Abstract
Autophagy is mostly a nonselective bulk degradation system within cells. Recent reports indicate that autophagy can act both as a protector and killer of the cell depending on the stage of the disease or the surrounding cellular environment (for review see Cuervo, A.M. 2004. Trends Cell Biol. 14:70–77). We found that cytoplasmic vacuoles induced in pancreatic acinar cells by experimental pancreatitis were autophagic in origin, as demonstrated by microtubule-associated protein 1 light chain 3 expression and electron microscopy experiments. To analyze the role of macroautophagy in acute pancreatitis, we produced conditional knockout mice lacking the autophagy-related 5 gene in acinar cells. Acute pancreatitis was not observed, except for very mild edema in a restricted area, in conditional knockout mice. Unexpectedly, trypsinogen activation was greatly reduced in the absence of autophagy. These results suggest that autophagy exerts devastating effects in pancreatic acinar cells by activation of trypsinogen to trypsin in the early stage of acute pancreatitis through delivering trypsinogen to the lysosome.
Introduction
There are at least three types of autophagy: (1) macroautophagy, (2) chaperone-mediated autophagy, and (3) microautophagy (Mizushima, 2005). Although autophagy is mostly a nonselective bulk degradation system within cells, chaperone-mediated autophagy is a selective degradation system in which cytoplasmic proteins are refolded and transported into the lysosome lumen across the membrane (for review see Cuervo, 2004). In microautophagy, only a small portion of cytoplasm is taken up directly by invagination of lysosome membrane into the lumen. As macroautophagy is believed to be the primary means for cytoplasm to lysosome delivery, it is most commonly referred to simply as autophagy. Genetic studies on yeast have identified >20 autophagy-related (ATG) genes that are required for autophagosome formation (Klionsky et al., 2003; Klionsky, 2005). Autophagy-defective yeast mutants are not able to survive during nitrogen starvation (Tsukada and Ohsumi, 1993). Similarly, most of the Atg5−/− and Atg7−/− mice died within 1 d after birth (Kuma et al., 2004; Komatsu et al., 2005). Thus, autophagy is thought to be important for the cellular response to starvation and the normal turnover of cytoplasmic constituents.
Acute pancreatitis has long been considered to be an autodigestive disorder in which inappropriate activation of trypsinogen to trypsin within pancreatic acinar cells leads to the development of pancreatitis (Hirota et al., 2006). However, the mechanisms responsible for intracellular activation of trypsin have not been elucidated with certainty. There are two major hypotheses: the colocalization hypothesis (van Acker et al., 2006) and the autoactivation hypothesis (Leach et al., 1991). According to the former hypothesis, digestive enzymes become colocalized with lysosomal hydrolases, such as cathepsin B, and activate trypsinogen in cytoplasmic vacuoles of acinar cells (Steer and Meldolesi, 1987). The latter hypothesis suggests that trypsinogen is autoactivated under low pH conditions in the presence of serine protease (Leach et al., 1991). However, the mode of trypsinogen delivery to the lysosomes or cellular compartments has been the subject of investigation. There are three possible mechanisms for delivery of trypsinogen to the cellular compartment where activation occurs. One is fusion of zymogen granules with lysosomes (crinophagy; Koike et al., 1982). The second is perturbation of normal intracellular trafficking of zymogen granules and lysosomal hydrolases. The third is endocytic vacuole formation through uptake of secreted digestive enzymes by acinar cells via endocytosis, transportation to endosomes, and fusion of endosomes with lysosomes (Sherwood et al., 2007). One important clue to distinguish between these possibilities is the appearance of cytoplasmic vacuoles within pancreatic acinar cells (Watanabe et al., 1984). This is an early feature of acute pancreatitis. EM and immunohistochemical studies suggested that many vacuoles observed in both experimental and human acute pancreatitis were autophagic in origin (Helin et al., 1980; Adler et al., 1985). Our previous study suggested that autophagy was induced in the acinar cells of mice with experimental pancreatitis induced by cerulein (cholecystokinin [CCK] analogue; Ohmuraya et al., 2005). Collectively, these results indicate that vacuoles are autophagic in origin and that autophagy is somehow involved in the development of pancreatitis.
In this study, we report that cytoplasmic vacuoles induced in experimental acute pancreatitis are autophagic in origin and that absence of autophagy in the Atg5 conditional knockout mouse results in greatly reduced acute pancreatitis caused by loss of trypsinogen activation in pancreatic acinar cells. Our results suggest that trypsinogen is delivered to the endosome or lysosome through autophagosome/autolysosome formation.
