Abstract
Interleukin-1 (IL-1) and tumor necrosis factor (TNF) mediate bone resorption in a variety of diseases affecting bone. Like TNF, IL-1 is secreted by osteoclast precursors (OCPs), but unlike TNF, it does not induce osteoclast formation directly from OCPs in vitro. TNF induces IL-1 expression and activates c-Fos, a transcription factor required in OCPs for osteoclast formation. Here, we examined whether IL-1 can induce osteoclast formation directly from OCPs overexpressing c-Fos and whether interaction with bone matrix affects OCP cytokine expression. We infected OCPs with c-Fos or green fluorescent protein retrovirus, cultured them with macrophage colony-stimulating factor and IL-1 on bone slices or plastic dishes, and assessed osteoclast and resorption pit formation and expression of IL-1 by OCPs. We used a Transwell assay to determine whether OCPs secrete IL-1 when they interact with bone matrix. IL-1 induced osteoclast formation directly from c-Fos-expressing OCPs on plastic. c-Fos-expressing OCPs formed osteoclasts spontaneously on bone slices without addition of cytokines. OCPs on bone secreted IL-1, which induced osteoclast formation from c-Fos-expressing OCPs in the lower Transwell dishes. The bone matrix proteins dentin sialoprotein and osteopontin, but not transforming growth factor-β, stimulated OCP expression of IL-1 and induced c-Fos-expressing OCP differentiation into osteoclasts. Osteoclasts eroding inflamed joints have higher c-Fos expression compared with osteoclasts inside bone. We conclude that OCPs expressing c-Fos may induce their differentiation directly into osteoclasts by an autocrine mechanism in which they produce IL-1 through interaction with bone matrix. TNF could induce c-Fos expression in OCPs at sites of inflammation in bone to promote this autocrine mechanism and thus amplify bone loss.
Increased osteoclast formation and activity are responsible for bone loss in common bone diseases such as postmenopausal osteoporosis, rheumatoid arthritis, periodontal disease, and orthopedic implant loosening (1). Osteoclast differentiation and function are controlled primarily by macrophage colony-stimulating factor (M-CSF)2 and RANKL (receptor activator of NF-κB ligand) expressed by osteoblast/stromal cells and by signaling downstream from their receptors in osteoclast precursors (OCPs) (2–5). M-CSF induces expression of RANK, the receptor for RANKL, in OCPs (6). Binding of RANKL to RANK completes the differentiation of OCPs into mature osteoclasts through sequential activation of the transcription factors NF-κB, c-Fos, and NFATc1 (nuclear factor of activated T cells c1) (7). Expression of each of these transcription factors is required in OCPs for osteoclastogenesis (1, 8, 9). c-Fos is a component of the dimeric transcription factor AP-1 (10, 11), which mediates RANKL-stimulated osteoclast formation by transcriptionally activating NFATc1 (12–14). Overexpression of c-Fos in fos-/- OCPs rescues the defect in RANKL-mediated osteoclast formation from these cells (12), although overexpression of c-Fos is not sufficient by itself for induction of OCP differentiation into osteoclasts on plastic culture plates (12–14). We have reported recently that c-Fos can substitute for NF-κB p50 and p52 in OCPs from mice lacking NF-κB p50 and p52 and that, under these circumstances, RANKL or tumor necrosis factor (TNF) can induce osteoclast formation from these cells, but not when the cells express green fluorescent protein (GFP) (7).
Interleukin-1 (IL-1) and TNF are pro-inflammatory cytokines that stimulate bone resorption and are expressed in abundance at sites of inflammation in and around bones (15–17). They are produced by a variety of cell types, including monocytes/macrophages, OCPs, and mature osteoclasts themselves (18, 19). Like TNF, IL-1 promotes RANKL expression by marrow osteoblastic stromal cells (20) and by this mechanism induces osteoclastogenesis indirectly. However, unlike TNF, IL-1 alone cannot directly mediate osteoclast formation from OCPs in vitro (21). OCPs and osteoclasts express IL-1 receptors, and IL-1 appears to enhance osteoclast differentiation by promoting fusion of OCPs (22) and supporting the survival of mature osteoclasts (23–25).
OCPs are attracted to bone surfaces by signaling, which appears to involve bone-lining cells (26) and osteocytes (27). Once they are attached to bone matrix, osteoclasts and OCPs receive survival-enhancing signals through integrin-mediated signaling (28), and they secrete cytokines such as IL-1 and TNF (29) as well as factors that can affect the differentiation of osteoblast precursors (30). Bone matrix contains several non-collagenous proteins, including transforming growth factor-β (TGFβ) (31, 32) and members of the SIBLING (small integrin-binding ligand, N-linked glycoprotein) family of proteins such as osteopontin (OPN), dentin sialoprotein (DSP), and bone sialoprotein (33, 34). These proteins are released from the matrix during bone resorption. TGFβ has multiple effects on bone cells and has been proposed to be involved in coupling osteoblasts to sites of bone resorption (31, 32). OPN appears to anchor osteoclasts to bone matrix through interaction with the vitronectin receptor on the osteoclast basolateral membrane (35) and is required for the activation of osteoclastic bone resorption in unloaded mice (36). Overexpression of bone sialoprotein in osteoblasts leads to bone loss indirectly in mice by enhancing RANKL expression by osteoblastic cells and thus inducing osteoclast differentiation (37). However, it is not known if any of the bone matrix proteins play a more direct role in osteoclast formation.
Here, we report that when OCPs interact with bone matrix or are treated with DSP or OPN, they increase their expression of IL-1. This induces differentiation of c-Fos-expressing OCPs directly into osteoclasts in the absence of RANKL by an autocrine/paracrine mechanism.
MATERIALS AND METHODS
Reagents and Animals—Recombinant human M-CSF, murine RANKL, IL-1, TNF, IL-1 receptor antagonist (IL-1Ra), TGFβ1, and a pan-specific TGFβ antibody were purchased from R&D Systems, Inc. (Minneapolis, MN). TNF receptor fusion protein (ENBREL) was from Amgen (Thousand Oaks, CA). DSP and dentin phosphoprotein (DPP) were extracted from rat dentin, and OPN was extracted from rat long bone cortex as we reported previously (38). Wild-type (WT) mice (C57Bl/6), NF-κB p50/p52 double knock-out (dKO) mice (C57Bl/6×129), and human TNF transgenic (TNF-Tg) mice (line 3647, CBA×C57Bl/6) were used as we described previously (7, 39). The Institutional Animal Care and Use Committee approved all animal studies.
