Abstract
Aims
To investigate the effects of cardiac resynchronization therapy (CRT) on survival in heart failure (HF) patients with permanent atrial fibrillation (AF) and the role of atrio-ventricular junction (AVJ) ablation in these patients.
Methods and results
Data from 1285 consecutive patients implanted with CRT devices are presented: 1042 patients were in sinus rhythm (SR) and 243 (19%) in AF. Rate control in AF was achieved by either ablating the AVJ in 118 patients (AVJ-abl) or prescribing negative chronotropic drugs (AF-Drugs). Compared with SR, patients with AF were significantly older, more likely to be non-ischaemic, with higher ejection fraction, shorter QRS duration, and less often received ICD back-up. During a median follow-up of 34 months, 170/1042 patients in SR and 39/243 in AF died (mortality: 8.4 and 8.9 per 100 person-year, respectively). Adjusted hazard ratios were similar for all-cause and cardiac mortality [0.9 (0.57–1.42), P = 0.64 and 1.00 (0.60–1.66) P = 0.99, respectively]. Among AF patients, only 11/118 AVJ-abl patients died vs. 28/125 AF-Drugs patients (mortality: 4.3 and 15.2 per 100 person-year, respectively, P < 0.001). Adjusted hazard ratios of AVJ-abl vs. AF-Drugs was 0.26 [95% confidence interval (CI) 0.09–0.73, P = 0.010] for all-cause mortality, 0.31 (95% CI 0.10–0.99, P = 0.048) for cardiac mortality, and 0.15 (95% CI 0.03–0.70, P = 0.016) for HF mortality.
Conclusion
Patients with HF and AF treated with CRT have similar mortality compared with patients in SR. In AF, AVJ ablation in addition to CRT significantly improves overall survival compared with CRT alone, primarily by reducing HF death.
Keywords: Cardiac resynchronization therapy, Heart failure, Atrial fibrillation, Atrio-ventricular junction ablation
Introduction
Cardiac resynchronization therapy (CRT) is an effective therapy in symptomatic, optimally treated heart failure (HF) patients with prolonged QRS duration and low ejection fraction (EF).1–4 Long-term results of CRT on exercise tolerance and disease progression, as evaluated by reversal of maladaptive remodelling process, are rather limited5,6 and mostly reported in patients with sinus rhythm (SR). Permanent atrial fibrillation (AF) is a common arrhythmia in HF patients and is associated with increased morbidity and mortality.7,8 Some studies have reported significant acute9,10 and short-term5,6 benefits of CRT in AF patients with HF. We recently11 emphasized the importance of atrio-ventricular junction (AVJ) ablation in order to optimize CRT in patients with permanent AF. This approach appears to maximize CRT delivery, yielding a ‘pure’ resynchronization effect which translates into significant long-term improvement in left ventricular function and remodelling. Such a reverse remodelling effect has been shown to correlate with improved survival in HF patients with normal SR treated with CRT over mid-term follow-up.12 To date, there is no information regarding this aspect in HF patients with AF.
The aim of the present study was two-fold: first, to investigate the effect of CRT on long-term survival in HF patients with permanent AF compared with SR patients; second, to investigate whether the adjunct of AVJ ablation may influence long-term outcome in the AF patient subgroup.
Methods
Design
For the purpose of the present study, the data were derived from the Multicentre Longitudinal Observational Study (MILOS)13 and included all consecutive patients undergoing CRT pacemaker (CRT-P) or CRT defibrillator (CRT-D) device implant from 1 August 1995 to 1 August 2004.
Patients
Among the 1303 patients enrolled in the registry, 18 had incomplete data or were lost to follow-up: the remaining 1285 patients, who represent the study population, were regularly followed in outpatient clinic settings (at 2, 6, and every 6 months thereafter), where the physicians were ‘blinded’ to the AVJ ablation therapeutic scheme; all presented complete information about cardiac rhythm and survival status; the last follow-up was scheduled on November 2004.
Aetiology was assessed in all cases by coronary angiography, and causes of HF amenable to surgery or intervention were corrected at least 6 months before device implantation. Because indication for cardioverter–defibrillator therapy changed over time, patients received the most appropriate device on the basis of current available evidence and guidelines.
Two hundred and forty-three (19%) patients had permanent AF at the time of implant, whereas the remaining 1042 were in SR. Immediately after CRT device implantation, every AF patient received negative chronotropic drugs, and the rate adaptive mode plus specific pacing features (such as ventricular rate regularization and/or trigger function) were activated in order to maximize biventricular stimulation. AVJ ablation was performed in patients in whom suboptimal CRT delivery was observed. For all centres, the choice of performing AVJ ablation was mainly based on the detection of inadequate BVP% derived from device counters at 2 month post-implant control.11 In the earlier phases, the evidence of frequent fusion, pseudo-fusion, or competitive spontaneous beats detected from standard, dynamic, or stress test ECG monitoring was utilized to further indicate AVJ ablation.