Results and discussion
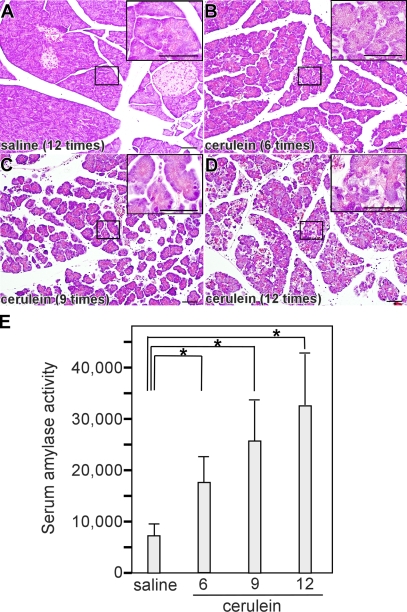
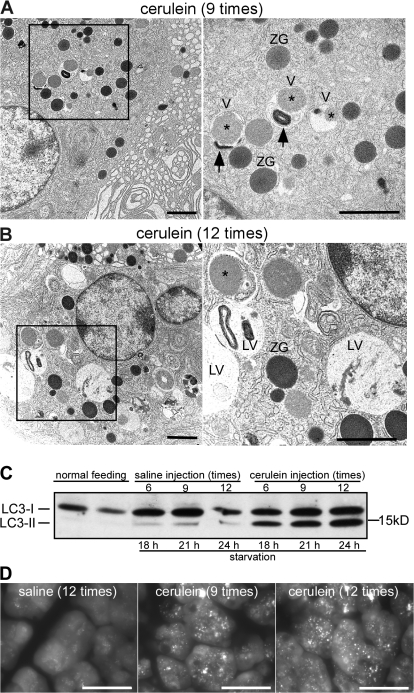
We first analyzed the origin of vacuoles in cerulein-induced experimental pancreatitis. No changes in pancreatic morphology were observed at any time in any sample obtained from saline-injected mice (Fig. 1 A). In contrast, acute pancreatitis was observed in cerulein-injected mice. The severity of acute pancreatitis increased with the number of cerulein injections. With six and nine cerulein injections, mild edema and acinar cell degeneration were observed (Fig. 1, B and C). With 12 cerulein injections, the pancreas showed severe acinar cell degeneration with significant edema and inflammatory cell infiltration in the interstitium (Fig. 1 D). In accordance with histological changes, we observed a significant increase in serum amylase activity (Fig. 1 E). The observed increase was proportional to the cerulein dose and the severity of acute pancreatitis. To examine the induction of autophagy, we performed EM examination, which revealed vacuoles containing zymogen granules (Fig. 2 A, asterisks) in acinar cells after nine cerulein injections (Fig. 2 A). Some vacuoles contained both zymogen granules and membrane-bound organelles (Fig. 2 A, arrows), suggesting nonselective uptake. EM images after 12 cerulein injections were characterized by an increase in vacuoles containing zymogen granules and organelles and by the appearance of large vacuoles with homogeneous material that seemed to be autolysosomes (Fig. 2 B). There are two forms of microtubule-associated protein 1 light chain 3 (LC3), a mammalian homologue of yeast Atg8. LC3-I is localized in the cytoplasm and is converted into LC3-II, which is associated with the autophagosome membrane in a phosphatidylethanolamine-conjugated form (Ichimura et al., 2000; Kabeya et al., 2000). The amount of LC3-II is thus correlated with the extent of autophagosome formation. In control mice, LC3-II was increased slightly by starvation but was virtually unchanged during saline treatment (Fig. 2 C). In mice treated with cerulein, a dose-dependent increase in LC3-II level was observed. Autophagy can also be monitored by detection of GFP fluorescence in GFP-LC3 mice (Mizushima et al., 2004). After 24-h fasting, some dots that represent autophagosomes were detected in the cytoplasm in saline-injected mice (Fig. 2 D). After cerulein injection, many dots were observed, and the number of GFP-LC3 dots from mice receiving 12 cerulein injections was larger than from mice with nine cerulein injections (Fig. 2 D). Together with EM examination, these results suggest that vacuoles are autophagic in origin.
Figure 1.
Autophagy induction in pancreatic acinar cells of cerulein-induced acute pancreatitis. Overnight-starved mice were treated by saline (A) or cerulein 6 (B), 9 (C), or 12 (D) times, and pancreatic sections were analyzed by H&E staining. Insets show higher magnifications of areas indicated in A–D. (E) Serum amylase activity in mice with cerulein-induced pancreatitis. Data are shown as mean ± SEM (error bars). *, P < 0.05. Bars, 50 μm.
