Abstract
Platelet aggregation requires agonist-induced αIIbβ3 activation, a process mediated by Rap1 and talin. To study mechanisms, we engineered αIIbβ3 Chinese hamster ovary (CHO) cells to conditionally express talin and protease-activated receptor (PAR) thrombin receptors. Human PAR1 or murine PAR4 stimulation activates αIIbβ3, which was measured with antibody PAC-1, indicating complete pathway reconstitution. Knockdown of Rap1–guanosine triphosphate–interacting adaptor molecule (RIAM), a Rap1 effector, blocks this response. In living cells, RIAM overexpression stimulates and RIAM knockdown blocks talin recruitment to αIIbβ3, which is monitored by bimolecular fluorescence complementation. Mutations in talin or β3 that disrupt their mutual interaction block both talin recruitment and αIIbβ3 activation. However, one talin mutant (L325R) is recruited to αIIbβ3 but cannot activate it. In platelets, RIAM localizes to filopodia and lamellipodia, and, in megakaryocytes, RIAM knockdown blocks PAR4-mediated αIIbβ3 activation. The RIAM-related protein lamellipodin promotes talin recruitment and αIIbβ3 activity in CHO cells but is not expressed in megakaryocytes or platelets. Thus, talin recruitment to αIIbβ3 by RIAM mediates agonist-induced αIIbβ3 activation, with implications for hemostasis and thrombosis.
Introduction
Integrins are α/β-heterodimeric receptors that mediate cell–matrix and cell–cell adhesion through interactions with extracellular adhesive ligands and cellular counterreceptors (Hynes, 2002). Ligand binding to many, if not most, integrins is regulated by inside-out signaling. In this process, occupancy of cell surface agonist receptors (e.g., G protein–coupled receptors and receptor protein tyrosine kinases) initiates intracellular signaling to activate integrins. Ultimately, activation signals are thought to converge on the integrin α and/or β cytoplasmic domains, leading to conformational changes that are propagated to the transmembrane and extracellular domains to increase receptor affinity (Calderwood, 2004; Ginsberg et al., 2005; Luo et al., 2007). In platelets, affinity modulation regulates the interaction of αIIbβ3 with cognate ligands, including fibrinogen, von Willebrand factor, and fibronectin, leading to platelet spreading and aggregation at sites of vascular damage (Shattil and Newman, 2004; Jackson, 2007). In humans, abnormalities in inside-out signaling may cause either reduced platelet adhesive function and bleeding (Chen et al., 1992; Wang et al., 1997; Pasvolsky et al., 2007) or heightened platelet function and thrombosis (Ruggeri, 2002; Michiels et al., 2006). Consequently, substantial efforts have been made to unravel the molecular mechanisms of inside-out signaling in platelets and other cells, with recent studies focusing on candidate proteins that may interact with the integrin cytoplasmic domains to flip the activation switch (Liu et al., 2000; Ginsberg et al., 2005; Leisner et al., 2007).
Talin1 (talin) is an ∼270-kD protein with an N-terminal globular head and a C-terminal rodlike tail (Critchley, 2004). The talin head contains a FERM domain capable of engaging several proteins, including integrin β cytoplasmic domains, and the talin rod contains a second integrin-binding site and binding sites for filamentous actin and vinculin (Knezevic et al., 1996; Critchley, 2004). Overexpression of the talin head or FERM domain promotes activation of αIIbβ3 and other integrins in model cell systems (Calderwood et al., 1999; Bouaouina et al., 2008). Talin knockout in the mouse is embryonic lethal (Monkley et al., 2000). However, knockdown of talin in megakaryocyte platelet precursors with short hairpin RNA (shRNA; Tadokoro et al., 2003) and hematopoietic- or platelet/megakaryocyte-specific talin knockout in mice (Nieswandt et al., 2007; Petrich et al., 2007b) cause severe impairment of agonist-induced αIIbβ3 activation and a bleeding diathesis. In unstimulated platelets, talin resides in the cytoplasm (Bertagnolli et al., 1993), presumably in an autoinhibited conformation, with the head and tail interacting in an intra- or intermolecular fashion to limit access to binding proteins (Calderwood, 2004; Critchley, 2005; Ginsberg et al., 2005). Platelet stimulation by thrombin or other agonists is hypothesized to relieve this autoinhibition and promote talin recruitment to and activation of αIIbβ3. However, several critical details of talin's function in platelets and other cells remain sketchy, particularly the process of talin recruitment to activate integrins.
Among the proteins that have been implicated in integrin-mediated cell adhesion are the Rap1 GTPase (Bos, 2005) and Rap1-GTP–interacting adaptor molecule (RIAM), a Rap1 effector (Lafuente et al., 2004). RIAM is a member of the MRL family of adaptor molecules that includes lamellipodin and its Caenorhabditis elegans orthologue, Mig-10 (Krause et al., 2004; Lafuente et al., 2004; Chang et al., 2006). Each contains an N-terminal coiled-coil region, central Ras association and pleckstrin homology domains, a proline-rich C-terminal region, and multiple FPPPP motifs capable of interacting with the EVH1 domains of the actin regulatory proteins Ena/vasodilator-stimulated phosphoprotein (VASP). RIAM but not lamellipodin interacts with Rap1-GTP to promote the adhesion of Jurkat T cells through β1 and β2 integrins (Krause et al., 2004; Lafuente et al., 2004). Lamellipodin, on the other hand, promotes lamellipodia formation through Ena/VASP (Krause et al., 2004), and Mig-10 promotes axon guidance and outgrowth through the Ena/VASP homologue, unc-34 (Chang et al., 2006).
Recent evidence supports roles for both Rap1 and RIAM in αIIbβ3 affinity modulation. Knockdown or knockout of Rap1b (Chrzanowska-Wodnicka et al., 2005) or of CalDAG-GEFI, a Rap1 exchange factor (Eto et al., 2002; Crittenden et al., 2004), is associated with defective agonist-induced αIIbβ3 activation. Furthermore, overexpression of activated Rap1a (V12) or RIAM in CHO cells leads to talin-dependent αIIbβ3 activation as measured with the ligand-mimetic antibody PAC-1. In contrast, knockdown of either protein by shRNA has the opposite effect (Han et al., 2006). Interestingly, Rap1a (V12) promotes RIAM association with talin, as determined indirectly by coimmunoprecipitation and immunofluorescence colocalization techniques. However, it remains to be determined whether RIAM promotes talin recruitment directly to αIIbβ3 in living cells, whether it mediates inside-out signaling in response to a platelet agonist, and whether it regulates integrin activation in platelets or megakaryocytes. Moreover, a role for lamellipodin, if any, in αIIbβ3 activation has not been addressed. In this study, we have resolved these issues by using bimolecular fluorescence complementation (BiFC; Kerppola, 2008) to monitor talin recruitment to αIIbβ3 in living cells and by studying RIAM–talin–αIIbβ3 relationships in platelets and megakaryocytes. The results establish that RIAM but not lamellipodin is a central player in agonist-induced recruitment of talin to αIIbβ3. Furthermore, a specific interaction between the talin FERM domain and β3 is required for the recruited talin to activate αIIbβ3.
Results
Talin recruitment to αIIbβ3 in living cells
To study the process of talin recruitment to αIIbβ3, we developed a method to monitor specific, proximal interactions between full-length talin and αIIbβ3 in living cells based on BiFC (Shyu et al., 2006; Kerppola, 2008). Two chimeras were constructed for this purpose (Fig. 1 A). One, VN-talin, consists of the N-terminal half of the Venus fluorophore fused to the N terminus of mouse talin. The other chimera, αIIb-VC, consists of the C-terminal half of Venus fused to the C terminus of human αIIb. The latter was cotransfected with human β3 to establish stable cell lines expressing αIIb-VCβ3. We reasoned that after transfection of VN-talin into these cells, this protein would be recruited from the cytoplasm to αIIb-VCβ3 in response to an inside-out stimulus. Assuming proper orientation, the split Venus moieties associated with talin and αIIbβ3 would then interact and refold to complement Venus fluorescence (Fig. 1 A). On the other hand, fluorescence complementation would be reduced in cells that express a β3 deletion mutant (β3 Δ724) that lacks the NPXY interaction site for the talin FERM domain (Wegener et al., 2007). Therefore, each experiment included cells expressing αIIb-VCβ3 (Δ724) as a control for fluorescence unrelated to this specific talin–β3 interaction.
