Abstract
A team of physicians, pharmacists, and informatics professionals developed a CDSS added to a commercial electronic medical record system to provide prescribers with patient-specific maximum dosing recommendations based on renal function. We tracked the time spent by team members and used US national averages of relevant hourly wages to estimate costs. The team required 924.5 hours and $48,668.57 in estimated costs to develop 94 alerts for 62 drugs. The most time intensive phase of the project was preparing the contents of the CDSS (482.25 hours, $27,455.61). Physicians were the team members with the highest time commitment (414.25 hours, $25,902.04). Estimates under alternative scenarios found lower total cost estimates with the existence of a valid renal dosing database ($34,200.71) or an existing decision support add-on for renal dosing ($23,694.51). Development of a CDSS for a commercial computerized prescriber order entry system requires extensive commitment of personnel, particularly among clinical staff.
Introduction
Renal insufficiency has an impact on the elimination of renally excreted drugs, leading to drug accumulation and the potential for serious adverse events. For patients with renal insufficiency, maximum dosing recommendations for many drugs should be based on assessment of the current level of renal function. This problem is particularly prevalent in nursing homes, where nearly half of residents have been found to have substantial levels of renal impairment. 1 In a previous study we found that nursing home residents are taking, on average, 9 regularly scheduled medications per day. 2 This combination of high prevalence of renal impairment and exposure to multiple medications places nursing home residents at high risk for medication-related problems.
Patient-specific dosing required for adults with renal impairment has been found to be a challenging aspect of medication prescribing in many settings. Several hospital-based studies have succeeded in improving prescribing for these patients through the use of computerized clinical decision support systems (CDSS) incorporated in computerized prescriber order entry systems (CPOE). 3,4
Many of the assessments of successful CDSS are based on locally developed systems designed to support CDSS. 3,5 The success of these experiments in improving the safety of medication use has inspired many healthcare systems to consider adding this tool to their commercially purchased CPOE systems. 6 However, the impact on staff time and the potential costs of developing CDSS in this situation have not been clear. 7
We developed and implemented a CDSS to provide prescribers with recommended maximum doses of 62 drugs for patients with renal insufficiency in the long-term care setting. The CDSS was built on a commercially purchased CPOE system. As we developed the CDSS we tracked the process and the time involvement of all participants, as well as any external costs.
Methods
This study was conducted in the long-stay units of a large, academically-affiliated long-term care facility in Canada with four years of experience using CPOE that incorporated a basic level CDSS. The system was a Meditech electronic medical record based on the MAGIC platform with CPOE using Provider Order Management (POM 4.9). To increase the likelihood that physicians personally entered medication orders using the CPOE system, the facility had added wireless capabilities and the option for physicians to access the system from their off-site offices and homes. 8,9 Within a randomized trial of the potential impact of advanced CDSS on the quality of prescribing and monitoring medications for long-term care residents, we developed a renal dosing CDSS that is representative of computerized programs for support of patient-specific dosing, including multiple types of alerts based on calculation of combinations of patient characteristics. As we developed and implemented this CDSS, we performed a sub-study estimating the time and costs involved. The study was approved by the institutional review boards of the University of Massachusetts Medical School and the participating facility.
CDSS and the Underlying Software
Not all commercially available CPOE systems are designed to support the elements required to deliver advanced decision support. 10,11,12 A guide developed by the early developers of successful CDSS described the components recommended for an advanced CDSS to support medication prescribing: quick system responsiveness, the capacity to provide information to clinicians when they need it, integration of suggestions with actual practice, avoidance of requests to users that they obtain and enter additional information, making it easy to do the right thing, provision of alternative actions, and a focus on aspects of the care process that clinicians are most willing to change. 13 In the case of medication dosing based on renal function, including these components requires that the underlying system and the CPOE software be prompt in responding to clinician requests, able to interact with locally written programming code to allow assessment of medication orders in real time, allow calculation of total daily dose of medication ordered, include linkages to electronic sources of lab test results and patients' weights in real time for programmed calculations, include the capacity to show specifically designed alerts to prescribers during the medication ordering process based on results of these calculations, support insertion of information from lab results into alerts, and be capable of identifying and acting on missing information. There also appear to be substantial advantages for CDSS in which alerts include revised medication orders that prescribers can generate simply by clicking on the alert.
