Abstract
Proteolytic processing of laminin-332 by matrix metalloproteinase (MMP)-2 and MMP-14 has been shown to yield fragments that are promigratory for epithelial cells. During acute and chronic inflammation, proteases are elaborated by neutrophils and macrophages that can degrade basement membranes. We investigated the susceptibility of laminin-332 to degradation by the following neutrophil and macrophage proteases: neutrophil elastase (NE), cathepsin G, proteinase-3, and MMPs-2, -8, -9, and -12. Protease-specific differences were seen in the capacity to cleave the individual chains of laminin-332. NE and MMP-12 showed the greatest activity toward the γ2 chain, generating a fragment similar in size to the γ2x fragment generated by MMP-2. The digestion pattern of laminin-332 by degranulated neutrophils was nearly identical to that generated with NE alone. Digestion by supernatants of degranulated neutrophils was blocked by an inhibitor of NE, and NE-deficient neutrophils were essentially unable to digest laminin-332, suggesting that NE is the major neutrophil-derived protease that degrades laminin-332. In vivo, laminin γ2 fragments were found in the bronchoalveolar lavage fluid of wild-type mice treated with lipopolysaccharide, whereas that obtained from NE-deficient mice showed a different cleavage pattern. In addition, NE cleaved a synthetic peptide derived from the region of human laminin γ2 containing the MMP-2 cleavage site, suggesting that NE may generate laminin-332 fragments that are also promigratory. Both laminin-332 fragments generated by NE digestion and NE-digested laminin γ2 peptide were found to be chemotactic for neutrophils. Collectively, these data suggest that degradation of laminin-332 by NE generates fragments with important biological activities.
Laminins are heterotrimeric proteins composed of α, β, and γ chains that are essential components of all basement membranes. Five α, four β, and three γ chains have been identified, which associate to form at least 15 laminin isoforms (1). Laminin-332 (formerly designated laminin-5) is composed of the α3, β3, and γ2 chains and is a critical adhesive component of hemidesmosomes, specialized basement membrane structures underlying certain epithelia (2). Mutations of the LAMA3 (α3), LAMB3 (β3), or LAMC2 (γ2) genes result in the severe blistering disease epidermolysis bullosa (3, 4). Laminin-332 is presumably an essential laminin, as mice lacking expression of the γ2 chain, which is unique to laminin-332, die a few days after birth (5).
The α3, β3, and γ2 chains are thought to self-associate via a central coiled-coil domain structure, with the short N-terminal arms of each extending from the coiled region to form a cruciform structure. Additionally, the C terminus of the α3 chain extends beyond the coiled region exposing five large globular (LG)2 domains that are important in modulating the phenotype of attached cells. The unprocessed form of the α3 chain is thought to bind the α3β1 integrin via the LG2 and LG3 domains, facilitating haptotactic migration of multiple cell types (6–8). Proteolytic processing of laminin-332 has been shown to regulate migration of overlying epithelial cells. Cleavage of the α3 chain of laminin-332 between the LG3 and LG4 domain by plasmin facilitates hemidesmosome formation through integrin α6β4 and decreases motility (9, 10). Inhibition of cell migration by the α6β4 integrin also involves up-regulation of E-cadherin, which mediates cell-cell adhesion (11).
Although limited data suggest that processing of the β3 chain of laminin-332 by matrix metalloproteinase (MMP)-14 promotes migration of prostate carcinoma cells (12), significantly more is known regarding processing of the γ2 chain and how its processing affects cell motility. The rat γ2 chain was shown to be cleaved by MMP-2 after the Ala-586 residue at the junction of the short arm and coiled-coil domain, removing the entire N-terminal short arm and resulting in an 80-kDa C-terminal fragment (γ2x) that remains bound to the α3 and β3 subunits (13). This cleavage is thought to expose a cryptic site on the α3 chain that is promigratory for breast epithelial cells (13). Furthermore, the 80-kDa γ2x form has been associated with tissues undergoing remodeling, whereas full-length γ2 is found in quiescent tissues (13–16). Other MMPs, including MMP-3, -12, -13, -14, -19, and -20, have also been shown to cleave the γ2 chain, generating the γ2x fragment, inducing epithelial cell migration (15, 17, 18). In addition to this cleavage site near the coiled-coil region, MMP-14 cleaves the short arm of the γ2 chain at an additional site (15, 19), releasing an ∼20-kDa D3 domain fragment containing three EGF repeats. Cleavage at this site alone by MMP-14 without removal of the D3 domain generates a 100-kDa fragment still bound to the α3 and β3 subunits known as γ2′. BMP-1, the enzyme that processes the C terminus of type I procollagen, also has also been shown to cleave near the N-terminal side of the D3 domain in the γ2 chain (20). A recombinant D3 fragment has been shown to bind the EGF receptor, stimulating activation of MAPK and cell migration (14).
In this study, we demonstrate that the serine protease neutrophil elastase (NE) cleaves all three chains of laminin-332 and generates a γ2 fragment similar in size to the γ2x fragment generated by MMPs. The similarity between the digestion patterns of laminin-332 induced by degranulated neutrophil supernatant and with NE alone, the ability of an inhibitor of NE to block these digestions, and the lack of laminin-332 digestion by NE-deficient neutrophil supernatants collectively suggest that NE is the major enzyme in neutrophils capable of digesting laminin-332. In addition, laminin γ2 fragments were detected in the bronchoalveolar lavage (BAL) fluid of mice treated with lipopolysaccharide (LPS), a potent inducer of neutrophil inflammation, but the fragmentation pattern of the γ2 chain is different in NE-deficient mice, suggesting a role of NE in the digestion of laminin-332 in vivo. A peptide containing the MMP-2 cleavage site in the γ2 chain is cleaved by NE at the same location as MMP-2, suggesting that NE cleavage of laminin-332 may also result in the generation of promigratory fragments. Digestion of purified laminin-332 with NE produces fragments that are chemotactic for neutrophils, in contrast to the intact trimer, revealing a new activity for cryptic sites in laminin-332 in addition to effects on epithelial cell migration.
EXPERIMENTAL PROCEDURES
Reagents—Rat laminin-332 was purchased from Chemicon International (Temecula, CA). Mouse laminin-111 and human laminin-511 were purchased from Sigma. Human MMP-2, -8, -9, and -12 were purchased from R & D Systems. Human NE, proteinase-3 (PR3), and cathepsin G (CG) were purchased from Elastin Products Co. (Owensville, MO).
Mouse Strains—Six- to eight-week-old C57BL/6 mice were obtained from Taconic Farms (Hudson, NY). NE-deficient mice (21) were obtained from Dr. C. Pham (Washington University, St. Louis, MO). Mice were housed in a barrier facility under the veterinary care of the Department of Comparative Medicine, Washington University School of Medicine. All procedures involving mice were approved by the Washington University School of Medicine Animal Studies Committee and were performed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Cleavage of Laminin-332—Purified laminin-332 (100 ng) was digested at protease concentrations ranging from 15 pm to 50 nm in 15 mm Tris (pH 7.5), 150 mm NaCl, and 5 mm CaCl2 for 6 h at 37 °C in a final volume of 13.3 μl. Prior to digestion, MMP-2, -8, and -9 were activated with 1 mm aminophenylmercuric acetate (1, 2, and 8 h, respectively) at 37 °C. MMP-12 was auto-activated by incubation at 37 °C for 12 h. Activation was confirmed prior to incubation with laminin-332 (data not shown). Digestion products of laminin-332 were separated on 4–15% gradient SDS-PAGE under reducing conditions and analyzed by silver staining or immunoblotting. Digestions of laminin-111 and laminin-511 were performed similarly using 1500 and 250 ng, respectively, of purified laminin.