Osteoclastogenesis and Viral Infection—Splenocytes were extracted from spleens through a fine wire mesh and cultured with conditioned medium from a M-CSF-producing cell line (1:20 dilution) (40) for 3 days in α-modified essential medium with 10% fetal calf serum (HyClone Laboratories, Logan, UT) to enrich for osteoclast precursors, which we named M-CSF-dependent splenocytes (MDS). The cells were then infected with retroviral supernatants of c-Fos-, NFATc1-, or GFP control-infected Plat-E packaging cells in the presence of M-CSF (10 ng/ml) and Polybrene (8 μg/ml) as we described previously (7).
For Transwell assays, GFP or c-Fos retrovirus-infected WT MDS were seeded directly on bovine cortical bone slices (4 × 4 × 0.2 mm) in 96-well plates or on plastic in the wells of the lower chamber of a 24-well Transwell dish and cultured in the presence of M-CSF for 2 days. The bone slices were then transferred to the upper chamber of the Transwell culture plates and co-cultured with the GFP- or c-Fos-infected MDS in the lower chamber for an additional 8 days in the presence of M-CSF. When multinucleated cell formation was identified under an inverted microscope, the cells were fixed with 10% formalin and stained for tartrate-resistant acid phosphatase (TRAP) activity to identify osteoclasts as TRAP+ cells containing three or more nuclei, and these cells were counted as described previously (7).
For cytokine blockade experiments using different inhibitors, GFP- or c-Fos-infected MDS were cultured directly on plastic plates or bone slices for 2 days in the presence of M-CSF and then treated with cytokines ± inhibitors. When primary spleen cells were used, they were cultured with M-CSF for 3 days prior to inhibitor treatment.
For functional assays, infected cells were cultured on bone slices for 10 days under the same conditions as described above. Osteoclasts were then removed; resorption pits were visualized after 0.1% toluidine blue staining; and the mean pit area (mm2/slice) was measured as described previously (7).
Quantitative Real-time Reverse Transcription (RT)-PCR—RNA from MDS or retrovirus-infected cells cultured on either plastic dishes or bone slices was extracted using TRIzol reagent (Invitrogen), and cDNA synthesis was performed using the GeneAmp RNA PCR core kit (Applied Biosystems, Foster City, CA). Quantitative PCR amplification was performed with gene-specific primers using an iCycler real-time PCR machine and iQ SYBR Green Supermix (both from Bio-Rad) according to the manufacturer's instruction. The murine primer sequences used were as follows: TNFα, 5′-CACACTCAGATCATCTTCTCAA-3′ (forward) and 5′-AGTAGACAAGGTACAACCCATC-3′ (reverse); IL-1β, 5′-ATTAGACAGCTGCACTACAGG-3′ (forward) and 5′-GGAGAATATCACTTGTTGGTTG-3′ (reverse); c-fos, 5′-CTGTCAACACACAGGACTTTT-3′ (forward) and reverse 5′-AGGAGATAGCTGCTCTACTTTG-3′ (reverse); and β-actin, 5′-ACCCAGATCATGTTTGAGAC-3′ (forward) and 5′-GTCAGGATCTTCATGAGGTAGT-3′ (reverse). The relative standard curve method was used to calculate the amplification difference for each primer set (41). A standard curve was made from four points corresponding to 5-fold cDNA serial dilutions for each gene. For each sample, the relative amount was calculated from its respective standard curve. The quantity of the target gene mRNA was then obtained by division of each value by the actin value. Standards and samples were run in triplicate.
Immunohistochemistry—Long bones from 4-month-old TNF-Tg mice and WT littermates (three/group) were fixed in 10% phosphate-buffered formalin, decalcified in 10% EDTA, and embedded in paraffin wax. Deparaffinized sections were quenched with 3% hydrogen peroxide, treated for antigen retrieval for 30 min, and stained with a rabbit anti-c-Fos antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and a biotinylated goat anti-rabbit secondary antibody (Dako, Carpinteria, CA). The biotin was detected using standard avidin-biotin peroxidase technology (Dako) and diaminobenzidine (Sigma) as the chromogen. An adjacent section was used for TRAP staining to identify mature osteoclasts as described previously (42). c-Fos-positive and -negative osteoclasts on the joint surfaces and in the primary spongiosa of the metaphyses were counted, and the number of c-Fos-positive osteoclasts was expressed as a percentage of the total number of osteoclasts at each site.
IL-1 Protein Measurement—Culture medium from GFP- or c-Fos-infected cells grown on plastic or bone slices in 96-well plates or in the Transwell assay system was collected at the time when osteoclasts could be seen on the plastic of the culture wells or around bone slices using an inverted microscope (generally after 7–9 days). A mouse IL-1β enzyme-linked immunosorbent assay kit (eBioscience, San Diego, CA) was used to measure IL-1 concentration according to the manufacturer's instructions.
Statistics—All results are given as the mean ± S.D. Comparisons between two groups were analyzed using Student's two-tailed unpaired t test. One-way analysis of variance and Dunnett's post hoc multiple comparisons were used for comparisons among three or more groups. p values <0.05 were considered statistically significant. Each experiment was repeated at least twice with similar results.
RESULTS
IL-1 Induces Osteoclast Formation Directly from Osteoclast Precursors Overexpressing c-Fos or NFATc1 and Synergizes with RANKL—To determine whether IL-1 can induce osteoclast formation from WT OCPs overexpressing c-Fos, we infected WT MDS with c-Fos retrovirus and cultured them on plastic culture plates in the presence of M-CSF. c-Fos-expressing MDS alone did not give rise to osteoclasts on plastic plates, but they formed numerous TRAP+ osteoclasts when they were treated with IL-1 (Fig. 1A). These cells also formed resorption pits when they were cultured on bone slices with IL-1 (data not shown). Furthermore, like TNF and RANKL (7), IL-1 induced osteoclast formation directly from NF-κB dKO OCPs overexpressing c-Fos (Fig. 1A). In addition, suboptimal doses of IL-1 and RANKL induced osteoclast formation synergistically (Fig. 1E).