A BVP% ≤ 85 was considered ‘insufficient’ for effective CRT delivery. Therefore, in patients with BVP% ≤ 85, AVJ ablation was performed; digoxin and amiodarone were subsequently suspended (amiodarone was continued only if significant ventricular tachyarrhythmias were present), whereas beta-blocker therapy was maintained. On the contrary, patients with BVP% > 85 continued throughout the follow-up the ‘rate-control’ treatment regimen combining chronotropic negative drugs with activation of device features.
Because AVJ ablation was not a standardized clinical care, formal approval was obtained from the review boards of each institution. All patients gave their written informed consent to undergo the ablation procedure under the understanding that irreversible complete atrio-ventricular block would be created with subsequent pacemaker dependency.
Adjudicated classification of deaths
Outcome data were collected at each participating centre by reviewing outpatient clinical files or by phone interviews with relatives and/or family doctors. At each centre, an adjudication committee was established to perform chart review and assign, by consensus, the mode of cardiac death. The adjudication committee was blinded to the strategy undertaken to achieve rate control in AF patients as well as to the type of implanted device. Deaths were classified as cardiac, non-cardiac, or unknown. Cardiac deaths were classified as sudden (not preceded by HF or ischaemic symptoms) or due to HF according to Epstein et al.14 Patients undergoing left ventricle assist device or urgent heart transplant were classified as HF deaths. When the cause of death could not be determined using all available sources, it was classified as unknown.
Device implantation and programming
Conventional ventricular leads were positioned in the right ventricular apex in all patients implanted with CRT systems, and conventional atrial leads were used only in SR patients. In those patients in whom implantable defibrillator was necessary, the defibrillation lead was positioned in the right ventricular apex. Transvenous left ventricular lead positioning was guided by coronary sinus venogram, preferring a lateral or postero-lateral vein as implantation site. In case of failure or technical difficulties by the transvenous approach (phrenic nerve stimulation, inability to cannulate the coronary sinus, etc.), epicardial screw-in or steroid-eluting passive lead was implanted through limited thoracotomy. The latter was the elective approach used before transvenous leads became routinely available.
In patients with preserved SR, devices were programmed in DDD modality, usually with a minimum heart rate of 50 b.p.m., and atrio-ventricular interval optimization was performed.15 In patients with AF, minimum heart rate was usually set at 70 b.p.m. (≥80 b.p.m. for 2 weeks after AVJ ablation).16 Rate adaptive response was activated in any AF patient and the maximum rate was set at 85% of the theoretical maximum heart rate; moreover, in order to achieve the higher biventricular pacing percentage, ventricular rate stabilization and/or ventricular rate regulation (VRR) and trigger mode were activated when available.17
Statistical analysis
Descriptive statistics were computed as mean and standard deviation for continuous variables and as counts and percentage for categorical variables. Patients’ characteristics were compared between groups with unpaired t-test or one-way analysis of variance and with Fisher's exact test, respectively. Median follow-up and its 25–75th percentiles were computed by means of the inverse Kaplan–Meier method.18 The rates of fatal and of cardiac events per 100 person-year and their 95% confidence intervals (95% CIs) were calculated. Kaplan–Meier method was used to compute cumulative event-free survival. Cox regression was used to assess the prognostic role of the presence of AF when compared with SR and, among AF patients, the presence or absence of AVJ ablation, while adjusting for a series of potential baseline confounders (age, gender, left ventricular EF, aetiology, QRS duration, functional NYHA class, and the type of implanted device—CRT-P or CRT-D), in a multivariable model stratified for centre. Hazard ratios (HRs) and their 95% CI were reported. The proportional hazard assumption was verified, based on Schoenfeld residuals. AVJ ablation was treated as a time-dependent variable. To better characterize the prognostic role of AF and AVJ ablation on the different causes and modes of death, separate Cox models were fitted for cardiac death, death from HF, and sudden death. In these cases, to avoid overfitting, confounding was addressed by including the quintiles of the propensity score (PS) in the Cox model stratified by centre. The PS is a measure of the likelihood for a patient to undergo AVJ, given its presenting characteristics, derived from a multivariable logistic model. To build the PS, we included in the model all the demographic and clinical variables collected (age, gender, left ventricular EF, aetiology, QRS duration, functional NYHA class, the type of implanted device, and centre), together with their two-way interactions, as well as the drug regimen administered (Table 1). The balancing property of the PS was satisfied and the c-statistic for the model was computed to 0.80. Moreover, the ability of the PS to control for confounding in the subsequent Cox models was confirmed by its lack of statistical significance.
Table 1.