The electronic medical record and CPOE software for which we developed the CDSS included most of these recommended capabilities, but was not able to present alerts from which prescribers could directly submit medication orders and did not calculate total daily doses automatically.
Process of Developing and Implementing the Renal Dosing Alerts
The CDSS was developed by a team that included physicians, pharmacists, informatics professionals, project coordinators, and a health services researcher. The physicians and pharmacists selected drugs for inclusion by reviewing published guidelines 14,15 and lists from previous hospital-based renal dosing alert systems 3 with updates for newer medications and/or recent evidence. The focus was on drugs primarily eliminated by the kidney with known potential nephrotoxic effects. We limited the review to oral drugs commonly prescribed in the long-term care setting. The resulting list was then compared to frequency of use of these therapies within the facility and the potential severity of the adverse effects. Final selection was based on team consensus and included 62 medications (▶). Decisions on dosing recommendations were based on specific recommendations for dose adjustment in geriatric and psychotropic drug dosing handbooks 14,15 and the MicroMedex® online knowledge base. We also took into consideration the availability on formulary of specific dosages and potential problems in splitting some drug dose forms. Where recommended frequency was 18 to 24 hours, we rounded to 24 hours.
Table 1.
Table 1 Medications Included in the Renal Dosing CDSS
| Acarbose | Acyclovir | Allopurinol |
| Amantadine | Amoxicillin | Amoxicillin/clavulanate |
| Ampicillin | Cefaclor | Cefprozil |
| Cefuroxime | Cephalexim | Cetirizine |
| Chloroquine | Chlorpropamide | Ciprofloxacin |
| Clarithromycin | Clodronate | Colchicine |
| Co-trimoxazole | Diclofenac | Digoxin |
| Erythromycin | Famciclovir | Famotidine |
| Fenofibrate | Fluconazole | Gabapentin |
| Gatifloxacin | Glyburide | Ibuprofen |
| Indomethacin | Ketoprofen | Levofloxacin |
| Lithium | Loratidine | Meloxicam |
| Memantine | Metformin | Methenamine |
| Methotrexate | Methyldopa | Metoclopramide |
| Metronidazole | Naproxen | Nitrofurantoin |
| Nizatidine | Norfloxacin | Oxaprozin |
| Penicillamine | Penicillin VK | Pentoxifylline |
| Piroxicam | Pramipexole | Primidone |
| Ranitidine | Rifampin | Sulfinpyrazone |
| Sulindac | Tetracycline | Topiramate |
| Trimethoprim | Venlafaxine |
Type and wording of alerts was determined by the team with subsequent review by the facility's Pharmacy and Therapeutics Committee. Four types of alerts were developed for various levels of creatinine clearance for residents with impaired renal function: 1) alerts recommending maximum total daily dose; 2) alerts recommending maximum frequency of administration; 3) alerts recommending that the medication be avoided; and 4) alerts notifying prescribers that no creatinine clearance could be calculated for this resident (due to missing creatinine test results or weight.) Examples of wording are provided in ▶. ▶ Ultimately, 94 alerts were developed within these categories.
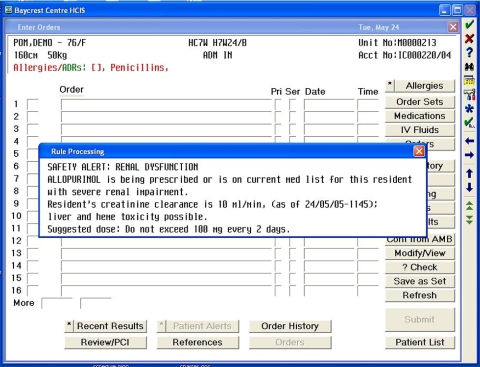
Figure 1.
Screen shot of an alert that will display if the physician orders allopurinol for a resident with severe renal impairment.