Figure 2.
Appearance of autophagic vacuoles in cerulein-induced pancreatitis. (A and B) Mice were injected with cerulein 9 (A) or 12 (B) times, and the pancreas was analyzed by EM. The right panels show higher magnification of the boxed areas. V, autophagic vacuole; ZG, zymogen granule; asterisk, zymogen granule contained in vacuole; arrow, membrane-bound organelle contained in vacuole; LV, large vacuole. (C) LC3 conversion in acute pancreatitis. Pancreas homogenates were prepared from cerulein-injected mice and subjected to immunoblotting using anti-LC3 antibody. The cytosolic LC3-I protein (16 kD) was converted into LC3-II (14 kD), and the amount of LC3-II was correlated with the extent of autophagosome formation. (D) GFP-LC3 mice were treated with cerulein and analyzed by fluorescence microscopy. Bars: (A and B) 2 μm; (D) 10 μm.
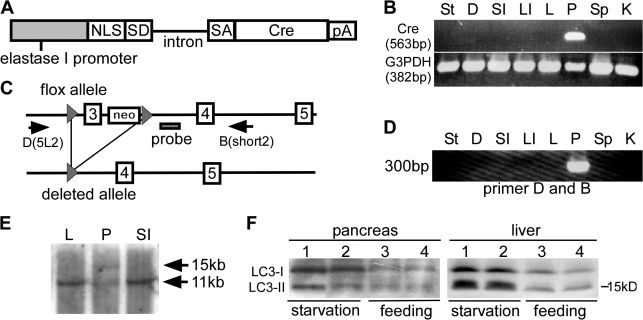
To analyze the role of autophagy in acute pancreatitis, we established conditional knockout mice in which the Atg5 gene was deleted in pancreatic acinar cells. We obtained an EL (rat elastase I promoter/enhancer)-Cre mouse line (EL-Cre2) in which Cre was specifically expressed in the pancreas under the control of EL (Fig. 3, A and B). This line was crossed with Atg5flox/flox (Hara et al., 2006) to produce Atg5flox/flox;EL-Cre2 mice (Fig. 3 C). Cre-mediated excision of exon 3 of the Atg5 gene was detected in the pancreas but not in other organs of Atg5flox/flox;EL-Cre2 (Fig. 3 D). Southern blot analysis using genomic DNA from the pancreas showed that recombination occurred in about half of the pancreatic cells (Fig. 3 E). As the pancreas contains not only acinar cells but also endocrine and duct cells, this ratio suggests that recombination occurred in most Atg5flox alleles of the acinar cells. The expression of Cre in acinar cells was also assessed by expression of lacZ in mice obtained by mating EL-Cre2 mice with a reporter line for monitoring Cre expression, R26R (Soriano, 1999). All acinar cells examined were stained with X-gal, suggesting that recombination occurred in all Atg5flox alleles of the acinar cells (unpublished data). To further demonstrate the inactivation of Atg5 in acinar cells, we analyzed induction of autophagy under starving conditions. Atg5flox/flox;EL-Cre2 mice and Atg5flox/flox mice were starved for 24 h and examined for induction of autophagy. Autophagy was greatly induced in the liver and slightly induced in the pancreas of Atg5flox/flox;EL-Cre2 after starvation (Fig. 3 F). As Atg5 was present in nonacinar cells, autophagy may be induced in these cells.
Figure 3.
Establishment of EL-Cre transgenic mouse. (A) Structure of the EL-Cre gene. NLS, nuclear localization signal domain; SD, splice donor; SA, splice acceptor; Cre, Cre recombinase; pA, polyadenylation signal. (B) RT-PCR analysis detecting Cre recombinase in each organ at 2 mo of age. (C) The deletion of exon 3 flanked by loxP in the flox allele by Cre recombinase results in a loss of function mutation. (D) PCR analysis showing the 300-bp band lacking exon 3 only in the pancreas, not in the stomach, intestine, liver, spleen, and kidney. (E) Southern blot analysis showing the presence of two bands, the 15-kb deleted allele and 11-kb wild allele. (F) Western blot analysis of LC3 in the pancreas and liver in Atg5flox/flox mice (lanes 1 and 3) and Atg5flox/flox;EL-Cre2 mice (lanes 2 and 4). Mice were starved for 24 h and examined for induction of autophagy. St, stomach; D, duodenum; SI, small intestine; LI, large intestine; L, liver; P, pancreas; Sp, spleen; K, kidney.