Baseline characteristics of global permanent atrial fibrillation patient population compared with sinus rhythm patients
| SR (n = 1042) | AF (n = 243) | P-value | |
|---|---|---|---|
| Age (years) | 63.4 (9.5) | 66.2 (8.9) | <0.001 |
| Female | 262 (25.1%) | 44 (18.1%) | 0.020 |
| Aetiology | |||
| Non-ischaemic | 545 (52.3%) | 146 (60.0%) | 0.032 |
| Ischaemic | 497 (47.7%) | 97 (40.0%) | |
| NYHA | |||
| II | 60 (5.8%) | 10 (4.1%) | 0.196 |
| III | 826 (79.3%) | 193 (79.4%) | |
| IV | 146 (14.9%) | 40 (16.5%) | |
| QRS (ms) | 170 (28) | 161 (32) | <0.001 |
| Left ventricular EF (%) | 24.4 (7.3) | 26.0 (8.0) | 0.005 |
| Mitral regurgitation (grade 3–4) | 594 (57%) | 185 (76%) | <0.001 |
| Left atrial diameter (mm) | 51 (7) | 56 (6) | <0.001 |
| CRT-D | 604 (57.8%) | 117 (48.2%) | 0.006 |
| ACE-inhibitors/ARBs | 948 (90.9%) | 228 (93.7%) | 0.162 |
| Beta-blockers | 833 (79.9%) | 194 (79.7%) | 1.000 |
| Aldosterone antagonists | 548 (52.6%) | 140 (57.6%) | 0.175 |
| Diuretics | 938 (90.0%) | 224 (92.2%) | 0.335 |
| Digitalis | 550 (52.9%) | 171 (70.3%) | <0.001 |
| Amiodarone | 259 (24.9%) | 108 (44.4%) | <0.001 |
| Negative chronotropic drugs | 907 (87.0%) | 236 (97.1%) | <0.001 |
| Intravenous inotropic drugs | 12 (1.2%) | 5 (2.0%) | 0.344 |
Mean (SD) for continuous variables; n (%) for categorical variables.
SR, sinus rhythm; AF, atrial fibrillation; CRT-D, cardiac resynchronization therapy pacemaker with defibrillator; ARBs, angiotensin receptor blockers.
Stata 9 (StataCorp, College Station, TX, USA) was used for computation. A two-sided P-value <0.05 was considered statistically significant.
Results
Long-term survival stratified according to baseline rhythm
The characteristics of the 1042 patients in SR are compared with those of the 243 patients with permanent AF in Table 1. The two groups had similar severity of symptoms as shown by their NYHA class and need of intravenous inotropic therapy. Patients with AF were older, with a greater prevalence of female gender, less coronary artery disease, slightly higher EF, and marginally shorter QRS duration. AF patients received less CRT-D devices and were more often treated with digoxin, amiodarone, and negative chronotropic drugs (to achieve a higher percentage of biventricular pacing time).
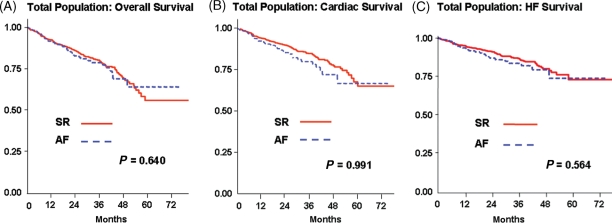
During a median follow-up of 34 months (interquartile range: 10–40 months), 170/1042 and 39/243 patients in the SR and AF groups died, with a mortality rate of 8.4 (95% CI 7.2–9.8) and 8.9 (95% CI 6.5–12.2) per 100 person-year (Figure 1A), respectively. Multivariable analysis confirmed similar mortality of SR and AF patients (HR 0.90, 95% CI 0.57–1.42, P = 0.64).
Figure 1.
Comparison of Kaplan–Meier estimates of overall (A), cardiac (B), and heart failure (C) survival between sinus rhythm and the global atrial fibrillation population. The P-values presented derive from the adjusted hazards ratio analysis stratified according to the corresponding cause of death.
The cause of death could not be established in only six cases (four patients in SR and two in AF) and were therefore classified as unknown. One hundred and sixty-nine patients died from cardiac death, 135/1042 and 34/243 in the SR and AF groups, respectively. Cardiac death rates were 6.7 (95% CI 5.6–7.9) and 7.7 (95% CI 5.5–10.8) per 100 person-year in SR and AF patients, respectively (Figure 1B). No difference in cardiac mortality was found between patients in SR and AF both at univariable and multivariable analyses (adjusted HR 1.00, 95% CI 0.60–1.66, P = 0.991).