Figure 1.
Talin recruitment to αIIbβ3 can be visualized in living cells by BiFC. (A) Principle of BiFC. The top panel depicts the two fusion proteins, αIIb-VC and VN-talin, used for BiFC. Also depicted are wild-type β3 and β3 (Δ724), which were cotransfected into CHO cells with αIIb-VC to enable the expression of αIIb-VCβ3 and αIIb-VCβ3 (Δ724), respectively. TM, transmembrane domain. The bottom panel illustrates the concept of BiFC when VN-talin successfully interacts with αIIb-VCβ3 as the result of inside-out signaling. See Results for further discussion. (B) Flow cytometry with antibody D57 was used to determine αIIbβ3 surface expression in cells stably transfected with αIIbβ3, αIIb-VCβ3, or αIIb-VCβ3 (Δ724). Parental CHO cells were used as a negative control. Expression of the chimeric integrins was equivalent to that of wild-type αIIbβ3. (C) Western blotting illustrates the molecular sizes of the αIIb and β3 subunits obtained from cells shown in B. (D) VN-talin complements Venus fluorescence better than talin-VC. The N-terminal half of Venus was fused to either the talin N terminus (VN-talin) or the talin C terminus (talin-VN) and transiently transfected into αIIb-VCβ3 cells. BiFC was then measured by flow cytometry. The same experiment was performed in parallel with αIIb-VCβ3 (Δ724) cells, which lack a major talin interaction site in β3. Data are means ± SEM (error bars) of three experiments. (E) αIIb-VCβ3–CHO cells and αIIb-VCβ3 (Δ724)–CHO cells were cotransfected with VN-talin and Rap1a (V12). 48 h later, cells were plated on fibrinogen for 60 min, fixed, and analyzed by deconvolution microscopy. Note that in the αIIb-VCβ3 cells, BiFC fluorescence (green) was colocalized with vinculin (red) to lamellipodial edges (arrow), focal complexes (arrowheads), and focal adhesions (double arrowhead). Only minor colocalization was noted in the αIIb-VCβ3 (Δ724) cells. Bars, 10 μm.
Cell lines expressing αIIb-VCβ3 or αIIb-VCβ3 (Δ724) showed integrin surface expression profiles that were equivalent to that of a cell line expressing αIIbβ3 (Fig. 1 B). Furthermore, the αIIb-VC, β3, and β3 (Δ724) subunits each migrated as expected in Western blots of cell lysates (Fig. 1 C). We chose VN-talin for these studies rather than placing VN at the C terminus of talin (talin-VN) because in preliminary studies, overexpression of VN-talin in αIIb-VCβ3 cells consistently yielded greater fluorescence complementation than did talin-VN (Fig. 1 D). This may be explained by the fact that in VN-talin, the split fluorophore is contiguous with the talin FERM domain rather than with the rodlike tail. The fluorescence complementation we observed between VN-talin and αIIb-VCβ3 in the absence of an inside-out stimulus is likely the result of overexpression and/or stabilization of the VN/VC pair, as seen in a previous study using BiFC (Kerppola, 2008). To further validate the BiFC approach, cells transiently transfected with VN-talin were allowed to bind to immobilized fibrinogen, which activates αIIbβ3 directly (Du et al., 1991) and promotes cell spreading. Fixed and stained αIIb-VCβ3–CHO cells were well spread, and BiFC was prominent at vinculin-rich lamellipodial edges, focal complexes, and focal adhesions (Fig. 1 E). In contrast, αIIb-VCβ3 (Δ724) cells spread poorly and exhibited only minor colocalization of BiFC and vinculin at focal adhesions (Fig. 1 E). No BiFC was observed if VN-talin or αIIb-VCβ3 was expressed independently. These results indicate that VN-talin can interact specifically with αIIb-VCβ3 and at sufficient proximity to monitor talin interaction with αIIbβ3.
Next, we asked whether inside-out signals can lead to activation of αIIbβ3 by recruiting talin to the integrin. When αIIb-VCβ3 cells were cotransfected with constitutively active Rap1a (V12) and VN-talin, they exhibited significant increases in BiFC and PAC-1 binding when compared with the same cells not transfected with VN-talin (Fig. 2 A). In contrast, αIIb-VCβ3 (Δ724) cells transfected with Rap1a (V12) and VN-talin showed lower fluorescence complementation and no increase in PAC-1 binding. VN-talin expression was similar in both cell lines (Fig. 2 A). BiFC involving VN-talin and αIIb-VCβ3 did not require the presence of an αIIbβ3 ligand, such as fibrinogen or PAC-1. Thus, Rap1a induces both talin recruitment and αIIbβ3 activation. To determine whether talin recruitment is necessary and sufficient for αIIbβ3 activation, we tested talin FERM domain mutants (L325R and W359A) and a β3 cytoplasmic domain mutant (Y747A), all of which are reported to be defective in supporting αIIbβ3 activation (Tadokoro et al., 2003; Petrich et al., 2007a). However, when cotransfected with Rap1a (V12), VN-talin (L325R) was recruited normally to αIIb-VCβ3 (Fig. 2 A). In contrast, both the recruitment of VN-talin (W359A) to αIIb-VCβ3 (Fig. 2 A) and recruitment of VN-talin to αIIbβ3 (Y747A; Fig. 2 B) were significantly impaired (P < 0.001). All three mutants failed to support Rap1a (V12)-mediated PAC-1 binding to αIIbβ3 (P < 0.001) despite levels of talin expression similar to that of αIIb-VCβ3 cells transfected with VN-talin (Fig. 2, A and B). Thus, talin recruitment appears to be necessary for αIIbβ3 activation in response to an inside-out stimulus such as that imparted by activated Rap1a. However, the results with VN-talin (L325R) indicate that events subsequent to talin recruitment are also needed. Crystallographic experiments (Wegener et al., 2007) have shown that the talin FERM domain makes contact with membrane-distal residues in the NPXY region of β3, a contact disrupted by the talin (W359A) substitution. In contrast, talin (L325R) disrupts interaction with the more membrane-proximal region of β3. The present results in living cells support speculation from the crystallographic experiments (Wegener et al., 2007) that after initial talin recruitment to β3 NPXY, additional talin interactions with the membrane-proximal region of β3 are required for integrin activation to proceed.
Figure 2.
Structural requirements in talin and β3 for talin recruitment and inside-out αIIbβ3 activation in living cells. (A) Effects of talin mutation on BiFC and PAC-1 binding. VN-talin (either wild-type, L325R, or W359A talin) was cotransfected with Rap1a (V12) into CHO cells expressing αIIb-VCβ3 or αIIb-VCβ3 (Δ724). As a control, cells were transfected with Rap1a (V12) but not VN-talin (white bars). 48 h later, BiFC fluorescence, PAC-1 binding, and talin expression in transfected cells were quantified by flow cytometry as described in Materials and methods. Responses are expressed as fold increase compared with cells not transfected with VN-talin. (B) Effects of β3 mutation on BiFC and PAC-1 binding. VN-talin was cotransfected with Rap1a (V12) into CHO cells expressing αIIb-VCβ3, αIIb-VCβ3 (Y747A), or αIIb-VCβ3 (Δ724). As a control, cells were transfected with empty vector (mock). 48 h later, BiFC fluorescence, PAC-1 binding, and talin expression in transfected cells were quantified by flow cytometry. Responses are expressed as fold increase compared with mock controls. (A and B) Data are means ± SEM (error bars) of four experiments.
