Abstract
We examined the role of glycogen synthase kinase-3β (GSK-3β) inhibition in airway smooth muscle hypertrophy, a structural change found in patients with severe asthma. LiCl, SB216763, and specific small interfering RNA (siRNA) against GSK-3β, each of which inhibit GSK-3β activity or expression, increased human bronchial smooth muscle cell size, protein synthesis, and expression of the contractile proteins α-smooth muscle actin, myosin light chain kinase, smooth muscle myosin heavy chain, and SM22. Similar results were obtained following treatment of cells with cardiotrophin (CT)-1, a member of the interleukin-6 superfamily, and transforming growth factor (TGF)-β, a proasthmatic cytokine. GSK-3β inhibition increased mRNA expression of α-actin and transactivation of nuclear factors of activated T cells and serum response factor. siRNA against eukaryotic translation initiation factor 2Bε (eIF2Bε) attenuated LiCl- and SB216763-induced protein synthesis and expression of α-actin and SM22, indicating that eIF2B is required for GSK-3β-mediated airway smooth muscle hypertrophy. eIF2Bε siRNA also blocked CT-1- but not TGF-β-induced protein synthesis. Infection of human bronchial smooth muscle cells with pMSCV GSK-3β-A9, a retroviral vector encoding a constitutively active, nonphosphorylatable GSK-3β, blocked protein synthesis and α-actin expression induced by LiCl, SB216763, and CT-1 but not TGF-β. Finally, lungs from ovalbumin-sensitized and -challenged mice demonstrated increased α-actin and CT-1 mRNA expression, and airway myocytes isolated from ovalbumin-treated mice showed increased cell size and GSK-3β phosphorylation. These data suggest that inhibition of the GSK-3β/eIF2Bε translational control pathway contributes to airway smooth muscle hypertrophy in vitro and in vivo. On the other hand, TGF-β-induced hypertrophy does not depend on GSK-3β/eIF2B signaling.
Increased smooth muscle mass is the most prominent pathologic change observed in the airways of patients with asthma. Clinical studies examining the underlying cellular mechanism are limited but suggest that both hypertrophy (1, 2) and hyperplasia (1, 3) play a role. Despite evidence that smooth muscle hypertrophy contributes to airway remodeling in asthma, little is known about the biochemical mechanisms regulating this process.
Glycogen synthase kinase (GSK)-3β2 is a serine/threonine kinase that is constitutively active in unstimulated cells and becomes inactivated upon phosphorylation at Ser9 (4). The serine/threonine kinase Akt is the major GSK-3β kinase, but others exist, including mitogen-activated protein kinase kinase 1 (5) and protein kinase A (6). GSK-3β activity is also inhibited by Wingless/Wnt signaling, independently of phosphorylation at serine 9 (7). Accumulated evidence suggests that GSK-3β negatively regulates cardiac (8-11) and skeletal muscle (12, 13) hypertrophy. The mechanisms underlying GSK-3β-mediated inhibition of hypertrophy are not completely understood. GSK-3β negatively regulates transcription factors involved in muscle-specific gene expression, including nuclear factors of activated T cells (NFAT), GATA4, and β-catenin (9, 11, 12, 14-16). Phosphorylation of the GTPase-activating protein tuberous sclerosis complex-2 by GSK-3β increases the ability of tuberous sclerosis complex-2 to inhibit mammalian target of rapamycin signaling (17). Other downstream targets of GSK-3β regulate muscle hypertrophy via the translational process. One of the critical steps controlling the initiation of protein translation is formation of the 43 S preinitiation complex. Eukaryotic initiation factor-2 (eIF2), a multimer consisting of α, β, and γ subunits, functions to recruit methionyl-tRNA and conduct it as a tRNA-eIF2-GTP ternary complex to the 40 S ribosomal subunit. eIF2 GTP loading is determined by the activity of eIF2B, a guanine nucleotide exchange factor. eIF2Bε Ser539 phosphorylation by GSK-3β inhibits its GDP/GTP exchange activity, thereby limiting binding of methionyl-tRNA to the 40 S ribosomal subunit. However, phosphorylation of GSK-3β by Akt inactivates it, leading to eIF2B dephosphorylation and activation and a general enhancement of translation initiation (18, 19). Accordingly, overexpression of a nonphosphorylatable eIF2Bε increases cardiac myocyte cell size and abolishes the antihypertrophic effect of GSK-3β (19).
The role of GSK-3β in smooth muscle hypertrophy has not been studied. Unlike cardiac myocytes, which withdraw from the cell cycle early in development, smooth muscle cells may proliferate or hypertrophy, depending on the stimulus. A number of peptide growth factors and bronchoconstrictor agonists have been shown to induce airway smooth muscle proliferation in vitro (20). More recently, cardiotrophin (CT)-1, a member of the IL-6 superfamily present in human lungs, has been shown to induce protein synthesis and cell enlargement, but not DNA synthesis, in cultured human bronchial smooth muscle cells (21) and guinea pig airway explants (22). In addition, we showed that transforming growth factor (TGF)-β, a proasthmatic cytokine (23-26), increased human bronchial smooth muscle cell size, protein synthesis, expression of α-smooth muscle actin and smooth muscle myosin heavy chain (smMHC), formation of actomyosin filaments, and cell shortening to acetylcholine (27). Further, TGF-β induced the phosphorylation of eIF-4E-binding protein (4E-BP), and inhibitors of 4E-BP phosphorylation blocked TGF-β-induced α-actin expression and cell enlargement, suggesting that eIF4E-, cap-dependent translation is necessary for TGF-β-induced hypertrophy.
In this report, we investigate the contribution of the GSK-3β translational control pathway to airway smooth muscle hypertrophy. We found that inhibition of GSK-3β is sufficient for human airway smooth hypertrophy and that inhibition of GSK-3β/eIF2B signaling is required for CT-1- but not TGF-β-induced hypertrophy. Finally, we provide evidence for airway smooth muscle GSK-3β phosphorylation in a mouse model of asthma.
EXPERIMENTAL PROCEDURES
Cell Culture—Primary human airway smooth muscle cells were isolated by enzymatic digestion from lung donor tissue unsuitable for transplantation (from Julian Solway, University of Chicago). This protocol was approved by the relevant institutional review boards. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and penicillin/streptomycin. Cells were seeded on uncoated plastic culture plates at ∼50% confluence. Prior to experiments, cells were serum-deprived for 24 h. Cells were treated with LiCl (10 mm), SB216763 (50 nm), CT-1 (10 ng/ml), or TGF-β (10 ng/ml) for 6 days. Fresh medium and chemicals were added 48 h after initial treatment. Experiments were performed in the absence of serum. Finally, for selected experiments, A7R5 rat vascular smooth muscle cells (American Type Culture Collection, Manassas, VA) were studied.
Immunoblotting—Human bronchial smooth muscle cell lysates were matched for protein concentration, resolved by SDS-PAGE, and transferred to nitrocellulose or polyvinylidene difluoride membrane. Membranes were blocked in 5% milk for 1 h and probed with mouse anti-α-smooth muscle actin (Calbiochem), mouse anti-myosin light chain kinase (MLCK) (Sigma), mouse anti-SM-22 (Abcam, Cambridge, MA), mouse anti-smMHC (Sigma), rabbit anti-extracellular signal regulated kinase (ERK) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), rabbit anti-phospho-Ser9 GSK-3β, rabbit anti-GSK-3β, rabbit anti-eIF2Bε (each from Cell Signaling, Danvers, MA), or rabbit anti-phospho-Ser539 eIF2Bε (Biosource, Camarillo, CA). Antibody binding was detected with a peroxidase-conjugated anti-rabbit or anti-mouse IgG and chemiluminescence.
