Abstract
Culture-expanded human mesenchymal stem cells (hMSCs) are increasingly used in a variety of preclinical and clinical studies. However, these cells have a low rate of engraftment to bone marrow or damaged tissues. Several laboratories have shown that during isolation and subculturing mesenchymal stem cells quickly lose the expression of CXCR4, the key receptor responsible for lymphocytes and hematopoietic stem cell homing. Here we show that culturing of hMSCs as three-dimensional aggregates (hMSC spheroids) restores CXCR4 functional expression. Expression of CXCR4 inversely correlates with the secretion of SDF-1 by hMSCs. Cells from hMSC spheroids up-regulate expression of CD49b, the α2 integrin subunit, and suppress the expression of CD49d, the α4 integrin subunit. Transfer of cells from the spheroids back to a monolayer suppresses the expression of CXCR4 and CD49b and restores the expression of CD49d. Treatment of cells from the spheroids with SDF-1 leads to CXCR4 internalization and activation of ERK-1,2. Adhesion of hMSCs to human umbilical vein endothelial cells (HUVECs) was investigated. SDF-1, AMD-3100, or exposure of HUVECs to hypoxia did not affect adhesion of hMSCs from a monolayer to HUVECs. Adhesion of cells from hMSC spheroids to HUVECs was stimulated by SDF-1, AMD-3100, or by exposure of HUVECs to hypoxia. Stimulatory effects of hypoxia and addition of SDF-1 or AMD-3100 were not additive. Overall, our data indicate that the expression of CXCR4 by hMSCs regulates hMSC adhesion to endothelial cells.
Human mesenchymal stem cells (hMSCs)2 isolated from the bone marrow support self-renewal and differentiation of human mesenchymal stem cells within the bone marrow (1) and have the potential to differentiate into mesenchymal and non-mesenchymal lineages (2, 3). These cells are increasingly used as a therapeutic tool to assist HSC engraftment in the bone marrow (4–6) and to treat bone and cartilage disorders (7–9). When infused into the peripheral circulation, mesenchymal stem cells (MSCs) are attracted to an irradiated bone marrow or injured tissues (10, 11). Systemic administration of MSCs also improves functions of ischemic tissues after stroke (12) or myocardial infarction (13). However, the majority of the systemically delivered cells were found primarily in the lung, with some in the liver and other organs (14–16). The limited targeting capabilities of hMSCs require the use of a high number of cells to achieve therapeutic benefits. In most studies as many as 1–5 × 106 cells/kg have been delivered (5–7). Improvement in hMSC engraftment in bone marrow and ischemic tissues might help to decrease the number of infused cells and be beneficial for clinical applications.
The homing mechanisms that guide recruitment of MSCs are still unclear. MSCs are believed to migrate via the bloodstream to seed the sites of hematopoiesis during embryonic development (17) or sites of injury in adult life (10, 11). Over the past decade, significant advances have been made in characterizing homing of HSCs to the bone marrow. The distribution of HSCs circulating in the bloodstream is controlled by the ability of these cells to adhere and roll on bone marrow sinusoidal endothelial cells, transmigrate through an endothelial barrier, and be retained in bone marrow niches. These processes are controlled by cell adhesion molecules and chemokines (18). Initially discovered as a co-receptor for human immunodeficiency virus entry (19), the chemokine receptor 4 (CXCR4) and its ligand, the stromal cell-derived factor-1 (SDF-1/CXCL12), are believed to be key players in colonization of bone marrow by HSCs (20). The interaction between SDF-1 and CXCR4 mediates engraftment of cancer stem cells to the bone marrow (21), chemotaxis of endothelial (22) and neuronal cells (23), trafficking of rat MSCs to the sites of injury, and the migration of MSCs in vitro (24). SDF-1 is constantly produced by bone marrow stromal cells, immature osteoblasts, and endothelial cells (25). Knock outs of CXCR4 or SDF-1 are lethal, resulting in severe bone marrow failure, a significant defect in colonization of embryonic bone marrow by HSCs, and abnormal development of heart and brain (26, 27).