Worsening HF was the most important mode of death in both groups accounting for 105/1042 deaths in SR patients and for 28/243 deaths in patients with AF, a mortality rate of 5.2 (95% CI 4.3–6.3) and 6.3 (95% CI 4.4–9.2) per 100 person-year, respectively (Figure 1C). No difference in adjusted HR for HF death (1.18, 95% CI 0.67–2.06, P = 0.564) was found.
Long-term survival of atrial fibrillation patients stratified according to atrio-ventricular junction ablation
The AF population was subdivided depending on whether the modality used to control heart rate was by negative chronotropic drugs (AF-Drugs) or AVJ ablation (AVJ-abl). The two groups were similar with respect to some of their main baseline characteristics (Table 2), including age, gender, aetiology, NYHA class, and pharmacological therapy. Patients in the AVJ-abl group had a shorter QRS duration and less frequently received CRT-D. Moreover, the use of negative chronotropic drugs, in the first 2–3 months after CRT implant, was similar between the two groups and dosages were adequate. Of the 243 patients, 240 were still alive at the 2 month follow-up: three AF patients died before this scheduled visit (two on drug therapy and one extremely compromised patient, who underwent AVJ ablation soon after CRT implantation and died from refractory HF before discharge).
Table 2.
Baseline characteristics at the time of cardiac resynchronization therapy implant of the permanent atrial fibrillation patient population divided according to atrio-ventricular junction ablation (AVJ-abl) or drugs (AF-Drugs) for rate control
| AF-Drugs (n = 125) | AVJ-abl (n = 118) | P-valuea | |
|---|---|---|---|
| Age (years) | 65.9 (8.6) | 66.5 (9.2) | 0.893 |
| Female | 28 (22.4%) | 16 (13.6%) | 0.074 |
| Aetiology | |||
| Non-ischaemic | 77 (61.6%) | 69 (58.5%) | 0.694 |
| Ischaemic | 48 (38.4%) | 49 (41.5%) | |
| NYHA | |||
| II | 4 (3.2%) | 6 (5.1%) | 0.733 |
| III | 101 (80.8%) | 92 (78.0%) | |
| IV | 20 (16.0%) | 20 (17.0%) | |
| QRS (ms) | 168 (29) | 155 (34) | 0.010 |
| Left ventricular EF (%) | 24.8 (7.6) | 27.0 (12.0) | 0.087 |
| Mitral regurgitation (grade 3–4) | 97 (78%) | 88 (75%) | 0.652 |
| Left atrial diameter (mm) | 57 (8) | 55 (9) | 0.068 |
| Mean resting heart rate (b.p.m.) | 73 (7.2) | 77 (9.1) | <0.001 |
| CRT-D | 69 (55.2%) | 48 (40.7%) | 0.029 |
| ACE-inhibitors/ARBs | 117 (93.5%) | 111 (94.1%) | 1.000 |
| Beta-blockers | 102 (81.6%) | 92 (78.0%) | 0.524 |
| Aldosterone antagonists | 67 (49.6%) | 73 (61.9%) | 0.198 |
| Diuretics | 118 (96.6%) | 106 (89.8%) | 0.234 |
| Digitalis | 92 (73.6%) | 79 (66.9%) | 0.265 |
| Amiodarone | 60 (48.0%) | 48 (40.7%) | 0.302 |
| Negative chronotropic drugs | 123 (98.4%) | 113 (96.6%) | 0.270 |
| Intravenous inotropic drugs | 2 (1.6%) | 3 (2.7%) | 0.676 |
Mean (SD) for continuous variables; n (%) for categorical variables.
AVJ-abl, atrial fibrillation patients who underwent atrio-ventricular junction ablation; AF-Drugs, atrial fibrillation patients who did not undergo atrio-ventricular junction ablation; CRT-D, cardiac resynchronization therapy pacemaker with defibrillator; ARBs, angiotensin receptor blockers.
aFor post hoc comparisons, Scheffé's test was used after one-way anova; after Fisher's exact test, the level of significance was set to 0.017 for Bonferroni correction.
At the 2-month control, 123 patients reached BVP% > 85 (mean 89.4 ± 2.4%) and continued negative chronotropic drugs throughout the follow-up to maintain adequate BVP% (AF-Drugs group). The other 117 AF patients with BVP% ≤ 85 at 2 months (mean 74.2 ± 4.2%) underwent AVJ ablation within 3 months from device implant (AVJ-abl group). AVJ ablation was effective in 98.4% of cases, no major complications occurred. Once ablation of the AVJ was performed, digoxin and amiodarone were discontinued (amiodarone was continued only in cases presenting relevant ventricular tachyarrhythmias), whereas beta-blockers were maintained. At the following control after AVJ ablation, device counters revealed full biventricular pacing effectiveness, with BVP% nearing 100 (mean 98.7 ± 1.8%). The evaluation of drug therapy modifications in the AF group was performed after 1 year of CRT. Dosage of beta-blockers increased compared with baseline (carvedilol increased from 14.6 to 19.5 mg/day, P < 0.001). No differences were detected in either beta-blocker or ACE-inhibitor usage between the two AF patient groups.