FIGURE 1.
c-Fos overexpression in osteoclast precursors directly induces their differentiation into osteoclasts on bone slices. GFP, c-Fos, or NFATc1 retrovirus-infected WT and NF-κB p50/p52 dKO MDS were cultured on plastic culture plates ± IL-1 or on bone slices + M-CSF to form osteoclasts. Osteoclasts were identified by TRAP staining, and resorption pits were visualized by toluidine blue staining. The number of osteoclasts (Ocls) that formed on plastic dishes from GFP- or c-Fos-infected (A) or NFATc1-infected (B) cells was assessed. TRAP+ osteoclasts in c-Fos-overexpressing cells on bone slices (C, panels a–d) and TRAP+ cells that formed on the plastic dish around bone slices (panels e and f) and the number of osteoclasts on bone slices and pit area (D) were assessed. c-Fos retrovirus-infected WT MDS were cultured with various doses of IL-1 and RANKL on plastic plates to form osteoclasts. The numbers of osteoclasts were assessed (E). Values are the mean ± S.D. of four plastic culture wells or bone slices. *, p < 0.05 versus the respective value for GFP-infected cells or phosphate-buffered saline (PBS)-treated cells.
c-Fos induces expression of NFATc1, which is critical for osteoclastogenesis (12). We found that expression of NFATc1 was up-regulated in cells overexpressing c-Fos when they were treated with IL-1 for 72–96 h, the time when osteoclasts were forming in these cultures (data not shown). IL-1 induced osteoclast formation from both WT and NF-κB dKO MDS expressing NFATc1 (Fig. 1B), and notably, NFATc1 induced osteoclast formation 1 day earlier than c-Fos, although both ultimately induced similar numbers of osteoclasts (data not shown).
Overexpression of c-Fos in Osteoclast Precursors on Bone Slices Promotes Osteoclast Differentiation through Production of Soluble Factors—When we cultured c-Fos-expressing WT or NF-κB dKO MDS on bone slices without IL-1, we found unexpectedly that they gave rise to osteoclasts, and these cells also formed resorption pits (Fig. 1C, panels a–d, and D). Interestingly, osteoclasts also formed from c-Fos-expressing OCPs on the plastic around the bone slices (Fig. 1C, panels e and f). Because there was no other cytokine added to these bone slice cultures except M-CSF and because c-Fos-overexpressing OCPs cultured on plastic did not form osteoclasts in the absence of IL-1, we hypothesized that c-Fos-expressing OCPs grown on bone produce and release a soluble factor(s) to drive differentiation of the precursors on the adjacent plastic into mature osteoclasts or that osteoclasts or OCPs migrate from the bone onto the plastic.
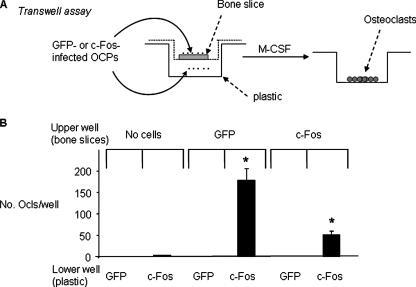
To examine whether c-Fos-expressing OCPs grown on bone secrete a soluble factor(s), we used a Transwell assay system. GFP- or c-Fos-expressing OCPs were cultured on bone slices in the upper chamber, and either GFP- or c-Fos-infected cells were cultured on plastic in the lower chamber of the Transwell culture system (Fig. 2A). These cultures also contained M-CSF. We found that c-Fos-expressing cells grown on the bone slices in the upper chamber induced mature osteoclasts from c-Fos-but not GFP-infected cells in the lower chamber (Fig. 2B). Furthermore, GFP-expressing OCPs cultured on bone slices in the upper chamber induced more osteoclasts in the lower chambers compared with c-Fos-expressing OCPs (Fig. 2B). Bone slices placed alone in the upper chamber without cells did not induce osteoclast formation from c-Fos-expressing OCPs.
FIGURE 2.
Osteoclast precursors grown on bone slices induce osteoclast formation from c-Fos-overexpressing precursors on plastic below them. A, GFP or c-Fos retrovirus-infected WT MDS were cultured on bone slices or plastic in 24-well plates for 2 days in the presence of added M-CSF. Bone slices with either GFP- or c-Fos-infected cells or control slices with no cells were moved into the upper chambers of the Transwells and co-cultured with cells on plastic in the lower chambers as indicated in the presence of added M-CSF. B, the cells in the lower chambers were stained for TRAP activity, and the number of osteoclasts (Ocls) was counted. Values are the mean ± S.D. of three wells. *, p < 0.05 versus the co-culture of c-Fos-infected cells in the lower chamber with blank bone.
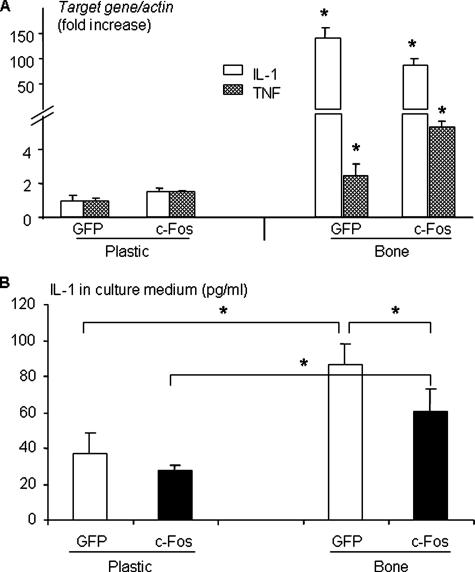
IL-1 Is Produced by Osteoclast Precursors Interacting with Bone and Promotes Osteoclastogenesis from c-Fos-expressing Precursors—TNF and IL-1 are pro-inflammatory cytokines known to be secreted by osteoclasts and OCPs (29). Thus, we considered both as candidate factors that could be produced by OCPs on bone slices to induce osteoclast formation. Both GFP- and c-Fos-expressing OCPs grown on bone slices expressed markedly more IL-1 mRNA (100–150-fold higher) compared with similar OCPs cultured on plastic dishes after 9 days of culture (Fig. 3A), the time when osteoclast formation begins. In contrast, the expression of TNF mRNA by the OCPs was increased by only 3–5-fold on bone slices. Furthermore, GFP-expressing OCPs expressed significantly higher levels of IL-1 mRNA on bone slices (Fig. 3A) and secreted more IL-1 protein into the culture medium compared with c-Fos-expressing OCPs (Fig. 3B).
