Abstract
Background
Lower respiratory tract infection is a differentiating feature of children with poorly controlled asthma. Objective: Given the role of alveolar macrophages (AMs) in innate immunity, we hypothesized that AM phagocytosis might be impaired in poorly controlled asthma.
Methods
Bronchoalveolar lavage fluid AMs were isolated from 28 asthmatic children (moderate asthma, n = 12; severe asthma, n = 16), 10 nonasthmatic children with chronic cough treated with inhaled corticosteroids, and 10 healthy adult control subjects. AMs were stimulated with LPS and exposed to fluorescein isothiocyanate–conjugated Staphylococcus aureus for 2 hours. Phagocytosis was quantified by using a phagocytic index (PI) calculated from the percentage of phagocytic cells multiplied by the relative fluorescence (RFU) units of S aureus per cell. Apoptosis was determined from the percentage of cells positive for poly (adenosine diphosphate–ribose) polymerase.
Results
Phagocytosis as measured by using the unstimulated PI was decreased in subjects with poorly controlled asthma (healthy control subjects, 9330 ± 3992 RFU; chronic cough, 9042 ± 5976 RFU; moderate asthma, 4361 ± 2536 RFU; severe asthma, 3153 ± 1886 RFU; P < .001) and remained unchanged with LPS stimulation. Children with severe asthma also had increased AM apoptosis, both the unstimulated and LPS-simulated states (P < .001), which correlated with the PI.
Conclusions
AM function is compromised in children with poorly controlled asthma and is characterized by decreased phagocytosis and increased apoptosis.
Keywords: Asthma, children, innate immunity, alveolar macrophage, respiratory infection
Children with poorly controlled asthma remain symptomatic despite treatment with inhaled corticosteroids (ICSs).1 Symptoms are thought to result from persistent airway inflammation, with viral respiratory tract infection as a major trigger.2 In children atypical bacteria and viruses have been implicated in more than 50% of acute exacerbations and hospitalizations.2–4 Thus respiratory tract infection can be an important risk factor for poor symptom control. The mechanisms linking asthma and respiratory tract infection are not well defined.
Alveolar macrophages (AMs) are the primary airway cells responsible for innate immune defense, including phagocytosis of respiratory pathogens. Although other inflammatory disorders, such as chronic alcoholism5 and particulate matter inhalation,6 have been linked with AM dysfunction, AM function has not been extensively evaluated in asthma. Recently, increased AM activation and decreased phagocytosis of apoptotic cells were reported in asthmatic adults.7 Although that study provided insight into decreased clearance of eosinophils and neutrophils, no study to date has examined phagocytosis of infectious particles in asthmatic children. The purpose of this study was to characterize unstimulated and stimulated phagocytosis in children with moderate versus severe poorly controlled asthma.
METHODS
Sample
Children 5 to 17 years of age undergoing flexible bronchoscopy with bronchoalveolar lavage (BAL) for clinical indications were recruited from the outpatient pulmonary clinic at Emory University. Indications for bronchoscopy included (1) poor asthma control despite maximum ICS doses, (2) suspected aspiration, (3) suspected atypical infection, or (4) confirmation of habitual cough or vocal cord dysfunction. This study was approved by the Emory University Institutional Review Board. Informed consent and assent were obtained from all caregivers and their children, respectively.
Poorly controlled asthma was diagnosed by a pediatric pulmonologist and was defined by 12% or greater FEV1 reversibility after short-acting β2-agonist administration and daily asthma symptoms (minimum of 5 of 7 days) requiring bronchodilator use. Asthmatic children were further classified as having severe disease if they had a baseline FEV1 of less than 80% of predicted value and an asthma-related hospitalization within the previous year. All asthmatic subjects received ICSs for at least 8 weeks before bronchoscopy. Children with immunodeficiency or other pulmonary morbidities, such as cystic fibrosis or bronchopulmonary dysplasia, were excluded.
Control subjects for this study were recruited from 2 populations: (1) children with suspected habitual cough or vocal cord dysfunction undergoing clinical bronchoscopy for definitive diagnosis and (2) healthy nonsmoking adults undergoing bronchoscopy for research purposes. Children and adults serving as control subjects had no historical evidence of 12% or greater FEV1 reversibility8 and exhaled offline nitric oxide (FENO) concentrations of less than 10 ppb.9 Adult control subjects were asymptomatic and were not taking ICSs. By contrast, pediatric control subjects were symptomatic and were empirically treated with ICSs.
Procedures
Spirometry was performed with a portable spirometer (KoKo Legend; Ferraris, Louisville, Colo) according to American Thoracic Society criteria for reproducibility,10 and results were interpreted by using reference standards.11 FENO was collected with a reservoir bag at a fixed exhaled flow rate of 0.35 L/s12 and analyzed offline by means of chemiluminescence (Sievers NOATM 280-I; Ionic Instruments, Boulder, Colo).
Clinical bronchoscopy was performed in children by pediatric pulmonologists using a laryngeal airway mask (n = 37) or endotracheal tube (n = 3). All subjects received inhaled general anesthesia (fluothane or sevoflurane). BAL fluid was collected from the right middle lobe through three 10-mL saline lavages (37°C, pH 6.8–7.0) flushed through the suction channel of a flexible bronchoscope (Olympus BF-3C160 [3.7 mm] or BF-P160 [4.9 mm]; Olympus America, Inc, Melville, NY) by using hand pressure on a syringe. BAL fluid was collected and pooled under continuous low-pressure suction. Half of the return volume was used for this research.
Research bronchoscopy was performed in healthy adults by a pulmonary specialist with a flexible bronchoscope (Olympus BF-1T20D; Olympus American, Inc) passed transnasally into the right middle lobe. Subjects received intravenous sedation for the procedure (midazolam and fentanyl). Three 50-mL saline aliquots were instilled and immediately aspirated into 50-mL suction traps under continuous low-pressure suction. BAL fluid was pooled for analysis.