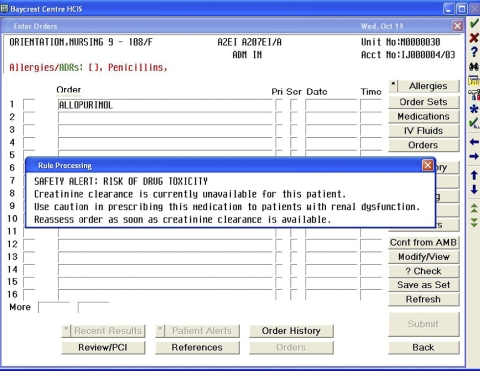
Figure 2.
Screen shot of an alert that will display if the physician orders any of the 62 included drugs when creatinine clearance is not available. This occurs if data for either a serum creatinine lab test or weight for a resident are missing from the electronic medical record.
Based on these decisions, the project coordinators prepared a “blueprint” for each alert that included scenario, alert message, mnemonics for all drugs and range of creatinine clearance that would trigger that alert. To identify mnemonics for all strengths of each included drug, pharmacists reviewed the facility's formulary and medication usage history. The facility's pharmacists had previously developed an underlying calculation of creatinine clearance using the Cockcroft-Gault equation based on age, weight, sex and serum creatinine 16 and this calculation had already been programmed within the CPOE system. Pre-testing proceeded with the selection of four prototypical drugs which were programmed and fully tested off-line with a cycle of test and revision of programming until all problems were eliminated. The remaining alerts were then programmed and tested off-line. A message was sent to prescribers to inform them of the new CDSS messages before the alerts were transferred to the live system. The facility had a history of including alerts within their CPOE system so training requirements were minimal and few user problems were encountered. The CDSS also included programming that outputs audit trails of all alerts. This component enables on-going tracking of the usage and impact of the system.
Tracking Costs and Personnel Time
Because the alerts were added to an existing CPOE system within an electronic medical record that included laboratory test results and nursing notes, no additional hardware or software were required. Costs for developing and implementing the system resulted entirely from personnel time. Six categories of personnel were required: physician, pharmacist, informatics project manager, project coordinator, health services researcher, and specialized computer programmer. The programmer was external to the facility and was paid hourly as a consultant; estimates of the time and costs of programming are based on tracking of submitted bills. Time tracking for the remaining personnel is based on weekly reports that required participants to specifically categorize the time spent on the project. Optional categories are provided in ▶. For analyses, we collapsed categories into: project management, preparation of the contents of the CDSS, preparation of blueprints and instructions for the programmer, programming, and testing and implementing. For this report, we include data collection through 2 weeks following the go live date and do not include personnel time for on-going maintenance and upgrades.
Table 2.
Table 2 Project Activities
| Project Management |
| Identifying staff and introducing them to the project |
| Scheduling and managing related conference calls, meetings |
| Travel time |
| Meetings and conference calls with management and department heads |
| Designing process and procedures for developing CDSS components |
| Preparation of the contents of the CDSS |
| Thinking about and drafting preliminary descriptions of potential drug issues for inclusion |
| Reviewing proposed drug issues for feasibility |
| Constructing and reviewing rules for inclusion in the CDSS |
| Meetings and conference calls related to rule development and wording of alerts |
| Informatics Project Management (this collapsed category also included all activities of the informatics project manager) |
| Reviewing the current CPOE system and making decisions about up-grades and other changes |
| Preparation of blueprints and instructions for the programmer |
| Meetings and conference calls related to work on programming and CPOE system issues |
| Designing computer programming for CDSS implementation |
| Designing the audit trail for tracking usage of the CDSS and developing programming specifications |
| Extracting information from the CPOE system for developing programming (e.g., drug utilization, drug mnemonics) |
| Programming |
| Programming the CDSS |
| Testing and implementing |
| Testing the CDSS |
| Reviewing the CDSS with groups of users |
| Training users |
Cost Analysis
Our goal was to produce cost estimates that would be of use to clinicians considering development of CDSS within their own facilities. Therefore, we did not collect facility-specific costs for this project, such as actual wages of the participants, fringe benefits or overhead costs. Rather, we based estimates on the reported hours for each individual combined with US national average hourly wages for their personnel category, obtained from the Bureau of Labor Statistics, National Compensation Survey (▶). 17 Costs for the specialized computer programmer were the exception to this approach; they are based on the actual billed hours converted from Canadian to US currency. We produced summary tables by personnel categories as well as activity categories.
Table 3.