FIGURE 3.
Osteoclast precursors grown on bone slices express markedly more IL-1 than those cultured on plastic plates. GFP or c-Fos retrovirus-infected WT MDS were cultured on plastic or bone slices in the presence of M-CSF for 9 days. A, total RNA was extracted and subjected to real-time RT-PCR to determine the expression levels of IL-1 and TNF. The -fold changes were calculated by dividing the value of the target gene by that of the GFP-infected samples on plastic. Values are the mean ± S.D. of triplicate loadings. *, p < 0.05 versus cells grown on plastic. B, IL-1β protein concentrations were measured by enzyme-linked immunosorbent assay in the culture medium collected from the cultures described in A at the end of the culture. Data are the mean ± S.D. of five wells from one independent experiment representative of three. *, p < 0.05 between the two groups indicated.
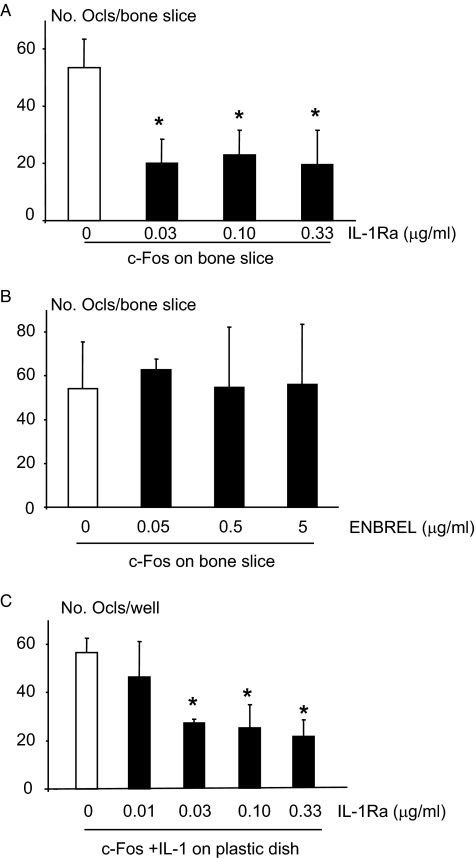
To determine whether IL-1 or TNF is responsible for the differentiation of c-Fos-expressing OCPs on bone slices and functions as a soluble factor, OCPs were infected with c-Fos retrovirus and cultured on bone slices in the presence of IL-1Ra or the TNF inhibitor ENBREL. IL-1Ra reduced c-Fos-induced osteoclast formation by ∼50% (Fig. 4A), an effect similar to that reported as the maximal effect of this protein to inhibit IL-1-induced osteoclast activity (29). In contrast, ENBREL at doses that completely blocked TNF-induced osteoclast formation on plastic had no inhibitory effect on c-Fos-induced osteoclast formation on bone slices (Fig. 4B). Furthermore, IL-1Ra reduced osteoclast formation (Fig. 4C) induced by IL-1 in c-Fos-expressing OCPs cultured on plastic to a similar extent as in c-Fos-expressing OCPs without IL-1 on bone slices (Fig. 4A).
FIGURE 4.
IL-1Ra decreases c-Fos expression-induced osteoclast formation on bone slices. c-Fos retrovirus-infected WT MDS were cultured on bone slices and treated with the indicated doses of IL-1Ra (A) or ENBREL (B) or cultured on plastic plates and treated with IL-1 (10 ng/ml) plus the indicated doses of IL-1Ra (C) in the presence of M-CSF. The number of TRAP+ osteoclasts (Ocls) was counted. Values are the mean ± S.D. of four bone slices or wells. *, p < 0.05 versus the non-blocker control.
Bone Matrix Proteins Stimulate Osteoclast Precursors to Produce IL-1 and Induce Their Differentiation into Osteoclasts When c-Fos Is Overexpressed—Bone matrix contains at least 20 non-collagenous proteins, several of which have been reported to stimulate macrophages to produce cytokines such as IL-1 (43). To examine whether this is also the case for OCPs, we cultured OCPs on plastic, treated them with the bone matrix proteins (DSP, DPP, OPN, or TGFβ), and determined their effects on the levels of IL-1 and TNF mRNA expression by real-time RT-PCR. DSP in particular and also OPN significantly increased IL-1 and to a lesser extent TNF mRNA expression, whereas DPP and TGFβ had no effect (Fig. 5A).
FIGURE 5.
Bone matrix proteins stimulate osteoclast precursors to express IL-1, which induces c-Fos-expressing precursors to form osteoclasts. A, WT MDS were treated with DSP, DPP, OPN, or TGFβ for 4 h at the indicated doses. The expression levels of IL-1 mRNA were evaluated by real-time RT-PCR. The -fold changes were calculated by dividing the value of the treated cells by that of the phosphate-buffered saline (PBS)-treated samples. Values are the mean ± S.D. of triplicate loadings. B, GFP or c-Fos retrovirus-infected WT MDS were treated with DSP (3 μg/ml) or OPN (3 μg/ml) in the absence or presence of IL-1Ra (0.1 μg/ml) for 6 days. The number of TRAP+ osteoclasts (Ocls) was counted. Values are the mean ± S.D. of four wells. *, p < 0.05 versus the non-blocker control (Ctrl).
To determine whether IL-1 induced by DSP or OPN can substitute for exogenous IL-1 and stimulate c-Fos-expressing OCPs to differentiate into osteoclasts, we treated GFP- or c-Fos-infected cells with DSP or OPN on plastic. As expected, these proteins did not induce osteoclast formation from GFP-infected cells. However, they induced osteoclast formation from c-Fos-expressing precursors, which was partially blocked by IL-1Ra (Fig. 5B) to the same extent as observed in cultures on bone slices (Fig. 4A) or in IL-1-induced osteoclast formation (Fig. 4C).