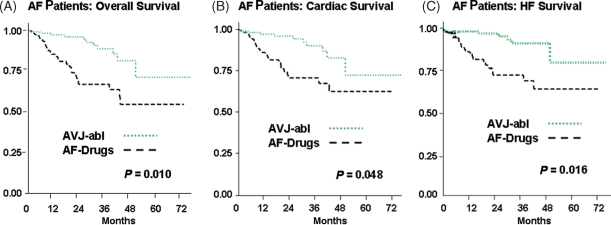
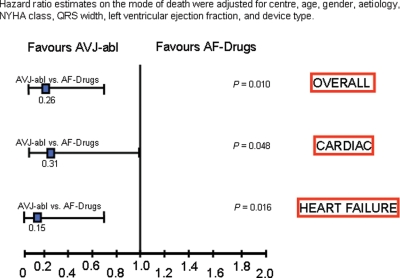
A total of 39 deaths occurred in AF patients. Of these events, 28/125 patients were observed in the AF-Drugs group and 11/118 patients in AVJ-abl group (Figure 2A), with mortality rates of 14.2 (95% CI 9.7–20.5) and 4.6 (95% CI 2.5–8.2) per 100 person-year, respectively (Figure 2A). Total mortality was therefore significantly better in the AVJ-abl group compared with the AF-Drugs group (adjusted HR 0.26, 95% CI 0.09–0.73, P = 0.010, Figure 3).
Figure 2.
Comparison of Kaplan–Meier estimates of overall (A), cardiac (B), and heart failure (C) survival between atrial fibrillation patients who underwent atrio-ventricular junction ablation (AVJ-abl) and atrial fibrillation patients treated only with negative chronotropic drugs (AF-Drugs). The P-values presented derive from the adjusted hazards ratio analysis stratified according to the corresponding cause of death.
Figure 3.
Hazard ratio estimates stratified according to cause of death between atrial fibrillation patients who underwent atrio-ventricular junction ablation (AVJ-abl) and atrial fibrillation patients treated with negative chronotropic drugs (AF-Drugs); hazard ratio estimates were adjusted for centre, age, gender, aetiology, NYHA class, QRS width, left ventricular ejection fraction, and device type. Corresponding hazard ratio values for each cause of death are indicated with a square, the bar represents 95% confidence interval range, and the P-value for each estimate is presented on the right of the figure.
With respect to the cause of death, cardiac deaths occurred in 24/125 patients of the AF-Drugs group compared with 10/118 patients in the AVJ-abl group (Figure 2B), with cardiac mortality rates of 12.1 (95% CI 8.1–18.1) and 4.1 (95% CI 2.2–7.7) per 100 person-year, respectively (Figure 2B), and an adjusted HR of 0.31 (95% CI 0.10–0.99, P = 0.048) for AVJ-abl vs. AF-Drugs patients (Figure 3).
A total of 28 events (death or urgent heart transplant or left ventricular assist device positioning) occurred as a result of worsening HF with an incidence of 22/125 in the AF-Drugs group, as opposed to only 6/118 in the AVJ-abl group, with a mortality rate of 11.1 per 100 person-year (95% CI 7.3–16.9) in the AF-Drugs group and of only 2.5 (95% CI 1.1–5.5) per 100 person-year in the AVJ-abl group (Figure 2C) (adjusted HR 0.15, 95% CI 0.03–0.70, P = 0.016) (Figure 3).
The occurrence of sudden cardiac death was very rare and accounted for only two events in the AF-Drugs (1.01, 95% CI 0.3–4.1 per 100 person-year) and four events in the AVJ-abl group (1.7, 95% CI 0.6–4.4 per 100 person-year). Adjusted HR was 2.59 (95% CI 0.22–30.15, P = 0.370).
Discussion
CRT confers significant reductions in left ventricular volumes and improvement of left ventricular EF in HF patients.3,11 Such favourable changes have shown to correlate with mortality reduction over a mid-term follow-up in SR patients.12 We recently11 described significant long-term improvements in left ventricular EF and left ventricular reversal of maladaptive remodelling in AF patients treated with the combined CRT and AVJ approach. In AF patients with preserved AVJ conduction, however, no such improvements were observed. No consistent correlation has been reported until now between reverse remodelling and mortality reduction after CRT in AF patients. The present study may be considered an extension of the previous one11 and aimed to evaluate, in a much larger patient cohort, whether the effect of the combined AVJ ablation and CRT strategy may also translate into favourable long-term survival of HF patients with permanent AF.