RIAM and lamellipodin in talin recruitment and αIIbβ3 activation
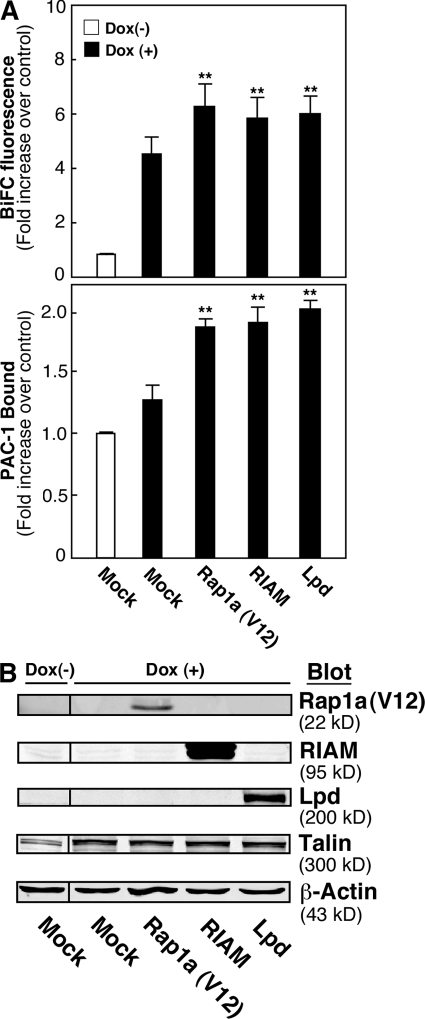
RIAM has been implicated as a Rap1 effector in the αIIbβ3 activation pathway (Han et al., 2006). Therefore, we asked whether RIAM promotes talin recruitment to αIIbβ3 in living cells and compared its effects with that of the structurally related MRL protein, lamellipodin (Krause et al., 2004). To facilitate these studies, VN-talin was expressed in αIIb-VCβ3 cells under the control of a tetracycline-regulated promoter. Induction of talin by doxycycline caused an increase in BiFC and PAC-1 binding compared with noninduced cells. However, the transient expression of Rap1a (V12), RIAM, or lamellipodin caused further increases in BiFC and PAC-1 binding when compared with doxycycline-induced but mock-transfected cells (P ≤ 0.01; Fig. 3 A). Western blotting of the doxycycline-induced cells showed appropriate expression of the transfected recombinant proteins and equivalent levels of VN-talin (Fig. 3 B). Thus, when overexpressed, activated Rap1a, RIAM, and lamellipodin each have the capacity to promote talin recruitment and αIIbβ3 activation.
Figure 3.
RIAM and lamellipodin promote talin recruitment and αIIbβ3 activation. (A) αIIb-VCβ3 CHO cells were engineered to conditionally express VN-talin under the control of a tetracycline-regulated promoter as described in Materials and methods. Cells were then incubated for 24 h in the absence (Dox (−)) or presence (Dox (+)) of 0.75 ng/ml doxycycline, a concentration that induced approximately half-maximal expression of VN-talin. Concomitantly, cells were transiently transfected as indicated with empty vector (mock) or vectors for Rap1a (V12), RIAM, or lamellipodin (Lpd). Then, BiFC and PAC-1 binding were analyzed and expressed as fold increase compared with Dox (−) mock controls. Data represent means ± SEM (error bars) of four experiments. Double asterisks indicate means that were significantly different from the Dox (+) mock control at P ≤ 0.01. (B) Protein expression was analyzed in cell lysates by Western blotting. Immunoblotting for β-actin was used to assess gel loading. A representative Western blot is shown.
To evaluate the function of endogenous RIAM or lamellipodin in VN-talin–expressing αIIb-VCβ3 cells, each protein was knocked down by shRNA. Compared with control shRNA, RIAM or lamellipodin shRNA reduced levels of their respective target proteins by ∼70% without affecting levels of talin (Fig. 4 A). This partial knockdown of RIAM but not lamellipodin inhibited both basal and Rap1a (V12)-mediated BiFC and PAC-1 binding in talin-expressing cells (P ≤ 0.05; Fig. 4 B). These results suggest that talin recruitment and αIIbβ3 activation mediated by Rap1a (V12) require RIAM but not lamellipodin. The inability of lamellipodin knockdown to block the effects of Rap1a (V12) may be explained by the fact that, unlike RIAM, lamellipodin does not interact with Rap1-GTP as a typical effector (Krause et al., 2004).
Figure 4.
Knockdown of RIAM but not lamellipodin blocks talin recruitment and αIIbβ3 activation induced by Rap1a (V12). αIIbβ3-CHO cells were transduced with lentivirus encoding RIAM-specific shRNA, lamellipodin-specific shRNA (Lpd), or an irrelevant control (CTRL) shRNA. 48 h later, some cells were transfected with Rap1a (V12) as indicated, and, 24 h after that, 0.75 ng/ml doxycycline was added to induce VN-talin expression. (A) Protein expression was analyzed in cell lysates by Western blotting. (B) BiFC and PAC-1 binding were measured and expressed as fold increase compared with noninduced cells (no VN-talin). Data are means ± SEM (error bars) of three experiments.
Requirements for reconstitution of agonist-induced αIIbβ3 activation
αIIbβ3-CHO cells, even those overexpressing talin, do not bind soluble αIIbβ3 ligands when stimulated with thrombin or other physiological agonists, indicating that, unlike platelets, agonist-response coupling is incomplete. In an attempt to engineer agonist responsiveness, the human protease-activated receptor 1 (PAR1) thrombin receptor and talin were coexpressed in a tetracycline-regulated fashion. After induction with doxycycline and transfection of cells with a control shRNA, addition of 0.5 or 50 μM SFLLRN, a PAR1 agonist peptide, resulted in increased PAC-1 binding (P < 0.0001; Fig. 5 A, Dox (+)). Similar results were obtained if these cells were stimulated with thrombin or if murine PAR4 was expressed instead of PAR1 and the cells stimulated with AYPGKF, a PAR4 agonist peptide (not depicted). However, no significant αIIbβ3 activation in response to SFLLRN was observed in the absence of PAR1 and talin induction (Fig. 5 A, Dox (−)) or if PAR1 was expressed without talin (not depicted). Thus, expression of platelet PAR receptors in CHO cells reconstitutes agonist-induced and talin-dependent αIIbβ3 activation.
Figure 5.
Effect of RIAM or lamellipodin knockdown on PAR1-mediated αIIbβ3 activation in CHO cells. (A) αIIbβ3-CHO cells were engineered to conditionally express PAR1 and talin under the control of a tetracycline-regulated promoter. Cells were then transduced with lentivirus encoding RIAM shRNA, lamellipodin (Lpd) shRNA, or control shRNA (CTRL). After PAR1 and talin induction for 24 h with doxycycline, induced (Dox (+)) and noninduced (Dox (−)) cells were incubated for 10 min at room temperature with PAC-1 in the absence or presence of a subsaturating (0.5 μM) or saturating (50 μM) concentration of PAR1 agonist peptide, SFLLRN. Then, specific PAC-1 binding to the lentiviral-transduced cells was determined. PAC-1 binding is expressed as fold increase compared with unstimulated Dox (−) cells transduced with control shRNA. Data are means ± SEM (error bars) of three experiments. (B) Protein expression was analyzed by Western blotting. (C) Flow cytometry histograms depicting surface expression of recombinant PAR1 before (open histograms) and after (closed histograms) doxycycline induction detected with an antibody specific for the Flag epitope.
To investigate the requirements for endogenous RIAM or lamellipodin in agonist-induced αIIbβ3 activation, each protein was knocked down with shRNA. As before, RIAM and lamellipodin shRNAs caused a specific ∼70% reduction in the target protein (Fig. 5 B). RIAM knockdown in doxycycline-induced cells inhibited PAC-1 binding in response to both low and high concentrations of SFLLRN (P < 0.0001; Fig. 5 A). A second RIAM shRNA had the same effect (not depicted). By comparison, lamellipodin knockdown appeared less effective in blocking PAR1-mediated αIIbβ3 activation, reducing PAC-1 binding in response to low but not high concentrations of SFLLRN (Fig. 5 A). These results indicate that endogenous RIAM is required to support full PAR1-mediated, talin-dependent αIIbβ3 activation in CHO cells.