In Vitro GSK-3β Kinase Assay—GSK-3β activity was measured by immunoprecipitating cell lysates with mouse anti-GSK-3β (clone GSK-4B; Sigma) and incubating immunoprecipitates with the GSK-3β substrate Tau (1 μg/μl; Sigma), ATP (1 mm), and [γ-32P]ATP (10 μCi) for 30 min at 30 °C, as described (28). Because immunoprecipitation of human airway smooth muscle cell lysates brought down a contaminating phosphorylated protein of similar molecular weight to the substrate, A7R5 vascular smooth muscle cells were used for these experiments. Cells were treated with LiCl, SB216763, CT-1, or TGF-β for 6 days. Cells were then washed with ice-cold PBS and then lysed. Reaction mixtures were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to film. Total GSK-3β in the immunoprecipitates was examined using rabbit anti-GSK-3β (Cell Signaling).
Transfection of GSK-3β and eIF2Bε siRNA—21-bp duplexes of either GSK-3β siRNA or eIF2Bε siRNA (both from Dharmacon, Lafayette, CO) were transfected into subconfluent primary human airway smooth muscle cells using Oligofectamine in OptiMEM (Invitrogen). For GSK-3β, a pool of double-stranded siRNAs containing equal parts of the following antisense sequences was used: 1, 5′-PUAUACCACACCAAAUGAUCUU-3′; 2, 5′-PUAUGUUACAGUGAUCUAGCUU-3′; 3, 5′-PAUAGGCUAAACUUCGGAACUU-3′; 4, 5′-PCAAAGAUCAACUCUGGUGCUU-3′. For eIF2Bε siRNA, a pool of double-stranded siRNAs containing equal parts of the following antisense sequences was used: 1, 5′-PUAAAUCAUAUCGAACCUCCUU; 2, 5′-PUUAGACUUAUGUUAUAGGCUU; 3, 5′-PCCAUAUUCCUUAGCUGUUAUU; 4, 5′-PUCUUGCGCAACUGCUGGCCUU. The corresponding nontargeting siRNA sequence was 5′-UAGCGACUAAACACAUCAA-3′. Six hours later, DMEM and FBS were added. The next morning, cells were incubated in fresh DMEM containing 10% FBS for 24 h. Finally, cells were treated with the relevant stimulus in serum-free medium for 2 days prior to harvest.
Cell Size Analysis—Human bronchial smooth muscle cell size was measured by fluorescence-activated cell sorting. Cells were treated with LiCl, SB216763, GSK-3β siRNA, CT-1, or TGF-β. Cells were collected and fixed with 75% ethanol and stored at -20 °C before staining. Cells were centrifuged and stained with propidium iodide (50 μg/ml), RNase (100 μg/ml) solution for 1 h. Cells in G0/G1 phase were gated for forward scatter measurement using a FACSCalibur flow cytometer (BD Biosciences).
Protein Synthesis—Cells were serum-starved for 24 h before experiments. Cells were plated at 5 × 105 cells/well (or 3 × 105 cells/well for experiments involving transfection) and incubated in [3H]leucine (0.5 μCi; PerkinElmer Life Sciences) for 48 h. Cells were lysed, and proteins were precipitated with 10% trichloroacetic acid. After washing with cold ethanol and solubilization with 1% Triton X-100 in 0.5 m NaOH, radioactivity was measured by a scintillation counter.
Fluorescence Microscopy—Human bronchial smooth muscle cells were grown on collagen-coated glass slides (BD Biosciences) and fixed in 1% paraformaldehyde. To stain filamentous actin, slides were incubated with Alexa Fluor 488-conjugated phalloidin (Molecular Probes, Eugene, OR). For immunocytochemistry, slides were probed with Cy3-conjugated mouse anti-α-smooth muscle actin-Cy3 (Sigma) or anti-MLCK, followed by Alexa Fluor 594-labeled goat anti-mouse IgG (Molecular Probes) and with phospho-GSK-3β antibody followed by Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes).
Retroviral Transduction of Human Bronchial Cell Lines—cDNA encoding a nonphosphorylatable GSK-3β (GSK-3β-A9), with Ser9 replaced by alanine, was provided by Dr. Anne Vojtek (University of Michigan). GSK-3β-A9 cDNA was subcloned into the pMSCVpuro retroviral vector (BD Biosciences). The Phoenix-GP retrovirus packaging cell line, a 293-cell derivative line that expresses only the gag-pol viral components (provided by G. Nolan, Stanford University) was transiently transfected with pHCMV-G, which contains the vesicular stomatitis virus envelope glycoprotein, and either pMSCVpuro-AA-GSK-3β-A9 or pMSCV alone. Viral supernatant was collected, filtered, and supplemented with Polybrene (8 μg/ml). Human bronchial smooth muscle cells were infected with viral supernatant (four times for 4 h each). Infected cells were selected with puromycin (2 μg/ml). After selection, cells were grown to confluence, split into 6-well plates, and incubated in the absence or presence of LiCl, CT-1, or TGF-β.
Cell Contraction—Individual cell length before and after contraction with KCl was measured by computerized image micrometry. Cells were seeded in 100-mm dishes and grown to confluence in serum-free medium or medium supplemented with LiCl, CT-1, or TGF-β. At confluence, cells were scraped off with a rubber policeman, triturated, and transferred to polypropylene tubes. At this stage, cells tend to maintain a contracted state due to mechanical stimulation. The cells were treated with 8-bromo-cAMP and then allowed to float freely and relax for 24 h with occasional swirling to prevent settling or sticking to the sides of the tube. During this period, cells regain a spindle shape and extend processes. Aliquots of cultured cell suspension (2.5 × 104 cells/0.5 ml) were stimulated with KCl (75 mm). The reaction was allowed to proceed for 4 min and stopped by the addition of 0.1 ml of glutaraldehyde at a final concentration of 1% (v/v). Fixed cells were allowed to settle and then transferred by wide mouth pipette to a microscope slide for analysis. The average length of cells before or after the addition of test agents was obtained from 20 cells encountered in successive microscopic fields.
Real Time PCR—Quantitative two-step real time PCR for human α-smooth muscle actin, human 18 S rRNA, mouse α-actin, mouse β-actin, and mouse CT-1 was conducted using specific primers and probes. Primers were from IDT (Coralville, IA) and used 6-carboxyfluorescien (FAM) as a reporter fluorochrome and either tetramethyl-6-carboxyrhodamine (TAMRA) or Iowa Black (IB) as fluorescent quenchers. Human α-actin forward primer was 5′-GAC CCT GAA GTA CCC GAT AGA AC-3′, reverse primer was 5′-GGG CAA CAC GAA GCT CAT TG-3′, and probe was 5′-FAM-TGG CAT CAT CAC CAA CTG GGA CG-IB-3′. Human 18 S rRNA forward primer was 5′-CGC CGC TAG AGG TGA AAT TCT-3′, reverse primer was 5′-CAT TCT TGG CAA ATG CTT TCG-3′, and probe was 5′-FAM-ACC GGC GCA AGA CGG ACC AGA-TAMRA-3′. Mouse α-actin forward primer was 5′-CCA GGC ATT GCT GAC AGG AT-3′, reverse primer was 5′-CCA CCG ATC CAG ACA GAG TAC-3′, and probe was 5′-FAM-AAG GAG ATC ACA GCC CTC GCA CC-IB-3′. Mouse β-actin forward primer was 5′-TGA CAG GAT GCA GAA GGA GAT-3′, reverse primer was 5′-GCG CTC AGG AGG AGC AAT-3′, and probe was 5′FAM-ACT GCT CTG GCT CCT AGC ACC AT-IB-3′. Mouse CT-1 forward primer was 5′-GCC TCA GCC TTT GAG AGG AAA-3′, reverse primer was 5′-AGC CCG GTC TGT CCA GTG A-3′, and probe was 5′-FAM-TGC AGA GGC TAC ATA GTG ACC CGA-IB-3′. Reactions were performed on an Eppendorf Realplex2 thermocycler (Westbury, NY).