Atg5flox/flox;EL-Cre2 mice were born healthy with no signs of developmental anomalies and grew without displaying any noticeable pathological phenotype after 2 mo (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200712156/DC1). There were no apparent abnormalities in blood biochemistry, such as total protein, glucose, total cholesterol, amylase, and lipase (unpublished data). Hematoxylin and eosin (H&E) staining and EM analysis revealed that acinar cells were normal (Fig. S1, B and C). The levels of amylase and trypsinogen were the same in the pancreas of Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice (Fig. S2). In addition, we examined whether the lack of Atg5 affected CCK-induced calcium signaling in acinar cells. Type 2 and type 3 inositol 1,4,5-trisphosphate receptors (IP3R2 and IP3R3) are the major Ca2+ release channels responsible for secretagogue-induced Ca2+ signaling in pancreatic acinar cells (Futatsugi et al., 2005). Basal levels of IP3R1, -2, and -3 in both Atg5flox/flox and Atg5flox/flox;El-Cre2 mice at 2 mo of age were the same as revealed by Western blot assay (Fig. S3 A). We next examined Ca2+ signaling induced by pancreatic exocrine secretagogues CCK octapeptide (CCK8) in fura-2–loaded enzymatically isolated pancreatic acinar cells. Again, Atg5-deficient pancreatic acinar cells show similar [Ca2+]i increases as those of Atg5flox/flox mice in response to 100-pM CCK8 stimulation (Fig. S3 B). Both Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice at 2 mo of age were used in the following experiments.
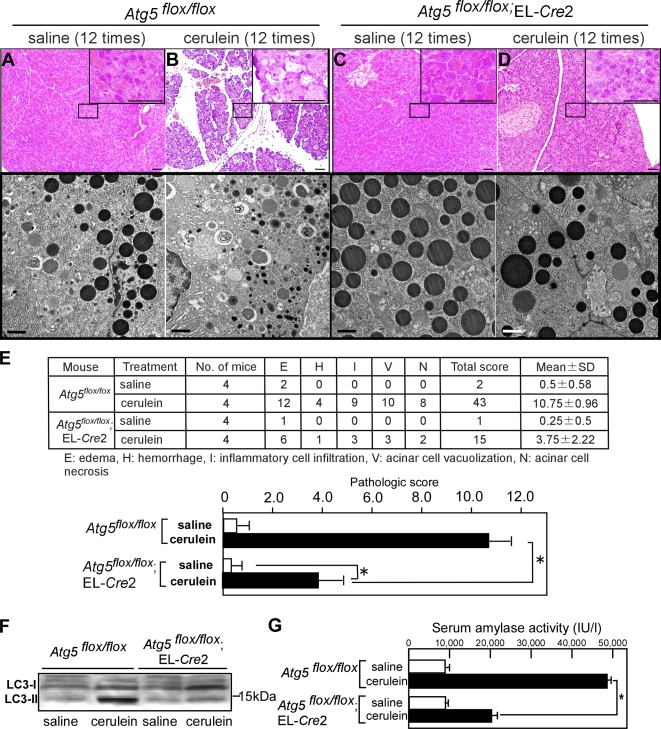
Saline injection did not cause any pathological changes in both Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice as revealed by histochemical and EM analyses (Fig. 4, A and C). We then induced acute pancreatitis by cerulein in Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice. Atg5flox/flox mice showed typical signs of acute pancreatitis, such as severe acinar cell degeneration, edema in connective tissue, and infiltration of inflammatory cells with H&E staining (Fig. 4 B). EM analysis revealed numerous autophagosomes and a decrease of zymogen granules in Atg5flox/flox mice (Fig. 4 B). In contrast, Atg5flox/flox;EL-Cre2 mice displayed normal histochemical characteristics except very mild edema in a small restricted region (Fig. 4 D). In fact, autophagosomes were not observed, and zymogen granules appeared to be intact in Atg5flox/flox;EL-Cre2 mice (Fig. 4 D). We quantified histological changes using a histological score (Hughes et al., 1996) by a pathologist and showed that there was statistical significant difference in pathological changes by a pathologist between Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice (Fig. 4 E). In accordance with histological and EM examination, LC3-II increased significantly in Atg5flox/flox mice but not in Atg5flox/flox;EL-Cre2 mice after cerulein injection (Fig. 4 F). In addition, the serum amylase level in Atg5flox/flox;EL-Cre2 mice with cerulein was significantly lower than in Atg5flox/flox mice (Fig. 4 G). There was a slight increase in serum amylase when Atg5flox/flox;EL-Cre2 mice were treated with cerulein (Fig. 4 G). This may be caused by stimulation of secretion of digestive enzymes by cerulein itself. Collectively, these results indicate that typical acute pancreatitis is not induced in the absence of autophagy.