BAL fluid was centrifuged at 1200 rpm for 7 minutes for recovery of the cell pellet. Bacterial cultures and RT-PCR for Mycoplasma pneumoniae and Chlamydia pneumoniae were performed on the supernatant by a clinical microbiology laboratory (Children’s Healthcare of Atlanta, Atlanta, Ga). Manual cell counts were performed with a hemocytometer, and differentials were obtained from 300 consecutive cells after Diff-Quik staining (Andwin Scientific, Addison, Ill). The cell pellet was resuspended in 10 mL of 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 solution containing 2% FBS, L-glutamine, 15 mmol/L HEPES, penicillin (10,000 U), streptomycin (10,000 mg/mL), amphotericin (25 mg/mL), and gentamicin (4 μg/mL). AMs (100,000 cells) were added to glass-chamber slides containing 100 μL of medium and 20 μL of PBS and were assessed before and after the addition of 20 μL of 1 mg/mL LPS. AMs were incubated at 37°C with 10% CO2 for 15 hours, after which 10 × 105 particles of fluorescein isothiocyanate–conjugated inactivated Staphylococcus aureus (Invitrogen, Carlsbad, Calif) were added (10:1 ratio of S aureus/AMs) to the cultures and incubated for 2 hours. Cells were fixed with 3.7% paraformaldehyde and stored at 4°C until analysis.
Bacterial phagocytosis was assessed with an Olympus confocal microscope (model BX51; Olympus America, Inc) containing an argon/krypton laser. AMs were analyzed from 10 experimental fields per set by using quantitative digital fluorescence imaging software (Olympus FluoView 300, Version 4.3). Laser confocal microscopy was performed at 50% of the AM depth by using identical background and gain settings to ensure internalization of S aureus. AMs with bacterial inclusions at 50% of the cell depth, regardless of the number, were considered positive for phagocytosis. AMs without inclusions that had nonspecific binding of S aureus to the plasma membrane were considered positive for external binding. The remaining AMs were classified as unresponsive to S aureus particles. The relative fluorescence units (RFU) of S aureus were determined for each phagocytic cell at 50% of the cell depth to further quantify phagocytic internalization. A phagocytic index (PI) was calculated as follows:
.13
Apoptosis was determined by means of immunostaining for cleavage of poly (adenosine diphosphate–ribose) polymerase (PARP) by using a 1:100 dilution of PARP-1 primary antibody (Santa Cruz Biotechnology, Santa Cruz, Calif) and a 1:200 dilution of secondary antibody (TRITC F[ab′]2, Santa Cruz Biotechnology).5 Cleavage of PARP was assessed in AMs immediately after isolation from the BAL fluid and after 15 hours of culture with or without 20 μg/mL LPS. Cellular fluorescence was determined by means of quantitative digital analysis with Image-Pro Plus for Windows (Version 3.1; Media Cybernetics, Inc, Silver Spring, Md). Data were expressed as the percentage of AMs fluorescently positive from 10 experimental fields per set.
Data analysis
Data analysis was performed with SPSS software (Version 15; SPSS, Inc, Chicago, Ill). FENO measurements were logarithmically transformed. Kruskal-Wallis tests and Wilcoxon signed-rank tests were performed for all between-group and within-group comparisons, respectively. Pearson correlation coefficients were calculated between the unstimulated and LPS-stimulated PI and all subject features. Stepwise forward linear regression analysis was then performed with the PI (unstimulated and LPS stimulated) as the dependent variables and severity, age, history of asthma-related hospitalization, FEV1/forced vital capacity ratio, FENO (log value), ICS dose, and history of abnormal chest radiograph as predictors. Multicolinearity between predictors was assessed with tolerance statistics. Two-tailed tests were used for all analyses, with significance determined by using an α value of less than .05.
RESULTS
Thirty-four children with poorly controlled asthma (severe asthma, n = 18) were recruited for the study. Four children (severe asthma, n = 2) had BAL fluid colonization with Streptococcus pneumoniae, Moraxella catarrhalis, or both and were excluded from data analyses. Ten children with chronic cough treated with ICSs served as control subjects. All children in this grouphadbeen receiving ICSs for at least 16 weeks before bronchoscopy (median, 30 weeks; range, 16–52 weeks). Postbronchoscopy diagnoses in this control group included habitual cough (n = 5), gastroesophageal reflux aspiration (n = 3), and vocal cord dysfunction (n = 2). Given the symptomatic nature of this group, 10 nonsmoking healthy adults were also recruited for comparison. Adults in this control group were free of asthma symptoms and medication use; however, they were significantly older (Table I). Features of the groups appear in Table I.
TABLE I.
Baseline characterization
| Healthy control subjects, no ICS (n = 10) | Chronic cough, ICS (n = 10) | Moderate asthma, ICS (n = 14) | Severe asthma, ICS (n = 16) | |
|---|---|---|---|---|
| Age (y)* | 37 ± 7 | 11 ± 4 | 9 ± 4 | 10 ± 5 |
| Male (%) | 5 (50) | 5 (50) | 10 (71) | 9 (56) |
| Ethnicity | ||||
| White | 5 (50) | 10 (100) | 10 (71) | 8 (50) |
| African American§ | 5 (50) | 0 | 4 (29) | 8 (50) |
| Daily medications | ||||
| Budesonide† | 0 | 2 (20) | 5 (36) | 4 (25) |
| Fluticasone/salmeterol* | 0 | 7 (70) | 6 (43) | 11 (69) |
| Fluticasone | 0 | 0 | 2 (14) | 1 (6) |
| Beclomethasone | 0 | 1 (10) | 1 (7) | 0 |
| Montelukast* | 0 | 6 (60) | 10 (71) | 16 (100) |
| Prednisone|| | 0 | 0 | 0 | 7 (44) |
| Daily ICS dose*|| (μg fluticasone equivalents/d) | 0 | 440 ± 357 | 515 ± 289 | 890 ± 329 |
| Pulmonary function | ||||
| FVC (% predicted)|| | 96 ± 18 | 102 ± 10 | 105 ± 21 | 78 ± 14 |
| FEV1 (% predicted)|| | 104 ± 20 | 99 ± 6 | 100 ± 21 | 65 ± 12 |
| FEV1/FVC ratio|| | 0.90 ± 0.06 | 0.85 ± 0.05 | 0.86 ± 0.08 | 0.74 ± 0.13 |
| FEF25–75 (% predicted)|| | 115 ± 27 | 93 ± 14 | 90 ± 27 | 47 ± 26 |
| FEV1 reversibility (%)|| | 2 ± 4 | 3 ± 4 | 13 ± 11 | 23 ± 13 |
| FENO (offline, ppb)|| | 5 ± 2 | 7 ± 2 | 6 ± 4 | 16 ± 13 |
| Abnormal chest radiograph# | ||||
| Peribronchial thickening*‡ | 0 | 0 | 7 (50) | 5 (31) |
| Lobar consolidation†§ | 0 | 0 | 4 (29) | 4 (25) |
| Atelectasis† | 0 | 1 (10) | 4 (29) | 7 (44) |
| Hyperinflation†§ | 0 | 0 | 1 (7) | 4 (25) |
| Asthma-related hospitalization‡# | 0 | 0 | 4 (29) | 16 (100) |
Data represent the mean ±SD or frequency (percentage).