Table 3 Hourly Wages Used for Cost Estimates
| Personnel Categories | Hourly Wage | Comments |
|---|---|---|
| Physician | $62.52 | |
| Master's level Pharmacist | $44.23 | |
| Baccalaureate level Pharmacist | $30.36 | Based on average of pharmacist ($44.23) and technician ($16.49) |
| Informatics Project Manager | $40.98 | Based on wages for managers and administrators, not elsewhere classified |
| Health Services Researcher | $28.60 | Based on wages for biological or life scientist/medical scientist |
| Baccalaureate level Project Coordination | $16.49 | Based on wages for health technologists and technicians |
| Computer Programmer | $30.89 | |
| Specialized Computer Programmer (Meditech) | $79.76 | Actual cost |
Several aspects of the project were likely to produce large costs, including the need to develop the contents of the CDSS and the use of a specialized and expensive computer programmer. To support estimates of the reduction in costs that might be attained with variations in these factors, we developed a series of alternative scenarios:
1 Availability of a pre-existing and updated database with recommended dosing for drugs according to level of renal impairment assessed by creatinine clearance and appropriate for frail elderly patients18,19,20
2 Availability of an off-the-shelf renal dosing program compatible with the CPOE system
3 Use of a CPOE system that is programmable by a less specialized programmer
The physicians and project coordinators estimated the reductions in hours for each category of personnel that would result from each of these scenarios and we estimated alternative total and activity category costs using these reduced estimates.
Results
The total estimate of costs for personnel involved in the production of the renal dosing CDSS is $48,178.11. The total time spent on the project across all personnel types (presented in ▶) was 924.5 hours with physicians providing nearly half of that time. The three participating physicians spent the majority of their project time (390 hours) preparing the content of the CDSS. The two pharmacists contributed 179.75 hours. Seventy-nine percent of their time was split between participating in the preparation of the content of the CDSS and performing extensive testing of each alert. The informatics project manager contributed nearly 122 hours. Her activities included managing interactions between the project and the Information Management department, selecting and overseeing the activities of the specialized computer programmer, and coordinating and supporting the process of testing and implementing the alerts. She also participated in all project meetings throughout the development process. Over the course of the project, several project coordinators participated. They attended all project meetings, maintained and distributed agendas and meeting minutes and handled communication flow among the various participants. They also prepared the alert blueprints under the direction of the health services researcher who also designed the audit trail system to allow on-going evaluation of the impact of the alerts. The project required a computer programmer with extensive expertise in programming within the Meditech electronic medical record system. The total programming time was 110.5 hours.
Table 4.
Table 4 Personnel Time and Estimated Costs
| Personnel Category | Hours | Cost ($) | % of Total Time |
|---|---|---|---|
| Master's level Pharmacist | 120 | 5,307.60 | 13 |
| Baccalaureate level Pharmacist | 59.75 | 1,814.01 | 6 |
| Physician | 414.25 | 25,902.04 | 45 |
| Project Coordinator | 79.75 | 1,315.08 | 9 |
| Health Services Researcher | 18.5 | 529.10 | 2 |
| Informatics Project Manager | 121.75 | 4,987.27 | 13 |
| Specialized Computer Programmer | 110.5 | 8,813.48 | 12 |
| Total | 924.5 | 48,668.57 |
▶ presents the estimated costs for personnel time across the collapsed categories of project activities. Fifty-six percent of the costs were associated with preparation of the contents of the CDSS. This reflects the extensive time required from physicians and pharmacists. Because the alerts were designed to guide dosing decisions, the process of selecting the drugs and deciding on the combinations of renal impairment and dose recommendations was painstakingly thorough, including reviews of geriatric dosing guidelines and the dosing recommendations used in hospital-based CDSS. The personnel time for physicians also includes meetings with the facility's Pharmacy and Therapeutics and Medical Advisory Committees. Eighteen percent of the project's costs were for programming. The other project activities accounted for the remaining 26% of project costs.
Table 5.