RIAM localization and function in platelets and megakaryocytes
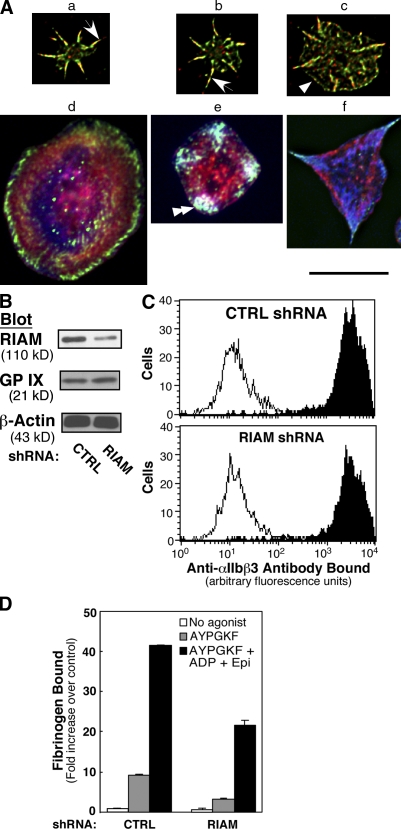
RIAM is expressed in platelets and megakaryocytes (Han et al., 2006). However, we have been unable to identify lamellipodin in human or mouse platelets or megakaryocytes by Western blotting. To study the subcellular localization of RIAM, human platelets were plated on fibrinogen for up to 45 min in the presence of ADP and epinephrine to enhance spreading, and cells were examined by immunofluorescence microscopy. As spreading proceeded, RIAM was localized to vinculin-rich filopodia and lamellipodial edges (Fig. 6 A, a–d). In fully spread platelets, RIAM localized with vinculin and actin in structures that may be the platelet equivalent of focal adhesions (Fig. 6 A, e and f; Nachmias and Golla, 1991; Leng et al., 1998; Bearer et al., 2002).
Figure 6.
Localization and function of RIAM in platelets and megakaryocytes. (A) RIAM localization in platelets. Washed human platelets were plated on fibrinogen for up to 45 min. Cells in a–c were stained with antibodies to RIAM (red) and vinculin (green). Cells in d–f were stained for RIAM (green), vinculin (blue), and F-actin (red). Platelets were imaged by deconvolution microscopy. Each panel represents a platelet representative of the range of platelets observed. As spreading proceeded, RIAM was prominent within vinculin-rich filopodia (arrows), lamellipodial edges (arrowhead), and focal adhesion-like structures (double arrowhead). Bar, 10 μm. (B–D) RIAM is required for agonist-induced αIIbβ3 activation in primary megakaryocytes. Murine megakaryocytes derived from embryonic stem cells were infected with lentivirus encoding control (CTRL) or RIAM shRNA. 7 d later, megakaryocytes were evaluated for RIAM expression in cell lysates by Western blotting (B), αIIbβ3 surface expression by flow cytometry using antibody D57 (closed histograms) and an IgG control (open histograms; C), and specific fibrinogen binding by flow cytometry (D). Megakaryocytes were either unstimulated (no agonist) or stimulated for 30 min at room temperature with 1 mM PAR4 agonist peptide (AYPGFK) or a combination of AYPGFK, 50 μM ADP, and 50 μM epinephrine. Fibrinogen binding is expressed as fold increase compared with unstimulated megakaryocytes transduced with control shRNA. Data are means ± SEM (error bars) of three experiments.
To determine whether RIAM is required for inside-out signaling in primary cells, megakaryocytes derived from murine embryonic stem cells (Eto et al., 2002) were transduced with lentivirus encoding RIAM shRNA. This resulted in an ∼60% reduction in immunoreactive RIAM but no change in levels of other megakaryocyte proteins, including glycoprotein IX, β-actin (Fig. 6 B), and αIIbβ3 (Fig. 6 C). Megakaryocytes transduced with control shRNA and stimulated with the PAR4 agonist peptide, AYPGKF, or a combination of AYPGKF, ADP, and epinephrine bound 10- and 42-fold more fibrinogen, respectively, than unstimulated megakaryocytes. RIAM knockdown inhibited each of these responses significantly (P < 0.0001; Fig. 6 D). Thus, RIAM is required for normal agonist-induced activation of αIIbβ3 in primary cells.
Discussion
In this study, BiFC was used to monitor talin recruitment to αIIbβ3 in living cells, and the potential mechanisms and consequences of this event were studied. Coupled with studies in primary cells and successful reconstitution of agonist-induced inside-out αIIbβ3 signaling in heterologous cells, the major conclusions are (1) αIIbβ3 activation initiated through platelet PAR thrombin receptors requires the recruitment of talin to αIIbβ3, a process regulated by the Rap1 effector, RIAM; (2) RIAM is required for agonist-induced activation of αIIbβ3 in primary megakaryocytes; (3) in platelets, RIAM is present in platelets within filopodia and lamellipodia, subcellular locales where integrin activation would be expected to take place; and (4) lamellipodin can promote talin recruitment and αIIbβ3 activation in CHO cells; however, unlike RIAM, it may not function downstream of Rap1 and is not detectable in megakaryocytes or platelets. Given the central role of αIIbβ3 activation in platelet function (Shattil and Newman, 2004; Jackson, 2007), these findings have implications for hemostasis or thrombosis.
Signal transduction from PARs or other agonist receptors to αIIbβ3 involves a network of protein–protein interactions and posttranslational modifications (Brass, 2004). The inherent complexity of mammalian signaling networks need not preclude eventual identification of all essential elements. Global analyses of the platelet transcriptome and proteome are contributing toward this goal (Garcia et al., 2005; Maguire et al., 2005; Gnatenko et al., 2006), but formal characterization of candidate proteins will require additional strategies. Our approach here was to attempt to fully reconstitute and analyze a thrombin-induced αIIbβ3 activation pathway in CHO cells and characterize proteins within this pathway in platelets and megakaryocytes. The starting point for this study was the observation that transient overexpression of full-length mouse talin in αIIbβ3-CHO cells is sufficient to induce αIIbβ3 activation in response to overexpression of activated Rap1a or RIAM (Han et al., 2006). However, CHO cells are inherently unresponsive to thrombin or other platelet agonists, leaving the role of RIAM in agonist-induced αIIbβ3 activation unclear.
This issue was resolved here by coexpressing talin in αIIbβ3-CHO cells with either human PAR1 or murine PAR4, the dominant platelet PARs in these species (Kahn et al., 1999; Sambrano et al., 2001). In some experiments, PAR1 and talin were expressed in a tetracycline-regulatable fashion to gain better control of overexpression levels and to limit prolonged contact of the PARs with thrombin or other proteases in serum to avoid desensitization (Hoxie et al., 1993; Kahn et al., 1999; Kuliopulos et al., 1999). Under these conditions, thrombin or highly specific agonist peptides for PAR1 or PAR4 (Vu et al., 1991; Faruqi et al., 2000) stimulated αIIbβ3 activation, as measured by binding of the ligand-mimetic antibody, PAC-1, the antibody generally used to study αIIbβ3 affinity modulation in human platelets (Michelson, 2006). Using shRNA to partially knock down specific proteins, RIAM was identified as a necessary distal element of the thrombin-mediated and talin-driven αIIbβ3 activation machinery both in the CHO model system and megakaryocytes. Thus, it should now be possible to identify and order other essential PAR pathway components, some of which may be Rap1 dependent and others Rap1 independent (Coughlin, 2000; Brass, 2004). PARs in platelets also signal to activate the integrin α2β1 collagen receptor (Jung and Moroi, 2001). Because PARs and α2β1 are widely expressed (Coughlin, 2000; Chen et al., 2002), the approaches taken here to probe αIIbβ3 activation may be useful in characterizing inside-out integrin signaling in other biological contexts.