Figure 4.
Absence of cerulein-induced acute pancreatitis in acinar cell–specific Atg5-deficient mice. Atg5flox/flox (A and B) or Atg5flox/flox;EL-Cre2 (C and D) mice were injected with saline or cerulein 12 times, and pancreatic sections were analyzed by H&E staining (A and C) and EM analysis (B and D). Insets show higher magnifications of areas indicated in A–D. EM analysis demonstrating the absence of autophagy in Atg5flox/flox;EL-Cre2 mice. (E) Histological changes were quantified using a histological score by a pathologist. There was statistically significant difference in pathological changes between Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice. Unpaired t tests were used to calculate p-values. P < 0.05 was considered a significant difference. (F) Increase of LC3-II in Atg5flox/flox but not in Atg5flox/flox;EL-Cre2 after cerulein treatment. (G) Serum amylase activity in cerulein-induced acute pancreatitis. Open bars, saline injection (n = 4); black bars, cerulein injection (n = 4). The asterisk indicates the statistical difference between amylase levels in Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice (*, P < 0.05). Error bars represent SEM. Bars: (A and C) 50 μm; (B and D) 2 μm.
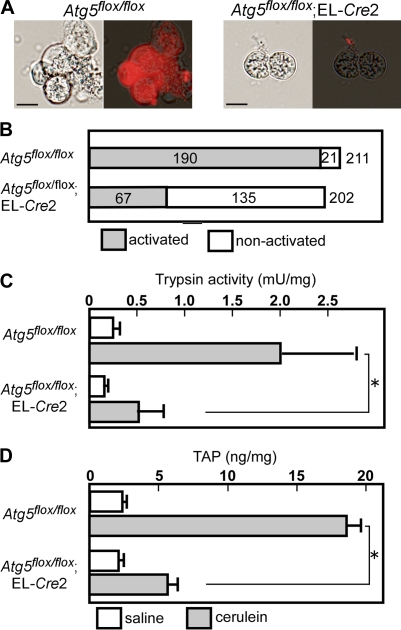
The mechanism for much milder acute pancreatitis in the absence of autophagy could be the low level of trypsinogen activation. To test this hypothesis, we analyzed trypsinogen activation by cerulein in primary cultured acinar cells using a highly sensitive method for detection of trypsin activity (Ohmuraya et al., 2006). Trypsinogen activation was observed in 90% (190/211 cells) and 33% (67/202 cells) of acinar cells from Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice, respectively (Fig. 5, A and B). We also used acinar cells isolated from Atg5-deficient newborn mice (Atg5−/−) in which Atg5 was completely inactivated in all cells (Kuma et al., 2004). Trypsinogen activation was observed in 91% (182/201 cells) and 5% (11/217 cells) of acinar cells from Atg5+/− and Atg5−/− mice, respectively. Trypsinogen activation in some acinar cells may be caused by the presence of an alternative pathway for trypsin activation.
Figure 5.
Loss of trypsin activation in Atg5-deficient pancreatic acinar cells. (A and B) Acinar cells derived from Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice were incubated with cerulein. Acinar cells were isolated at 2 mo of age by treatment of the pancreas with collagenase. After stimulation with 10 nM cerulein, cells were suspended in the medium with a synthetic trypsin substrate, (CBZ-Ile-Pro-Arg)2-rhodamine 110. Rhodamine fluorescence was detected in 90% of acinar cells of Atg5flox/flox mice but in 33% of acinar cells in Atg5flox/flox;EL-Cre2 mice. (C and D) The effects of autophagy and cerulein on the pancreatic trypsin activity (C) and content of TAP (D). Cerulein administration increased the pancreatic trypsin activity and content of TAP in Atg5flox/flox mice but not in Atg5flox/flox;EL-Cre2 mice. *, P < 0.01. Error bars represent SEM. Bars, 10 μm.