Immunoblotting Analysis—Laminin digestion products were analyzed by immunoblotting using laminin chain-specific antibodies after proteins were transferred onto Immobilon-P PVDF membranes (Millipore) and the membranes blocked with 5% nonfat dried milk in Tris-buffered saline containing 0.1% Tween 20 overnight at 4 °C. Monoclonal antibodies against the α3 (CM6) and β3 (FM3) chains were gifts from Dr. G. Plopper (Rensselaer Polytechnic Institute) (22) and were used at a 1:6000 dilution. A polyclonal antibody against the γ2 chain was a gift from Dr. G. Meneguzzi (INSERM U634, France) (23) and was used at 1:10,000. To detect laminin-111 chains, monoclonal antibodies to the human β1 (Chemicon, catalog number 1920) and γ1 chains (Chemicon, catalog number 1921) were used at 1:5000. A polyclonal antibody (8B3) against the α1 chain was a gift from Dr. D. Abrahamson (University of Kansas Medical Center) (24) and was used at 1:5000. A monoclonal antibody against the human α5 chain (Chemicon catalog number 1924) was used at 1:4000. Of note, this antibody was described by the vendor as effective for immunoblot analysis only under nonreducing conditions. Peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) were used at 1:20,000, and blots were developed using the ECL Plus Western blotting detection system (Amersham Biosciences) and subsequent autoradiography.
Isolation of Neutrophils from Human Peripheral Blood and Mouse Bone Marrow—Neutrophils were isolated from blood from healthy volunteers by dextran sedimentation, hypotonic lysis of red blood cells, and Histopaque density centrifugation. Procedures using human subjects were approved by the Washington University School of Medicine Human Studies Committee. Neutrophils were isolated from the bone marrow collected from the femurs and tibias of wild-type and NE-deficient mice as described previously (25). Briefly, distal and proximal tips were cut off and washed with cold PBS buffer using a 1-ml syringe and 27-gauge needle. After dispersing cell clumps with a 1-ml pipette on ice, the suspension was centrifuged (200 × g, 10 min, 4 °C), and the pellet was resuspended in 2 ml of sterile PBS. The cell suspension was carefully layered on the top of a discontinuous Percoll gradient (72, 63, and 50%; 2 ml each). After centrifugation at 500 × g for 30 min, the lowest band was transferred into 10 ml of PBS in a new tube. Residual erythrocytes were eliminated by hypotonic lysis. The neutrophils were adjusted to a concentration of 5 × 106/ml and resuspended in Hanks' balanced salt solution.
Preparation of Degranulated Neutrophil Supernatants—Degranulation of neutrophils was performed by incubation for 15 min in cytochalasin B (5 μg/ml) followed by 30 min with N-formyl-methionyl-leucyl-phenylalanine (fMLP, 10 nm). Neutrophil supernatants were collected by centrifugation at 400 × g for 10 min at 4 °C. Specific colorimetric substrates (Sigma) were used to assess the activity of NE (N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide) and CG (N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide) in these supernatants. Briefly, 100 μl of supernatant was added to 100 μl of substrate mixture resulting in a final concentration of 0.5 mm substrate in 25 mm Tris (pH 7.5). Reactions were read at 405 nm every 30 s for 30 min. An NE standard was used to calculate Vmax/min. Supernatants were adjusted to contain ∼50 nm NE activity for incubation with laminin-332. In some experiments, digestions were performed in the presence of 0.1 mm N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, an NE-specific inhibitor.
Intranasal Instillation of LPS—Adult wild-type C57BL/6 or NE-deficient mice (21) were anesthetized, placed supine, and dropwise inoculated intranasally with 50 μl of PBS containing 100 μgof Escherichia coli LPS (Sigma). Following the inoculation, the mice were kept supine 1–2 min to ensure inhalation of the sample. After 24 h, the mice were euthanized by carbon dioxide narcosis, and BAL fluid was retrieved by injecting three times 0.7 ml of saline through the trachea and pooling the fractions. Protein concentrations of the BAL samples were determined using the DC protein assay kit (Bio-Rad). Protease inhibitor mixture (Sigma) was added to each BAL fluid to prevent ex vivo proteolysis. BAL samples were analyzed by immunoblotting using an antibody against the γ2 chain as described above. This experiment was performed using three mice per condition three independent times.
Digestion and Analysis of Human Laminin γ2 Peptide—A peptide derived from the region surrounding the MMP-2 cleavage site in human laminin γ2 (FGGPNCEHGAFSCPACYNQVKI) was synthesized on an ABI-431A synthesizer using Fast-Moc chemistry on Wang-capped resins. After cleavage, the peptide was dissolved in MilliQ water with 0.05% trifluoroacetic acid and purified with reverse phase HPLC on a Vydak 218TP1022 C-18 column using a linear 0–50% acetonitrile gradient. Peak fractions were dried by rotary evaporation, dissolved in MilliQ water, and lyophilized. The peptide was dissolved in water, and disulfide bonds were allowed to form before use. LC-mass spectrometry was performed to verify the identity of the purified peptide by the Protein and Nucleic Acid Chemistry Laboratory at Washington University School of Medicine. The concentration was determined by amino acid analysis using a Beckman 6300 high performance amino acid analyzer. The susceptibility of the peptide to NE cleavage was determined by incubation of the peptide (125 μg at 0.5 μg/μl) with 50 nm NE for 1 h at 37 °C. The reaction was terminated by the addition of phenylmethylsulfonyl fluoride to 0.8 mm, and the digests were analyzed by HPLC and electrospray mass spectrometry (Protein and Nucleic Acid Chemistry Laboratory, Washington University).
Neutrophil Chemotaxis Assay—Human neutrophils were isolated as described above. Chemotaxis was assayed in modified Boyden chambers as described previously (26). Intact laminin-332 (800 ng/ml; 1 nm), NE-digested laminin-332 (1 nm incubated with 50 nm NE, 6 h at 37 °C), or NE alone (50 nm) was placed in the lower compartment of the chambers. fMLP (10 nm) and IL-8 (5 nm) were used as positive controls. Neutrophils (1 × 105/well) were placed in the upper compartment. Upper and lower compartments were separated by polyvinylpyrrolidone-free polycarbonate filters (Osmonics, Liverpool, CA) with 3-μm pore size. Following a 90-min incubation at 37 °C in 5% CO2, the filter was stained with Wright's stain, and nonmigrated cells from the upper side of the filter were removed, and the number of migrated neutrophils on the underside of the filter were counted in 10 random high powered fields (×400) for each of the triplicate wells. Cell counts were averaged and standard errors calculated. Data are representative of three independent experiments.
RESULTS
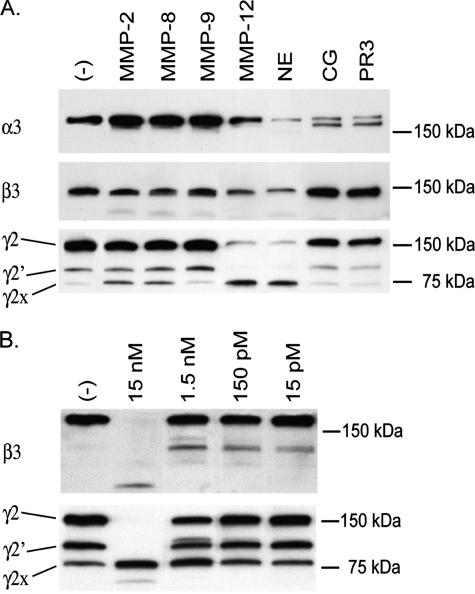
Laminin-332 Chains Are Differentially Susceptible to Cleavage by Neutrophil and Macrophage Proteases—The γ2 chain of laminin-332 is cleaved by a number of MMPs, leading to the production of fragments that are promigratory for epithelial cells (13). As neutrophilic inflammation is a major contributor to the acute inflammatory response that the basement membrane encounters following initial injury, we investigated whether the individual chains of laminin-332 are also susceptible to cleavage by serine proteases found in neutrophils. The serine proteases NE, CG, and PR3 were all capable of degrading the α3 chain of rat laminin-332, as indicated by alterations in banding pattern or reduction in the band intensities upon immunoblotting (Fig. 1A). The β3 and γ2 chains were substrates for cleavage by NE but were resistant to cleavage by CG and PR3 (Fig. 1A).