In model cells, including CHO cells, overexpression of the talin head or FERM domain leads to activation of β1, β2, and β3 integrins (Calderwood et al., 1999; Kim et al., 2003; Tadokoro et al., 2003; Ma et al., 2006; Bouaouina et al., 2008). Because talin normally resides in the cytoplasm of unstimulated platelets (Bertagnolli et al., 1993), agonist stimulation is thought to induce posttranslational and conformational modifications within talin to expose integrin-binding sites, resulting in talin recruitment to integrin cytoplasmic domains and inside-out integrin activation (Calderwood, 2004; Critchley, 2004; Ginsberg et al., 2005). We propose that it is RIAM that mediates talin recruitment to αIIbβ3 in platelets. First, when Rap1a (V12), RIAM, and talin are overexpressed in CHO cells, RIAM coimmunoprecipitates and colocalizes with talin (Han et al., 2006). Second, RIAM coimmunoprecipitates with talin after human platelets are stimulated by a PAR1 agonist (Han et al., 2006). Third, Rap1a (V12) and RIAM promoted talin recruitment to αIIbβ3 in living cells, as demonstrated here by BiFC (Figs. 2 and 3). Fourth, there was tight linkage between talin recruitment and αIIbβ3 activation, and mutants in either talin or β3 that abrogate their mutual interaction blocked both responses (Fig. 2). Finally, RIAM was strategically localized in platelets for a role in αIIbβ3 activation (Fig. 6 A), and RIAM knockdown inhibited agonist-induced αIIbβ3 activation in megakaryocytes (Fig. 6 D).
In fibroblasts, lamellipodin regulates lamellipodia formation and protrusion velocity through interactions with Ena/VASP and other effectors (Krause et al., 2004). Lamellipodin does not appear to be a Rap1 effector and may even function to reduce adhesion of Jurkat T lymphocytes via β1 integrins (Krause et al., 2004; Lafuente et al., 2004). The domain compositions of RIAM and lamellipodin are similar. We found that overexpression of lamellipodin in CHO cells increased talin recruitment and αIIbβ3 activation (Fig. 3), and lamellipodin knockdown reduced PAR1-mediated αIIbβ3 activation, albeit less effectively than RIAM knockdown (Fig. 5). Although we could not detect lamellipodin protein in platelets or megakaryocytes, further studies of lamellipodin/integrin relationships in other cells appear to be warranted.
We chose BiFC as a method to study talin recruitment to αIIbβ3 in living cells because of its ease of implementation, its facile adaptation to standard fluorescence microscopy and flow cytometry (Kerppola, 2008), and our previous success with the method in analyzing integrin–protein tyrosine kinase interactions (de Virgilio et al., 2004). Potential drawbacks of BiFC compared with other methods, such as FRET, are delayed maturation of the reconstituted split fluorophore and possible stabilization of the protein–protein interaction under study (Shyu et al., 2006; Kerppola, 2008). Although this makes BiFC unsuitable for assessing dynamic changes in protein interactions, any secondary stabilization of the talin–αIIbβ3 interaction may have proved useful in our case by also stabilizing the high-affinity state of αIIbβ3 and, thus, its detection with PAC-1. Recently, FRET was used to cleverly monitor the activation state of β1 integrins (Parsons et al., 2008). There, the β1 subunit was tagged with GFP, and the isolated talin rod domain was tagged with mRFP. Addition of β1 extracellular ligands induced the association of β1 integrin with the talin rod (Parsons et al., 2008), which is consistent with previous observations that the binding of adhesive ligands activates integrins (Du et al., 1991). Interestingly, interaction of the isolated talin head domain with β1 integrin could not be demonstrated by FRET, possibly because of fluorophore orientation. Although inside-out integrin signaling was not evaluated in the FRET study, it is apparent that FRET and BiFC will provide powerful complementary approaches to study integrin signaling and, in particular, to characterize newly identified effectors of integrin function (Moser et al., 2008).
Identification of a functional RIAM–talin–integrin axis in platelets may provide new insights into clinical disorders of hemostasis or thrombosis. On one hand, there are several poorly understood bleeding disorders associated with defects in αIIbβ3 activation (Rao et al., 2004). Some may now be explained by abnormalities in this axis. As a recent example, patients have been described with recurrent bleeding and infections and defective agonist-induced integrin activation in platelets and white blood cells attributable to a deficiency of the Rap1 exchange factor CalDAG-GEFI (Pasvolsky et al., 2007). On the other hand, excessive or inappropriate αIIbβ3 activation may complicate atherothrombosis and other diseases (Ruggeri, 2002; Michiels et al., 2006). Current oral antiplatelet agents, such as aspirin and ADP receptor antagonists, are only partially effective in reducing arterial thrombosis in humans (Bhatt and Topol, 2003). Thus, there is an unmet need for more effective, long-term platelet function blockade, and oral αIIbβ3 antagonists have not proven useful in this regard (Bhatt and Topol, 2003). Accordingly, RIAM and talin, by virtue of their central roles in a final common pathway of inside-out αIIbβ3 activation, provide potential targets for new antithrombotic drugs.
Materials and methods
Reagents and plasmid vectors
Agonist peptides specific for human PAR1 (SFLLRN; Vu et al., 1991) and murine PAR4 (AYPGKF; Faruqi et al., 2000) were synthesized and purified by the Scripps Center for Protein Sciences. Antibodies specific for RIAM and lamellipodin were described previously (Krause et al., 2004; Lafuente et al., 2004). R-phycoerythrin–conjugated rat anti–mouse CD41 antibody (MWReg30) was purchased from BD Biosciences. Antibody to human integrin β3 was obtained from Santa Cruz Biotechnology, Inc. Antibody to mouse glycoprotein IX (Xia.B4) was obtained from Emfret. Antibody to β-actin was purchased from Abcam. Antibodies to talin (8d4), vinculin (VIN-11-5), and the Flag epitope were purchased from Sigma-Aldrich. Antibody to the influenza hemagglutinin HA-1 epitope was purchased from Covance Research Products.
Plasmids encoding cDNAs for mouse talin1 (Han et al., 2006), human PAR1 (Kahn et al., 1999), and mouse PAR4 (Kahn et al., 1998) have been described previously. cDNAs were subcloned into the pcDNA4/TO tetracycline-inducible expression vector (Invitrogen). Plasmid encoding tdTomato (pREST/tdTomato; gift from R. Tsien, University of California, San Diego, La Jolla, CA; Shaner et al., 2004) was subcloned along with the internal ribosomal entry site (IRES) from pIRES-GFP2 (Invitrogen) into pEF4/Myc-His-A (Invitrogen) to construct the pEF4/IRES-tdTomato mammalian expression vector. Also, IRES-GFP from the pIRES-GFP2 vector was subcloned into pEF4/Myc-His-A to construct pEF4/IRES-GFP. Mammalian expression vectors for HA-Rap1a (V12) and HA-Rap1GAP were gifts from J. Bos (University Medical Center Utrecht, Utrecht, Netherlands) and A. Hall (Memorial Sloan-Kettering, New York, NY), respectively. The coding regions of HA-Rap1a (V12) and HA-Rap1GAP were amplified by PCR to place appropriate restriction sites, and PCR products were subcloned downstream of the EF-1α promoter into pEF4/IRES-tdTomato. Similarly, RIAM and lamellipodin cDNAs (Krause et al., 2004; Lafuente et al., 2004) were cloned into pEF4/IRES-tdTomato and pEF4/IRES-GFP.