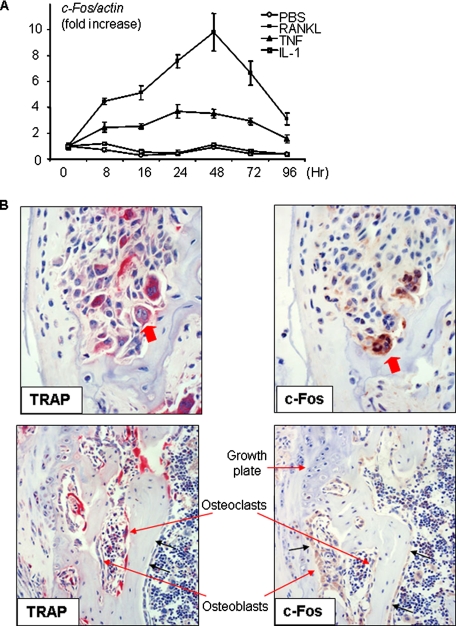
TNF, but Not IL-1, Increases c-Fos Expression in Osteoclast Precursors—Our experiments demonstrated that IL-1-induced osteoclast formation requires forced c-Fos expression in OCPs. We reasoned that IL-1 would not have this effect by itself, otherwise IL-1 alone should be able to induce osteoclast formation. TNF has been reported to induce osteoclast differentiation directly from OCPs in the absence of RANKL (7, 44). To examine whether TNF induces high c-Fos expression in OCPs in vitro, we first treated OCPs with TNF and compared its effects on the expression of c-Fos by OCPs with that of IL-1 and RANKL, which was included here as a positive control. TNF induced sustained c-Fos expression from 8 to 96 h and induced osteoclast formation. RANKL had a similar but less pronounced effect. In contrast, IL-1 did not have any stimulatory effect on c-Fos expression or induce osteoclast differentiation (Fig. 6A).
FIGURE 6.
TNF increases c-Fos expression in osteoclasts and precursors. A, WT MDS were treated with IL-1 (10 ng/ml), TNF (20 ng/ml), RANKL (10 ng/ml), or phosphate-buffered saline (PBS) for the indicated time points. Expression of c-Fos mRNA was measured by real-time RT-PCR. The -fold changes were calculated by dividing the value of the treated cells by that of the untreated base-line samples. Values are the mean ± S.D. of triplicate loadings. B, long bone sections from a knee joint of a 4-month-old TNF-Tg mouse showing severe synovial inflammation and adjacent bone and cartilage erosion were stained for TRAP activity. Adjacent sections were stained with anti-c-Fos antibody. The upper panels show TRAP+ osteoclasts actively resorbing subchondral bone and also strongly expressing c-Fos (arrows). The lower panels show the primary spongiosa of the same animal below the growth plate, where osteoclasts were actively resorbing bone as part of normal endochondral ossification, with these TRAP+ osteoclasts having low or no c-Fos protein expression. Data are representative of three TNF-Tg mice.
We then examined the effect of TNF on c-Fos expression by osteoclasts in vivo using TNF-Tg mice, which develop an inflammatory erosive arthritis similar to rheumatoid arthritis (45). In bone sections from TNF-Tg mice with erosive arthritis, we found a strong signal for c-Fos in the cytoplasm and nuclei of many osteoclasts (43 ± 10%) eroding the bone on the outer surfaces of inflamed joints (Fig. 6B), where local concentrations of IL-1 and TNF are increased (46, 47). In contrast, fewer osteoclasts (21 ± 1%) in the subjacent primary spongiosa away from the inflammation expressed c-Fos, and the intensity of the staining in the same sections was much weaker (Fig. 6B). In the primary spongiosa, c-Fos was expressed strongly in osteoblasts, cells known to up-regulate their expression of c-Fos in response to factors such as parathyroid hormone (48, 49), indicating that the lower intensity of the signal for c-Fos in osteoclasts inside the bones was not due to suboptimal specimen fixation or processing.
DISCUSSION
IL-1 is a potent stimulator of bone resorption in vitro and in vivo (50). It enhances osteoclast precursor fusion and osteoclast survival in vitro (23–25). However, unlike TNF and RANKL, IL-1 cannot induce osteoclast formation directly from OCPs in vitro. In this study, we have demonstrated that IL-1 can induce osteoclast formation directly from OCPs when they are overexpressing c-Fos and that these cells can resorb bone in vitro. Thus, under conditions in which c-Fos expression is increased in OCPs, IL-1 could potentially induce osteoclast formation directly to mediate bone loss in addition to its known effect to induce osteoclast formation indirectly through up-regulation of RANKL expression by accessory cells such as osteoblast/stromal cells or synoviocytes.
c-Fos plays an essential role in osteoclast formation. Its expression is required in OCPs for RANKL- and TNF-induced osteoclast formation (7, 12). c-Fos acts downstream from NF-κB in RANKL- and TNF-induced OCP differentiation into osteoclasts and can substitute for NF-κB p50 and p52 in this process (7). We found that TNF and RANKL, but not IL-1, increase c-Fos expression by OCPs in vitro. This failure of IL-1 to induce c-Fos expression in OCPs could explain its inability to induce osteoclast formation directly from these cells. We found that c-Fos expression is increased in osteoclasts on the eroded surfaces of joints and to a lesser extent in the metaphyseal bones of TNF-Tg mice. TNF and RANKL expression is increased in the joints of these mice and of patients with rheumatoid arthritis, where IL-1 expression is also increased (46). Thus, TNF and RANKL could increase the expression of c-Fos in OCPs in rheumatoid arthritis and other inflammatory bone diseases, and IL-1 could directly induce differentiation of these OCPs into osteoclasts. Our finding that IL-1 acts synergistically with RANKL at very low doses to induce osteoclast formation directly from c-Fos-expressing WT OCPs suggests that this osteoclast-inducing effect of IL-1 could be further enhanced by RANKL. This would represent a new role for IL-1 to increase osteoclast formation by a direct autocrine mechanism that would amplify the bone-resorbing effects of TNF and RANKL and contribute to the vicious cycle we have proposed for cytokine-induced bone loss (7). This effect would be similar to that reported previously for bone sialoprotein, which contributes to RANKL-mediated bone resorption by inducing osteoclastogenesis and osteoclast survival and decreasing osteoclast apoptosis (51).