FIGURE 1.
Susceptibility of laminin-332 to digestion by MMPs and serine proteases. A, 100 ng of purified laminin-332 was incubated with 50 nm human neutrophil elastase (NE), cathepsin G (CG), proteinase-3 (PR3), or matrix metalloproteinase (MMP) 2, 8, 9, or 12 for 6 h at 37 °C. B, laminin-332 was digested with NE for 24 h at enzyme concentrations ranging from 15 pm to 15 nm. Digestion products of laminin-332 were separated on 4–15% gradient SDS-polyacrylamide gels under reducing conditions, transferred to PVDF membranes, and analyzed by immunoblotting using antibodies directed against the lamininα3, β3, orγ2 chains.
The α3 and β3 chains of laminin-332 were marginally susceptible to cleavage by the MMPs tested. The widened appearance of the α3 band after digestion with MMPs-2, -8, and -9 likely resulted from a cleavage that slightly alters the size of the α3 chain. MMP-12 and NE had the most potent activity against the γ2 chain of those proteases tested. NE was found to have some activity against laminin-332, particularly the β3 chain, at enzyme concentrations as low as 15 pm (Fig. 1B), representing an enzyme to substrate ratio of ∼1:1000, supporting a physiologic role for NE in degrading laminin-332 in vivo. Importantly, like the MMPs that have been shown to cleave the γ2 chain liberating promigratory fragments, NE also generates a stable product of 80-kDa, the size of the γ2x fragment (13, 15, 18).
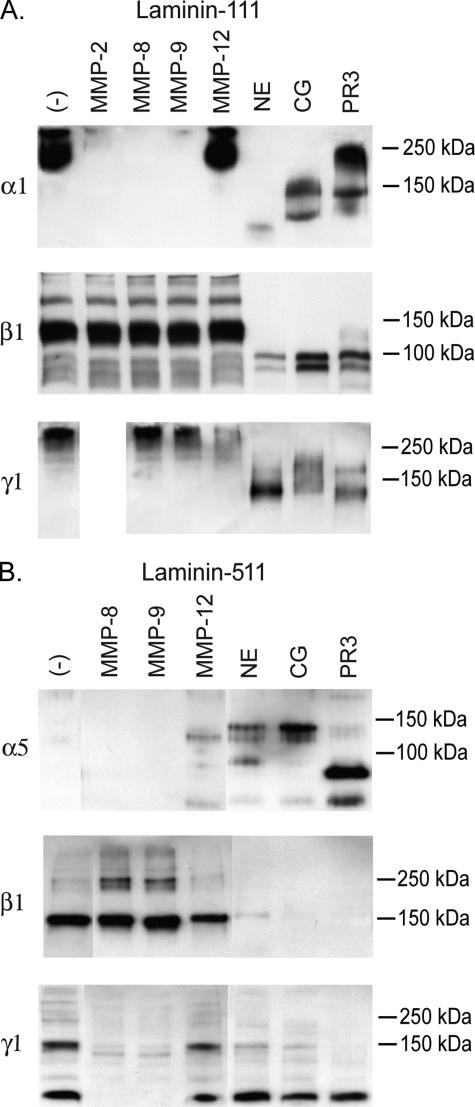
Laminins-332, -111, and -511 Are Differentially Susceptible to Cleavage by Neutrophil and Macrophage Proteases—The ability of individual proteases or classes of proteases to degrade different laminin isoforms is specific and unpredictable. For example, although the MMPs we tested were only marginally able to degrade the α3 chain of laminin-332 (Fig. 1A), most were very active against the α1 chain of laminin-111, with MMP-12 being the exception (Fig. 2A). In contrast, among the MMPs we tested, only MMP-12 showed the ability to degrade the α5 chain (Fig. 2B). The serine proteases as a group appear capable of degrading all three α chains. Of note, the immunoblot for the α5 chain was performed under nonreducing conditions, and only digested fragments were detected. No bands were detected when the gels were done in reducing conditions (data not shown), supporting that this α5 antibody does not work on reduced samples.
FIGURE 2.
Susceptibility of laminin-111 and laminin-511 to digestion by MMPs and serine proteinases. 150 ng of laminin-111 (A) or 250 ng of laminin-511 (B) was digested with 50 nm NE, CG, PR3, or MMP-2, -8, -9, or -12 for 6 h at 37 °C. Digestion products were separated on 4–15% gradient SDS-polyacrylamide gels, transferred to PVDF membranes, and analyzed by immunoblotting using antibodies directed against the laminin α1, α5, β1, or γ1 chains. Gels used for detection of the laminin α5 chain digestion products were performed under nonreducing conditions according to the manufacturer's recommendations for the anti-laminin α5 antibody. All other gels were run under reducing conditions.
Although MMP-12 and NE are curiously unique in their ability to degrade the β3 chain (Fig. 1A), striking differences exist between these MMPs and serine proteases in their capacity to cleave the β1 chain of laminin-111 (Fig. 2A) or laminin-511 (Fig. 2B). These MMPs showed little or no activity against the β1 chain of either of these laminin isoforms, whereas all serine proteases tested were active against this chain.
Unlike the unique ability of NE among the serine proteases to degrade the γ2 chain, all three serine proteases tested appear equally capable of degrading the γ1 chain of laminin-111 (Fig. 2A) or laminin-511 (Fig. 2B). Interestingly, MMP-8 and -9 are relatively unable to degrade the γ1 chain of laminin-111, whereas MMP-8 and -9 demonstrate significant activity against the same chain in the context of laminin-511. This raises the possibility that the context or complement of other chains present affects the susceptibility of the γ1 chain to degradation by these MMPs. Alternatively, the fact that laminin-511 is mildly digested with pepsin during its isolation may predispose it to degradation that does not occur in the native protein. MMP-12 displayed little or no activity against this chain in either of these laminin isoforms.
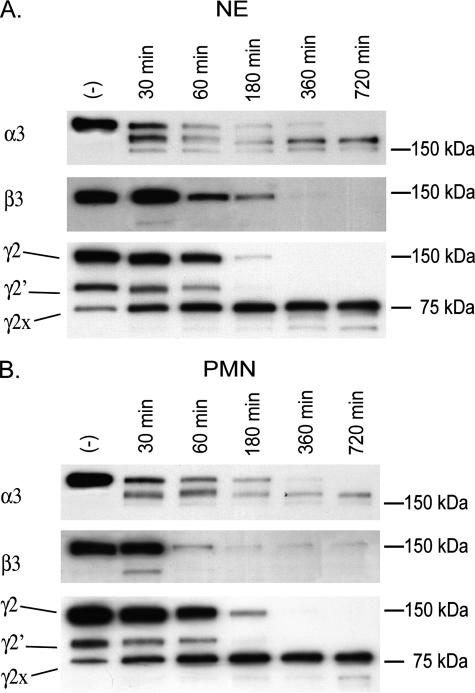
NE Is the Major Enzyme in Neutrophils Capable of Degrading Laminin-332—To determine the contribution of NE to total neutrophil-mediated degradation of laminin-332, we compared the digestion patterns observed when laminin-332 was cleaved by NE versus a degranulated neutrophil supernatant (Fig. 3). By immunoblotting, the digestion patterns for all three laminin-332 chains were very similar when produced by either NE alone or degranulated neutrophil supernatant. As the cleavage patterns of laminin-332 differ between NE and other neutrophil-derived serine proteinases (Fig. 1A), this suggested that NE is the primary enzyme released by neutrophil responsible for cleavage of laminin-332.