To our knowledge, this is the first study comparing outcomes among patients treated with CRT, between those in SR and those with AF, and, even more importantly, among patients with AF, based on whether or not these patients underwent AVJ ablation. The dramatic difference in mortality rate observed between AF-drugs and AF-abl could support the view that AVJ ablation may be strongly recommended to achieve effective CRT in AF patients. However, our data should be confirmed by prospective randomized trials, possibly comparing in these patients VVIR-ICD and rate control vs. CRT-D+AVJ ablation.19
Effects of cardiac resynchronization therapy in heart failure patients with permanent atrial fibrillation compared with sinus rhythm
Despite a 20% prevalence of AF among HF patients treated with CRT,13 randomized controlled CRT trials have generally included only patients in SR. Therefore, there are virtually no data investigating the effects of CRT on survival in HF patients with permanent AF.
Both ventricular conduction delay and AF are associated with a poor prognosis in HF patients.7,8 CRT may be therefore indicated in these patients. Small studies have shown beneficial effects of CRT also in patients with HF and AF, with an improvement in NYHA class, exercise capacity, and quality of life.6,20,21
In the present study, the multivariable analysis did not detect any significant differences in overall, cardiac, and HF long-term mortality rates between patients in SR and in permanent AF. Our recent contribution11 described similar long-term improvements in left ventricular EF and left ventricular reversal of maladaptive remodelling between SR and AF patients treated with CRT. In this study, mean left ventricular EF increase at 1 year was of 6.5% absolute units in both the SR group and the AF group, with a concomitant decrease in the left ventricular end-systolic volume of 20% in the SR patients compared with ∼15% in the AF group. These results are consistent with the similar mortality rates that we observed in our patients with either SR or AF.
Importance of atrio-ventricular junction ablation in patients with permanent atrial fibrillation
It is important to point out that studies5,11,20,21 reporting benefits of CRT in permanent AF included patients with either a low ventricular rate or a previously ablated AVJ. This situation is, however, not frequent in clinical practice and many patients with AF have a high ventricular rate (at least during exertion) despite concomitant treatment with negative chronotropic AF-Drugs (e.g. beta-blockers, digitalis, amiodarone). Moreover, the activation of rate-smoothing features such as VRR or trigger mode may effectively regularize heart rate, whereas forcing faster heart rates may become deleterious.
Hence, AVJ ablation may be the only procedure allowing a ‘complete’ heart rate control in these patients. Such a procedure offers the advantage of obtaining a regular ventricular rhythm and, probably more importantly, ensures effective CRT through pure and constant biventricular pacing. Achievement of a regular ventricular rhythm through AVJ ablation has been associated with an improvement in global cardiac mechanics.22 AVJ ablation may therefore find an elective indication in HF patients treated with CRT, as it should allow greater benefits compared with those observed in the general population in which the ‘ablate and pace’ strategy is conventionally used to obtain a more regular and slower heart rhythm.23,24 Another possible explanation for the impressive effects on survival obtained as a result of AVJ ablation may be related to the fact that, after AVJ ablation, most patients suspended both digoxin and amiodarone: data derived from randomized trials suggest that digoxin25 and amiodarone26 may have a negative impact on morbidity and mortality in HF patients.
Recently published data11 have suggested that in HF patients with AF, CRT confers long-term improvements of EF, end-systolic volume, NYHA class, and exercise capacity only in patients who undergo AVJ ablation. Such extensive improvements in EF and in left ventricular geometry have already been correlated to favourable prognosis in HF patients in SR.12 The previous findings advocating that AVJ ablation optimizes CRT delivery thus yielding a significant reverse remodelling effect11 are consistent with what has been described in the present study. The analysis of the different death modes, stratified on the propensity of a patient being ablated, given its presenting characteristics, allowed to elucidate the role of AVJ ablation in patients with permanent AF treated with CRT. Overall, cardiac and HF deaths were significantly lower in AF patients who underwent AVJ ablation compared with those who were not ablated. This survival benefit was mainly due to the protective effect of AVJ ablation on deaths for HF. AF patients who underwent AVJ ablation had a nine-fold lower HF mortality compared with non-ablated AF patients. Kaplan-Meier analysis revealed that the time pattern of survival for HF becomes apparent after 4 months of CRT; this time point, shortly after AVJ ablation, corresponds to the same period when the reverse remodelling process becomes manifest in the AVJ-abl population.11 Taken together, these findings suggest, as previously described in SR patients,12 that the reverse remodelling effect produced by CRT may translate to a lower incidence of HF death in the AVJ-abl group.
No clear statement may be made regarding possible effects on mortality from sudden cardiac death in AF patients treated with CRT, due to the low number of sudden death events that occurred in our patient cohort and due to the significant difference in patients receiving ICD back-up between the two groups.