Table 5 Costs of Activities
| Activity Category | Hours | Cost ($) | % of Total Cost |
|---|---|---|---|
| Project management | 80.25 | 2,220.17 | 5 |
| Preparing contents of the CDSS | 482.25 | 27,455.61 | 56 |
| Informatics project management | 121.7 | 4,987.27 | 10 |
| Preparing blueprints and instructions for programmer | 50.8 | 1,869.95 | 4 |
| Programming | 110.5 | 8,813.48 | 18 |
| Testing and implementing | 79.0 | 3,322.09 | 7 |
| Total | 924.5 | 48,668.57 |
The first alternative scenario was constructed to estimate the reduction in costs that would be attained if a standard database existed with recommended drug dosing for frail elderly patients with renal impairment based on the best evidence. We estimate a substantial reduction in the costs for developing the content of the CDSS of 50% and an accompanying 33% reduction in project management time (▶). Estimated reductions are limited by the need for a facility's physicians to carefully review and weigh a database's recommendations before enacting them within the CDSS. 18,19,21 Nevertheless, the total estimated cost is lowered by approximately 30% to $34,200.71 (▶).
Table 6.
Table 6 Costs for Alternative Scenarios
| Activity | Scenario 1 Renal Dosing Database Exists |
Scenario 2 CDSS Product Exists |
Scenario 3 CPOE System Does Not Require Special Programmer |
|||
|---|---|---|---|---|---|---|
| Hours | Cost ($) | Hours | Cost ($) | Hours | Cost ($) | |
| Project management | 53.5 | 1,480 | 53.5 | 1,480 | 80.25 | 2,220 |
| Preparing contents of the CDSS | 241.2 | 13,728 | 241.2 | 13,728 | 482.25 | 27,456 |
| Informatics project management | 121.7 | 4,987 | 60.9 | 2,494 | 121.7 | 4,987 |
| Preparing blueprints and instructions for progammer | 50.8 | 1,870 | 12.7 | 467 | 50.8 | 1,870 |
| Programming | 110.5 | 8,813 | 27.63 | 2,203 | 110.5 | 3,413 |
| Testing and implementing | 79.0 | 3,322 | 79.0 | 3,322 | 79.0 | 3,322 |
| Total | 656.7 | 34,201 | 474.93 | 23,695 | 924.5 | 43,268 |
The second alternative (▶) scenario further reduces costs to $23,694.51 by positing the existence of a CDSS renal dosing product compatible with the CPOE system. We estimate the same reductions in costs for developing the content of the system and managing the project as for the first scenario. Additional reductions include half of informatics project management time, and three-quarters of the time required for programming and preparing instructions for the programmer. If the CDSS product was truly “plug and play”, there could be further reductions in programming and informatics management time.
The third scenario produced a more modest reduction to $43,268.44 by assuming a CPOE system that did not require specialized programming skills. Estimates for this scenario do not reduce the hours involved in any of the activities but reduce the hourly cost for programming by using the average hourly wage for computer programmers in the United States in 2005 of $30.89.
Discussion
During development of a computerized clinical decision support system for renal dosing, we tracked 924.5 hours of personnel time at an estimated total cost of $48,668.57. We developed the CDSS for application in a long-term care setting and included 62 drugs and 94 different alerts. Other healthcare settings may involve a larger number of drugs and patient conditions of special concern. For example, in the hospital setting the CDSS would include intravenous drugs requiring extensive additional design time. In the ambulatory setting, the CDSS would need to be expanded to take into account additional patient-specific factors. We intend our experience to provide a baseline for considering the personnel time and costs that may be involved in developing CDSS. For facilities considering such a development, a particularly noteworthy finding was the many hours of physician and pharmacist time required. In most clinical settings, extensive involvement of these clinicians would place a heavy burden on the on-going functions of the facility. It is also clear that such a project would be difficult to undertake without many hours of time from an in-house informatics specialist with knowledge of the facility's electronic medical record and CPOE systems as well as an understanding of specific elements of their local implementation.
The goal of implementing a CDSS is to lower the rate of adverse events among patients and this will reduce the costs associated with treating adverse events. That cost reduction will offset a portion of the initial costs of implementing the system. Costs for treating adverse drug events have been estimated in the hospita 22–26 and ambulatory settings 27 but we have found no comparable evidence from long-term care. In all of these settings the savings only partly accrue to the providers who pay for the development and implementation of the CDSS. This is a particular issue in the ambulatory and long-term care setting where the most serious and costly adverse events involve hospitalizations, which are usually the responsibility of other payers. Thus, prediction of the extent to which savings downstream will offset the initial costs is a complex undertaking.
