Ago2/pWPI-Ago2 supernatants, which gave rise to identical results, verifying the specificity of the observed effects for Ago2 expression.
Additional experimental procedures can be found in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by the German Research Foundation (DFG) (Grant Di 1421/1-1, to S.D.) and the National Institutes of Health (Grant T32, to S.M.R.; R37 CA058596-15, to D.A.H.) and a DF/HCC Prostate SPORE Award (to S.M.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800803105/DCSupplemental.
References
- 1.Berns K, et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 2.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 3.Ebert BL, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moffat J, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Ngo VN, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 6.Paddison PJ, et al. A resource for large-scale RNA-interference-based screens in mammals. Nature. 2004;428:427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- 7.Silva JM, et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–1288. doi: 10.1038/ng1650. [DOI] [PubMed] [Google Scholar]
- 8.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci USA. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AL, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 10.Grimm D, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 11.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Yi R, Doehle BP, Qin Y, Macara IG, Cullen BR. Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA. 2005;11:220–226. doi: 10.1261/rna.7233305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Carroll D, et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakiyama M, Takimoto K, Ohara O, Yokoyama S. Let-7 microRNA-mediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev. 2007;21:1857–1862. doi: 10.1101/gad.1566707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 16.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 19.Meister G, et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 2005;19:2837–2848. doi: 10.1101/gad.1370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Leuschner PJ, Ameres SL, Kueng S, Martinez J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006;7:314–320. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echeverri CJ, et al. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat Methods. 2006;3:777–779. doi: 10.1038/nmeth1006-777. [DOI] [PubMed] [Google Scholar]
- 26.Soutschek J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 27.Diederichs S, Haber DA. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res. 2006;66:6097–6104. doi: 10.1158/0008-5472.CAN-06-0537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.