FVC, forced vital capacity; FEF25–75, forced expiratory flow.
P < .01, healthy control subjects versus other groups.
P < .05, healthy control subjects versus other groups.
P < .01, chronic cough versus other groups.
P < .05, chronic cough versus other groups.
P < .01, severe asthma versus other groups.
Within the previous 12 months.
Flexible bronchoscopy with BAL was well tolerated. Prolonged bronchospasm was observed in 1 child with severe asthma, which normalized with intraoperative albuterol and positive airway pressure. Postoperative cough was a common finding in more than 75% of participants and improved after airway clearance measures, albuterol administration, or both. There was no incidence of postoperative fever, and no participant required prolonged observation.
BAL fluid cellularity is presented in Table II. Cultures for fungus and viruses (adenovirus, influenza A/B, parainfluenza, and respiratory syncytial virus) were negative for all subjects. M pneumoniae and C pneumoniae were also undetectable by means of RT-PCR. Cellular composition was similar between groups, with AMs comprising at least 85% of the total cell count.
TABLE II.
BAL fluid composition
| Healthy control subjects (n = 10) | Chronic cough, ICS (n = 10) | Moderate asthma (n = 14) | Severe asthma (n = 16) | |
|---|---|---|---|---|
| BAL return (% of volume instilled) | 50 ± 16 | 41 ± 16 | 46 ± 11 | 38 ± 14 |
| BAL cell count (cells/mL, × 106)* | 8.66 ± 5.67 | 4.03 ± 2.42 | 4.16 ± 3.20 | 3.77 ± 2.47 |
| BAL cellularity (%) | ||||
| Macrophages/monocytes | 90.8 ± 3.8 | 91.5 ± 7.8 | 91.6 ± 4.2 | 88.5 ± 6.2 |
| Neutrophils | 3.0 ± 2.6 | 5.2 ± 6.2 | 4.7 ± 1.9 | 5.2 ± 4.5 |
| Eosinophils | 0.7 ± 0.5 | 0.4 ± 0.8 | 0.9 ± 1.6 | 1.9 ± 3.9 |
| Lymphocytes | 4.5 ± 2.2 | 2.6 ± 2.3 | 2.5 ± 2.7 | 4.2 ± 2.4 |
| Basophils | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.5 | 0.1 ± 0.3 |
| BAL total protein (μg/mL) | 138 ± 76 | 234 ± 155 | 224 ± 110 | 197 ± 55 |
Data represent the mean ±SD.
P < .01, healthy control subjects versus other groups.
Unstimulated phagocytosis
Children with poorly controlled asthma had impaired phagocytosis evidenced by fewer AMs with cytoplasmic S aureus inclusions at 50% of the AM depth (control, 88% ± 9%; chronic cough, 89% ± 10%; moderate asthma, 71% ± 11%; severe asthma, 58% ± 18%; P <.001). This was accompanied by decreased overall S aureus uptake as measured by the RFUs for each phagocytic cell (control, 9742 ± 4547 RFU/cell; chronic cough, 9789 ± 5947 RFU/cell; moderate asthma, 6235 ± 3820 RFU/cell; severe asthma, 5813 ± 3356 RFU/cell; P =.045). The resulting PI was also decreased in asthmatic children and was most impaired in children with severe asthma (Fig 1). External S aureus binding without internalization was increased in subjects with severe asthma compared with that seen in the other groups (Fig 2).
FIG 1.
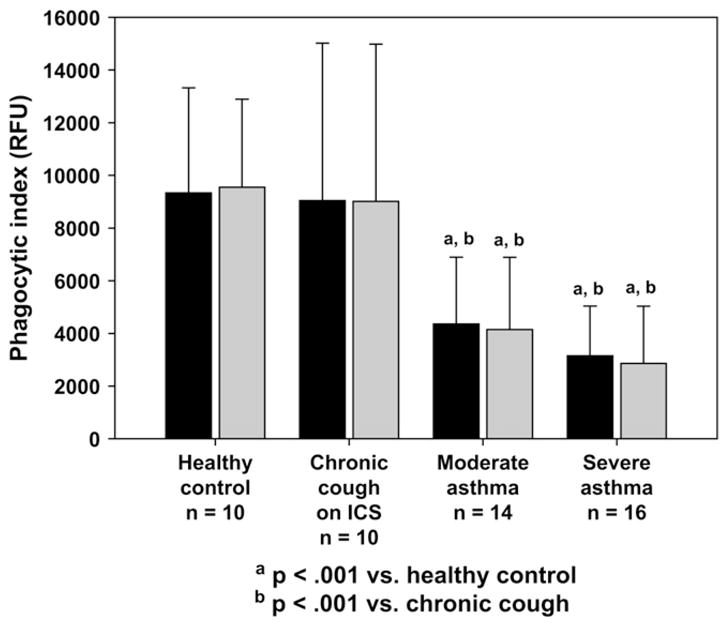
Percentage of AMs with bacterial inclusions.