FIGURE 3.
Neutrophil supernatants digest laminin-332 in a similar fashion as NE alone. 100 ng of laminin-332 was digested with 50 nm NE or with 50 nm NE equivalents of degranulated human neutrophil supernatants (standardization described under “Experimental Procedures”) for periods up to 12 h at 37 °C. Digestion products of laminin-332 were separated on 4–15% gradient SDS-polyacrylamide gels under reducing conditions, transferred to PVDF membranes, and analyzed by immunoblotting using antibodies directed against the laminin α3, β3, or γ2 chains.
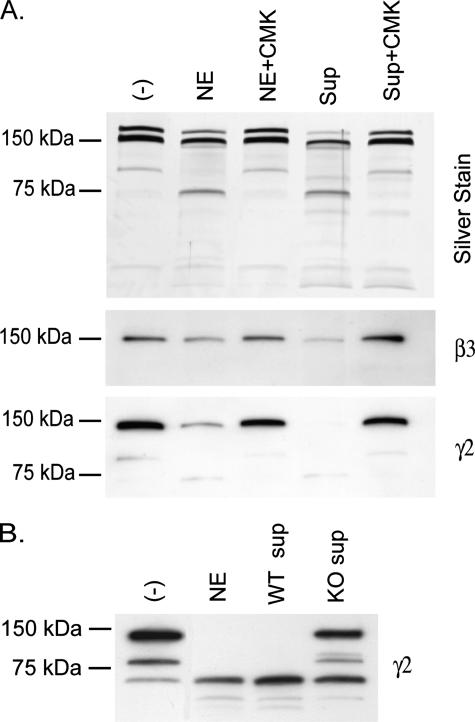
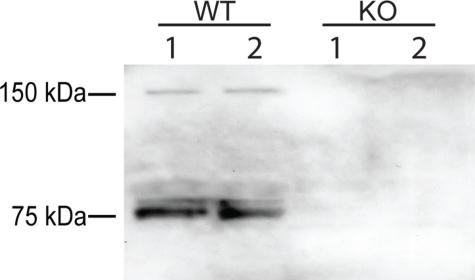
To investigate this further, we performed the experiment in the presence of a specific chloromethyl ketone inhibitor of NE (Fig. 4A). This inhibitor not only blocked digestion of laminin-332 by NE, but blocked digestion by degranulated neutrophil supernatants as well. The idea that NE is the major neutrophil-derived protease capable of degrading laminin-332 is most clearly supported in Fig. 4B, where neutrophil supernatants derived from NE-deficient mice were largely unable to cleave laminin-332.
FIGURE 4.
NE is the primary neutrophil protease capable of digesting laminin-332. A, 100 ng of laminin-332 was digested with 50 nm NE or with 50 nm NE equivalents of degranulated human neutrophil supernatants (Sup) (standardization described under “Experimental Procedures”) for 6 h at 37 °C in the presence or absence of 0.1 mm of chloromethyl ketone (CMK). Digestion reactions were separated on 4–15% gradient SDS-polyacrylamide gels, and digestion products of laminin-332 were visualized by silver staining (upper panel) or by immunoblotting using antibodies directed against the laminin β3(middle panel) or γ2(lower panel) chains. B, 100 ng of laminin-332 was digested with equivalent amounts of degranulated neutrophil supernatant protein of wild-type (WT) or NE-deficient (KO) bone marrow neutrophils for 6 h at 37 °C. Digestion products of laminin-332 were separated on 4–15% gradient SDS-polyacrylamide gels and analyzed by immunoblotting using antibodies directed against the laminin γ2 chain.
NE-dependent Cleavage of the Laminin γ2 Chain in Vivo—To determine whether NE contributes to laminin-332 degradation in vivo, we intranasally instilled LPS, a potent inducer of neutrophilic inflammation, into the lungs of wild-type and NE-deficient mice. BAL fluids were collected 24 h after LPS administration, a time point where there is significant neutrophil recruitment to the lungs, and assayed for the presence of laminin γ2 fragments by immunoblot analysis. The neutrophil counts in the BAL of NE-deficient mice treated with LPS were consistently higher than those of wild-type mice (3.3-, 2.9-, and 5.3-fold in three experiments), with higher doses of LPS associated with more neutrophils and laminin-332 degradation. In Fig. 5, two laminin γ2 bands were detected in the BAL fluid of LPS-treated wild-type mice, which were not detected in the BAL fluid of LPS-treated NE-deficient mice. These fragments correspond in size to the full-length ∼150- and ∼80-kDa γ2x species. The pattern of laminin-332 fragmentation varied between experiments; however, it was consistently different in wild-type and NE-deficient mice. These data indicate that NE can cleave the γ2 chain of laminin-332 both in vitro and in vivo.
FIGURE 5.
Reduced degradation of laminin γ2 in response to LPS in NE-deficient mice. Wild-type (WT) C57BL/6 or NE-deficient (KO) mice were intranasally instilled with 100 μgof E. coli LPS, and 24 h post-instillation, BAL fluids were collected. The cell types were determined by light microscopy of Wright stained cytospins. The BAL fluid supernatants were analyzed for γ2 chain fragments by immunoblotting using an antibody against the laminin γ2 chain. Data are representative of three separate experiments.
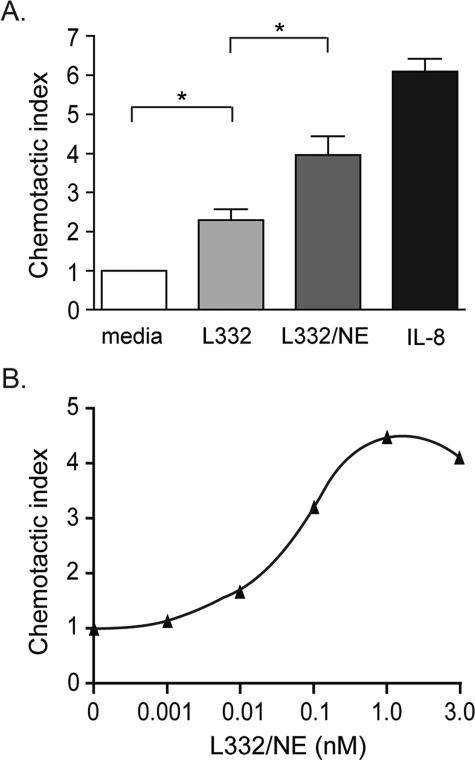
Cleavage of Laminin-332 by NE Generates Fragments That Are Chemotactic for Neutrophils—As fragmentation of the laminin γ2 chain by MMP-2 and other MMPs liberates peptides that are promigratory for epithelial cells (15, 17, 18), we investigated whether protease digestion of laminin-332 might also unmask cryptic sites that are chemotactic for inflammatory cells, particularly neutrophils. Using a modified Boyden chamber assay, we found that NE-digested laminin-332 (1 nm) was significantly more chemotactic for neutrophils than intact laminin-332 (Fig. 6A). Although “intact” laminin-332 has low, but significant, chemotactic activity for neutrophils, this is possibly due the presence of laminin-332 fragments in the laminin-332 preparation (Fig. 4A). When equal amounts of laminin-332 or digested laminin-332 were placed in the upper chamber of the Boyden chamber as in the lower chamber, the neutrophil migration observed was similar to that of the media alone control (data not shown). Thus, the laminin-332 fragments are chemotactic, not chemokinetic.
FIGURE 6.