Reporter Assays—A7R5 cells were used for these experiments because of their superior transfection efficiency. Cells were transiently transfected with the 200 ng of NFAT-luc (BD Biosciences) or serum response factor (SRF)-luc (29) (from J. Solway, University of Chicago). Three nanograms of the SV40 Renilla luciferase vector was used as transfection control. Cells were transfected using a Lipofectamine 2000 (Invitrogen). The following day, cells were serum-deprived for 2 h and treated with LiCl, SB216763, CT-1, or TGF-β for 48 h. Cells were subsequently lysed, and luciferase activity was measured using the Promega luciferase assay system (Madison, WI).
Ovalbumin Sensitization/Challenge Model and Isolation of Murine Airway Smooth Muscle Cells—BALB/c mice (Charles River Laboratories, Wilmington, MA) were sensitized and challenged to endotoxin-free ovalbumin (Pierce), as previously described (30). On day 0, mice were anesthetized and injected intraperitoneally with 200 μl of a suspension of 25% (w/v) alum (Pierce) in either PBS or ovalbumin (5 mg/ml). On day 11, animals were given an identical intraperitoneal injection as well as a 50-μl intranasal instillation of PBS or ovalbumin (20 mg/ml). On days 18, 21, 22, and 23, intranasal instillations were repeated. Twenty-four hours after the final challenge, mice were euthanized, and the lungs were processed for RNA quantitation or airway smooth muscle cell isolation. Cells were isolated by dissection of major bronchi from the lung, mincing well, and incubating for 1 h at 21 °C on a rotary mixer in DMEM with 0.1% trypsin and 0.1% collagenase. Proteolysis was stopped by the addition of 9 volumes of DMEM with 10% FBS. Intact cells and tissue debris were sedimented at 1000 × g for 5 min, dispersed with further mincing, and plated in growth media with antibiotics. Medium was changed every day for the next week to remove floating cells, and all tissue fragments were removed at the end of 1 week.
RESULTS
GSK-3β Inhibitors Decrease GSK-3β Activity in Intact Cells—LiCl attenuates GSK-3β activity by increasing the phosphorylation of the inhibitory Ser9 residue, probably by inhibiting a protein phosphatase, and by acting as a competitive inhibitor for Mg2+, which is needed for maximal kinase activity (31). LiCl has been shown to decrease cardiac myocyte GSK-3β activity and eIF2Bε phosphorylation (19) while increasing protein synthesis (9). SB216763 is a permeable, structurally distinct maleimide that inhibits GSK-3 activity (32). We examined the effect of these inhibitors, as well as CT-1 and TGF-β, on GSK-3β phosphorylation and kinase activity. First, early passage human bronchial smooth muscle cells were treated with LiCl (10 mm), SB216763 (50 nm), CT-1 (10 ng/ml), or TGF-β (10 ng/ml), and GSK-3β phosphorylation was assessed by immunoblotting with a phosphospecific antibody. LiCl, CT-1, and TGF-β each enhanced GSK-3β phosphorylation without affecting the expression of total GSK-3β (Fig. 1A). As expected, SB216763 had no effect on GSK-3β phosphorylation. Next, we examined the effects of LiCl, SB216763, CT-1, and TGF-β on GSK-3β kinase activity by in vitro assay. A7R5 cell lysates were immunoprecipitated with anti-GSK3β antibody and incubated with [γ-32P]ATP and Tau protein, a GSK-3β substrate. Treatment with LiCl, SB216763, CT-1, and TGF-β each inhibited GSK-3β activity, as indicated by a decrease in Tau phosphorylation (Fig. 1B).
FIGURE 1.
GSK-3β inhibitors increase GSK-3β phosphorylation and decrease GSK-3β activity. A, human airway smooth muscle cells were treated with LiCl (10 mm), CT-1 (10 ng/ml), and TGF-β (10 ng/ml). Representative immunoblots for phospho-GSK-3β and total GSK-3β are shown. LiCl, CT-1, and TGF-β increased the level of phospho-GSK-3β without affecting that of total GSK-3β. SB216763, a GSK-3β kinase inhibitor, had no effect. B, A7R5 vascular smooth muscle cells were treated with LiCl, SB216763, CT-1, or TGF-β and lysed. Lysates were immunoprecipitated and assayed for GSK-3β activity in a reaction containing the GSK-3β substrate Tau (1 μg/μl), ATP (1 mm), and [γ-32P]ATP (10 μCi). Total GSK-3β of immunoprecipitates is also shown. In the first example shown, the IgG heavy chain apparently migrated just behind GSK-3β, leaving a “ghost band” and creating the impression of a doublet. For this panel, the control and SB216763 bands are from different parts of the same gel. Results are representative of three separate experiments.
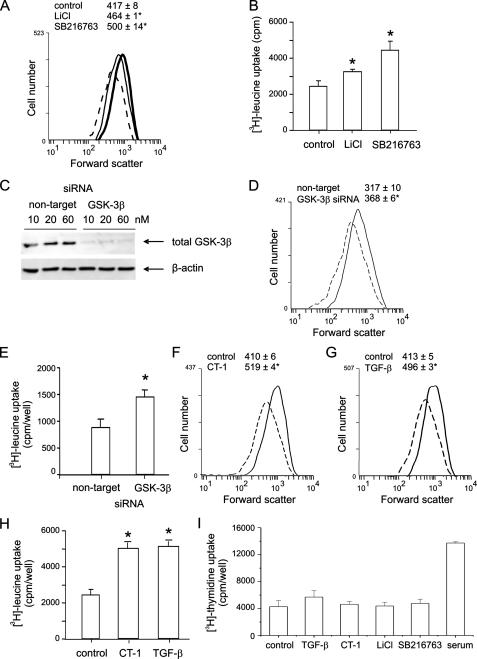
GSK-3β Inhibitors Increase Human Airway Smooth Muscle Cell Size and Protein Synthesis—We determined the effect of GSK-3β inhibition on human airway smooth muscle cell size, protein synthesis, and contractile protein expression. Flow cytometry data showed that cells treated with LiCl or SB216763 displayed a rightward shift in forward scatter, indicating an increase in cell size (Fig. 2A). Changes in cell size were accompanied by an increase in protein synthesis, since the [3H]leucine incorporation was enhanced (Fig. 2B). We also examined the effect of GSK-3β knockdown on cell size and protein synthesis using specific siRNA. Cells treated with GSK-3β siRNA exhibited decreased GSK-3β expression (Fig. 2C). GSK-3β siRNA-treated cells also exhibited a rightward shift in the forward scatter compared with the cells treated with nontargeting siRNA (Fig. 2D). Like LiCl and SB216763, treatment with GSK-3β siRNA also enhanced protein synthesis (Fig. 2E). CT-1 and TGF-β also induced cell hypertrophy, as indicated by a rightward shift in forward scatter and increased protein synthesis (Fig. 2, F-H). Finally, there was no effect of GSK-3β inhibition or CT-1 on human airway smooth muscle DNA synthesis (Fig. 2I).