hMSCss express a variety of adhesion molecules (3, 28, 29) and respond to CXCL12 (SDF-1), CX3CL1, CXCL16, CCL3, CCL19, and CCL21 chemokines (30, 31). Homing capabilities of MSCs are greatly influenced by cell culture conditions. Most of the freshly isolated MSCs were detected in the bone marrow of sublethally irradiated mice after systemic administration, whereas homing to the bone marrow of MSCs subcultured for as little as 24 h was significantly reduced (10). It was suggested that subculturing of MSCs leads to changes in their phenotype that affects MSC homing (32). The earliest passages of hMSCs express CXCR4 and chemotactically respond to SDF-1 (33, 34). Culturing of hMSCs for more than two passages was associated with a decrease in expression of adhesion molecules, the loss of chemokine receptors, including CXCR4, and lack of chemotactic response to chemokines (33, 35, 36). Because of the low abundance of MSCs in bone marrow isolates, only culture-expanded hMSCs are likely to be used in clinic. The loss of the chemokine response results in impairment of homing and represents a substantial challenge for therapeutic application of hMSCs. Attempts to increase functional CXCR4 expression in MSCs have included transduction of hMSCs with a retroviral vector encoding CXCR4 (37) and treatment of the cells with tumor necrosis factor-α, interferon-β and -γ, and copaxone (38) or insulin-like growth factor (39).
We have previously reported that transfer of hMSCs from a monolayer to hanging drops results in spontaneous association of cells leading to formation of spheroids. Culturing hMSCs as three-dimensional aggregates caused substantial changes in the pattern of gene expression (40). These changes included up-regulation of CXCR4 and the α2 integrin subunit mRNAs. We hypothesized that the expression of CXCR4 and the α2 integrin subunit at the protein level will be affected as well. In this study we investigated effects of spheroid formation on the expression of cell surface markers by hMSCs and adhesion of hMSCs to HUVECs.
EXPERIMENTAL PROCEDURES
Cell Culture— hMSCs and HUVECs were purchased from Cambrex BioScience. hMSCss were cultured at 37 °C in a humidified atmosphere of 5% CO2 in mesenchymal stem cell growth medium (Lonza). HUVECs were cultured in EGM-2 basal medium containing growth factors supplied by the manufacturer (Lonza). Passages 2–5 of hMSCs or HUVECs were used.
Spheroids were formed from hMSCs as previously described (40). Briefly, hMSCs grown to confluence were washed with Dulbecco's phosphate-buffered saline (PBS) (Sigma) and dissociated with 0.25% trypsin-EDTA solution (Lonza). The digestion was stopped by addition of trypsin inhibitor solution (Lonza). hMSCs were collected by centrifugation and resuspended in high glucose Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with penicillin-streptomycin (Sigma) and 5% fetal bovine serum (Sigma). The cells (250,000 cells in 40 μl) were kept for 3 days in hanging drops. Growth medium was changed every day.
Flow Cytometric Analysis of Cell Surface Antigens—Analysis of cell surface antigens was performed as described (29) with some modifications. Cells were dissociated from a monolayer of hMSCs or hMSC spheroids with 0.25% trypsin-EDTA solution (Lonza) for 90 min at room temperature. Trypsinization was stopped by addition of trypsin inhibitor solution (Lonza). Cells were collected by centrifugation and washed with flow cytometry buffer made from PBS containing 2% bovine serum albumin (Sigma) and 0.1% sodium azide (Fluka). Cells (2 × 105) were stained using manufacturer-suggested concentrations of fluorochrome-conjugated monoclonal antibodies for 30 min at room temperature in the dark. Anti-human HLA class I, CD31, CD34, CD55, and CD105 were purchased from Diaclone. Anti-human c-met was obtained from eBioscience. Antibodies to human CD28, CD29, CD38, CD44, CD49b, CD49d, CD54, CD73, CD90, CD117, CD166, CD184, and CD209 were from BD Biosciences. After staining, cells were washed with 5 ml of the flow cytometry buffer and resuspended in the flow cytometry buffer supplemented with 1% paraformaldehyde (Electron Microscopy Sciences). Background staining was assessed by incubation of cells with mouse fluorochrome- and isotype-matched immunoglobulins (isotype controls). Flow cytometric analysis of hMSCs grown as a monolayer or isolated from the spheroids was performed using identical instrumental settings by analyzing 10,000 events on a FACScan flow cytometer (BD Biosciences). Signals from subcellular debris were eliminated during data acquisition by gating. The CellQuest™ software package was used to process the data.