We then measured trypsin activity in pancreatic homogenates from Atg5flox/flox;EL-Cre2 and Atg5flox/flox mice treated with cerulein or saline. In saline controls, there was no difference in the levels of trypsin activities between Atg5flox/flox (0.25 ± 0.09 mU/mg; n = 3) and Atg5flox/flox;EL-Cre2 mice (0.16 ± 0.03 mU/mg; n = 3). After cerulein injection, trypsin levels significantly increased in Atg5flox/flox mice (1.97 ± 1.10 mU/mg; n = 3) but mildly increased in Atg5flox/flox;EL-Cre2 mice (0.52 ± 0.41 mU/mg; n = 3; Fig. 5 C). Activation of trypsinogen into active trypsin results in the production of trypsinogen activation peptide (TAP), which corresponds to the N-terminal region of the trypsinogen. TAP was quantified in pancreatic homogenates from Atg5flox/flox;EL-Cre2 and Atg5flox/flox mice treated with cerulein or saline. In saline controls, there was no difference in the levels of TAP between Atg5flox/flox (2.36 ± 0.22 ng/mg; n = 3) and Atg5flox/flox;EL-Cre2 mice (2.08 ± 0.18 ng/mg; n = 3). After cerulein injection, TAP levels significantly increased in Atg5flox/flox mice (18.54 ± 2.04; n = 3) but not in Atg5flox/flox;EL-Cre2 mice (5.65 ± 1.19; n = 3; Fig. 5 C). All of these data suggest that trypsinogen activation is considerably suppressed by reduced autophagy.
Our findings established that autophagy is induced by supramaximal stimulation of cerulein and is directly related to trypsinogen activation and onset of acute pancreatitis. This is the first example that autophagy plays a destructive role in the early stage of disease development. Although the mechanism for autophagy induction by cerulein is not yet clear, insufficient recruitment of zymogene granule membranes under supramaximal stimulation may account for it. In physiological conditions, digestive enzymes are targeted to the secretory compartment, and mixing of lysosomes with digestive zymogens does not occur in the exocrine pancreas (Gorelick et al., 1992). In autophagy, autophagosomes containing intracellular components fuse with endosomes and lysosomes to form autolysosomes (Dunn, 1990). Thus, trypsinogen could be hydrolyzed to trypsin by a lysosomal enzyme such as cathepsin B in autolysosomes. Thus, we propose the autophagy theory for activation of trypsin in acute pancreatitis, and this can explain both the colocalization hypothesis and autoactivation hypothesis.
Materials and methods
Cerulein-induced pancreatitis
After overnight fasting, mice were given hourly intraperitoneal injections of saline or saline containing 50 μg/kg cerulein (Sigma-Aldrich) for 6, 9, and 12 h. 1 h after the last injection, mice were killed, and serum and pancreas samples were rapidly obtained.
Histological analysis
Tissue was fixed overnight in 10% formalin, embedded in paraffin, sectioned, and stained with H&E. For EM analysis, the pancreas was fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 h. Conventional EM was performed as previously described (Yamamoto et al., 1991).
Fluorescence microscopic analysis
The pancreas from GFP-LC3 mice was dissected, fixed with 4% PFA, and sectioned, and GFP fluorescence was observed using a fluorescence microscope (IX81; Olympus) equipped with a CCD camera (ORCA ER; Hamamatsu; Mizushima et al., 2004).
Trypsin assay in acinar cells
Isolated acinar cells were cultured with 10 nM cerulein, and trypsin activity was examined by using a synthetic trypsin substrate, (CBZ-Ile-Pro-Arg)2-rhodamine 110 (Invitrogen; Ohmuraya et al., 2006).
Measurement of trypsin activity
Measurement of trypsin activity was performed as described previously (Towatari et al., 2002).
TAP
Pancreas specimens were boiled at 100°C for 15 min in 0.2 M Tris (hydroxymethyl) aminomethane (Tris)-HCl buffer, pH 7.3, containing 20 mM EDTA. Samples were homogenized on ice for 30 s and centrifuged at 1,500 g for 10 min at 4°C. TAP was quantified in an aliquot of each supernatant using an enzyme immunoassay kit (Oriental Yeast Co.). The total protein concentration was determined, and pancreatic tissue TAP levels were expressed as nanograms/milligrams of total protein.
Western blot analysis
Conventional Western blot analysis was performed as previously described (Ohmuraya et al., 2005). Rabbit anti-LC3 antibody (MBL International), goat antiamylase antibody (Santa Cruz Biotechnology, Inc.), rabbit antitrypsinogen antibody (Nordic Immunological Laboratories), and rabbit anti–IP3R1-3 antibody (Chemicon) were used at 1:2,000, 1:1,000, 1:1,000, and 1:2,000 dilutions, respectively.
Generation of pancreatic acinar cell–specific Cre recombinase expression mice
pNintCre contained five fragments: NLS derived from SV40, splice donor and intron derived from rabbit β globin, intron and splice acceptor derived from mouse En2, the cre recombinase gene, and the polyadenylation signal derived from SV40. The elastase I gene is selectively expressed in pancreatic acinar cells (Hammer et al., 1987). The DNA fragment containing EL was obtained by PCR using the primers 5′-TGGTGGGAGACATTCCAACAACA-3′ for s1 and 5′-TGTGGAGAGAGTAGACCACTGCC-3′ for a2. A fragment containing EL was digested and inserted into pNintCre to generate pEL-Cre (Fig. 3 A). Fragments excised from the pEL-Cre plasmid were separated and used for microinjection. Transgenic founder mice (EL-Cre) were backcrossed with C57BL/6J mice.