FIGURE 2.
GSK-3β inhibitors increase human airway smooth muscle cell size and protein synthesis. A, changes in human airway smooth muscle cell size after treatment with LiCl or SB216763. Cells were sorted by forward scatter. B, overall protein synthesis of human airway smooth muscle cells treated with LiCl or SB216763, as assessed by [3H]leucine incorporation (cpm/well). C, immunoblots for GSK-3β expression in cells transfected with GSK-3β or nontargeting siRNA (60 nm). D, changes in human airway smooth muscle cell size after treatment with GSK-3β siRNA (dashed line, nontargeting siRNA; solid line, GSK-3β siRNA). E, changes in protein synthesis with GSK-3β siRNA. F-H, CT-1 and TGF-β also induced cell hypertrophy, as indicated by a rightward shift in forward scatter and increased protein synthesis (dashed lines, control; solid lines, CT-1 or TGF-β; n = 3-5 for each group, mean ± S.E.; *, p < 0.05 relative to unstimulated cells or nontargeting siRNA, one-way ANOVA). I, effects of TGF-β, CT-1, and inhibitors of GSK-3β on human airway smooth muscle [3H]thymidine uptake, a measure of DNA synthesis (n = 3). 10% FBS was employed as a positive control.
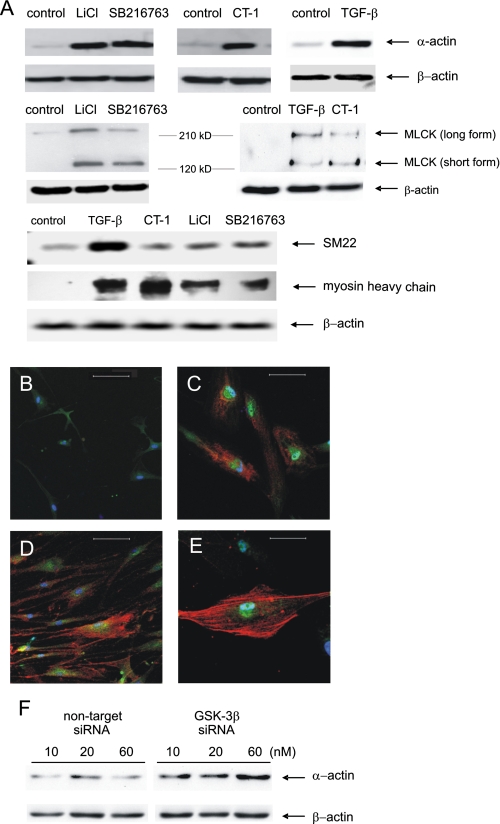
GSK-3β Inhibition Increases Contractile Protein Expression—Cellular proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for α-smooth muscle actin, MLCK, smMHC, and SM22. LiCl, SB216763, CT-1, and TGF-β each increased α-actin but not β-actin expression (Fig. 3A). Immunocytochemical stains showed increased α-smooth muscle actin and phospho-Ser9-GSK-3β content (Fig. 3, B-E). LiCl, SB216763, CT-1, and TGF-β also increased MLCK, smMHC, and SM22 expression (Fig. 3A). For MLCK, there was increased induction of both the long and short forms of MLCK, the latter of which is associated with enhanced contractile function (33). Inhibition of GSK-3β expression by specific siRNA also increased α-actin expression, with the maximum effect at 60 nm (Fig. 3G).
FIGURE 3.
GSK-3β inhibition increases expression of contractile proteins. A, representative immunoblots for α-actin, MLCK, SM22, and smMHC. β-Actin expression was employed as a control. B-E, cells were stained with Cy3-conjugated anti-α-actin (red channel) and anti-phospho-Ser9 GSK-3β antibody followed by Alexa Fluor 488-conjugated secondary antibody (green channel). Nuclei were stained with bis-benzimide (blue channel). Cells were untreated (B) or stimulated with 10 mm LiCl (C), 10 ng/ml CT-1 (D), or 10 ng/ml TGF-β (E). Bar, 100 μm. F, representative immunoblots for MLCK. Both the low molecular weight (contractile) and high molecular weight forms are induced by GSK-3β inhibition. G, effect of GSK-3β siRNA on α-smooth muscle actin expression. For this panel, the two sets of bands are from different parts of the same gel. The results shown are representative of three separate experiments.
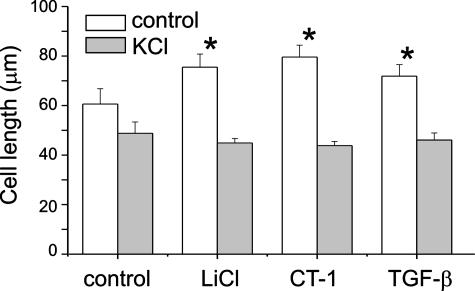
LiCl, CT-1, and TGF-β Increase Cell Shortening in Response to KCl—To determine whether cellular hypertrophy is accompanied by increased contractile function, cells were grown to confluence in either serum-free medium or medium containing LiCl, CT-1, or TGF-β. At confluence, cells were gently scraped off the plate with a rubber policeman, triturated, transferred to polypropylene tubes, and relaxed with 8-bromo-cAMP. Selected aliquots were treated with KCl (75 mm). After 4 min, the cells were fixed with glutaraldehyde, and cell length was measured by computerized image micrometry. Cells treated with LiCl, CT-1, and TGF-β were longer at rest compared with control cells (Fig. 4). Based on length comparisons between unstimulated and KCl-stimulated cells, LiCl-, CT-1-, and TGF-β-treated cells demonstrated increased mean fractional shortening (control, 19%; LiCl, 41%; CT-1, 45%; TGF-β, 36%). These data support the notion that GSK-3β-mediated increases in cell size and contractile protein expression are linked to increased contractility.
FIGURE 4.
LiCl, CT-1, and TGF-β increase cell shortening in response to KCl. Compared with control cells, treatment with LiCl, CT-1, and TGF-β each increased resting length and fractional shortening in response to KCl (75 mm) (n = 20 for each group, mean ± S.D.; *, p < 0.05 relative to control cells without KCl treatment, ANOVA).
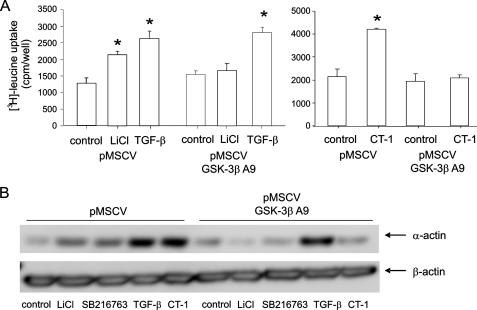
Phosphorylation of GSK-3β Is Required for LiCl- and CT-1-induced but Not TGF-β-induced Hypertrophy—To address the question of whether inhibition of GSK-3β is necessary for airway smooth muscle hypertrophy, we expressed GSK-3βA9, which cannot be phosphorylated at Ser9, in human airway smooth muscle cells via retroviral gene transfer and determined its effect on LiCl-, CT-1-, and TGF-β-induced hypertrophy. Expression of GSK-3βA9 significantly attenuated LiCl- and CT-1-induced protein synthesis (Fig. 5A) and LiCl-, SB216763-, and CT-1-induced α-actin expression (Fig. 5B). However, expression of GSK-3βA9 did not affect protein synthesis and α-actin expression in TGF-β-treated cells. These data suggest that phosphorylation of GSK-3β is required for CT-1 but not TGF-β-induced hypertrophy.