Exposure of Cells to Hypoxia—HUVECs were exposed to hypoxia in a BD GasPak EZ Anaerobe Gas-generating Pouch System with an Indicator (BD Biosciences) as described (40). As certified by the manufacturer, the Anaerobe Gas Generating Pouch System produces an atmosphere containing 10% carbon dioxide and 1% oxygen.
Preparation of Conditioned Medium—DMEM containing 5% fetal bovine serum was conditioned by a confluent monolayer of hMSCs or by hMSC spheroids for 24 h as described (40). Conditioned medium was collected, passed through Acrodisc 0.2 μm HT Tuffryn Membrane Low Protein Binding Filters (Pall Corp.), and stored at -80 °C until use.
SDF-1 Enzyme-linked Immunosorbent Assay (ELISA)—The SDF-1 content in conditioned medium was measured with an ELISA kit for human SDF-1 (R&D Systems). The ELISA was performed according to the manufacturer's protocol.
Immunocytochemistry—Cells were dissociated from a monolayer of hMSCs or 3-day old hMSC spheroids as described above. Cells were collected by centrifugation, washed with PBS, plated in DMEM containing 5% fetal bovine serum on Lab-Tek II chamber CC2 glass slides (Nalge Nunc International), and allowed to adhere for 8 h. Then the cells were serum-starved for 2 h in Hanks' balanced salt solution (HBSS) and treated with or without 1 μg/ml SDF-1α in HBSS for 45 min in a humidified atmosphere of 5% CO2 at 37 °C.
Cells were fixed with buffered formalin (1:10 dilution) (Fisher Diagnostic) for 10 min, washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. Slides were washed three times with PBS and blocked with 5% bovine serum albumin in PBS for 30 min. Bovine serum albumin was then removed, slides were washed again with PBS, and cells were stained overnight at 4 °C with 1 μg/ml anti-human CXCR4 antibody (R&D Systems; catalog number MAB172). Unbound CXCR4 antibodies were removed by washing slides three times with PBS. Slides were incubated with anti-mouse Alexa Fluor 488-conjugated F(ab′)2 secondary antibodies (1:1000; Molecular Probes) dissolved in PBS containing 0.165 μm Alexa Fluor 594 phalloidin (Molecular Probes) and 1% bovine serum albumin for 2 h at room temperature in the dark. Slides were washed three times with PBS and mounted on coverslips in VectaShield mounting medium containing 4,6-diamidino-2-phenylindole (VectaShield-DAPI; Vector Laboratories).
Immunofluorescence was analyzed by deconvolution microscopy using an Axiovert 200 M fluorescence microscope (Carl Zeiss). Cross-sectional images were obtained with 250 nm Z-stack steps and processed using a constrained iterative algorithm of the AxioVision 4.1 software package (Carl Zeiss).