Digestion of laminin-332 with NE generates fragments that are chemotactic for neutrophils. Chemotaxis assays were performed using human neutrophils placed in the upper compartment of a modified Boyden chamber. A, NE only (50 nm), intact laminin-332 (1 nm), NE-digested laminin-332 (1 nm), or IL-8 (5 nm) was placed in the lower compartment as the chemoattractant. B, 0–3 nm of NE-digested laminin-332 was used as chemoattractant in the lower compartment. The average number of migrated neutrophils per high powered field in triplicate wells from three separate experiments was determined. The chemotactic index refers to the fold change in the number of neutrophils when compared with NE alone. *, p < 0.05, Mann-Whitney test.
At 1 nm, NE-digested laminin-332 was half as active as 5 nm IL-8 (Fig. 6A) and 10–15% as active as 10 nm fMLP (not shown), other known potent neutrophil chemoattractants. NE-digested laminin-332 was still chemotactic at 0.01 nm (Fig. 6B), but maximal neutrophilic chemotactic activity was detected at 1 nm.
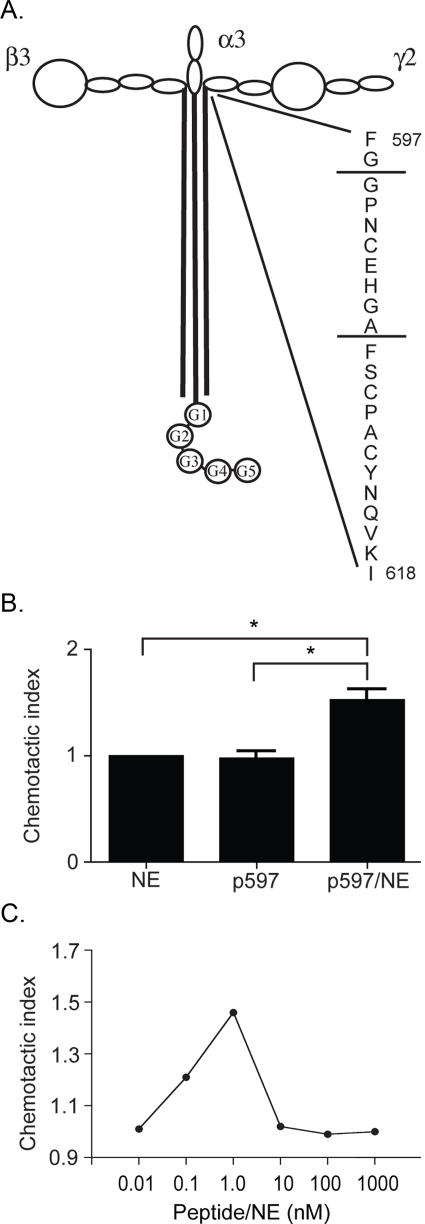
NE Cleaves the Hinge Region of γ2 Chain of Laminin-332 in a Manner Similar to MMP-2—MMP-2 has been shown to cleave the γ2 chain of laminin-332 C-terminal to an Ala residue in the hinge region located at the junction of the short arm and coiled-coil domain (13, 15), leaving behind the γ2x fragment. Because cleavage of laminin-332 by NE results in a similar sized laminin γ2 fragment, we examined whether cleavage of the γ2 chain by NE also occurs at this site. To do this, a peptide was generated corresponding to the region in the human laminin γ2 chain surrounding the known MMP-2 cleavage site, FGGPNCEHGAFSCPACYNQVKI, and was subjected to NE. Following NE digestion of the peptide, two major fragments were detected by HPLC. Analysis of the digest by electrospray mass spectrometry identified a peak with a mass corresponding to the peptide GPNCEHGA (Table 1). The C-terminal alanine residue of this fragment corresponds with Ala-586, the site of MMP-2 cleavage of rat laminin γ2 (13). These data suggest that cleavage of the γ2 chain of laminin-332 by NE results in a fragmentation pattern similar to that produced by MMP-2, and that γ2 fragments produced by digestion with NE may also facilitate epithelial cell migration in a manner similar to that resulting from MMP-2 or MMP-14 cleavage. The location of this peptide and the NE cleavage sites within the γ2 chain are depicted in Fig. 7A.
TABLE 1.
Cleavage of the human laminin γ 2 peptide, FGGPNCEHGAFSCPACYNQVKI, with NE
The p597 peptide derived from the region in the human laminin γ2 chain surrounding the known MMP-2 cleavage site was digested with 50 nm NE for 1 h at 37 °C, and the digestion mixture was analyzed by electrospray mass spectrometry. A major fragment with a mass corresponding to the peptide GPNCEHGA was identified, indicating cleavage sites after Gly-598 and Ala-666. The location of this peptide and the NE cleavage sites are depicted in Fig. 7A.
| Peptide | Predicted mass | Observed mass |
|---|---|---|
| Da | Da | |
| GPNCEHGA | 783.2 | 783.2 |
FIGURE 7.
Digestion of laminin γ2 peptide with NE generates fragments that are chemotactic for neutrophils. A peptide (p597) was generated corresponding to the region in the human laminin γ2 chain surrounding the known MMP-2 cleavage site. The location of this peptide and the NE cleavage sites within the peptide are depicted in A. This peptide was assessed for chemotactic activity using human neutrophils and a modified Boyden chamber. B, media containing 50 nm NE only, 1 nm undigested p597, or 1 nm NE-digested p597 was used as chemoattractant. C, 10 pm to 1 μm of NE-digested p597 was used as the chemoattractant. The average number of migrated neutrophils per high powered field in triplicate wells from three separate experiments was determined. The Chemotactic Index refers to the fold change in the number of neutrophils when compared with NE alone. *, p < 0.05, Mann-Whitney test.
NE-cleaved Laminin γ2 Peptide Is Chemotactic for Neutrophils—We also determined whether the laminin γ2 peptide derived from the region surrounding the MMP-2 cleavage site was chemotactic toward neutrophils. Although the intact peptide was not chemotactic for neutrophils, digestion of the peptide with NE produced fragments that were chemotactic (Fig. 7B), with maximal neutrophilic chemotactic activity at 1 nm (Fig. 7C). Collectively, these data suggest that localized injury and limited digestion of laminin-332-containing basement membranes may perpetuate the inflammatory response by recruitment of neutrophils.
DISCUSSION
As processing of laminin-332 by MMPs unmasks cryptic sites with important biological activities, we sought to investigate whether serine proteases are also capable of processing laminin-332, and whether this processing results in biological consequences. Indeed, laminin-332 is susceptible to proteolysis by the neutrophil-derived serine proteases NE, CG, and PR3. Although all of these enzymes are capable of degrading the α3 chain, NE is unique among this class in its ability to degrade the β3 and γ2 chains. Intriguingly, cleavage of the γ2 chain by NE generates a fragment that appears identical in size to the γ2x fragment generated by a number of MMPs, which release an EGF-containing fragment from the short arm of this chain that binds the EGF receptor and facilitates epithelial cell migration (14). Our data also suggest that NE cleaves in this same region, as it cleaves a synthetic peptide derived from this region of the human laminin γ2 chain at the site cleaved by MMP-2. Whether NE cleavage of laminin-332 results in increased epithelial cell migration remains to be determined.
NE appears to be the major neutrophil protease capable of degrading laminin-332, as digestion of laminin-332 using a degranulated neutrophil supernatant produces a digestion pattern of all three chains essentially identical to that seen with NE alone. More importantly, an inhibitor of NE prevented cleavage of laminin-332 by NE and neutrophil supernatants, and NE-deficient neutrophil supernatants were largely unable to cleave laminin-332. Similarly, NE was found by others to be one of two major enzymes in neutrophils capable of degrading laminin-111, as monospecific antibodies against NE blocked degradation of laminin-111 (27). Cleavage of laminin-332 by NE is likely physiologically relevant, as it can be produced by NE concentrations as low as 15 pm, representing an enzyme:substrate ratio of ∼1:1000. In comparison, we find that NE is ∼10-fold more potent than MMP-12 (data not shown), which was the most active of the MMPs we tested against laminin-332.