NFATc1, the master transcription factor regulating late-stage osteoclast differentiation, is activated downstream from c-Fos (7, 12). In NF-κB p50/p52 dKO OCPs treated with RANKL or TNF, NFATc1 rescues the defect in osteoclast formation as effectively as c-Fos (7). We found that NFATc1 also rescues this defect in dKO OCPs treated with IL-1 and that it mediates osteoclast formation from WT OCPs treated with IL-1. These findings indicate that although IL-1 is unable to induce osteoclast formation on its own, its downstream signaling can lead to osteoclast formation in a pathway that requires c-Fos and NFATc1.
Surprisingly, we also found that when OCPs overexpressing c-Fos were cultured on bone slices along with M-CSF, they released sufficient amounts of IL-1 to promote their differentiation into mature bone-resorbing osteoclasts in the absence of other cytokines. This induction of osteoclast formation was inhibited significantly by IL-1Ra, but not by the TNF inhibitor ENBREL, indicating that IL-1 largely mediated the effect. The failure of the IL-1Ra to completely prevent osteoclast formation in any of our assays, including IL-1-treated c-Fos-expressing OCPs, suggests that osteoclast interaction with bone matrix may induce the expression by OCPs of another factor(s). However, a previous study using this reagent (29) and our experience with it indicate that these reagents do not completely prevent IL-1-induced effects on osteoclasts or OCPs. Thus, further studies will be required to resolve this issue.
Our Transwell culture assays showed that GFP-expressing OCPs express more IL-1 and thus support more osteoclast formation from precursors in the lower chamber compared with c-Fos-expressing OCPs. RANKL induces c-Fos expression in OCPs, but it also induces expression of interferon-β, which limits osteoclast formation by down-regulating c-Fos expression (52). However, we found that c-Fos- and GFP-expressing OCPs express similar levels of interferon-β (data not shown). At this time, we do not have a definitive explanation for this difference between these culture conditions. It may represent another autoregulatory mechanism within OCPs to limit excess osteoclast differentiation.
Interaction of osteoclasts with bone matrix is known to increase osteoclast survival (53) through integrin-mediated signaling (28). Our findings indicate a previously unknown effect of interaction of osteoclastic cells with bone matrix, viz. release of IL-1 and differentiation of c-Fos-expressing precursors into mature bone-resorbing osteoclasts. We found that the bone matrix proteins DSP and OPN stimulate expression of IL-1 by OCPs. A previous study has shown that dental matrix proteins stimulate macrophages to release cytokines, including IL-1, TNF, and MCP-1 (monocyte chemoattractant protein-1) (43). Furthermore, we found that DSP and OPN stimulate differentiation of c-Fos-expressing OCPs into osteoclasts and that IL-1Ra significantly inhibits this effect. It could be concluded from these observations that when OCPs adhere to bone matrix, they begin to resorb it and so cause release of these proteins, which in turn could stimulate the OCPs to produce IL-1. However, GFP-expressing OCPs also express increased amounts of IL-1, and they do not form resorption pits even after they have been on the matrix for >10 days. Thus, resorption does not seem to be required for OCPs to increase their expression of IL-1. Furthermore, neither NF-κB p50 nor NF-κB p52 expression appears to be required for OCPs to produce IL-1 because c-Fos-overexpressing NF-κB dKO OCPs also form osteoclasts on bone slices (Fig. 1C). Nevertheless, release of DSP and OPN from bone matrix by osteoclasts during resorption could augment the effect of this interaction.
It is becoming increasingly clear that osteoclasts are involved in more complex processes than simply resorption of bone. Their precursors are formed in the marrow cavity, where they increase their proliferation and expression of c-Fms, the receptor for M-CSF, in response to cytokines such as TNF (54). OCPs circulate in the blood and are attracted to sites of inflammation, where pro-inflammatory cytokine concentrations are high (18), and to sites destined for resorption. OCPs enter resorption lacunae through blood vessels, where they appear to be separated from the adjacent marrow cells by a membrane inside which osteoclasts, osteoblasts, and other cells interact (55). OCPs and osteoclasts secrete cytokines to modulate the activities of cells around them and, in that respect at least, can be considered to be immune cells at sites of inflammation in bone (8, 18, 56). Recent studies indicate that OCPs not only respond to osteoblasts to have their formation regulated either positively or negatively (1) but also regulate osteoblast formation both positively and negatively (30, 57). Our studies indicate that OCPs also react to signals coming from bone matrix by secreting IL-1. It is likely that this interaction between OCPs and osteoblastic cells is more complicated than these recent studies suggest and that other proteins released from the matrix will affect the function of osteoclasts and other cells in the resorption lacunae. IL-1 has multiple ways of promoting bone resorption, including mediating the pro-osteoclastogenic effects of TNF through osteoblast/stromal cells (29). IL-1 is generally a more powerful stimulator of bone resorption than TNF, and this direct action mediated through interaction with bone matrix may be one of the mechanisms that accounts for these differences.
In conclusion, our findings have defined novel interactions among OCPs, bone matrix, and cytokines at sites of bone resorption. These involve an autocrine role for IL-1 to induce osteoclast formation from OCPs in which c-Fos expression has been up-regulated by cytokines such as TNF and RANKL to amplify their effects at sites in bone where their concentrations are increased. They also support involvement of a paracrine loop in which matrix proteins such as DSP and OPN could induce increased secretion of IL-1 by OCPs to induce the differentiation of c-Fos-expressing OCPs into bone-resorbing osteoclasts. Collectively, these processes could account for the aggressive resorption observed in common inflammatory bone diseases.
Acknowledgments
We thank Dr. Toshio Kitamura for the Plat-E cell line and Bianai Fan for technical assistance with histology.
This work was supported by National Institutes of Health Grants AR43510 (to B. F. B.) and AR48697 (to L. X.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: M-CSF, macrophage colony-stimulating factor; OCPs, osteoclast precursors; TNF, tumor necrosis factor; GFP, green fluorescent protein; IL-1, interleukin-1; TGFβ, transforming growth factor-β; OPN, osteopontin; DSP, dentin sialoprotein; IL-1Ra, IL-1 receptor antagonist; DPP, dentin phosphoprotein; WT, wild-type; dKO, double knock-out; TNF-Tg, TNF transgenic; MDS, M-CSF-dependent splenocytes; TRAP, tartrate-resistant acid phosphatase; RT, reverse transcription.