FIGURE 5.
Phosphorylation of GSK-3β is required for LiCl- and CT-1-induced but not TGF-β-induced protein synthesis and α-actin expression. GSK-3βA9 was expressed in human airway smooth muscle cells via retroviral gene transfer. A, effect of GSK-3βA9 on protein synthesis (n = 3 for each group, mean ± S.E.; *, p < 0.05 relative to unstimulated cells). B, effect of GSK-3βA9 on α-smooth muscle α-actin expression in the presence of GSK-3β inhibitors, TGF-β or CT-1. Results are representative of three separate experiments.
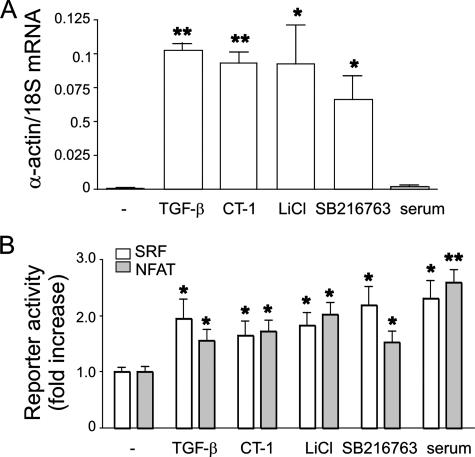
Effect of GSK-3β Inhibition on α-Smooth Muscle Actin mRNA Expression—As noted above, GSK-3β may regulate cell size and contractile protein expression by negatively regulating transcription factors involved in muscle-specific gene expression or by negatively regulating the initiation of protein translation via eIF2Bε. We examined the effect of GSK-3β inhibition on α-smooth muscle actin mRNA expression by real time PCR. TGF-β, CT-1, LiCl, and SB216763 each increased α-actin mRNA expression (Fig. 6A). Based on previous work showing that GSK-3β negatively regulates NFAT and other transcription factors involved in muscle-specific gene expression, we examined the effect of LiCl and SB216763 on the transactivation of NFAT and SRF, a regulator of smooth muscle-specific gene expression (29) (Fig. 6B). TGF-β, CT-1, LiCl, and SB216763 each increased NFAT and SRF activity, consistent with the notion that inhibition of GSK-3β induces human airway smooth muscle hypertrophy, at least in part, via transcriptional mechanisms.
FIGURE 6.
Effect of GSK-3β inhibition on α-smooth muscle actin mRNA expression. A, quantitative two-step real time PCR for human α-smooth muscle actin and human 18 S rRNA was conducted using specific primers and probes. TGF-β, CT-1, LiCl, and SB216763 each increased α-actin mRNA expression (B). A7R5 cells were transiently transfected with SV40 Renilla luciferase vector and either NFAT-luc or SRF-luc. After treatment with TGF-β, CT-1, LiCl, or SB216763 for 48 h, cells were lysed, and luciferase activity was determined. Each stimulus increased NFAT and SRF activity (n = 8, mean ± S.E.; different from unstimulated cells; *, p < 0.05; **, p < 0.001, ANOVA).
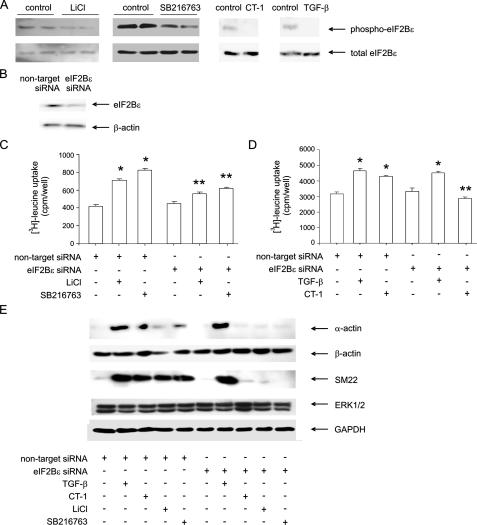
Cell Hypertrophy Induced by GSK-3β Inhibition Is Mediated by eIF2B—We also assayed changes in the phosphorylation of eIF2B, a downstream target of GSK-3β that regulates translation initiation. Treatment with LiCl, SB216763, CT-1, and TGF-β each conferred a marked reduction in eIF2Bε phosphorylation, consistent with their inhibitory effect on GSK-3β (Fig. 7A).
FIGURE 7.
Induction of protein synthesis via inhibition of GSK-3β is mediated by eIF2B. A, immunoblots of phospho-eIF2Bε and total eIF2Bε from LiCl- and SB216763-treated human bronchial smooth muscle cells. The results shown are representative of three experiments. Cells were pretreated with nontargeting or eIF2B siRNA (B). Next, cells were incubated with LiCl (10 mm), SB216763 (50 nm), CT-1 (10 ng/ml), or TGF-β (10 ng/ml). Protein synthesis was assessed by [3H]leucine incorporation (C and D). In cells pretreated with nontargeting siRNA, each stimulus induced protein synthesis. Pretreatment with eIF2B siRNA attenuated LiCl-, SB216763-, and CT-induced protein synthesis. TGF-β-induced protein synthesis was unaffected (n = 6, mean ± S.E.; *, different from unstimulated cells, p < 0.05; **, different from nontargeting siRNA; p < 0.05, ANOVA). E, siRNA targeting eIF2B decreased LiCl-, SB216763-, and CT-1-induced α-smooth muscle actin and SM22 protein expression. eIF2B siRNA did not significantly reduce TGF-β-induced responses. siRNA against eIF2B had no effects on β-actin, ERK, or GAPDH protein abundance.
Next, we pretreated cells with either siRNA targeting eIF2B or nontargeting siRNA (Fig. 7B) and then stimulated with LiCl or SB216763. LiCl and SB216763 each increased protein synthesis in cells pretreated with nontargeting siRNA, but the increase was significantly reduced by siRNA against eIF2B (Fig. 7C). Similar to the effects of GSK-3βA9, eIF2B siRNA decreased CT-1 but not TGF-β-induced protein synthesis (Fig. 7D). In addition, siRNA targeting eIF2B decreased LiCl-, SB216763-, and CT-1-induced α-smooth muscle actin and SM22 protein expression (Fig. 7E). Again, siRNA against eIF2B had no effect on TGF-β-induced contractile protein expression. siRNA against eIF2B had no effects on β-actin, ERK, or GAPDH protein abundance. Taken together with the data presented in Fig. 6, these results suggest that GSK-3β inhibition induces human airway smooth muscle hypertrophy via an enhancement of translation, leading to increased protein synthesis and cell size, as well as by specific effects on contractile protein expression, which are mediated at both the transcriptional and translational levels.
Effects of Ovalbumin Sensitization and Challenge on Airway Smooth Muscle Mass and GSK-3β Phosphorylation—Ovalbumin sensitization and challenge is a commonly used mouse model of asthma. After a 3-week sensitization and challenge protocol, airways exhibited an increased amount of α-actin immunostaining compared with PBS-treated controls (not shown), as others have observed (30, 34). Ovalbumin treatment also increased lung CT-1 mRNA expression (Fig. 8A). Bronchial smooth muscle cells were isolated from selected control and ovalbumin-sensitized and -challenged mice. Cells from allergen-sensitized and -challenged mice were larger, as evidenced by greater amounts of α-smooth muscle actin (Fig. 8, B and C) and increased forward scatter (Fig. 8D). Cells from treated mice also demonstrated greater levels of phospho-Ser9-GSK-3β and decreased GSK-3β kinase activity (Fig. 8, E and F).