Cell culture and transfection
CHO-K1, 293T, and NIH3T3 cells were cultured in DME and supplemented with antibiotics, nonessential amino acids, l-glutamine, and 10% FBS. For transient transfections, Lipofectamine (Invitrogen) was used according to the manufacturer's recommendations. To produce stable cell lines, CHO cells were transfected with the appropriate expression plasmids. 48 h later, antibiotics for selection were added, and cells were cultured for ∼2 wk. Clones were selected further by single-cell sorting using MoFlo (Dako), and stable expression of recombinant proteins was confirmed by flow cytometry or Western blotting. In some cases, stable CHO cell clones capable of tetracycline-inducible expression of PAR1 and/or talin were produced (Yao et al., 1998).
Lentivirus production and infection
Plasmids encoding shRNA for mouse RIAM and the human U6 promoter (Han et al., 2006) were amplified by PCR to place XbaI and XhoI sites, and PCR products were cloned into lentiviral vector FG12 (Qin et al., 2003). Promoter and shRNA sequences were confirmed by DNA sequencing. shRNAs for mouse and rat lamellipodin with mouse U6 promoter fragments (Krause et al., 2004) were removed at XbaI and XhoI sites from the pLL3.7 vector and cloned into FG12. Control shRNA was obtained from a scrambled sequence of mRNA derived from Rock1 (Tadokoro et al., 2003).
Viruses were produced by calcium phosphate–mediated transient transfection of 293T cells. The cells were triple transfected with plasmid FG12 encoding shRNA, envelope plasmid pCI–vesicular stomatitis virus G protein, and packaging plasmid pCMVΔR8.91. Viruses were collected as conditioned medium 3 d after transfection, and viral titers were determined by measuring GFP expression in infected NIH3T3 cells. Titers ranged from 0.3 to 107 U/ml. To achieve protein knockdown, CHO cells were incubated for 16 h with 8 μg/ml Polybrene (Sigma-Aldrich) and lentivirus-containing medium at a multiplicity of infection of five. After further culture in complete DME, cells were studied as described in each experiment.
Flow cytometry
αIIbβ3 activation was assessed by ligand-mimetic antibody PAC-1 (Shattil et al., 1985) 96 h after lentiviral infection with shRNAs and 48 h after transient transfection with expression plasmids. For tetracycline-inducible expression of PAR1 and/or talin, cells were incubated with doxycycline (Sigma-Aldrich) for 24 h. PAC-1 binding and αIIbβ3 surface expression were determined by incubating cells with 0.4% PAC-1 ascites or 0.25% antibody D57, respectively, for 10 min at room temperature. To study agonist-induced αIIbβ3 activation in CHO cells expressing PAR1, the cells were incubated in duplicate or triplicate with PAR1 agonist peptide SFLLRN during the PAC-1 incubation phase. After washing the cells, AlexaFluor647-conjugated anti–mouse IgM (for PAC-1) or anti–mouse IgG (for D57) was added for 20 min at 4°C, and antibody binding to living (propidium iodide negative) cells was determined by flow cytometry using a dual-laser FACSCalibur and Cell Quest software (BD Biosciences; O'Toole et al., 1994). Cells transiently transfected with expression plasmids or transduced with shRNAs were typically identified and gated in the flow cytometer by their GFP or td-Tomato fluorescence. Specific PAC-1 binding was normalized for αIIbβ3 expression and defined as (sample PAC-1 binding) − (PAC-1 binding with 5 mM EDTA)/(sample D57 staining). Specific PAC-1 binding generally represented >85% of total binding. Surface expression of recombinant PAR1 or PAR4 was measured by flow cytometry using an antibody specific for the Flag epitope. Talin expression was assessed by flow cytometry after cell fixation, permeabilization, and intracellular staining with antibody 8d4 as described previously (Tadokoro et al., 2003).
Interactions between αIIbβ3 and talin in living cells
The chimeric proteins used for BiFC experiments and the rationale for these experiments are depicted in Fig. 1 A. BiFC Venus vectors were provided by C.-D. Hu (Purdue University, West Lafayette, IN; Shyu et al., 2006). A plasmid template encoding the C-terminal half of Venus (VC) was amplified by PCR to place appropriate restriction sites, and the PCR product was inserted in-frame at the 3′ end of αIIb in pcDNA3.1 (Invitrogen) to form αIIb-VC. The N-terminal half of Venus (VN) was amplified to place appropriate restriction sites, and the PCR product was inserted in-frame into the 5′ end of talin cDNA in pcDNA3.1 to form VN-talin. For inducible expression, VN-talin was subcloned into pcDNA4/TO. cDNAs encoding wild-type β3 and β3 (Δ724) were cloned into pcDNA3.1. VN-talin mutants (L325R and W359A) and the β3 (Y747A) mutant were created from wild-type cDNAs by mutagenesis (QuikChange Mutagenesis kit; QIAGEN). All coding sequences were confirmed by DNA sequencing. Plasmid encoding αIIb-VC was cotransfected with β3, β3 (Δ724), or β3 (Y747A) into CHO cells with Lipofectamine. 48 h later, cells were cultured in the presence of G418 (Invitrogen) for 2 wk. Clones stably expressing the recombinant integrin were obtained by single-cell FACS sorting using anti-αIIbβ3 antibody D57. For some experiments, cells capable of the inducible expression of VN-talin were obtained by transfection of stable αIIb-VCβ3–CHO cells with pcDNA6/TR (repressor plasmid) and pcDNA4/TO/VN-talin and selection with Zeocin and Blasticidin (Invitrogen) for 2 wk. Then, cells were incubated with or without 1 μg doxycycline for 24 h and selected by single-cell FACS sorting for BiFC fluorescence resulting from high coexpression of VN-talin and αIIb-VCβ3. BiFC experiments were typically conducted by flow cytometry to enable simultaneous measurement of PAC-1 binding.
Deconvolution microscopy
αIIb-VCβ3–CHO and αIIb-VCβ3 (Δ724) cells were cotransfected with VN-talin and Rap1a (V12) and, 48 h later, were allowed to spread for 1 h on fibrinogen-coated glass coverslips (100-μg/ml coating concentration). Washed human platelets were plated on fibrinogen-coated coverslips for up to 45 min. After washing with PBS, pH 7.4, adherent cells were fixed with 4% PFA in PBS, permeabilized with 0.2% Triton X-100 in PBS, and blocked with 10% goat serum in PBS. Cells were stained sequentially with antivinculin antibody and AlexaFluor548-conjugated secondary antibody. Images were captured at room temperature on a deconvolution microscope (DeltaVision; Applied Precision) using a charged-coupled device camera system (CoolSNAP HQ; Photometrics) attached to an inverted wide-field fluorescence microscope (Eclipse TE200; Nikon) equipped with 40× and 100× oil-immersion objectives (Nikon). Identical software settings were used for image acquisition of all samples in a given experiment, and images were deconvoluted with an iterative-constrained algorithm. Cell fluorescence intensity range was standardized using the Softworks Analysis Program (Applied Precision). Any further adjustments of color balance made with Photoshop CS3 software (Adobe) were applied to the entire image in a figure, and no nonlinear adjustments were made.
Western blotting
Cells were lysed with NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM NaF, 0.5 mM sodium vanadate, 12.5 μg/ml leupeptin, and Complete EDTA free [Roche]). After clarification, cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Membranes were blocked with 3% BSA in TBS with 0.1% Tween 20 and incubated for 1 h each with primary antibodies and IRDye 680– or IRDye 800CW–conjugated secondary antibodies (LI-COR Biotechnology). Antibody binding was detected with the Odyssey system (LI-COR Biotechnology). Band density was quantified on a Pro computer (Macintosh) with ImageJ 1.39m software (National Institutes of Health).