The relative specificity of proteases for individual laminin chains was unpredictable. MMP-12 was unique relative to the other MMPs tested in its activity against the different laminin chains. MMP-12 was the most potent of the MMPs tested against each of the laminin-332 chains. In addition, obvious differences were observed when comparing the susceptibilities of the laminin chains of laminins-111 and -511, which only differ in their α chain composition. Although the α1 chain of laminin-111 was very susceptible to digestion by MMPs-2, -8, and -9, it was resistant to digestion with MMP-12. The opposite was true for the α5 chain of laminin-511, which was resistant to cleavage by MMP-8 and -9 but susceptible to digestion by MMP-12. Interestingly, there were also differences in protease susceptibility among the MMPs for the same chain, the γ1 chain, in the two different laminin isoforms. Although the γ1 chain of laminin-111 was resistant to digestion by MMP-8 and -9, it was susceptible to digestion by MMP-12. In contrast, the γ1 chain of laminin-511 was resistant to only MMP-12 of the MMPs tested. These data raise the possibility that the context of chains present affects the susceptibility of the laminin isoforms to degradation by these MMPs. MMP-12 was similar to the other MMPs tested only in that all of these MMPs were largely unable to degrade the β1 chain.
Digestion of laminin-332 with MMP-2 (13), MMP-14 (15, 19), and MMPs-3, -13, and -20 (18) has been shown to facilitate epithelial cell migration. This has been proposed to occur via two different ways. First, cleavage of laminin-332 by these MMPs results in the generation of the γ2x form of the γ2 chain that remains bound to the α3 and β3 subunits, exposing a promigratory cryptic site on the α3 chain (13). Second, MMP digestion of laminin-332 releases the D3 domain of the γ2 chain, which contains three EGF repeats, and this fragment engages the EGF receptor, leading to stimulation of MAPK and an increase in cell migration (14). NE cleavage of laminin-332 also generates a fragment of the same size as the γ2x fragment, presumably releasing a fragment containing the EGF repeats. However, as EGF receptors are not expressed by neutrophils, it is almost certain that the effect we see on neutrophil chemotaxis is mechanistically distinct from that controlling migration of epithelial cells in response to proteolytic degradation of laminin-332. Neutrophils do express α6 integrins (28) and α3β1 integrin (29) known to bind laminin-332, so one of these receptors might mediate chemotaxis in response to laminin-332 fragments. It will be interesting to determine whether NE digestion of laminin-332 facilitates epithelial cell migration and conversely whether MMP-mediated proteolysis of laminin-332 generates fragments that are chemotactic for neutrophils.
Laminin-332 is not unique among basement membrane or even hemidesmosomal proteins in which its proteolysis results in fragments that are chemotactic for various cell types. Laminin-111 is a substrate for both NE and CG (27), and digestion of laminin-111 by human neutrophils, NE, or CG liberated fragments that were chemotactic for human neutrophils (30). A number of synthetic peptides derived from laminin-111 have also been shown to facilitate either neutrophil chemotaxis or chemokinesis (migration in the absence of a gradient) (31). However, the biological significance of this is unknown because laminin-111 is not present in most adult tissues (32–34). BP180 (collagen XVII), a cell surface structural component of hemidesmosomes, contains a collagenous ectodomain that can be shed by members of the ADAMs protease family (35, 36) and by NE (37, 38). This ectodomain has been shown to be chemotactic for squamous cell carcinoma cells but not for normal keratinocytes, involving the αIIB integrin that is present on the tumor cells but not present on normal keratinocytes (39).
Proteolytic fragments of other extracellular matrix proteins also exhibit chemotactic activity for inflammatory cells. Elastin fragments are chemotactic for monocytes in vitro (40, 41) and in vivo (42), and antibodies against the repeating unit VGVAPG block monocytic inflammation in response to elastin fragments in vivo. Recently, proteolytic degradation of collagen was found to result in the release of PGP-containing peptides, which share structural homology with CXC chemokines (43). Administration of PGP-containing peptides caused neutrophil recruitment into the lungs of wild-type mice, but not in CXCR2-deficient mice. Furthermore, digestion of fibronectin with thermolysin releases a 120-kDa fragment that is chemotactic for monocytes, whereas intact fibronectin does not have this activity (44).
Although proteolysis of laminins exposes cryptic sites that are chemotactic for neutrophils, cryptic laminin fragments have other functions as well. A cryptic site within the α1 chain of laminin-111 (SRARKQAASIKVAVSADR) was shown to cause up-regulation of MMP-9 and urokinase in RAW264.7 macrophages through a MAPK-dependent pathway, whereas intact laminin-111 was inactive in this regard (45). Similarly, a cryptic peptide sequence from the region of homology within the laminin α5 chain, AQARSAASKVKVSMKF, was also found to induce MMP-9 synthesis by RAW264.7 macrophages, in addition to MMP-14, whereas intact laminin-511 did not exhibit these activities (45). Interestingly, the corresponding peptide from the laminin α3 chain of laminin-332 did not induce MMP-9 expression. This α5 peptide was also found to induce macrophage expression of several cytokines and was chemotactic for neutrophils and macrophages both in vitro and in vivo (46, 47). Cryptic sites within laminin-111 mediate binding to heparin (48, 49) and tumor necrosis factor-α (50). Osteoclasts bind poorly to intact laminin-111 but much more strongly to pepsin-treated laminin-111 or to the purified P1 fragment, both in an RGD-dependent manner, supporting other evidence that the RGD-containing integrin-binding site in laminin-111 is cryptic (51). Similar RGD-dependent preferential binding of the P1 fragment over intact laminin to purified αVβ3 integrin underscores the cryptic nature of the RGD site in laminin-111 (52).
There are little data to address the role that NE-mediated proteolysis of laminin-332 may have in vivo. In response to challenge with Pseudomonas aeruginosa (53), LPS (53), or intratracheal bleomycin (54), neutrophilic inflammation in the lung appears uncompromised in NE-deficient mice compared with wild-type controls. It is important to note that in our LPS experiments, differences in the fragmentation pattern of laminin-332 in NE-deficient mice were not because of impaired neutrophil recruitment, as we observed more neutrophils in the BAL fluid of NE-deficient mice than wild-type mice (2.9–5.3-fold in three experiments). However, adhesion and transmigration of neutrophils were reduced in NE-deficient mice in response to particulate zymosan (55), and NE-deficient mice are partially protected from cigarette smoke-induced emphysema, demonstrating impaired recruitment of both neutrophils and monocytes (56). Thus, neutrophils from NE-deficient mice appear to be differentially affected in their ability to respond to various chemotactic stimuli. In a murine model of the blistering skin disease bullous pemphigoid, passive transfer of antibodies against the hemidesmosomal protein BP180 causes an acute inflammatory reaction in which neutrophils migrate to the injection site and effect dermal-epidermal separation. NE is the most important enzyme in this model, with NE-deficient mice failing to form blisters (37). Although NE has been shown to cleave the hemidesmosomal protein BP180 both in vitro and in vivo (37), it is likely that NE-mediated cleavage of laminin-332, another structural component of hemidesmosomes connecting the epithelium to the basement membrane, contributes to blister formation in this model. Similarly, leukocyte-mediated dermal-epidermal detachment can be elicited in human skin cryosections using BP180 auto-antibodies derived from patients with bullous pemphigoid, and selective inhibition of NE in this model suppresses this separation (57). Thus, in the context of blistering skin disease, it is likely that NE plays an important role in degrading laminin-332, and that liberated fragments would be involved in neutrophil recruitment during the acute phase.