FIGURE 8.
Ovalbumin sensitization and challenge increases airway smooth muscle cell size and phospho-Ser9 GSK-3β content in airway smooth muscle. A, α-smooth muscle actin and CT-1 mRNA expression in lung homogenates was determined by quantitative RT-PCR. Ovalbumin treatment increased α-actin and CT-1 expression compared with PBS-treated controls. α-Actin and CT-1 values were initially normalized for β-actin expression. Murine airway smooth muscle cells were isolated from control (B) and ovalbumin-treated animals (C). Cells from ovalbumin-sensitized and -challenged mice showed greater α-smooth muscle actin expression (red) and phospho-Ser9 GSK-3β content (green). D, cells from ovalbumin-sensitized and -challenged mice also showed greater cell size, as assessed by forward scatter (dashed line, PBS; solid line, ovalbumin; n = 3, mean ± S.E.; *, different from cells from PBS-treated mice, p < 0.05, ANOVA). E, typical immunoblots showing phospho-Ser9 GSK-3β and total GSK-3β from PBS and ovalbumin-sensitized (OVA) mice. In addition, immunoprecipitates were tested for GSK-3β activity using recombinant Tau protein as the substrate. The total GSK-3β content of immunoprecipitates was also examined. F, group mean data of experiments described in E (n = 3, mean ± S.E.; *, different from cells from PBS-treated mice, p < 0.05, ANOVA).
DISCUSSION
Excess smooth muscle mass is present in patients with fatal and nonfatal asthma. Using advanced stereologic techniques, Ebina et al. (1) examined the airways of patients with fatal asthma and found two subgroups, one in which smooth muscle hyperplasia was present only in the central bronchi and another in which smooth muscle cell size was increased throughout the airway tree. Subsequently, Benayoun et al. (2) found that the airways of patients with nonfatal severe asthma had larger smooth muscle cell diameter than control subjects, patients with mild asthma, or patients with chronic obstructive pulmonary disease. Expression of α-smooth muscle actin and MLCK was also increased. Further, there was no evidence of airway smooth muscle cell proliferation, as evidenced by the lack of staining for Ki67, a nuclear marker of cell cycle traversal. Finally, Woodruff et al. (3) found that airway smooth muscle cell number was nearly 2-fold higher in subjects with mild to moderate asthma, whereas there was no increase in cell size between groups. Also, although α-smooth muscle actin immunoreactivity increased by 50-83% in these patients, the mRNA expression of contractile protein genes was not increased, consistent with the notion that contractile protein expression may be regulated in a post-transcriptional manner.
Despite evidence that smooth muscle hypertrophy contributes to airway remodeling in asthma, little is known about the biochemical mechanisms regulating this process. Two models employing cell cycle arrest (long term serum deprivation and withdrawal of SV40 large T antigen) demonstrate increases in cell size and contractile protein accumulation without a corresponding increase in mRNA (35, 36), again suggestive of post-transcriptional control. In the latter model, as with TGF-β-treated cells, hypertrophy did not occur in cells infected with a retrovirus encoding a phosphorylation site mutant of 4E-BP1 that dominantly inhibits eIF4E, suggesting that phosphorylation of 4E-BP, eIF4E release, and cap-dependent protein synthesis are required for hypertrophy of human airway smooth muscle cells (27, 37).
In the present study, we examined the contribution of GSK-3β signaling to airway smooth muscle hypertrophy. GSK-3β has been identified previously as a negative regulator of cardiac and skeletal muscle hypertrophy (8-13). Selective activation of Akt induces cellular hypertrophy and atrial natriuretic factor expression in cardiac myocytes, and isoproterenol-induced atrial natriuretic factor expression is blocked by expression of GSK-3β S9A (8). Phosphorylation of GSK-3β is required for endothelin-1-, phenylephrine-, and Fas ligand-induced cardiomyocyte sarcomere organization and atrial natriuretic factor expression (9, 10). Tissue-specific activation of GSK-3β suppresses cardiac hypertrophy in response to activated calcineurin, chronic β-adrenergic stimulation, and pressure overload in vivo (11). In C2C12 myotubes, constitutive activation of GSK-3β blocks insulin-like growth factor-induced NFAT-inducible gene expression, a marker of myotube hypertrophy, whereas inhibition of GSK-3β by LiCl restores myoblast gene expression (12, 13). However, in general, these studies failed to consider the entire range of hypertrophic responses (cell size, protein synthesis, contractile gene expression), and only one study considered the role of GSK-3β in the regulation of contractile function, either at the tissue or cellular level (11). Further, the role of GSK-3β in smooth muscle cells capable of reentering the cell cycle has not been studied. In the present report, we found that GSK-3β chemical inhibitors, as well as GSK-3β siRNA, increased cell size, protein synthesis, and KCl-induced cell shortening. Interestingly, inhibition of GSK-3β induced the relative expression of specific contractile proteins (α-actin, MLCK, smMHC, and SM22) as well as a global increase in protein synthesis. We also studied the effects of CT-1 and TGF-β, each of which have been shown to induce human airway smooth muscle hypertrophy but not hyperplasia (21, 26), on GSK-3β phosphorylation and activity. CT-1 and TGF-β each increased protein synthesis, cell size, contractile protein expression, fractional shortening, and GSK-3β phosphorylation while inhibiting GSK-3β kinase activity. Finally, GSK-3β phosphorylation and CT-1 mRNA expression were increased in the lungs of allergen-challenged and -sensitized mice with airway hyperresponsiveness and increased airway smooth muscle mass. Detailed morphometric studies have shown that increased airway smooth muscle mass in ovalbumin-treated mice is due to cellular hypertrophy as well as hyperplasia.3 Taken together, these data demonstrate that inhibition of GSK-3β is sufficient for airway smooth muscle hypertrophy and suggest that GSK-3β may play an important physiologic role in the regulation of smooth muscle contractile function. These data contrast with a previous study in which CT-1-treated airway explants showed increased smooth muscle mass but paradoxically reduced maximal isometric stress in response to acetylcholine (22), suggesting that hypertrophic muscle may not function normally. However, in the former study, CT-1 increased extracellular matrix deposition and passive tension, suggesting that the effects of CT-1 on contractility may have been counterbalanced by parallel increases in elastic load.
We examined the mechanisms by which GSK-3β regulates airway smooth muscle cell size and contractile protein expression. Based on the preferential effects of GSK-3β inhibition on α-smooth muscle actin, MLCK, smMHC, and SM22 protein abundance relative to β-actin, we examined the effects of GSK-3β inhibition on α-smooth muscle actin mRNA expression and NFAT promoter activity. GSK-3β negatively regulates NFAT transactivation, and NFAT has been implicated in muscle-specific gene expression (11, 12). NFAT cytosolic component 1 potentiates GATA-6-activated smMHC transcription in primary human aortic vascular smooth muscle cells (38), and NFAT and SRF cooperatively regulate the activity of an α-actin intronic enhancer in the rat A7R5 aortic smooth muscle cell line (39). We found that inhibition of GSK-3β increased α-smooth muscle actin mRNA expression as well as NFAT and SRF transactivation, consistent with the notion that inhibition of GSK-3β induces human airway smooth muscle hypertrophy, at least in part, via transcriptional mechanisms.