Fibrinogen binding to murine megakaryocytes
Mouse R1 embryonic stem cells were cultured with OP9 stromal cells under conditions that yield megakaryocytes capable of binding fibrinogen in response to platelet agonists (Eto et al., 2003). After5 d in culture, nonadherent hematopoietic progenitors were exposed for 16 h to lentivirus encoding RIAM shRNA or control shRNA and were cultured on a fresh OP9 layer for an additional 7 d as described previously (Eto et al., 2003) to induce megakaryocyte differentiation. Megakaryocytes were collected by sedimentation and incubated with biotin-fibrinogen and R-phycoerythrin–streptavidin (Invitrogen) in the presence or absence of platelet agonists for 30 min (Eto et al., 2003). Intermediate-sized and large megakaryocytes were gated in a flow cytometer on the basis of forward light scatter, and specific fibrinogen binding, which is defined as binding inhibitable with 5 mM EDTA, was measured (Shiraga et al., 1999; Eto et al., 2003).
Acknowledgments
We are grateful to Johannes Bos, Alan Hall, Cheng-Deng Hu, and Roger Tsien for reagents.
This work was supported by research grants HL56595 and HL57900 from the National Institutes of Health.
Abbreviations used in this paper: BiFC, bimolecular fluorescence complementation; IRES, internal ribosomal entry site; PAR, protease-activated receptor; RIAM, Rap1-GTP–interacting adaptor molecule; shRNA, short hairpin RNA; VASP, vasodilator-stimulated phosphoprotein.
References
- Bearer, E.L., J.M. Prakash, and Z. Li. 2002. Actin dynamics in platelets. Int. Rev. Cytol. 217:137–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagnolli, M.E., S.J. Locke, M.E. Hensler, P.F. Bray, and M.C. Beckerle. 1993. Talin distribution and phosphorylation in thrombin-activated platelets. J. Cell Sci. 106:1189–1199. [DOI] [PubMed] [Google Scholar]
- Bhatt, D.L., and E.J. Topol. 2003. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2:15–28. [DOI] [PubMed] [Google Scholar]
- Bos, J.L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123–128. [DOI] [PubMed] [Google Scholar]
- Bouaouina, M., Y. Lad, and D.A. Calderwood. 2008. The N-terminal domains of talin co-operate with the PTB-like domain to activate beta 1 and beta 3 integrins. J. Biol. Chem. 283:6118–6125. [DOI] [PubMed] [Google Scholar]
- Brass, L.F. 2004. Molecular basis for platelet activation. In Hematology: Basic Principles and Practice. R. Hoffman, E. Benz, S. Shattil, B. Furie, H. Cohen, L. Silberstein, and P. McGlave, editors. Churchill-Livingstone, New York. 1899–1914.
- Calderwood, D.A. 2004. Integrin activation. J. Cell Sci. 117:657–666. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A., R. Zent, R. Grant, D.J.G. Rees, R.O. Hynes, and M.H. Ginsberg. 1999. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274:28071–28074. [DOI] [PubMed] [Google Scholar]
- Chang, C., C.E. Adler, M. Krause, S.G. Clark, F.B. Gertler, M. Tessier-Lavigne, and C.I. Bargmann. 2006. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr. Biol. 16:854–862. [DOI] [PubMed] [Google Scholar]
- Chen, J., T.G. Diacovo, D.G. Grenache, S.A. Santoro, and M.M. Zutter. 2002. The α2 integrin subunit-deficient mouse—a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am. J. Pathol. 161:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.-P., I. Djaffar, D. Pidard, B. Steiner, A.-M. Cieutat, J.P. Caen, and J.-P. Rosa. 1992. Ser-752 → Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbß3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc. Natl. Acad. Sci. USA. 89:10169–10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., S.S. Smyth, S.M. Schoenwaelder, T.H. Fischer, and G.C. White II. 2005. Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Invest. 115:680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin, S.R. 2000. Thrombin signalling and protease-activated receptors. Nature. 407:258–264. [DOI] [PubMed] [Google Scholar]
- Critchley, D.R. 2004. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 32:831–836. [DOI] [PubMed] [Google Scholar]
- Critchley, D.R. 2005. Genetic, biochemical and structural approaches to talin function. Biochem. Soc. Trans. 33:1308–1312. [DOI] [PubMed] [Google Scholar]
- Crittenden, J.R., W. Bergmeier, Y. Zhang, C.L. Piffath, Y. Liang, D.D. Wagner, D.E. Housman, and A.M. Graybiel. 2004. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 10:982–986. [DOI] [PubMed] [Google Scholar]
- de Virgilio, M., W.B. Kiosses, and S.J. Shattil. 2004. Proximal, selective and dynamic interactions between integrin αIIbß3 and protein tyrosine kinases in living cells. J. Cell Biol. 165:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X.P., E.F. Plow, A.L. Frelinger III, T.E. O'Toole, J.C. Loftus, and M.H. Ginsberg. 1991. Ligands “activate” integrin αIIbß3 (platelet GPIIb-IIIa). Cell. 65:409–416. [DOI] [PubMed] [Google Scholar]
- Eto, K., R. Murphy, S.W. Kerrigan, A. Bertoni, H. Stuhlmann, T. Nakano, A.D. Leavitt, and S.J. Shattil. 2002. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc. Natl. Acad. Sci. USA. 99:12819–12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto, K., A.L. Leavitt, T. Nakano, and S.J. Shattil. 2003. Development and analysis of megakaryocytes from murine embryonic stem cells. In Methods in Enzymology. Differentiation of Embryonic Stem Cells. Vol. 365. P.M. Wassarman and G.M. Keller, editors. Academic Press, New York. 142–158. [DOI] [PubMed]
- Faruqi, T.R., E.J. Weiss, M.J. Shapiro, W. Huang, and S.R. Coughlin. 2000. Structure-function analysis of protease-activated receptor 4 tethered ligand peptides. Determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 275:19728–19734. [DOI] [PubMed] [Google Scholar]
- Garcia, A., S.P. Watson, R.A. Dwek, and N. Zitzmann. 2005. Applying proteomics technology to platelet research. Mass Spectrom. Rev. 24:918–930. [DOI] [PubMed] [Google Scholar]
- Ginsberg, M.H., A. Partridge, and S.J. Shattil. 2005. Integrin regulation. Curr. Opin. Cell Biol. 17:509–516. [DOI] [PubMed] [Google Scholar]
- Gnatenko, D.V., P.L. Perrotta, and W.F. Bahou. 2006. Proteomic approaches to dissect platelet function: half the story. Blood. 108:3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., C.J. Lim, N. Watanabe, A. Soriani, B. Ratnikov, D.A. Calderwood, W. Puzon-McLaughlin, E.M. Lafuente, V.A. Boussiotis, S.J. Shattil, and M.H. Ginsberg. 2006. Reconstructing and deconstructing agonist-induced activation of integrin αIIbß3. Curr. Biol. 16:1796–1806. [DOI] [PubMed] [Google Scholar]
- Hoxie, J.A., M. Ahuja, E. Belmonte, S. Pizarro, R. Parton, and L.F. Brass. 1993. Internalization and recycling of activated thrombin receptors. J. Biol. Chem. 268:13756–13763. [PubMed] [Google Scholar]
- Hynes, R.O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- Jackson, S.P. 2007. The growing complexity of platelet aggregation. Blood. 109:5087–5095. [DOI] [PubMed] [Google Scholar]
- Jung, S.M., and M. Moroi. 2001. Platelet collagen receptor integrin α2β1 activation involves differential participation of ADP-receptor subtypes P2Y1 and P2Y12 but not intracellular calcium change. Eur. J. Biochem. 268:3513–3522. [DOI] [PubMed] [Google Scholar]
- Kahn, M.L., Y.W. Zheng, W. Huang, V. Bigornia, D.W. Zeng, S. Moff, R.V. Farese Jr., C. Tam, and S.R. Coughlin. 1998. A dual thrombin receptor system for platelet activation. Nature. 394:690–694. [DOI] [PubMed] [Google Scholar]