Study limitations
This observational study has several limitations, being a non-randomized study; to consider, however, our cohort of 1285 patients with a median follow-up of 34 months represents one of the largest series evaluating the effects of CRT reported so far. It may have been useful to have an AF control group not treated with CRT, but the data are derived from a pre-defined CRT registry of consecutively implanted patients. Also, outcome may have been biased by patients’ selection and changes in both pharmacological and non-pharmacological therapies over the study period. One limitation may be the lack of a ‘direct’ correlation between remodelling effect and mortality data. Unfortunately, only two of the four centres rigorously collected follow-up echocardiographic data. For this reason, the observations of the present study were interpreted and integrated with the findings of the previous contribution.11
The limits posed by the differences in the baseline characteristics between the study groups were mitigated by the statistical analysis which considered possible confounding effects of centre, gender, age, aetiology, QRS duration, functional NYHA class, baseline left ventricular EF, and device type in the fitted models. Moreover, to avoid over-fitting, confounding was addressed by adjusting the Cox models by the quintiles of the PS. Given the longitudinal, observational design of the study, even these adjusted models may not guarantee complete suppression of some confounders, hence the need for confirmatory randomized studies.
Although the AF group was numerically inferior to the SR group, the subdivision of AF patients in AVJ-abl and AF-Drugs resulted in two numerically balanced groups with similar baseline characteristics. Finally, the declining number of patients over the follow-up may represent a limitation, but this is the case of any observational cohort study. All the available patients were included and only few patients were either lost to follow-up or had incomplete outcome data; the latter were excluded from the study.
Conclusions
In our large multicentre series, the long-term overall survival of drug refractory HF patients with permanent AF and left ventricular conduction delay treated with CRT was similar to that of SR patients. However, in HF patients with permanent AF, AVJ ablation in addition to CRT appears to improve long-term overall mortality compared with CRT alone, primarily by reducing HF death. AVJ ablation seems to be a fundamental adjunct to ensure adequate CRT delivery and thus reducing mortality in patients with permanent AF. Prospective randomized studies are necessary to further confirm these findings.
Conflict of interest: none declared.
Funding
Funding to pay the Open Access publication charges for this article was provided by MT Tedeschi, IRCCS Istituto Clinico Humanitas, Clinical Research Department, Rozzano (MI), Italy.
Appendix
Other investigators participating in the Multicentre Longitudinal Observational Study (MILOS) Group are as follows.
Department of Cardiology, IRCCS Istituto Clinico Humanitas, Rozzano-Milan, Italy: Paola Galimberti, M.D.; Carlo Ceriotti, M.D.; Edoardo Gronda, M.D.; Renato Bragato, M.D.; Daniela Pini, M.D.; Maurizio Mangiavacchi, M.D.
Division of Cardiology, University Hospital Magdeburg, Germany: Santi Raffa, M.D.; Michael Kloss, M.D.; Silke M. Trautmann, M.D.; Francesca Pastori, M.D.; Simona Fratini, M.D.; Helmut U. Klein, M.D.; Christof Huth, M.D.; Andrea Friedl, M.D.
Section of Cardiovascular Diseases, Department of Experimental and Applied Medicine, Unit of Cardiology, University and Spedali Civili, Brescia, Italy: Giosuè Mascioli, M.D.; Livio dei Cas, M.D.
Department of Cardiology, Heart and Diabetes Center NRW, Bad Oeynhausen, Germany: Helga Buschler, M.D.; Anja Dorszewski, M.D.; Anke Schmidt, M.D.; Bert Hansky, M.D.; Johannes Heintze, M.D.; Dieter Horstkotte, M.D.