References
- 1.Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003) Nature 423 337-342 [DOI] [PubMed] [Google Scholar]
- 2.Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., Elliott, R., Colombero, A., Elliott, G., Scully, S., Hsu, H., Sullivan, J., Hawkins, N., Davy, E., Capparelli, C., Eli, A., Qian, Y. X., Kaufman, S., Sarosi, I., Shalhoub, V., Senaldi, G., Guo, J., Delaney, J., and Boyle, W. J. (1998) Cell 93 165-176 [DOI] [PubMed] [Google Scholar]
- 3.Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S., Oliveira-dos-Santos, A. J., Van, G., Itie, A., Khoo, W., Wakeham, A., Dunstan, C. R., Lacey, D. L., Mak, T. W., Boyle, W. J., and Penninger, J. M. (1999) Nature 397 315-323 [DOI] [PubMed] [Google Scholar]
- 4.Li, J., Sarosi, I., Yan, X. Q., Morony, S., Capparelli, C., Tan, H. L., McCabe, S., Elliott, R., Scully, S., Van, G., Kaufman, S., Juan, S. C., Sun, Y., Tarpley, J., Martin, L., Christensen, K., McCabe, J., Kostenuik, P., Hsu, H., Fletcher, F., Dunstan, C. R., Lacey, D. L., and Boyle, W. J. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1566-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapur, R. P., Yao, Z., Iida, M. H., Clarke, C. M., Doggett, B., Xing, L., and Boyce, B. F. (2004) J. Bone Miner. Res. 19 1689-1697 [DOI] [PubMed] [Google Scholar]
- 6.Arai, F., Miyamoto, T., Ohneda, O., Inada, T., Sudo, T., Brasel, K., Miyata, T., Anderson, D. M., and Suda, T. (1999) J. Exp. Med. 190 1741-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita, T., Yao, Z., Li, F., Zhang, Q., Badell, I. R., Schwarz, E. M., Takeshita, S., Wagner, E. F., Noda, M., Matsuo, K., Xing, L., and Boyce, B. F. (2007) J. Biol. Chem. 282 18245-18253 [DOI] [PubMed] [Google Scholar]
- 8.Takayanagi, H. (2007) Nat. Rev. Immunol. 7 292-304 [DOI] [PubMed] [Google Scholar]
- 9.Boyce, B. F., Yamashita, T., Yao, Z., Zhang, Q., Li, F., and Xing, L. (2005) J. Bone Miner. Metab. 23, (suppl.) 11-15 [DOI] [PubMed] [Google Scholar]
- 10.Karsenty, G., and Wagner, E. F. (2002) Dev. Cell 2 389-406 [DOI] [PubMed] [Google Scholar]
- 11.Matsuo, K., Owens, J. M., Tonko, M., Elliott, C., Chambers, T. J., and Wagner, E. F. (2000) Nat. Genet. 24 184-187 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo, K., Galson, D. L., Zhao, C., Peng, L., Laplace, C., Wang, K. Z., Bachler, M. A., Amano, H., Aburatani, H., Ishikawa, H., and Wagner, E. F. (2004) J. Biol. Chem. 279 26475-26480 [DOI] [PubMed] [Google Scholar]
- 13.Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T. (2002) Dev. Cell 3 889-901 [DOI] [PubMed] [Google Scholar]
- 14.Asagiri, M., Sato, K., Usami, T., Ochi, S., Nishina, H., Yoshida, H., Morita, I., Wagner, E. F., Mak, T. W., Serfling, E., and Takayanagi, H. (2005) J. Exp. Med. 202 1261-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan, C., and Finnegan, A. (2003) Front. Biosci. 8 d1018-d1029 [DOI] [PubMed] [Google Scholar]
- 16.Sato, K., and Takayanagi, H. (2006) Curr. Opin. Rheumatol. 18 419-426 [DOI] [PubMed] [Google Scholar]
- 17.Teitelbaum, S. L. (2000) J. Bone Miner. Metab. 18 344-349 [DOI] [PubMed] [Google Scholar]
- 18.Xing, L., Schwarz, E. M., and Boyce, B. F. (2005) Immunol. Rev. 208 19-29 [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum, S. L. (2006) Arthritis Res. Ther. 8 201-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofbauer, L. C., Lacey, D. L., Dunstan, C. R., Spelsberg, T. C., BRiggs, B. L., and Khosla, S. (1999) Bone 25 255-259 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, K., Takahashi, N., Jimi, E., Udagawa, N., Takami, M., Kotake, S., Nakagawa, N., Kinosaki, M., Yamaguchi, K., Shima, N., Yasuda, H., Morinaga, T., Higashio, K., Martin, T. J., and Suda, T. (2000) J. Exp. Med. 191 275-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimi, E., Nakamura, I., Duong, L. T., Ikebe, T., Takahashi, N., Rodan, G. A., and Suda, T. (1999) Exp. Cell Res. 247 84-93 [DOI] [PubMed] [Google Scholar]
- 23.Lee, Z. H., Lee, S. E., Kim, C. W., Lee, S. H., Kim, S. W., Kwack, K., Walsh, K., and Kim, H. H. (2002) J. Biochem. (Tokyo) 131 161-166 [DOI] [PubMed] [Google Scholar]
- 24.Jimi, E., Akiyama, S., Tsurukai, T., Okahashi, N., Kobayashi, K., Udagawa, N., Nishihara, T., Takahashi, N., and Suda, T. (1999) J. Immunol. 163 434-442 [PubMed] [Google Scholar]
- 25.Jimi, E., Shuto, T., and Koga, T. (1995) Endocrinology 136 808-811 [DOI] [PubMed] [Google Scholar]
- 26.Chambers, T. J. (1981) J. Pathol. 135 1-7 [DOI] [PubMed] [Google Scholar]
- 27.Tatsumi, S., Ishii, K., Amizuka, N., Li, M., Kobayashi, T., Kohno, K., Ito, M., Takeshita, S., and Ikeda, K. (2007) Cell Metab. 5 464-475 [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum, S. L. (2006) Ann. N. Y. Acad. Sci. 1068 95-99 [DOI] [PubMed] [Google Scholar]