This paradigm remains to be investigated in other organs such as the lung where laminin-332 is expressed, but evidence is accumulating that laminin-332 fragments are generated during pulmonary inflammatory processes. In dogs with pulmonary eosinophilia, elevated levels of γ2 fragments have been identified in the BAL fluid (58). In response to LPS, we observed γ2 fragments in the BAL of wild-type mice and altered processing of the laminin γ2 chain in NE-deficient mice. Bleomycin-induced lung injury in mice also results in damage to basement membrane proteins, as BAL fluid from bleomycin-treated mice contained laminin γ2 fragments that were not detected in the BAL fluid of mice treated with PBS alone (data not shown). These results suggest that NE-mediated degradation of laminin-332 may be an important and common facet of the acute inflammatory response in multiple settings. It remains to be determined whether digestion of laminin-332 by NE in vivo results in the recruitment of neutrophils to sites of injury.
Acknowledgments
We thank Drs. Christine Pham and Steven Shapiro for providing NE-deficient mice.
This work was supported by National Institutes of Health NHLBI Grant HL29594 and the Alan A. and Edith L. Wolff Charitable Trust/Barnes-Jewish Hospital Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LG, large globular; MMP, matrix metalloproteinase; NE, neutrophil elastase; fMLP, formyl-methionyl-leucyl-phenylalanine; PBS, phosphate-buffered saline; PVDF, polyvinylidene difluoride; LPS, lipopolysaccharide; HPLC, high pressure liquid chromatography; EGF, epidermal growth factor; MAPK, mitogen-activated protein kinase; BAL, bronchoalveolar lavage.
References
- 1.Aumailley, M., Bruckner-Tuderman, L., Carter, W. G., Deutzmann, R., Edgar, D., Ekblom, P., Engel, J., Engvall, E., Hohenester, E., Jones, J. C., Kleinman, H. K., Marinkovich, M. P., Martin, G. R., Mayer, U., Meneguzzi, G., Miner, J. H., Miyazaki, K., Patarroyo, M., Paulsson, M., Quaranta, V., Sanes, J. R., Sasaki, T., Sekiguchi, K., Sorokin, L. M., Talts, J. F., Tryggvason, K., Uitto, J., Virtanen, I., von der Mark, K., Wewer, U. M., Yamada, Y., and Yurchenco, P. D. (2005) Matrix Biol. 24 326–332 [DOI] [PubMed] [Google Scholar]
- 2.Borradori, L., and Sonnenberg, A. (1999) J. Investig. Dermatol. 112 411–418 [DOI] [PubMed] [Google Scholar]
- 3.Kivirikko, S., McGrath, J. A., Baudoin, C., Aberdam, D., Ciatti, S., Dunnill, M. G., McMillan, J. R., Eady, R. A., Ortonne, J. P., Meneguzzi, G., Ultto, J., and Christiano, A. M. (1995) Hum. Mol. Genet. 4 959–962 [DOI] [PubMed] [Google Scholar]
- 4.Pulkkinen, L., Christiano, A. M., Gerecke, D., Wagman, D. W., Burgeson, R. E., Pittelkow, M. R., and Uitto, J. (1994) Genomics 24 357–360 [DOI] [PubMed] [Google Scholar]
- 5.Meng, X., Klement, J. F., Leperi, D. A., Birk, D. E., Sasaki, T., Timpl, R., Uitto, J., and Pulkkinen, L. (2003) J. Investig. Dermatol. 121 720–731 [DOI] [PubMed] [Google Scholar]
- 6.Mizushima, H., Takamura, H., Miyagi, Y., Kikkawa, Y., Yamanaka, N., Yasumitsu, H., Misugi, K., and Miyazaki, K. (1997) Cell Growth & Differ. 8 979–987 [PubMed] [Google Scholar]
- 7.Hirosaki, T., Mizushima, H., Tsubota, Y., Moriyama, K., and Miyazaki, K. (2000) J. Biol. Chem. 275 22495–22502 [DOI] [PubMed] [Google Scholar]
- 8.Shang, M., Koshikawa, N., Schenk, S., and Quaranta, V. (2001) J. Biol. Chem. 276 33045–33053 [DOI] [PubMed] [Google Scholar]
- 9.Goldfinger, L. E., Stack, M. S., and Jones, J. C. (1998) J. Cell Biol. 141 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, S., Brown, R., Jones, J. C., Ellerbroek, S. M., and Stack, M. S. (2000) J. Biol. Chem. 275 23869–23876 [DOI] [PubMed] [Google Scholar]
- 11.Hintermann, E., Yang, N., O'Sullivan, D., Higgins, J. M., and Quaranta, V. (2005) J. Biol. Chem. 280 8004–8015 [DOI] [PubMed] [Google Scholar]
- 12.Udayakumar, T. S., Chen, M. L., Bair, E. L., Von Bredow, D. C., Cress, A. E., Nagle, R. B., and Bowden, G. T. (2003) Cancer Res. 63 2292–2299 [PubMed] [Google Scholar]
- 13.Giannelli, G., Falk-Marzillier, J., Schiraldi, O., Stetler-Stevenson, W. G., and Quaranta, V. (1997) Science 277 225–228 [DOI] [PubMed] [Google Scholar]
- 14.Schenk, S., Hintermann, E., Bilban, M., Koshikawa, N., Hojilla, C., Khokha, R., and Quaranta, V. (2003) J. Cell Biol. 161 197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koshikawa, N., Giannelli, G., Cirulli, V., Miyazaki, K., and Quaranta, V. (2000) J. Cell Biol. 148 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seftor, R. E., Seftor, E. A., Koshikawa, N., Meltzer, P. S., Gardner, L. M., Bilban, M., Stetler-Stevenson, W. G., Quaranta, V., and Hendrix, M. J. (2001) Cancer Res. 61 6322–6327 [PubMed] [Google Scholar]
- 17.Sadowski, T., Dietrich, S., Koschinsky, F., Ludwig, A., Proksch, E., Titz, B., and Sedlacek, R. (2005) Cell. Mol. Life Sci. 62 870–880 [DOI] [PubMed] [Google Scholar]
- 18.Pirila, E., Sharabi, A., Salo, T., Quaranta, V., Tu, H., Heljasvaara, R., Koshikawa, N., Sorsa, T., and Maisi, P. (2003) Biochem. Biophys. Res. Commun. 303 1012–1017 [DOI] [PubMed] [Google Scholar]
- 19.Koshikawa, N., Minegishi, T., Sharabi, A., Quaranta, V., and Seiki, M. (2005) J. Biol. Chem. 280 88–93 [DOI] [PubMed] [Google Scholar]
- 20.Amano, S., Scott, I. C., Takahara, K., Koch, M., Champliaud, M. F., Gerecke, D. R., Keene, D. R., Hudson, D. L., Nishiyama, T., Lee, S., Greenspan, D. S., and Burgeson, R. E. (2000) J. Biol. Chem. 275 22728–22735 [DOI] [PubMed] [Google Scholar]
- 21.Belaaouaj, A., McCarthy, R., Baumann, M., Gao, Z., Ley, T. J., Abraham, S. N., and Shapiro, S. D. (1998) Nat. Med. 4 615–618 [DOI] [PubMed] [Google Scholar]
- 22.Plopper, G., Falk-Marzillier, J., Glaser, S., Fitchmun, M., Giannelli, G., Romano, T., Jones, J. C., and Quaranta, V. (1996) J. Cell Sci. 109 1965–1973 [DOI] [PubMed] [Google Scholar]