FIG 2.
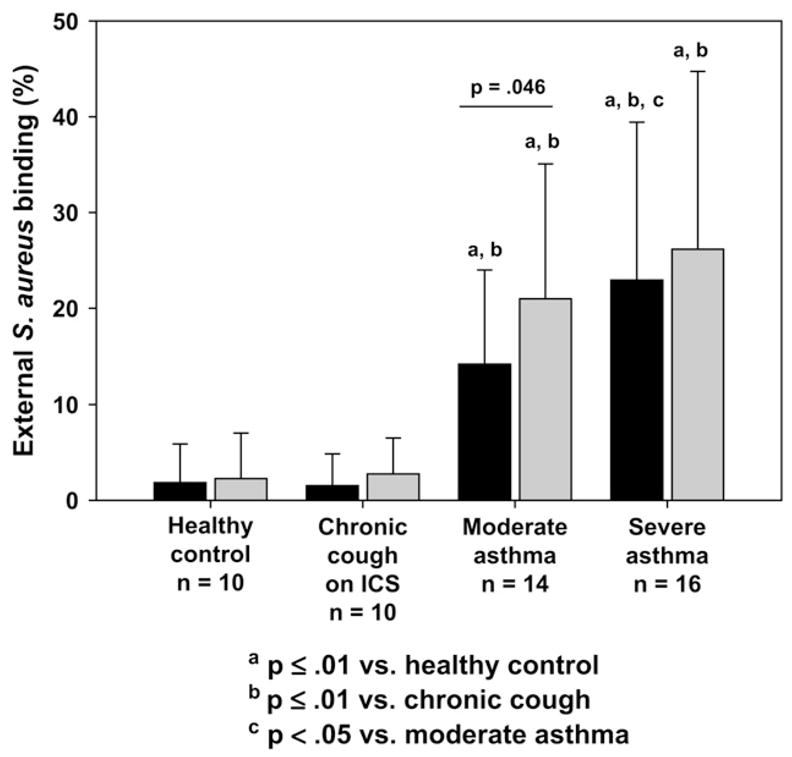
Percentage of AMs with nonspecific external S aureus binding (no internalization).
LPS-stimulated phagocytosis
With LPS stimulation, the PI remained lower in children with severe asthma (Fig 1). Whereas LPS stimulation did not alter phagocytosis in control subjects (percentage of phagocytosis for unstimulated vs LPS stimulated: control, 88% ± 9% vs 86% ± 10%, P = not significant; chronic cough, 89%± 10% vs 85%± 13%, P = not significant), it further decreased phagocytosis in subjects with poorly controlled asthma (moderate asthma, 71% ± 11% vs 65% ± 17%, P =.028; severe asthma, 58% ± 18% vs 48% ± 21%, P =.003). AMs from asthmatic subjects were also characterized by fewer S aureus RFUs than control subjects after LPS treatment (control, 10,583 ± 4116 RFU/cell; chronic cough, 10,022 ± 6068 RFU/cell; moderate asthma, 6550 ± 5273 RFU/cell; severe asthma, 6321 ± 4306 RFU/cell; P = .05), but these were not different from the unstimulated values (P = not significant). Although LPS increased the extent of nonspecific external binding of S aureus in subjects with moderate asthma (Fig 2), no differences were observed in the other groups with LPS stimulation. Representative images of AM phagocytosis are presented in Fig 3.
FIG 3.
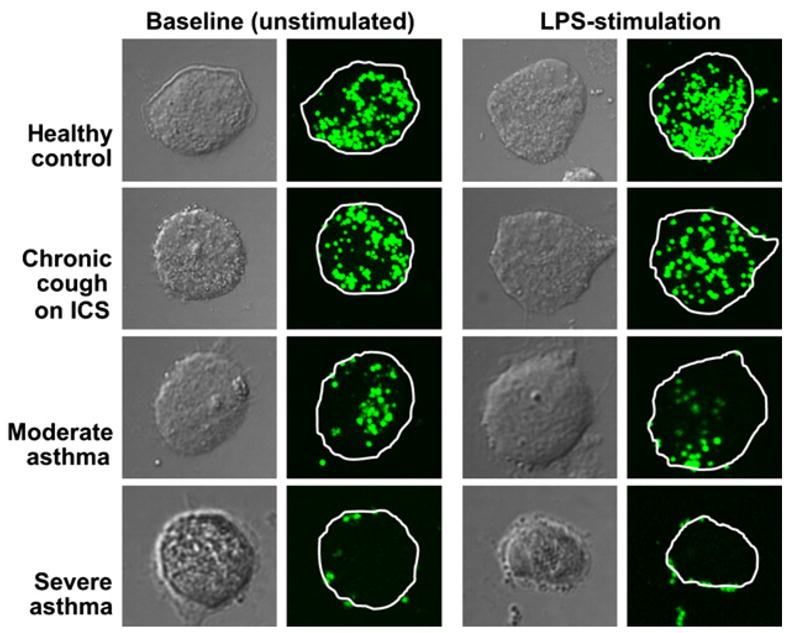
Representative cross-sectional confocal images of AMs at 50% of the cell depth depicting internalization of S aureus (green particles).
AM apoptosis
Compared with control subjects, subjects with severe asthma had greater AM apoptosis at isolation (severe asthma, 15% ± 6%; moderate asthma, 8% ± 4%; chronic cough, 7% ± 4%; control, 5% ± 3%; p = .05) and after culture with and without LPS (Fig 4). Apoptotic indices from freshly isolated and cultured AMs were associated (r = 0.55, P = .034), suggesting that the cell culture conditions were not sufficient to influence the results. In the combined sample apoptosis was correlated with phagocytosis in the unstimulated state (r = −20.45, P =.003) and after LPS stimulation (r = −0.43, P =.008; Fig E1) and further accounted for approximately 20% of the variance in phagocytosis (unstimulated R2 = 0.205; LPS stimulated R2 = 0.183). Isolation of the apoptotic AMs from each subject in the unstimulated state revealed that the majority of the apoptotic cells underwent phagocytosis regardless of severity classification (percentage phagocytic: control, 86% ± 16%; chronic cough, 89% ± 10%; moderate asthma, 83% ± 12%; severe asthma, 78% ± 9%; P = not significant). Furthermore, no differences in the ability of apoptotic cells to ingest S aureus were observed after LPS stimulation (percentage phagocytic: control, 84% ± 15%; chronic cough, 81% ± 8%; moderate asthma, 83% ± 12%; severe asthma, 81% ± 11%; P = not significant). Representative images of apoptotic AMs are provided in Fig E2.