One of the critical steps controlling the initiation of protein translation is formation of the 43 S preinitiation complex. Phosphorylation and inactivation of GSK-3β by Akt lead to eIF2B dephosphorylation and activation and an increase in eIF2 GTP loading. eIF2, in turn, functions to recruit methionyl-tRNA and conduct it as a tRNA-eIF2-GTP ternary complex to the 40 S ribosomal subunit, thereby promoting a global increase in mRNA translation. In the present study, LiCl, SB216763, and CT-1 not only decreased GSK-3β kinase activity but also reduced phosphorylation of the GSK-3β downstream target eIF2Bε. Further, eIF2B siRNA attenuated LiCl-, SB216763-, and CT-1-induced protein synthesis. Finally, siRNA against eIF2Bε also inhibited LiCl-, SB216763-, and CT-1-induced protein expression of α-actin and SM22 but not β-actin, ERK, or GAPDH. Together, these data demonstrate that CT-1 induces airway smooth hypertrophy via the GSK-3β/eIF2Bε translational control pathway as well as by transcriptional pathways. We speculate that the preferential inhibitory effect of eIF2ε siRNA on α-actin and SM22 translation relates to the relatively high levels of contractile apparatus mRNA in the cell, compared with β-actin, ERK, or GAPDH. We have previously found that steady-state α-actin and SM22 mRNA levels are high in unstimulated airway smooth muscle cells (35, 36). This level would be further increased following GSK-3β inhibition, which, as noted above, stimulates α-actin transcription. Finally, it is conceivable that, once assembled into filaments, α-actin, MLCK, and other contractile proteins are less susceptible to proteolytic turnover, as has been observed for calpain and smooth muscle myosin (40, 41).
In cells overexpressing GSK-3βA9, a nonphosphorylable, constitutively active form of GSK-3β, LiCl, SB216763, and CT-1 failed to increase protein synthesis or α-smooth muscle actin expression. These data suggest that GSK-3β phosphorylation is required for LiCl-, SB216763-, and CT-1-induced airway smooth muscle hypertrophy. However, protein synthesis and α-actin expression induced by the pleotrophic growth factor TGF-β were not blocked by either GSK-3βA9 or eIF2B siRNA, suggesting that, in the context of TGF-β treatment, an alternative pathway or pathways are sufficient for the hypertrophic response. As noted above, besides phosphorylation and inhibition of GSK-3β, TGF-β treatment is also followed by phosphorylation of 4E-BP, which facilitates eIF4E-dependent translation of 7-methylguanine-capped mRNAs (27). Overexpression of AA-4E-BP, a nonphosphorylatable mutant, inhibited TGF-β-induced airway smooth muscle hypertrophy. Together, these results suggest that TGF-β induces hypertrophy via phosphorylation of 4E-BP and GSK-3β but that only the 4E-BP translational control pathway is required for the response. Finally, TGF-β has robust stimulatory effects on the transcription of contractile apparatus mRNAs, which may be independent of GSK-3β inhibition.
In conclusion, we have demonstrated that inhibition of GSK-3β activity or expression is sufficient to evoke a hypertrophic phenotype in human airway smooth muscle cells, as demonstrated by morphological, biochemical, and functional criteria. Inhibition of GSK-3β activity increases airway smooth muscle cell size, protein synthesis, contractile protein expression, and shortening. Our data show that GSK-3β inhibition induces human airway smooth muscle hypertrophy via an eIF2B-dependent translational control pathway as well as by specific effects on smooth muscle-specific gene transcription. Inhibition of GSK-3β is required for CT-1- but not TGF-β-induced airway smooth muscle hypertrophy. Finally, airway smooth muscle GSK-3β phosphorylation and CT-1 expression are increased in a mouse model of asthma. Although the GSK-3β null mouse is embryonic lethal (42), future studies utilizing tissue-specific knock-out mice could determine whether GSK-3β is required for airway smooth muscle hypertrophy in vivo. Additional studies examining the role of GSK-3β and eIF2B in airway smooth muscle growth will provide insight into the pathogenesis of asthma and perhaps lead to novel therapies.
Acknowledgments
We thank Dr. Julian Solway (University of Chicago) for human airway smooth muscle cells and SRF-luc, Dr. Michael Uhler (University of Michigan) for NFAT-luc, Dr. Anne Vojtek (University of Michigan) for GSK-3βA9, and Dr. Gary Nolan (Stanford University) for the retrovirus packaging cell line.
This work was supported by National Institutes of Health Grant HL79339. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GSK, glycogen synthase kinase; NFAT, nuclear factors of activated T cells; eIF, eukaryotic initiation factor; CT, cardiotrophin; TGF, transforming growth factor; 4E-BP, eIF-4E-binding protein; DMEM, Dulbecco's modified Eagle's medium; SRF, serum response factor; smMHC, smooth muscle myosin heavy chain; MLCK, myosin light chain kinase; ERK, extracellular signal-regulated kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PBS, phosphate-buffered saline; siRNA, small interfering RNA; FBS, fetal bovine serum; IB, Iowa Black; ANOVA, analysis of variance; FAM, 6-carboxyfluorescein; TAMRA, tetramethyl-6-carboxyrhodamine.
J. K. Bentley, unpublished data.
References
- 1.Ebina, M., Takahashi, T., Chiba, T., and Motomiya, M. (1993) Am. Rev. Respir. Dis. 148 720-726 [DOI] [PubMed] [Google Scholar]
- 2.Benayoun, L., Druilhe, A., Dombret, M. C., Aubier, M., and Pretolani, M. (2003) Am. J. Respir. Crit. Care Med. 167 1360-1368 [DOI] [PubMed] [Google Scholar]
- 3.Woodruff, P. G., Dolganov, G. M., Ferrando, R. E., Donnelly, S., Hays, S. R., Solberg, O. D., Carter, R., Wong, H. H., Cadbury, P. S., and Fahy, J. V. (2004) Am. J. Respir. Crit. Care Med. 169 1001-1006 [DOI] [PubMed] [Google Scholar]
- 4.Cohen, P., and Frame, S. (2001) Nat. Rev. Mol. Cell. Biol. 2 769-776 [DOI] [PubMed] [Google Scholar]
- 5.Shaw, M., and Cohen, P. (1999) FEBS Lett. 461 120-124 [DOI] [PubMed] [Google Scholar]
- 6.Fang, X., Yu, S. X., Lu, Y., Bast, R. C., Jr., Woodgett, J. R., and Mills, G. B. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 11960-11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez, I., and Green, J. B. (2001) Dev. Biol. 235 303-313 [DOI] [PubMed] [Google Scholar]
- 8.Morisco, C., Zebrowski, D., Condorelli, G., Tsichlis, P., Vatner, S. F., and Sadoshima, J. (2000) J. Biol. Chem. 275 14466-14475 [DOI] [PubMed] [Google Scholar]