- 23.Spirito, F., Chavanas, S., Prost-Squarcioni, C., Pulkkinen, L., Fraitag, S., Bodemer, C., Ortonne, J. P., and Meneguzzi, G. (2001) J. Biol. Chem. 276 18828–18835 [DOI] [PubMed] [Google Scholar]
- 24.Abrahamson, D. R., Prettyman, A. C., Robert, B., and St. John, P. L. (2003) Kidney Int. 63 826–834 [DOI] [PubMed] [Google Scholar]
- 25.Bylund, J., Macdonald, K. L., Brown, K. L., Mydel, P., Collins, L. V., Hancock, R. E., and Speert, D. P. (2007) Eur. J. Immunol. 37 1087–1096 [DOI] [PubMed] [Google Scholar]
- 26.Senior, R. M., Hinek, A., Griffin, G. L., Pipoly, D. J., Crouch, E. C., and Mecham, R. P. (1989) Am. J. Respir. Cell Mol. Biol. 1 479–487 [DOI] [PubMed] [Google Scholar]
- 27.Heck, L. W., Blackburn, W. D., Irwin, M. H., and Abrahamson, D. R. (1990) Am. J. Pathol. 136 1267–1274 [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, S., Dangerfield, J. P., Young, R. E., and Nourshargh, S. (2005) J. Cell Sci. 118 2067–2076 [DOI] [PubMed] [Google Scholar]
- 29.Gresham, H. D., Graham, I. L., Griffin, G. L., Hsieh, J. C., Dong, L. J., Chung, A. E., and Senior, R. M. (1996) J. Biol. Chem. 271 30587–30594 [DOI] [PubMed] [Google Scholar]
- 30.Steadman, R., Irwin, M. H., St John, P. L., Blackburn, W. D., Heck, L. W., and Abrahamson, D. R. (1993) J. Leukocyte Biol. 53 354–365 [DOI] [PubMed] [Google Scholar]
- 31.Harvath, L., Brownson, N. E., Fields, G. B., and Skubitz, A. P. (1994) J. Immunol. 152 5447–5456 [PubMed] [Google Scholar]
- 32.Pierce, R. A., Griffin, G. L., Miner, J. H., and Senior, R. M. (2000) Am. J. Respir. Cell Mol. Biol. 23 742–747 [DOI] [PubMed] [Google Scholar]
- 33.Virtanen, I., Gullberg, D., Rissanen, J., Kivilaakso, E., Kiviluoto, T., Laitinen, L. A., Lehto, V. P., and Ekblom, P. (2000) Exp. Cell Res. 257 298–309 [DOI] [PubMed] [Google Scholar]
- 34.Falk, M., Ferletta, M., Forsberg, E., and Ekblom, P. (1999) Matrix Biol. 18 557–568 [DOI] [PubMed] [Google Scholar]
- 35.Franzke, C. W., Tasanen, K., Schacke, H., Zhou, Z., Tryggvason, K., Mauch, C., Zigrino, P., Sunnarborg, S., Lee, D. C., Fahrenholz, F., and Bruckner-Tuderman, L. (2002) EMBO J. 21 5026–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franzke, C. W., Tasanen, K., Borradori, L., Huotari, V., and Bruckner-Tuderman, L. (2004) J. Biol. Chem. 279 24521–24529 [DOI] [PubMed] [Google Scholar]
- 37.Liu, Z., Shapiro, S. D., Zhou, X., Twining, S. S., Senior, R. M., Giudice, G. J., Fairley, J. A., and Diaz, L. A. (2000) J. Clin. Investig. 105 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Z., Zhou, X., Shapiro, S. D., Shipley, J. M., Twining, S. S., Diaz, L. A., Senior, R. M., and Werb, Z. (2000) Cell 102 647–655 [DOI] [PubMed] [Google Scholar]
- 39.Parikka, M., Nissinen, L., Kainulainen, T., Bruckner-Tuderman, L., Salo, T., Heino, J., and Tasanen, K. (2006) Exp. Cell Res. 312 1431–1438 [DOI] [PubMed] [Google Scholar]
- 40.Senior, R. M., Griffin, G. L., and Mecham, R. P. (1980) J. Clin. Investig. 66 859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunninghake, G. W., Davidson, J. M., Rennard, S., Szapiel, S., Gadek, J. E., and Crystal, R. G. (1981) Science 212 925–927 [DOI] [PubMed] [Google Scholar]
- 42.Houghton, A. M., Quintero, P. A., Perkins, D. L., Kobayashi, D. K., Kelley, D. G., Marconcini, L. A., Mecham, R. P., Senior, R. M., and Shapiro, S. D. (2006) J. Clin. Investig. 116 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weathington, N. M., van Houwelingen, A. H., Noerager, B. D., Jackson, P. L., Kraneveld, A. D., Galin, F. S., Folkerts, G., Nijkamp, F. P., and Blalock, J. E. (2006) Nat. Med. 12 317–323 [DOI] [PubMed] [Google Scholar]
- 44.Clark, R. A., Wikner, N. E., Doherty, D. E., and Norris, D. A. (1988) J. Biol. Chem. 263 12115–12123 [PubMed] [Google Scholar]
- 45.Khan, K. M., and Falcone, D. J. (2000) J. Biol. Chem. 275 4492–4498 [DOI] [PubMed] [Google Scholar]
- 46.Adair-Kirk, T. L., Atkinson, J. J., Broekelmann, T. J., Doi, M., Tryggvason, K., Miner, J. H., Mecham, R. P., and Senior, R. M. (2003) J. Immunol. 171 398–406 [DOI] [PubMed] [Google Scholar]
- 47.Adair-Kirk, T. L., Atkinson, J. J., Kelley, D. G., Arch, R. H., Miner, J. H., and Senior, R. M. (2005) J. Immunol. 174 1621–1629 [DOI] [PubMed] [Google Scholar]
- 48.Sung, U., O'Rear, J. J., and Yurchenco, P. D. (1993) J. Cell Biol. 123 1255–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung, U. (1997) Mol. Cells 7 272–277 [PubMed] [Google Scholar]
- 50.Hershkoviz, R., Goldkorn, I., and Lider, O. (1995) Immunology 85 125–130 [PMC free article] [PubMed] [Google Scholar]
- 51.Horton, M. A., Spragg, J. H., Bodary, S. C., and Helfrich, M. H. (1994) Bone 15 639–646 [DOI] [PubMed] [Google Scholar]
- 52.Pfaff, M., Gohring, W., Brown, J. C., and Timpl, R. (1994) Eur. J. Biochem. 225 975–984 [DOI] [PubMed] [Google Scholar]
- 53.Hirche, T. O., Atkinson, J. J., Bahr, S., and Belaaouaj, A. (2004) Am. J. Respir. Cell Mol. Biol. 30 576–584 [DOI] [PubMed] [Google Scholar]
- 54.Chua, F., Dunsmore, S. E., Clingen, P. H., Mutsaers, S. E., Shapiro, S. D., Segal, A. W., Roes, J., and Laurent, G. J. (2007) Am. J. Pathol. 170 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young, R. E., Thompson, R. D., Larbi, K. Y., La, M., Roberts, C. E., Shapiro, S. D., Perretti, M., and Nourshargh, S. (2004) J. Immunol. 172 4493–4502 [DOI] [PubMed] [Google Scholar]
- 56.Shapiro, S. D., Goldstein, N. M., Houghton, A. M., Kobayashi, D. K., Kelley, D., and Belaaouaj, A. (2003) Am. J. Pathol. 163 2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimanovich, I., Mihai, S., Oostingh, G. J., Ilenchuk, T. T., Brocker, E. B., Opdenakker, G., Zillikens, D., and Sitaru, C. (2004) J. Pathol. 204 519–527 [DOI] [PubMed] [Google Scholar]
- 58.Rajamaki, M. M., Jarvinen, A. K., Sorsa, T. A., Tervahartiala, T. I., and Maisi, P. S. (2006) Vet. J. 171 562–565 [DOI] [PubMed] [Google Scholar]