FIG 4.
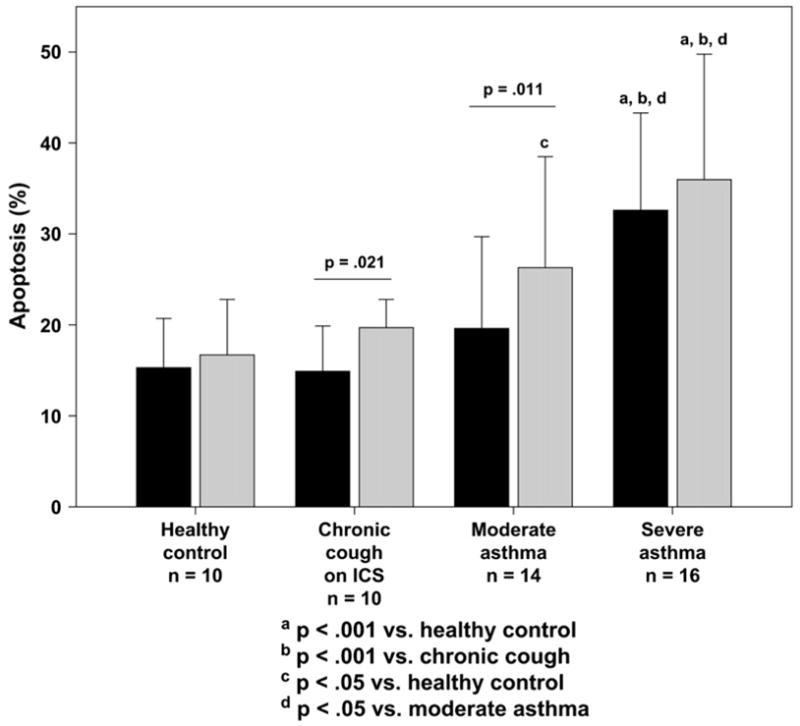
Percentage of apoptotic AMs, as indicated by positive staining for cleaved PARP.
FIG E1.
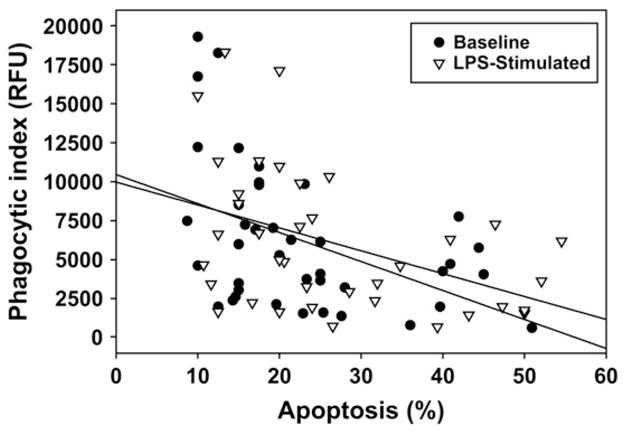
Scatterplot depicting the relationship between apoptosis and phagocytosis in the combined sample.
FIG E2.
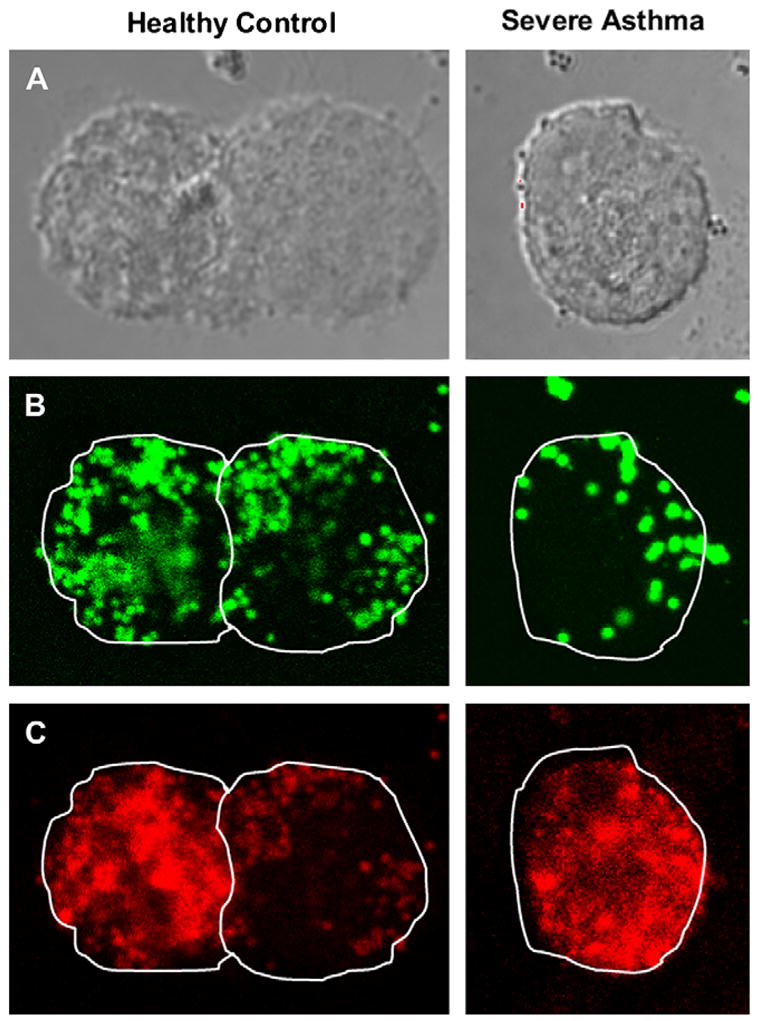
A-C, Representative cross-sectional confocal images of AMs from a child with severe asthma and a healthy adult control subject depicting phagocytosis of S aureus (green particles) despite cellular apoptosis (red staining). Images were taken at 50% of the cell depth.