Several of the alternative scenarios that we posited led to substantially lower cost and time estimates. Because over half of the time and costs were devoted to preparing the contents of the CDSS, the existence of an off-the-shelf CDSS product appropriate to the patient population had the largest impact on the costs, leading to an estimated total of $23,694.51. However, even in this scenario our team estimated 241 hours of preparation time that would be spent reviewing and validating the CDSS rules, assessing the appropriateness of the alerts, and evaluating the system's acceptability to affiliated physicians and pharmacists. Commercial entities developing CDSS assume some legal responsibility for the use of their products so they often include an excessive number of alerts. 18,19 The display of excess alerts and those perceived by prescribers as unnecessary or irrelevant have been found to lower the response of prescribers 28 so successful implementations usually include review by local clinicians and decisions to de-activate some alerts. Commercial CDSS products are also likely to be directed at broad patient populations so their rules and alerts will require careful local review and editing. Protection of the safety of patients requires complete testing of the alerts, with substantial time needed from pharmacists and possibly physicians. Thus, this scenario would reduce costs but the time required of clinicians would continue to burden the facility.
These estimates are based wholly on tracked personnel time combined with average U.S. hourly wages for categories of personnel. To allow these estimates to be of maximum use for sites considering parallel development, we did not include any costs specific to the facility or its personnel. Inclusion of overhead costs would add considerable expense. Cost estimates for personnel would differ by the level and experience of the staff members involved in the project. We also began the project within a fully implemented electronic medical record and CPOE system and a facility with a previous history of developing and using prescribing alerts. Thus, very little time was required for training and supporting users. Even for sites with an existing CPOE system in place, instituting a CDSS application for users with no previous experience would require substantial training.
Our time tracking process included only the development and initial implementation of the renal dosing CDSS. There are likely to be substantial additional costs for maintaining the system over time. For example, six months after implementation there was a major upgrade to the underlying CPOE software that replaced components that the CDSS used. The alerts ceased appearing. Extensive time was required to trouble-shoot the problem, communicate with the vendor, and re-program the CDSS rules to function within the revised software. In addition, we project future changes to both the facility's formulary and the evidence that underlies our decisions about the CDSS content, both of which will require edits to the system. New clinicians will be added to the facility who will require training and support. As we continue to track utilization and its impact on prescribing behavior, we expect to find areas requiring revision. We also hope that future upgrades to the software will allow us to develop further enhancements to the CDSS. Each of these are important aspects of long-term maintenance that will add to the overall costs of developing and using CDSS, with cost and time implications that may surpass the initial outlay.
As the implementation of CPOE systems spreads through hospital, out-patient and long-term care settings, the opportunity to add clinical decision support to the prescribing process is considered one of the major advantages. 12 Currently, implementation of a CDSS that is truly responsive to the needs of a facility's patients and the practice style of its clinical staff appears to require individual site-specific adaptation. The estimates of time and cost found in our study should provide some guidance to plans for such development. The significantly lower estimated costs for implementation of a pre-constructed CDSS suggests one advantage for this option, although we project extensive time involvement from clinicians even for this alternative. The widespread use of CDSS may depend on the development of CDSS products that are based on the best evidence for specific patient populations and are compatible with a variety of popular CPOE software.
Footnotes
Supported by grants from the Agency for Healthcare Research and Quality (HS010481 and HS15430).
References
- 1.Papaioannou A, Ray JG, Ferko NC, Clarke JA, Campbell G, Adachi JD. Estimation of creatinine clearance in elderly persons in long-term care facilities Am J Med 2001;11:569-573. [DOI] [PubMed] [Google Scholar]
- 2.Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, et al. The incidence of adverse drug events in two large academic long-term care facilities Am J Med 2005;118:251-258. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, et al. Guided medication dosing for inpatients with renal insufficiency JAMA 2001;286:2839-2844. [DOI] [PubMed] [Google Scholar]
- 4.Galanter WL, Didominico RJ, Polikaitis A. A trial of automated decision support alerts for contraindicated medications using computerized physician order entry J Am Med Inform Assoc 2005;12:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review Arch Intern Med 2003;163:1409-1416. [DOI] [PubMed] [Google Scholar]
- 6.The Leapfrog Group Factsheet: Computer physician order entryhttp://www.leapfroggroup.org/media/file/Leapfrog-Computer_Physician_Order_Entry_Fact_Sheet.pdf 2003. accessed June 26, 20007.