ERK-1,2 Activation Assay—Cells were dissociated from a monolayer of hMSCs or 3-day-old hMSC spheroids as described above, collected by centrifugation, and washed with PBS. Dissociated cells were plated in 24-well tissue culture dishes (5 × 104/well) in DMEM containing 5% fetal bovine serum and allowed to adhere for 8 h. Cells were then serum-starved for 4 h and treated with and without 1 μg/ml SDF-1α in HBSS for 0–20 min at 37 °C in a humidified atmosphere of 5% CO2. Cells were lysed with 1 ml/well of lysis buffer containing 0.025 m Tris-HCl, pH 7.4, 0.15 m NaCl, 5 mm EDTA, 1% Triton X-100, 0.5% Nonidet P-40, and a set of protease inhibitors (Roche Applied Science) and phosphatase inhibitors (cocktails type 1 and 2) (Sigma) for 15 min at 4 °C. The extract was cleared by centrifugation at 15,000 × g at 4 °C for 30 min. Proteins (25 μg) were separated in Bis-Tris 12% Criterion gel using XT MOPS running buffer (Bio-Rad) and transferred onto nitrocellulose membranes (Bio-Rad). Western blot was performed using p44/42 mitogen-activated protein kinase and phospho-p44/42 mitogen-activated protein kinase (Thr-202/Tyr-284) (197G2) antibodies (Cell Signaling Technology). Immunoreactive bands were visualized using affinity-purified peroxidase-labeled goat anti-rabbit F(ab′)2 fragment antibody (Kirkegaard and Perry Laboratories) and ECL Western blotting detection reagents (GE Healthcare).
Adhesion Assay—Adhesion of hMSCs to endothelial cells was studied using hMSCs isolated from a monolayer or 3-day-old hMSC spheroids. Cells were dissociated using 0.25% trypsin-EDTA solution (Lonza) for 15 min in the case of hMSC monolayers or for 90 min in the case of the spheroids. Trypsinization was stopped by addition of trypsin inhibitor solution (Lonza). Isolated cells were washed with PBS, labeled with 2 μg/ml Calcein AM in HBSS for 45 min at 37 °C in a humidified atmosphere of 5% CO2, washed with HBSS, and resuspended in DMEM supplemented with 5% fetal bovine serum. HUVECs were plated in 24-well plates and maintained in EBM-2 growth medium for 2–3 days until they reached confluence. For the adhesion assay, EBM-2 medium was replaced with 0.5 ml of DMEM supplemented with 5% fetal bovine serum. hMSCs (10,000 cells/well) were added to endothelial cells. The adhesion assay was performed in the presence of 0.5 μg/ml SDF-1 or 10 μm AMD-3100 or without additives. Plates were placed on a rotation platform (30 rotations/min) and incubated for 30 min at 37 °C. Wells were washed three times with 1 ml of DMEM supplemented with 5% fetal bovine serum followed by addition of 0.5 ml/well of the same medium. Fluorescence was acquired from the bottom of the plates using a POLARstar OPTIMA microplate reader at excitation/emission wavelengths of 485/520 nm. Background fluorescence was determined in the wells containing no hMSCs but a monolayer of endothelial cells. Total cell load was estimated as the fluorescence from non-washed wells with added hMSCs. A minimum of six wells was used to estimate background fluorescence, total cell load, or fluorescence from adhered cells. Percent of bound cells was calculated as a ratio between fluorescence of adhered cells and total cell load after subtraction of background fluorescence. Data from five independent preparations of hMSC spheroids and four independent preparations of hMSCs grown as a monolayer were statistically processed with the SigmaStat software package (Sigma).
RESULTS
Expression of Cell Surface Markers by hMSCs—According to flow cytometry, cells dissociated from a monolayer or the spheroids gave rise to homogeneous populations. Cells from 3-day-old spheroids had smaller size and higher granularity (supplemental Fig. S1). We tested expression of 19 markers commonly used to characterize hMSCs by flow cytometry (supplemental Fig. S2). hMSCs from a monolayer and the spheroids were positive for CD29, CD44, CD54, CD55, CD73, CD90, CD105, CD166, and HLA-I. Neither cells from a monolayer nor cells from the spheroids were positive for c-met, CD28, CD31, CD34, CD38, CD117, or CD209. We also analyzed effects of trypsinization on the expression of cell surface markers by hMSCs. Treatment with trypsin for 90 min did not affect the expression of CD49d and CD166 in hMSCs. Detection of CD29, CD44, CD54, CD55, CD90, CD105, and HLA-I was sensitive to trypsin-EDTA treatment. Nevertheless, all antigens that tested positive after 5 min of trypsinization remained positive after 90 min of incubation with trypsin (supplemental Fig. S3).