- 29.Wei, S., Kitaura, H., Zhou, P., Ross, F. P., and Teitelbaum, S. L. (2005) J. Clin. Investig. 115 282-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, S. H., Rho, J., Jeong, D., Sul, J. Y., Kim, T., Kim, N., Kang, J. S., Miyamoto, T., Suda, T., Lee, S. K., Pignolo, R. J., Koczon-Jaremko, B., Lorenzo, J., and Choi, Y. (2006) Nat. Med. 12 1403-1409 [DOI] [PubMed] [Google Scholar]
- 31.Mundy, G. R., and Bonewald, L. F. (1990) Ann. N. Y. Acad. Sci. 593 91-97 [DOI] [PubMed] [Google Scholar]
- 32.Zwerina, J., Hayer, S., Tohidast-Akrad, M., Bergmeister, H., Redlich, K., Feige, U., Dunstan, C., Kollias, G., Steiner, G., Smolen, J., and Schett, G. (2004) Arthritis Rheum. 50 277-290 [DOI] [PubMed] [Google Scholar]
- 33.Qin, C., Baba, O., and Butler, W. T. (2004) Crit. Rev. Oral Biol. Med. 15 126-136 [DOI] [PubMed] [Google Scholar]
- 34.Fisher, L. W., Torchia, D. A., Fohr, B., Young, M. F., and Fedarko, N. S. (2001) Biochem. Biophys. Res. Commun. 280 460-465 [DOI] [PubMed] [Google Scholar]
- 35.Reinholt, F. P., Hultenby, K., Oldberg, A., and Heinegard, D. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 4473-4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishijima, M., Rittling, S. R., Yamashita, T., Tsuji, K., Kurosawa, H., Nifuji, A., Denhardt, D. T., and Noda, M. (2001) J. Exp. Med. 193 399-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valverde, P., and Chen, J. (2006) J. Bone Miner. Res. 21 Suppl. 1, S167 (abstr.) [Google Scholar]
- 38.Qin, C., Brunn, J. C., Cadena, E., Ridall, A., Tsujigiwa, H., Nagatsuka, H., Nagai, N., and Butler, W. T. (2002) J. Dent. Res. 81 392-394 [DOI] [PubMed] [Google Scholar]
- 39.Li, P., Schwarz, E. M., O'Keefe, R. J., Ma, L., Looney, R. J., Ritchlin, C. T., Boyce, B. F., and Xing, L. (2004) Arthritis Rheum. 50 265-276 [DOI] [PubMed] [Google Scholar]
- 40.Takeshita, S., Kaji, K., and Kudo, A. (2000) J. Bone Miner. Res. 15 1477-1488 [DOI] [PubMed] [Google Scholar]
- 41.Johnson, M. R., Wang, K., Smith, J. B., Heslin, M. J., and Diasio, R. B. (2000) Anal. Biochem. 278 175-184 [DOI] [PubMed] [Google Scholar]
- 42.Xing, L., Bushnell, T. P., Carlson, L., Tai, Z., Tondravi, M., Siebenlist, U., Young, F., and Boyce, B. F. (2002) J. Bone Miner. Res. 17 1200-1210 [DOI] [PubMed] [Google Scholar]
- 43.Silva, T. A., Lara, V. S., Silva, J. S., Oliveira, S. H., Butler, W. T., and Cunha, F. Q. (2005) J. Dent. Res. 84 79-83 [DOI] [PubMed] [Google Scholar]
- 44.Kim, N., Kadono, Y., Takami, M., Lee, J., Lee, S. H., Okada, F., Kim, J. H., Kobayashi, T., Odgren, P. R., Nakano, H., Yeh, W. C., Lee, S. K., Lorenzo, J. A., and Choi, Y. (2005) J. Exp. Med. 202 589-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keffer, J., Probert, L., Cazlaris, H., Georgopoulos, S., Kaslaris, E., Kioussis, D., and Kollias, G. (1991) EMBO J. 10 4025-4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldring, S. R. (2003) Calcif. Tissue Int. 73 97-100 [DOI] [PubMed] [Google Scholar]
- 47.Sfikakis, P. P., and Kollias, G. (2003) Curr. Opin. Rheumatol. 15 380-386 [DOI] [PubMed] [Google Scholar]
- 48.Lee, E., Miura, M., Yoshinari, M., Iwai, H., and Kariya, K. (1994) Biochem. Biophys. Res. Commun. 202 128-134 [DOI] [PubMed] [Google Scholar]
- 49.Clohisy, J. C., Scott, D. K., Brakenhoff, K. D., Quinn, C. O., and Partridge, N. C. (1992) Mol. Endocrinol. 6 1834-1842 [DOI] [PubMed] [Google Scholar]
- 50.Boyce, B. F., Aufdemorte, T. B., Garrett, I. R., Yates, A. J., and Mundy, G. R. (1989) Endocrinology 125 1142-1150 [DOI] [PubMed] [Google Scholar]
- 51.Valverde, P., Tu, Q., and Chen, J. (2005) J. Bone Miner. Res. 20 1669-1679 [DOI] [PubMed] [Google Scholar]
- 52.Takayanagi, H., Kim, S., Matsuo, K., Suzuki, H., Suzuki, T., Sato, K., Yokochi, T., Oda, H., Nakamura, K., Ida, N., Wagner, E. F., and Taniguchi, T. (2002) Nature 416 744-749 [DOI] [PubMed] [Google Scholar]
- 53.Hughes, D. E., Wright, K. R., Uy, H. L., Sasaki, A., Yoneda, T., Roodman, G. D., Mundy, G. R., and Boyce, B. F. (1995) J. Bone Miner. Res. 10 1478-1487 [DOI] [PubMed] [Google Scholar]
- 54.Yao, Z., Li, P., Zhang, Q., Schwarz, E. M., Keng, P., Arbini, A., Boyce, B. F., and Xing, L. (2006) J. Biol. Chem. 281 11846-11855 [DOI] [PubMed] [Google Scholar]
- 55.Hauge, E. M., Qvesel, D., Eriksen, E. F., Mosekilde, L., and Melsen, F. (2001) J. Bone Miner. Res. 16 1575-1582 [DOI] [PubMed] [Google Scholar]
- 56.Baron, R. (2004) Nat. Med. 10 458-460 [DOI] [PubMed] [Google Scholar]
- 57.Zhao, C., Irie, N., Takada, Y., Shimoda, K., Miyamoto, T., Nishiwaki, T., Suda, T., and Matsuo, K. (2006) Cell Metab. 4 111-121 [DOI] [PubMed] [Google Scholar]