- 9.Haq, S., Choukroun, G., Kang, Z. B., Ranu, H., Matsui, T., Rosenzweig, A., Molkentin, J. D., Alessandrini, A., Woodgett, J., Hajjar, R., Michael, A., and Force, T. (2000) J. Cell Biol. 151 117-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badorff, C., Ruetten, H., Mueller, S., Stahmer, M., Gehring, D., Jung, F., Ihling, C., Zeiher, A. M., and Dimmeler, S. (2002) J. Clin. Invest. 109 373-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antos, C. L., McKinsey, T. A., Frey, N., Kutschke, W., McAnally, J., Shelton, J. M., Richardson, J. A., Hill, J. A., and Olson, E. N. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 907-912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vyas, D. R., Spangenburg, E. E., Abraha, T. W., Childs, T. E., and Booth, F. W. (2002) Am. J. Physiol. 283 C545-C551 [DOI] [PubMed] [Google Scholar]
- 13.Rochat, A., Fernandez, A., Vandromme, M., Moles, J.-P., Bouschet, T., Carnac, G., and Lamb, N. J. C. (2004) Mol. Biol. Cell 15 4544-4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisco, C., Seta, K., Hardt, S. E., Lee, Y., Vatner, S. F., and Sadoshima, J. (2001) J. Biol. Chem. 276 28586-28597 [DOI] [PubMed] [Google Scholar]
- 15.Haq, S., Michael, A., Andreucci, M., Bhattacharya, K., Dotto, P., Walters, B., Woodgett, J., Kilter, H., and Force, T. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 4610-4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong, D. D., and Esser, K. A. (2005) Am. J. Physiol. 289 C853-C859 [DOI] [PubMed] [Google Scholar]
- 17.Inoki, K., Ouyang, H., Zhu, T., Lindvall, C., Wang, Y., Zhang, X., Yang, Q., Bennett, C., Harada, Y., Stankunas, K., Wang, C., He, X., MacDougald, O. A., You, M., Williams, B. O., and Guan, K.-L. (2006) Cell 126 955-968 [DOI] [PubMed] [Google Scholar]
- 18.Welsh, G. I., Miller, C. M., Loughlin, A. J., Price, N. T., and Proud, C. G. (1998) FEBS Lett. 421 125-130 [DOI] [PubMed] [Google Scholar]
- 19.Hardt, S. E., Tomita, H., Katus, H. A., and Sadoshima, J. (2004) Circ. Res. 94 926-935 [DOI] [PubMed] [Google Scholar]
- 20.Zhou, L., and Hershenson, M. B. (2003) Respir. Physiol. Neurobiol. 137 295-308 [DOI] [PubMed] [Google Scholar]
- 21.Zhou, D., Zheng, X., Wang, L., Stelmack, G., Halayko, A. J., Dorscheid, D., and Bai, T. R. (2003) Br. J. Pharmacol. 140 1237-1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng, X., Zhou, D., Seow, C. Y., and Bai, T. R. (2004) Am. J. Physiol. 287 L1165-L1171 [DOI] [PubMed] [Google Scholar]
- 23.Ohno, I., Nitta, Y., Yamauchi, K., Hoshi, H., Honma, M., Woolley, K., O'Byrne, P., Tamura, G., Jordana, M., and Shirato, K. (1996) Am. J. Respir. Cell Mol. Biol. 15 404-409 [DOI] [PubMed] [Google Scholar]
- 24.Minshall, E. M., Leung, D. Y. M., Matin, R. J., Song, Y. L., Cameron, L., Ernst, P., and Hamid, Q. (1997) Am. J. Respir. Cell Mol. Biol. 17 326-333 [DOI] [PubMed] [Google Scholar]
- 25.Tillie-Leblond, I., Pugin, J., Marquette, C.-H., Lamblin, C., Saulnier, F., Brichet, A., Wallaert, B., Tonnel, A.-B., and Gosset, P. (1999) Am. J. Respir. Crit. Care Med. 159 487-494 [DOI] [PubMed] [Google Scholar]
- 26.Nomura, A., Uchida, Y., Sakamoto, T., Ishii, Y., Masuyama, K., Morishima, Y., Hirano, K., and Sekizawa, K. (2002) Clin. Exp. Allergy 32 860-865 [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith, A. M., Bentley, J. K., Zhou, L., Jia, Y., Bitar, K. N., Fingar, D. C., and Hershenson, M. B. (2006) Am. J. Respir. Cell Mol. Biol. 34 247-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland, C., Leighton, I. A., and Cohen, P. (1993) Biochem. J. 296 15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camoretti-Mercado, B., Fernandes, D. J., Dewundara, S., Churchill, J., Ma, L., Kogut, P. C., McConville, J. F., Parmacek, M. S., and Solway, J. (2006) J. Biol. Chem. 281 20383-20392 [DOI] [PubMed] [Google Scholar]
- 30.Leigh, R., Ellis, R., Wattie, J., Southam, D. S., De Hoogh, M., Gauldie, J., O'Byrne, P. M., and Inman, M. D. (2002) Am. J. Respir. Cell Mol. Biol. 27 526-535 [DOI] [PubMed] [Google Scholar]
- 31.Jope, R. S. (2003) Trends Pharmacol. Sci. 24 441-443 [DOI] [PubMed] [Google Scholar]
- 32.Coghlan, M. P., Culbert, A. A., Cross, D. A., Corcoran, S. L., Yates, J. W., Pearce, N. J., Rausch, O. L., Murphy, G. J., Carter, P. S., Roxbee Cox, L., Mills, D., Brown, M. J., Haigh, D., Ward, R. W., Smith, D. G., Murray, K. J., Reith, A. D., and Holder, J. C. (2000) Chem. Biol. 7 793-803 [DOI] [PubMed] [Google Scholar]
- 33.Bao, J., Oishi, K., Yamada, T., Liu, L., Nakamura, A., Uchida, M. K., and Kohama, K. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 9556-9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho, J. Y., Miller, M., Baek, K. J., Han, J. W., Nayar, J., Rodriguez, M., Lee, S. Y., McElwain, K., McElwain, S., Raz, E., and Broide, D. H. (2004) Am. J. Respir. Cell Mol. Biol. 30 651-661 [DOI] [PubMed] [Google Scholar]
- 35.Zhou, L., Li, J., Goldsmith, A. M., Newcomb, D. C., Giannola, D. M., Vosk, R. G., Eves, E. M., Rosner, M. R., Solway, J., and Hershenson, M. B. (2004) Am. J. Respir. Crit. Care Med. 169 703-711 [DOI] [PubMed] [Google Scholar]
- 36.Halayko, A. J., Kartha, S., Stelmack, G. L., McConville, J., Tam, J., Camoretti-Mercado, B., Forsythe, S. M., Hershenson, M. B., and Solway, J. (2004) Am. J. Respir. Cell Mol. Biol. 31 266-275 [DOI] [PubMed] [Google Scholar]
- 37.Zhou, L., Goldsmith, A. M., Bentley, J. K., Jia, Y., Rodriguez, M. L., Abe, M. K., Fingar, D. C., and Hershenson, M. B. (2005) Am. J. Respir. Cell Mol. Biol. 33 195-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada, H., Hasegawa, K., Morimoto, T., Kakita, T., Yanazume, T., Abe, M., and Sasayama, S. (2002) J. Cell Biol. 156 983-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Bosc, L. V., Layne, J. J., Nelson, M. T., and Hill-Eubanks, D. C. (2005) J. Biol. Chem. 280 26113-26120 [DOI] [PubMed] [Google Scholar]
- 40.Silver, D. L., Vorotnikov, A. V., Watterson, D. M., Shirinsky, V. P., and Sellers, J. R. (1997) J. Biol. Chem. 272 25353-25359 [DOI] [PubMed] [Google Scholar]
- 41.Tsunekawa, S., Takahashi, K., Abe, M., Hiwada, K., Ozawa, K., and Murachi, T. (1989) FEBS Lett. 250 493-496 [DOI] [PubMed] [Google Scholar]
- 42.Hoeflich, K. P., Luo, J., Rubie, E. A., Tsao, M.-S., Jin, O., and Woodgett, J. R. (2000) Nature 406 86-90 [DOI] [PubMed] [Google Scholar]