Effect of corticosteroid treatment and other potential confounders
Given the clinical heterogeneity of children with severe asthma, a subanalysis was undertaken to determine whether corticosteroid treatment and other clinical features might account for impaired phagocytosis. We first compared subjects with severe asthma receiving daily oral corticosteroids and high-dose ICSs (n = 7) with subjects with severe asthma receiving high-dose ICSs alone (n = 9). Sex, ethnicity, pulmonary function, chest radiographic abnormalities, baseline FENO, and daily ICS dose (fluticasone equivalents) did not differ between groups, although subjects with severe asthma treated with oral corticosteroids were significantly older (median age, 15 vs 7 years; P <.001). Unstimulated phagocytosis and LPS-stimulated phagocytosis, as measured by the median PI, did not differ between groups (unstimulated: oral corticosteroids vs ICS only, 2991 vs 3044 RFU; LPS stimulated, 1981 vs 1767 RFU; P = not significant), although there was a trend toward decreased apoptosis in children treated with oral corticosteroids (unstimulated: oral corticosteroid vs ICS alone, 25% vs 40%, P =.05; LPS stimulated, 25% vs 45%, P =.05).
Stepwise forward linear regression analysis was performed by using the PI (unstimulated and LPS stimulated) as the dependent variable and severity (unstimulated: LPS stimulated, r = −0.58, −0.61), age (r = 0.31, 0.36), history of asthma-related hospitalization (r = −0.41, −0.47), FEV1/forced vital capacity ratio (r = 0.30, 0.35), log FENO (r = −0.36, −0.35), ICS dose (r = −0.41, −0.37), and history of abnormal chest radiograph (r = −0.43, −0.39) as predictors to control for the differing clinical features that might have influenced phagocytosis. Severity alone showed the most significant slope (unstimulated, P = .001; LPS stimulated, P =.001). Addition of the other predictors to the model was not statistically significant (Table E1), suggesting that cortico-steroid use and other clinical features could not sufficiently explain the impaired phagocytosis observed in asthmatic children.
TABLE E1.
Results of the stepwise forward linear regression of the PI in the unstimulated and stimulated states on selected clinical features
| Model | Regression coefficient | SE | t | P value |
|---|---|---|---|---|
| Unstimulated PI | ||||
| Included variables | ||||
| Constant | 9805.02 | 1216.33 | 8.061 | .000 |
| Severity* | − 2154.29 | 590.61 | − 3.648 | .001 |
| Excluded variables | ||||
| Age | − 0.37 | − 1.623 | .117 | |
| Hospitalization | 0.04 | 0.154 | .879 | |
| FEV1/FVC ratio | − 0.03 | − 0.126 | .901 | |
| Log FENO | − 0.19 | − 1.078 | .291 | |
| Daily ICS dose | − 0.14 | − 0.462 | .648 | |
| Abnormal chest radiograph | − 0.01 | − 0.029 | .977 | |
| LPS-stimulated phagocytosis | ||||
| Included variables | ||||
| Constant | 9956.42 | 1272.54 | 7.824 | .000 |
| Severity† | − 2365.03 | 605.08 | − 3.909 | .001 |
| Excluded variables | ||||
| Age | − 0.14 | − 0.609 | .548 | |
| Hospitalization | − 0.03 | − 0.091 | .929 | |
| FEV1/FVC ratio | 0.08 | 0.410 | .685 | |
| Log FENO | − 0.17 | − 0.871 | .393 | |
| Daily ICS dose | − 0.21 | − 0.718 | .480 | |
| Abnormal chest radiograph | 0.25 | 0.705 | .488 | |
FVC, Forced vital capacity.
Sum of squares for severity = 1.9 × 108/3.8 × 108, R2 = 0.330; 95% CI for the regression coefficient = − 3366 to − 942.
Sum of squares for severity = 2.1 × 108/3.3 × 108, R2 = 0.389; 95% CI for the regression coefficient = − 3614 to − 1116.
DISCUSSION
This is the first study to demonstrate impairment of AM phagocytosis in children with poorly controlled asthma. In subjects with moderate and severe asthma, phagocytosis was decreased by more than 50% compared with that seen in adult and pediatric control subjects. AM apoptosis was also greater in asthmatic subjects and increased further with LPS stimulation. These data suggest that the airway innate immune response might be impaired in children with poorly controlled asthma, a finding that might account for the aberrant response to respiratory tract infection commonly observed in this population.14,15
AMs internalize foreign airway particles through a variety of mechanisms, including pinocytosis, receptor-mediated endocytosis, and phagocytosis. Whereas pinocytosis refers to the nonspecific uptake of fluid and solutes, receptor-mediated endocytosis is a specific process in which small particles (typically <0.5 μm) enter cells. In the presence of larger particles (>0.5 μm), phagocytosis occurs either through opsonization of the antigen or unopsonized nonspecific uptake.16 In this study phagocytosis was assessed by adding inactivated S aureus (particle size, 0.8–1.1 μm) to AMs in culture media containing 2% FBS. Therefore our results do not permit conclusion as to the specific type of phagocytosis observed. Our findings might reflect impairment in both opsonized and unopsonized phagocytosis in children with poorly controlled asthma, although further studies are needed to define the specific factors responsible for our observations.