- Kahn, M.L., M. Nakanishi-Matsui, M.J. Shapiro, H. Ishihara, and S.R. Coughlin. 1999. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 103:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola, T.K. 2008. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol. 85:431–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M., C.V. Carman, and T.A. Springer. 2003. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 301:1720–1725. [DOI] [PubMed] [Google Scholar]
- Knezevic, I., T.M. Leisner, and S.C.T. Lam. 1996. Direct binding of the platelet integrin αIIbß3 (GPIIb-IIIa) to talin - evidence that interaction is mediated through the cytoplasmic domains of both αIIb and β3. J. Biol. Chem. 271:16416–16421. [DOI] [PubMed] [Google Scholar]
- Krause, M., J.D. Leslie, M. Stewart, E.M. Lafuente, F. Valderrama, R. Jagannathan, G.A. Strasser, D.A. Rubinson, H. Liu, M. Way, et al. 2004. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell. 7:571–583. [DOI] [PubMed] [Google Scholar]
- Kuliopulos, A., L. Covic, S.K. Seeley, P.J. Sheridan, J. Helin, and C.E. Costello. 1999. Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry. 38:4572–4585. [DOI] [PubMed] [Google Scholar]
- Lafuente, E.M., A.A. van Puijenbroek, M. Krause, C.V. Carman, G.J. Freeman, A. Berezovskaya, E. Constantine, T.A. Springer, F.B. Gertler, and V.A. Boussiotis. 2004. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell. 7:585–595. [DOI] [PubMed] [Google Scholar]
- Leisner, T.M., W. Yuan, J.C. DeNofrio, J. Liu, and L.V. Parise. 2007. Tickling the tails: cytoplasmic domain proteins that regulate integrin αIIbß3 activation. Curr. Opin. Hematol. 14:255–261. [DOI] [PubMed] [Google Scholar]
- Leng, L., H. Kashiwagi, X.-D. Ren, and S.J. Shattil. 1998. RhoA and the function of platelet integrin αIIbß3. Blood. 91:4206–4215. [PubMed] [Google Scholar]
- Liu, S., D.A. Calderwood, and M.H. Ginsberg. 2000. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113:3563–3571. [DOI] [PubMed] [Google Scholar]
- Luo, B.H., C.V. Carman, and T.A. Springer. 2007. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y.Q., J. Yang, M.M. Pesho, O. Vinogradova, J. Qin, and E.F. Plow. 2006. Regulation of integrin αIIbß3 activation by distinct regions of its cytoplasmic tails. Biochemistry. 45:6656–6662. [DOI] [PubMed] [Google Scholar]
- Maguire, P.B., M. Foy, and D.J. Fitzgerald. 2005. Using proteomics to identify potential therapeutic targets in platelets. Biochem. Soc. Trans. 33:409–412. [DOI] [PubMed] [Google Scholar]
- Michelson, A.D. 2006. Evaluation of platelet function by flow cytometry. Pathophysiol. Haemost. Thromb. 35:67–82. [DOI] [PubMed] [Google Scholar]
- Michiels, J.J., Z. Berneman, W. Schroyens, P.J. Koudstaal, J. Lindemans, H.A. Neumann, and H.H. van Vliet. 2006. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets. 17:528–544. [DOI] [PubMed] [Google Scholar]
- Monkley, S.J., X.H. Zhou, S.J. Kinston, S.M. Giblett, L. Hemmings, H. Priddle, J.E. Brown, C.A. Pritchard, D.R. Critchley, and R. Fassler. 2000. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 219:560–574. [DOI] [PubMed] [Google Scholar]
- Moser, M., B. Nieswandt, S. Ussar, M. Pozgajova, and R. Fassler. 2008. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14:325–330. [DOI] [PubMed] [Google Scholar]
- Nachmias, V.T., and R. Golla. 1991. Vinculin in relation to stress fibers in spread platelets. Cell Motil. Cytoskeleton. 20:190–202. [DOI] [PubMed] [Google Scholar]
- Nieswandt, B., M. Moser, I. Pleines, D. Varga-Szabo, S. Monkley, D. Critchley, and R. Fassler. 2007. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 204:3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, T.E., Y. Katagiri, R.J. Faull, K. Peter, R. Tamura, V. Quaranta, J.C. Loftus, S.J. Shattil, and M.H. Ginsberg. 1994. Integrin cytoplasmic domains mediate inside-out signaling. J. Cell Biol. 124:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, M., A.J. Messent, J.D. Humphries, N.O. Deakin, and M.J. Humphries. 2008. Quantification of integrin receptor agonism by fluorescence lifetime imaging. J. Cell Sci. 121:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasvolsky, R., S.W. Feigelson, S.S. Kilic, A.J. Simon, G. Tal-Lapidot, V. Grabovsky, J.R. Crittenden, N. Amariglio, M. Safran, A.M. Graybiel, et al. 2007. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J. Exp. Med. 204:1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich, B.G., P. Fogelstrand, A.W. Partridge, N. Yousefi, A.J. Ablooglu, S.J. Shattil, and M.H. Ginsberg. 2007. a. The antithrombotic potential of selective blockade of talin-dependent integrin αIIbß3 (platelet GPIIb-IIIa) activation. J. Clin. Invest. 117:2250–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich, B.G., P. Marchese, Z.M. Ruggeri, S. Spiess, R.A. Weichert, F. Ye, R. Tiedt, R.C. Skoda, S.J. Monkley, D.R. Critchley, and M.H. Ginsberg. 2007. b. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 204:3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X.F., D.S. An, I.S. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA. 100:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A.K., G. Jalagadugula, and L. Sun. 2004. Inherited defects in platelet signaling mechanisms. Semin. Thromb. Hemost. 30:525–535. [DOI] [PubMed] [Google Scholar]
- Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. [DOI] [PubMed] [Google Scholar]
- Sambrano, G.R., E.J. Weiss, Y.W. Zheng, W. Huang, and S.R. Coughlin. 2001. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 413:74–78. [DOI] [PubMed] [Google Scholar]
- Shaner, N.C., R.E. Campbell, P.A. Steinbach, B.N. Giepmans, A.E. Palmer, and R.Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22:1567–1572. [DOI] [PubMed] [Google Scholar]
- Shattil, S.J., and P.J. Newman. 2004. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 104:1606–1615. [DOI] [PubMed] [Google Scholar]
- Shattil, S.J., J.A. Hoxie, M. Cunningham, and L.F. Brass. 1985. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J. Biol. Chem. 260:11107–11114. [PubMed] [Google Scholar]
- Shiraga, M., A. Ritchie, S. Aidoudi, V. Baron, D. Wilcox, G. White, B. Ybarrondo, G. Murphy, A. Leavitt, and S. Shattil. 1999. Primary megakaryocytes reveal a role for transcription factor NF-E2 in integrin αIIbß3 signaling. J. Cell Biol. 147:1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu, Y.J., H. Liu, X. Deng, and C.D. Hu. 2006. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques. 40:61–66. [DOI] [PubMed] [Google Scholar]
- Tadokoro, S., S.J. Shattil, K. Eto, V. Tai, R.C. Liddington, J.M. de Pereda, M.H. Ginsberg, and D.A. Calderwood. 2003. Talin binding to integrin β cytoplasmic tails: a final common step in integrin activation. Science. 302:103–106. [DOI] [PubMed] [Google Scholar]
- Vu, T.-K., D.T. Hung, V.I. Wheaton, and S.R. Coughlin. 1991. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 64:1057–1068. [DOI] [PubMed] [Google Scholar]
- Wang, R., S.J. Shattil, D.R. Ambruso, and P.J. Newman. 1997. Truncation of the cytoplasmic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αIIbß3 complex. J. Clin. Invest. 100:2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener, K.L., A.W. Partridge, J. Han, A.R. Pickford, R.C. Liddington, M.H. Ginsberg, and I.D. Campbell. 2007. Structural basis of integrin activation by talin. Cell. 128:171–182. [DOI] [PubMed] [Google Scholar]
- Yao, F., T. Svensjo, T. Winkler, M. Lu, C. Eriksson, and E. Eriksson. 1998. Tetracycline repressor, tetR, rather than the tetR mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene Ther. 9:1939–1950. [DOI] [PubMed] [Google Scholar]