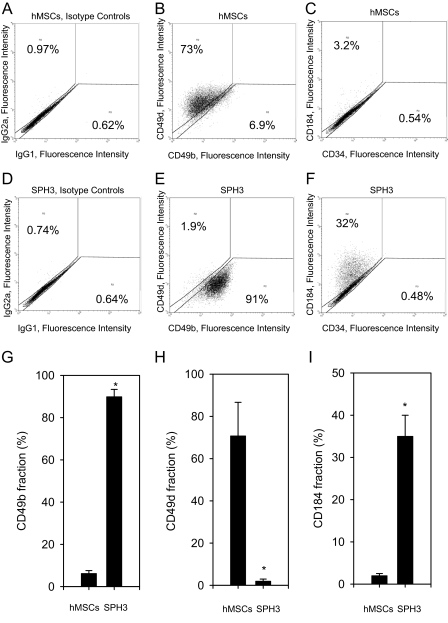
Cells from a monolayer and the spheroids showed differences in the expression of CD49d (α4 integrin subunit), CD49b (α2 integrin subunit), and CD184 (CXCR4). Changes in the expression of these antigens occur gradually from day 1 to day 3 of hMSC culturing as the spheroids (supplemental Fig. S4). Representative stainings of hMSCs from a monolayer and 3-day-old spheroids are shown in Fig. 1, A–F. Cells from the spheroids were CD49d-negative and CD49b-positive (Fig. 1E), whereas cells from a monolayer were CD49b-negative and CD49d-positive (Fig. 1B). hMSCs grown as a monolayer did not express CD184 (Fig. 1C). A significant fraction of cells isolated from the spheroids demonstrated positive staining for CD184 (Fig. 1F). On average, 6.19 ± 1.4% cells from a monolayer and 89.9 ± 3.5% cells from 3-day-old spheroids were positive for CD49b (Fig. 1G). The fraction of cells stained positive for CD49d was 70.77 ± 15.9% for cells from a monolayer and 1.95 ± 1% for cells from 3-day-old hMSC spheroids (Fig. 1H). Only 2 ± 0.5% cells from a monolayer were CD184-positive; 35 ± 5% cells from 3-day-old spheroids showed positive staining for CD184 (Fig. 1I). The differences in the expression levels of CD49b, CD49d, and CD184 were statistically significant (t-test, p value <0.05).
FIGURE 1.
Expression of CD184, CD34, CD49d, and CD49b by cells from a monolayer of hMSCs and hMSC spheroids. Cells were dissociated using trypsin-EDTA for 90 min, stained with IgG2a-PE - IgG1-FITC; CD184-PE - CD34-FITC; and CD49d-PE - CD49b-FITC antibody pairs and analyzed by flow cytometry. Representative two-color fluorescence dot plots are shown. Control stainings with isotype- and fluorochrome-matching antibody pairs are shown in A and D for hMSCs from a monolayer and 3-day-old spheroids (SPH3), accordingly. B and C show dot plots for a monolayer of hMSCs stained with CD49d-PE - CD49b-FITC (B) and CD184-PE - CD34-FITC (C) pairs. Corresponding stainings for cells from SPH3 are shown in E and F. G shows a mean value ± S.D. of a percentage of CD49b-positive cells from a monolayer of hMSCs and SPH3 as an average of three independent experiments. H shows a mean value ± S.D. of a percentage of CD49d-positive cells from a monolayer of hMSCs and SPH3 as an average of three independent experiments. I shows a mean value ± S.D. of a percentage of CD184-positive cells from a monolayer of hMSCs and SPH3 as an average of three independent experiments. To calculate a percentage of positively stained cells data were gated as shown. Statistically significant changes are denoted by asterisks (*, t-test, p value <0.05).
Overall, flow cytometric analysis demonstrated that the pattern of antigen expression by cells from the spheroids was similar to that found for hMSCs from a monolayer. Substantial differences, however, were detected in the expression of several proteins, CXCR4 and the α2 and α4 integrin subunits, responsible for cell adhesion and motility.