RNA analysis
Total RNA was isolated from each organ at 2 mo after birth with Sepasol (Nacalai Tesque). RT-PCR analysis was performed using the primers 5′-AATGCTTCTGTCCGTTTGCC-3′ and 5′-GATTTCCGTCTCTGGTGTAG-3′ for cre recombinase (563 bp) and 5′-GGAAAGCTGTGGCGTGATG-3′ and 5′-CTGTTGCTGTAGCCGTATTC-3′ for G3PDH (382 bp).
Generation of pancreatic acinar cell–specific Atg5-deficient mice
Mice bearing an Atg5 flox allele (Atg5flox) were crossed with a transgenic line, EL-Cre2. These mice caused deletion of the loxP-flanked exon 3 of the Atg5 gene. The deleted allele was detected by PCR with the primers 5′-CAGGGAATGGTGTCTCCCAC-3′ for D (5L2) and 5′-GTACTGCATAATGGTTTAACTCTTGC-3′ for B (short2). Southern blot analysis was performed as previously described (Hara et al., 2006).
Measurement of [Ca2+]i
CCK-8 was obtained from the Peptide Institute, and Fura-2AM was obtained from Dojindo. Measurement of intracellular Ca2+ concentration in pancreatic acinar cell suspensions was performed as previously described (Futatsugi et al., 2005) using an Aquacosmos ratio (Hamamatsu).
Online supplemental material
Fig. S1 shows the pancreas of Atg5flox/flox and Atg5flox/flox;EL-Cre2 mice. Fig. S2 shows Western blot analysis using antiamylase and antitrypsinogen antibodies at embryonic day 18.5, at 0.5 d after birth, and at 2 mo. Fig. S3 shows Western blot analysis of IP3R subtypes in the pancreas and [Ca2+]i changes induced by CCK8 receptor stimulation in pancreatic cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200712156/DC1.
Acknowledgments
We are grateful to Ms. Michiyo Nakata for technical assistance.
This work was supported, in part, by a KAKENHI (Grant in Aid for Scientific Research) in Priority Areas Integrative Research Toward the Conquest of Cancer, a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant from the Osaka Foundation of Promotion of Clinical Immunology, and a grant from the Pancreas Research Foundation of Japan.
D. Hashimoto and M. Ohmuraya contributed equally to this paper.
Abbreviations used in this paper: Atg, autophagy related; CCK, cholecystokinin; H&E, hematoxylin and eosin; LC3, light chain 3; TAP, trypsinogen activation peptide.
References
- Adler, G., C. Hahn, H.F. Kern, and K.N. Rao. 1985. Cerulein-induced pancreatitis in rats: increased lysosomal enzyme activity and autophagocytosis. Digestion. 32:10–18. [DOI] [PubMed] [Google Scholar]
- Cuervo, A.M. 2004. Autophagy: in sickness and in health. Trends Cell Biol. 14:70–77. [DOI] [PubMed] [Google Scholar]
- Dunn, W.A. Jr. 1990. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J. Cell Biol. 110:1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsugi, A., T. Nakamura, M.K. Yamada, E. Ebisui, K. Nakamura, K. Uchida, T. Kitaguchi, H. Takahashi-Iwanaga, T. Noda, J. Aruga, and K. Mikoshiba. 2005. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 309:2232–2234. [DOI] [PubMed] [Google Scholar]
- Gorelick, F.S., I.M. Modlin, S.D. Leach, R. Carangelo, and M. Katz. 1992. Intracellular proteolysis of pancreatic zymogens. Yale J. Biol. Med. 65:407–420 (discussion 437–440). [PMC free article] [PubMed] [Google Scholar]
- Hammer, R.E., G.H. Swift, D.M. Ornitz, C.J. Quaife, R.D. Palmiter, R.L. Brinster, and R.J. MacDonald. 1987. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol. Cell. Biol. 7:2956–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, T., K. Nakamura, M. Matsui, A. Yamamoto, Y. Nakahara, R. Suzuki-Migishima, M. Yokoyama, K. Mishima, I. Saito, H. Okano, and N. Mizushima. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 441:885–889. [DOI] [PubMed] [Google Scholar]
- Helin, H., M. Mero, H. Markkula, and M. Helin. 1980. Pancreatic acinar ultrastructure in human acute pancreatitis. Virchows Arch. A Pathol. Anat. Histol. 387:259–270. [DOI] [PubMed] [Google Scholar]