- 7.Kuperman GJ, Gibson RF. Computer physician order entry: benefits, costs, and issues Ann Intern Med 2003;139:31-39. [DOI] [PubMed] [Google Scholar]
- 8.Rochon P, Field TS, Bates DW, Lee M, Gavendo L, Erramuspe-Mainard J, et al. Computerized physician order entry with clinical decision support in the long-term care setting: Insights from the Baycrest Center for Geriatric Care J Am Geriatric Soc 2005;53:1780-1789. [DOI] [PubMed] [Google Scholar]
- 9.Rochon PA, Field TS, Bates DW, Lee M, Gavendo L, Erramuspe-Mainard J, et al. Clinical application of a computerized system for physician order entry with clinical decision support to prevent adverse drug events in long-term care CMAJ 2006;174:52-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell DS, Marken RS, Meili RC, Wang CJ, Rosen M, Brooke RH, RAND Electronic Prescribing Expert Advisory Panel Recommendations for comparing electronic prescribing systems: results of an expert consensus process Health Affairs 2004;W4:305-317. [DOI] [PubMed] [Google Scholar]
- 11.Wang CJ, Marken RS, Meili RC, Straus JB, Landman AB, Bell DS. Functional characteristics of commercial ambulatory electronic prescribing systems: a field study J Am Med Inform Assoc 2005;12:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teich JM, Osheroff JA, Pifer EEA, Sittig DF, Jenders RA, Expert Review Panel Clinical decision support in electronic prescribing: recommendations and an action plan J Am Med Inform Assoc 2005;12:365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality J Am Med Inform Assoc 2003;10:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aronoff GR, Berns JS, Brier ME. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults4th Edition. Amer College of Physicians; 1999.
- 15.Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs 16th Spi Rev edCambridge, MA: Hogrefe & Huber Publishing; 2006.
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine Nephron 1976;16:31-41. [DOI] [PubMed] [Google Scholar]
- 17.National Compensation Survey Occupational Wages in the United States, June 2005 Supplementary TablesWashington, DC: U.S. Department of Labor; 2006. July.
- 18.Reichley RM, Seaton TL, Resetar E, Micek ST, Scott KL, Fraser VJ, et al. Implementing a commercial rule base as a medication order safety net J Am Med inform Assoc 2005;12:383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuperman GJ, Reichley RM, Bailey TC. Using commercial knowledge bases for clinical decision support: opportunities, hurdles, and recommendations J Am Med Inform Assoc 2006;13:369-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, et al. Medication-related clinical decision support in computerized provider order entry systems: a review J Am Med Inform Assoc 2007;14:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller RA, Gardner RM. Summary recommendations for responsible monitoring and regulation of clinical software systems Ann Intern Med 1997;127:842-845. [DOI] [PubMed] [Google Scholar]
- 22.Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM. Costs of adverse events in intensive care units Crit Care Med 2007. epub ahead of print. [DOI] [PubMed]
- 23.Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay Ann Pharmacother 2007;41:400-406. [DOI] [PubMed] [Google Scholar]
- 24.Senst BL, Achusim LE, Genest RP, Cosentino LA, Ford CC, Little JA, et al. Practical approach to determining costs and frequency of adverse drug events in a health care network Am J Health Syst Pharm 2001;58:1126-1132. [DOI] [PubMed] [Google Scholar]
- 25.Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients JAMA 1997;277:207-311. [PubMed] [Google Scholar]
- 26.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277:301-306. [PubMed] [Google Scholar]
- 27.Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting Med Care 2005;43:1171-1176. [DOI] [PubMed] [Google Scholar]
- 28.Judge J, Field TS, DeFlorio M, LaPrino J, Auger J, Rochon P, Bates DW, Gurwitz JH. Prescribers' responses to alerts during medication ordering in the long term care setting JAMIA 2006;13:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]