References
- 1.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronisation therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2149. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronisation on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronisation in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 4.Cazeau S, Leclerq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 5.Leclercq C, Victor F, Alonso C, Pavin D, Revaulr d’Allones G, Bansard JY, Mabo P, Daubert C. Comparative effects of permanent biventricular pacing for refractory heart failure in patients with stable sinus rhythm. Am J Cardiol. 2000;85:1154–1156. doi: 10.1016/s0002-9149(00)00716-5. [DOI] [PubMed] [Google Scholar]
- 6.Molhoek SG, Bax JJ, Bleeker GB, Boersma E, van Erven L, Steendijk P, van der Wall EE, Schalij MJ. Comparison of response to cardiac resynchronisation therapy in patients with sinus rhythm versus chronic atrial fibrillation. Am J Cardiol. 2004;94:1506–1509. doi: 10.1016/j.amjcard.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 8.Baldasseroni S, De Biase L, Fresco C, Marchoinni N, Marini M, Masotti G, Orsini G, Porcu G, Pozzar F, Scherillo M, Maggioni AP. Italian Network on Congestive Heart Failure. Cumulative effect of complete left bundle-branch block and chronic atrial fibrillation on 1-year mortality and hospitalization in patients with congestive heart failure. A report from the Italian network on congestive heart failure (in CHF database) Eur Heart J. 2002;23:1692–1698. doi: 10.1053/euhj.2001.3157. [DOI] [PubMed] [Google Scholar]
- 9.Simantirakis EN, Vardakis KE, Kochiadakis GE, Manios EG, Igoumenidis NE, Brignole M, Vardas PE. Left ventricular mechanics during right ventricular apical or left ventricular-based pacing in patients with chronic atrial fibrillation after atrioventricular junction ablation. J Am Coll Cardiol. 2004;43:1013–1018. doi: 10.1016/j.jacc.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Hay I, Melenovsky V, Fetics BJ, Judge DP, Kramer A, Spinelli J, Reister C, Kass DA, Berger RD. Short-term effects of right–left heart sequential cardiac resynchronisation in patients with heart failure, chronic atrial fibrillation, and atrioventricular nodal block. Circulation. 2004;110:3404–3410. doi: 10.1161/01.CIR.0000148177.82319.C7. [DOI] [PubMed] [Google Scholar]
- 11.Gasparini M, Auricchio A, Regoli F, Fantoni C, Kawabata M, Galimberti P, Pini D, Ceriotti C, Gronda E, Klersy C, Fratini S, Klein HH. Four-year efficacy of cardiac resynchronisation therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48:734–743. doi: 10.1016/j.jacc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Bleeker GB, Fung JW, Zhang Q, Van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronisation therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 13.Auricchio A, Metra M, Gasparini M, Lamp B, Klersy C, Curnis A, Fantoni C, Gronda E, Vogt J. Long-term survival of patients with heart failure and ventricular conduction delay treated with cardiac resynchronisation therapy. Am J Cardiol. 2007;99:232–238. doi: 10.1016/j.amjcard.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ., Jr Classification of death in antiarrhythmic trials. J Am Coll Cardiol. 1996;27:433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 15.Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1:126–130. doi: 10.1053/eupc.1998.0032. [DOI] [PubMed] [Google Scholar]
- 16.Geelen P, Brugada J, Andries E, Brugada P. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol. 1997;20:343–348. doi: 10.1111/j.1540-8159.1997.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 17.Tse HF, Newman D, Ellenbogen KA, Buhr T, Lau CP. Effects of ventricular rate regularization pacing on the quality of life and symptoms in patients with atrial fibrillation (AF SYMPTOMS study) Am J Cardiol. 2004;94:938–941. doi: 10.1016/j.amjcard.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Schemper M, Smith TL. A note on quantifying follow-up studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 19.Hamdan MH, Freedman RA, Gilbert EM, DiMarco JP, Ellenbogen KA, Page RL. Atrioventricular Junction Ablation followed by Resynchronization Therapy in Patients with Congestive Heart Failure and Atrial Fibrillation (AVERT-AF) study design. PACE. 2006;29:1081–1088. doi: 10.1111/j.1540-8159.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 20.Leon AR, Greenberg JM, Kanuru N, Baker CM, Mera FV, Smith AL, Langberg JJ, DeLurgio DB. Cardiac resynchronisation in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J Am Coll Cardiol. 2002;39:1258–1263. doi: 10.1016/s0735-1097(02)01779-5. [DOI] [PubMed] [Google Scholar]
- 21.Garrigue S, Bordachar P, Reuter S, Jais P, Kobeissi A, Gaggini G, Haissaguerre M, Clementy J. Comparison of permanent left ventricular and biventricular pacing in patients with heart failure and chronic atrial fibrillation: prospective haemodynamic study. Heart. 2002;87:529–534. doi: 10.1136/heart.87.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melenovsky V, Hay I, Fetics BJ, Borlaug BA, Kramer A, Pastore JM, Berger R, Kass DA. Functional impact of rate irregularity in patients with heart failure and atrial fibrillation receiving cardiac resynchronisation therapy. Eur Heart J. 2005;26:705–711. doi: 10.1093/eurheartj/ehi066. [DOI] [PubMed] [Google Scholar]
- 23.Brignole M, Gianfranchi L, Menozzi C, Alboni P, Musso G, Bongiorni MG, Gasparini M, Raviele A, Lolli G, Paparella N, Aquarone S. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation. 1997;96:2617–2624. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan C, Jahangir A, Friedman PA, Patel PJ, Munger TM, Rea RF, Lloyd MA, Packer DL, Hodge DO, Gersh BJ, Mammill SC, Shen WK. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med. 2001;344:1043–1051. doi: 10.1056/NEJM200104053441403. [DOI] [PubMed] [Google Scholar]
- 25.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumbolz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 26.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]