- Hirota, M., M. Ohmuraya, and H. Baba. 2006. Genetic background of pancreatitis. Postgrad. Med. J. 82:775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, C.B., A.B. el-Din, M. Kotb, L.W. Gaber, and A.O. Gaber. 1996. Calcium channel blockade inhibits release of TNF alpha and improves survival in a rat model of acute pancreatitis. Pancreas. 13:22–28. [DOI] [PubMed] [Google Scholar]
- Ichimura, Y., T. Kirisako, T. Takao, Y. Satomi, Y. Shimonishi, N. Ishihara, N. Mizushima, I. Tanida, E. Kominami, M. Ohsumi, et al. 2000. A ubiquitin-like system mediates protein lipidation. Nature. 408:488–492. [DOI] [PubMed] [Google Scholar]
- Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D.J. 2005. The molecular machinery of autophagy: unanswered questions. J. Cell Sci. 118:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D.J., J.M. Cregg, W.A. Dunn, S.D. Emr, Y. Sakai, I.V. Sandoval, A. Sibirny, S. Subramani, M. Thumm, M. Veenhuis, and Y. Ohsumi. 2003. A unified nomenclature for yeast autophagy-related genes. Dev. Cell. 5:539–545. [DOI] [PubMed] [Google Scholar]
- Koike, H., M.L. Steer, and J. Meldolesi. 1982. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am. J. Physiol. 242:G297–G307. [DOI] [PubMed] [Google Scholar]
- Komatsu, M., S. Waguri, T. Ueno, J. Iwata, S. Murata, I. Tanida, J. Ezaki, N. Mizushima, Y. Ohsumi, Y. Uchiyama, et al. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma, A., M. Hatano, M. Matsui, A. Yamamoto, H. Nakaya, T. Yoshimori, Y. Ohsumi, T. Tokuhisa, and N. Mizushima. 2004. The role of autophagy during the early neonatal starvation period. Nature. 432:1032–1036. [DOI] [PubMed] [Google Scholar]
- Leach, S.D., I.M. Modlin, G.A. Scheele, and F.S. Gorelick. 1991. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J. Clin. Invest. 87:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima, N. 2005. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 12:1535–1541. [DOI] [PubMed] [Google Scholar]
- Mizushima, N., A. Yamamoto, M. Matsui, T. Yoshimori, and Y. Ohsumi. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 15:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmuraya, M., M. Hirota, M. Araki, N. Mizushima, M. Matsui, T. Mizumoto, K. Haruna, S. Kume, M. Takeya, M. Ogawa, et al. 2005. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 129:696–705. [DOI] [PubMed] [Google Scholar]
- Ohmuraya, M., M. Hirota, K. Araki, H. Baba, and K. Yamamura. 2006. Enhanced trypsin activity in pancreatic acinar cells deficient for serine protease inhibitor kazal type 3. Pancreas. 33:104–106. [DOI] [PubMed] [Google Scholar]
- Sherwood, M.W., I.A. Prior, S.G. Voronina, S.L. Barrow, J.D. Woodsmith, O.V. Gerasimenko, O.H. Petersen, and A.V. Tepikin. 2007. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 104:5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70–71. [DOI] [PubMed] [Google Scholar]
- Steer, M.L., and J. Meldolesi. 1987. The cell biology of experimental pancreatitis. N. Engl. J. Med. 316:144–150. [DOI] [PubMed] [Google Scholar]
- Towatari, T., M. Ide, K. Ohba, Y. Chiba, M. Murakami, M. Shiota, M. Kawachi, H. Yamada, and H. Kido. 2002. Identification of ectopic anionic trypsin I in rat lungs potentiating pneumotropic virus infectivity and increased enzyme level after virus infection. Eur. J. Biochem. 269:2613–2621. [DOI] [PubMed] [Google Scholar]
- Tsukada, M., and Y. Ohsumi. 1993. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333:169–174. [DOI] [PubMed] [Google Scholar]
- van Acker, G.J., G. Perides, and M.L. Steer. 2006. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J. Gastroenterol. 12:1985–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, O., F.M. Baccino, M.L. Steer, and J. Meldolesi. 1984. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am. J. Physiol. 246:G457–G467. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., H. Otsu, T. Yoshimori, N. Maeda, K. Mikoshiba, and Y. Tashiro. 1991. Stacks of flattened smooth endoplasmic reticulum highly enriched in inositol 1,4,5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells. Cell Struct. Funct. 16:419–432. [DOI] [PubMed] [Google Scholar]