AM phagocytosis is a complex process triggered by a variety of activation pathways.17 Here we focused on phagocytosis resulting from innate activation of the AM after a bacterial microbial stimulus (S aureus). With innate activation, microbes are recognized by pattern-recognition receptors, which induce proinflammatory cytokine production and promote phagocytosis.17 Although it is true that the majority of asthma exacerbations in young children and older school-age children are triggered by respiratory tract viruses and not by acute bacterial infections,2,18 innate AM activation is also important for respiratory viral clearance. Huber et al19 recently observed phagocytosis of influenza virus resulting from direct binding of the opsonized virus to Fc scavenger receptors on the AM surface. Furthermore, adenovirus and respiratory syncytial virus infection result in increased AM Fcγ scavenger receptor expression, suggesting that opsonization and phagocytic engulfment are important for viral clearance.20,21 Direct binding of rhinovirus to the AM cell surface has also been observed,22 although the associated receptors and mechanisms of engulfment remain unclear.22,23 Recently, a 3-fold increase in AM expression of the pattern-recognition Toll-like receptor 4 was observed in rhinovirus-infected children,24 which might contribute to AM engulfment of the virus. Although these studies highlight the importance of innate AM activation in respiratory tract virus clearance, the precise mechanisms involved with this process are far from understood and can vary between viral strains. Further studies of AM phagocytosis as it relates to viral respiratory tract infection in asthmatic children are needed.
Our findings of decreased AM phagocytosis in children with poorly controlled asthma are similar to those previously observed in other chronic airway disorders. In patients with cystic fibrosis25 and chronic obstructive pulmonary disease,26 phagocytosis of bacteria and apoptotic cells is reduced to half of the levels of healthy control subjects. In patients with chronic obstructive pulmonary disease, ex vivo treatment with broad anti-inflammatory agents, such as lovastatin and azithromycin, improves phagocytic activity by 50% or more.27,28 Although the precise mechanisms responsible for AM dysfunction can vary between disease states, these findings suggest that underlying inflammation might have an important effect on AM function in the human airway.
There are a limited number of studies on AM phagocytosis in patients with asthma, and few have targeted patients with poor asthma control. In one study phagocytosis did not differ between control subjects and subjects with mild intermittent asthma with good symptom control, although the total number of opsonized particles was lower in asthmatic subjects with airway eosinophilia compared with those without.29 In a similar sample the number of opsonized particles in airway macrophages was reduced by approximately 50% six hours after endotoxin (LPS) inhalation.30 These data suggest that even despite good symptom control, asthmatic subjects might be more susceptible to a secondary airway insult. This effect is more pronounced in those with severe disease. Recently, Huynh et al7 observed no phagocytic differences between adults with mild-to-moderate asthma and control subjects, but phagocytosis was impaired by nearly 50% in subjects with severe asthma. LPS stimulation further decreased phagocytosis in subjects with severe asthma, a finding that was reversed with ex vivo dexamethasone treatment.7 These data suggest that asthmatic AMs might be functionally modulated by airway inflammation, thus rendering the asthmatic patient more susceptible to a secondary airway insult. However, the focus of that study was to determine whether asthma compromised phagocytosis of apoptotic cells, a process critical for the clearance of neutrophils or eosin-ophils recruited to the airspace. In contrast, the goal of the present study was to assess phagocytosis in response to innate immune activation of the AM and clearance of infectious particles.
Although few studies have examined the capacity of asthmatic AMs to phagocytose foreign particles, previous studies have observed increased activation of AMs from asthmatic patients. Compared with control subjects, asthmatic subjects have increased basal spontaneous generation of superoxide anion,31 proinflammatory cytokines,32 and regulators of inflammatory gene expression, such as histone acetyltransferase.33 These alterations are further increased with antigen stimulation34 and are accompanied by decreased production of anti-inflammatory cytokines, such as IL-10.35 Taken together, these findings provide evidence that the respiratory burst of asthmatic AMs might be impaired, thus inhibiting microbe killing. However, it is important to note that the AM respiratory burst and phagocytosis are regulated by different cellular mechanisms and might not necessarily occur in parallel.35 Further study of the dynamic relationships between the respiratory burst and phagocytosis is warranted in asthmatic subjects to better define the mechanisms associated with respiratory infection in this population.
This study has a number of limitations. Because bronchoscopy cannot be performed on otherwise healthy children solely for research purposes, our pediatric control group was limited to children with symptomatic respiratory tract illnesses undergoing bronchoscopy for diagnostic purposes. Although this sample was sufficient to detect differences in AM phagocytosis and apoptosis between groups, the AMs isolated from these children might be phenotypically different from those of true pediatric control subjects. The fact that our healthy adult control subjects were significantly older than our pediatric sample also raises the question as to whether age is a determinant of AM function across the lifespan. Because AM function was similar between our adult and pediatric control subjects, it is unlikely that age contributed to our findings, particularly because age was treated as a potential confounder in multivariate analyses. However, additional studies are needed to more adequately address the effect of age on AM function in children.
Because pediatric bronchoscopies were not performed for research purposes, BAL samples from children were pooled before analysis. This practice is common at our institution and provides an increased sample yield for clinical laboratory analysis. Because pooling of the BAL fluid intermixes the bronchial and alveolar airway constituents, it is possible that the macrophages obtained from children for this study were not purely alveolar (or bronchial) in origin. It is also possible that bronchial macrophages and AMs have distinct functional abilities. The BAL samples from adult control subjects were similarly pooled to minimize this potential effect on our results. However, given the differences between adults and children with regard to airway structure and the lavage volumes used, we cannot exclude the possibility that the samples from healthy adults contained more alveolar cells. Further studies are needed to characterize the differences between the bronchial and alveolar cellular constituents, particularly in asthmatic subjects.
It is also possible the differences in AM function that we observed could be attributed to the confounding effects of asthma treatment or other unmeasured clinical variables. Although the effects of unmeasured clinical variables remain unknown, we do not believe that corticosteroid use sufficiently explains the discrepancies in AM function between asthmatic children and control subjects. Because our pediatric control subjects were symptomatic, all children in this group were treated empirically with ICSs for a minimum of 16 weeks, yet AM phagocytosis in this group was similar to that of healthy adult control subjects. A subgroup analysis of children with severe asthma receiving oral corticosteroids further revealed no differences in AM function compared with subjects with severe asthma treated with ICSs alone. Finally, statistical control of ICS dose and other potential confounding variables revealed that asthma severity alone, as defined by this study, was the most significant predictor of AM function. Although this evidence suggests a limited association between corticosteroid use and AM function, additional studies are needed to thoroughly examine this relationship in children with poorly controlled asthma.
Our findings of impaired AM phagocytosis and increased AM apoptosis warrant further study. Although apoptosis correlated with phagocytosis, the association was modest and did not sufficiently account for the differences in phagocytosis observed between groups. Furthermore, despite increased apoptosis in children with severe asthma, the apoptotic cells isolated from these children were not less likely to undergo phagocytosis compared with the other groups. These data suggest that other factors aside from apoptosis are related to the impairment in AM function that we observed.
In conclusion, the mechanisms associated with respiratory tact infection in asthmatic children are not well understood. These data support the hypothesis that AMs are functionally impaired in children with poorly controlled asthma and are characterized by increased apoptosis and decreased phagocytosis of pathogenic bacteria. Although there are limited studies on the epidemiology of bacterial infection in asthma, a recent study found that asthma was a risk factor for invasive pneumococcal disease.36 These findings, when taken into account with those demonstrating an increased prevalence of atypical mycobacteria in subjects with acute asthma,3 suggest that bacterial infection might play a role in asthma morbidity. However, the relationship between bacterial and respiratory tract viral infection in children with asthma is not clear. Further studies are needed to define how impairments in AM phagocytosis relate to bacterial phagocytosis and viral clearance in patients with poorly controlled asthma.
Acknowledgments
Supported with funds from National Institutes of Health (NIH)/National Institute of Nursing Research KO1 NR010548, NIH/National Center for Research Resources K12 RR017643 and KL2 RR025009, and NIH/National Heart, Lung, and Blood Institute SARP RO1 HL69170.
Abbreviations used
- AM
Alveolar macrophage
- BAL
Bronchoalveolar lavage
- FENO
Fraction of exhaled nitric oxide
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- PARP
Poly (adenosine diphosphate–ribose) polymerase
- PI
Phagocytic index
- RFU
Relative fluorescence unit
Footnotes
Disclosure of potential conflict of interest: W. G. Teague is on the speakers’ bureau for Merck. The rest of the authors have declared that they have no conflict of interest.
Clinical implications: These findings might account for the increased severity of lower respiratory tract infections in children with poorly controlled asthma.
References
- 1.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito S, Blasi F, Arosio C, Fioravanti L, Fagetti L, Droghetti R, et al. Importance of Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–6. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 4.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier TW, Ping X-D, Harris FL, Wong M, Elbahesh H, Brown LAS. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr Res. 2005;57:76–81. doi: 10.1203/01.PDR.0000149108.44152.D3. [DOI] [PubMed] [Google Scholar]
- 6.Yin XJ, Dong CC, Ma JYC, Antonini JM, Roberts JR, Stanley CF, et al. Suppression of cell-mediated immune responses to Listeria infection by repeated exposure to diesel exhaust particles in brown Norway rats. Toxicol Sci. 2004;77:263–71. doi: 10.1093/toxsci/kfh035. [DOI] [PubMed] [Google Scholar]
- 7.Huynh M-LN, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–9. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Lung function testing: selection of reference values and interpretive strategies. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 9.Jones SL, Kittleson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164:738–43. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society and the European Respiratory Society. ATS/ERS recommendations for standardizes procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 13.Berclaz P-Y, Zsengeller Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, et al. Endocytic internalization of adenovirus, nonspecific phagocytosis, and cytoskeletal organization are coordinately regulated in alveolar macrophages by GM-CSF and PU.1. J Immunol. 2002;169:6332–42. doi: 10.4049/jimmunol.169.11.6332. [DOI] [PubMed] [Google Scholar]
- 14.Corne JM, Marshall C, Smith S, Schreibner J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–4. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 15.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 16.Alderen A, Underhill DM. Mechanisms of phagocytosis in macrophages. Ann Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S. Alternative activation of macrophages. Nature Rev. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 18.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 20.Berclaz P-Y, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU. 1, regulates alveolar macrophage FcγR-mediated phagocytosis and the IL-18/IFN-γ-mediated molecular connection between innate and adaptive immunity. Blood. 2002;100:4193–200. doi: 10.1182/blood-2002-04-1102. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero-Plata A, Ortega E, Gomez B. Persistence of respiratory syncytial virus in macrophages alters phagocytosis and pro-inflammatory cytokine production. Viral Immunol. 2001;14:19–30. doi: 10.1089/08828240151061347. [DOI] [PubMed] [Google Scholar]
- 22.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, et al. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–7. [PubMed] [Google Scholar]
- 23.Laza-Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF-κB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–58. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grissell TV, Chang AB, Gibson PG. Reduced toll-like receptor 4 and substance P gene expression is associated with airway bacterial colonization in children. Pediatr Pulmonol. 2007;42:380–5. doi: 10.1002/ppul.20592. [DOI] [PubMed] [Google Scholar]
- 25.Vandivier RW, Fadok VA, Hoffman PR, Bratton DL, Penvari C, Brown KK, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–70. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–84. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–65. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 28.Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PM. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J. 2006;28:486–95. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 29.Alexis NE, Soukup J, Nierkens S, Becker S. Association between airway hyperre-activity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol. 2001;280:L369–75. doi: 10.1152/ajplung.2001.280.2.L369. [DOI] [PubMed] [Google Scholar]
- 30.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112:353–61. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 31.Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–6. [PubMed] [Google Scholar]
- 32.John H, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony-stimulating factor, and interferon-γ release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–62. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 33.Cosio B, Mann B, Ito K, Jazrawi E, Barnes PJ, Chung KF, et al. Histone acetylase and deacetylase activity in alveolar macrophages and blood monocytes in asthma. Am J Respir Crit Care Med. 2004;170:141–7. doi: 10.1164/rccm.200305-659OC. [DOI] [PubMed] [Google Scholar]
- 34.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–25. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 35.Iles KE, Forman HJ. Macrophage signaling and respiratory burst. Immunol Res. 2002;26:95–105. doi: 10.1385/IR:26:1-3:095. [DOI] [PubMed] [Google Scholar]
- 36.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–90. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]