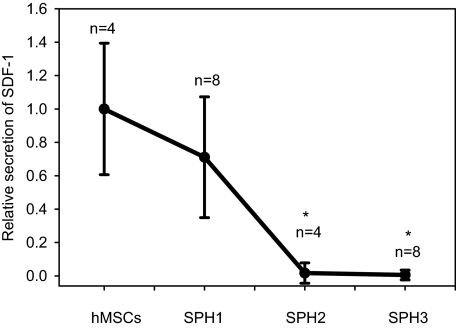
Secretion of SDF-1 by hMSCs and hMSC Spheroids—It was reported that the expression of CXCR4 by hMSCs inversely correlates with the expression of SDF-1 (41, 42). According to our Affymetrix microarray data, the expression of SDF-1 mRNA was 10-fold down-regulated in hMSC spheroids (40). We investigated how formation of the spheroids affects SDF-1 secretion by hMSCs. SDF-1 was measured in medium conditioned by a monolayer of hMSCs or 1-, 2-, or 3-day-old hMSC spheroids. The amount of SDF-1 in conditioned medium was normalized to the cell number. Relative changes in the secretion of SDF-1 are shown in Fig. 2. There was a statistically significant decline (t-test, p value <0.05) in SDF-1 secretion by 2- and 3-day-old hMSC spheroids in comparison with hMSCs from a monolayer.
FIGURE 2.
Secretion of SDF-1 by a monolayer of hMSCs and hMSC spheroids. Concentrations of SDF-1 were measured in medium conditioned by a monolayer of hMSCs and one- (SPH1), two- (SPH2), and three- (SPH3) day-old hMSC spheroids using enzyme-linked immunosorbent assay and normalized to the total number of cells. Mean values of relative secretion ± S.D. of SDF-1 by hMSCs (n = 4), SHP1 (n = 8), SPH2 (n = 4), and SPH3 (n = 4) are shown. Statistically significant changes in comparison with hMSCs are denoted by asterisks (*, t-test, p value <0.05).
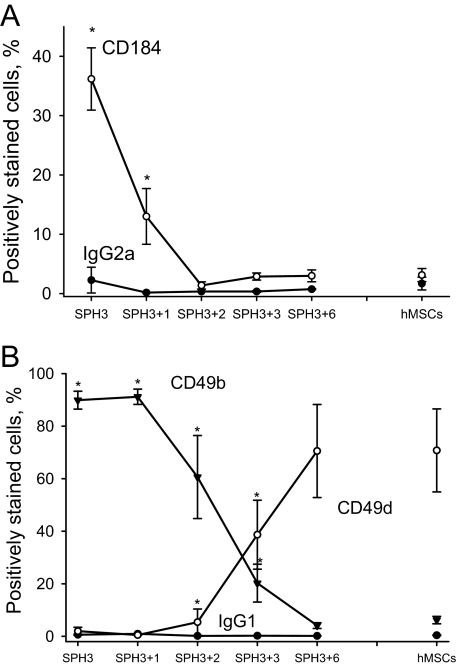
Transfer of hMSCs from the Spheroids to a Monolayer Restores the Expression Pattern of CXCR4 and the α2 and α4 Integrin Subunits—We investigated whether changes in the expression of CXCR4 and the α2 and α4 integrin subunits are reversible. For this, cells were isolated from 3-day-old hMSC spheroids and maintained in monolayer for 1–7 days. Expression of CXCR4 (CD184) and the α2 (CD49b) and α4 (CD49d) integrin subunits was analyzed by flow cytometry. Fig. 3A shows that the expression of CXCR4 (CD184) was down-regulated. Expression of the α2(CD49b) and α4(CD49d) integrin subunits also returned to the levels characteristic of a monolayer of hMSCs (Fig. 3B). Changes in the expression of CXCR4 and the α2 and α4 integrin subunits were robust and occurred within 48 h.
FIGURE 3.
Changes in CD184, CD49d, and CD49b expression by cells from the spheroids after plating them as a monolayer. Cells were dissociated from 3-day-old hMSC spheroids (SPH3) and cultured in a monolayer for 1–6 days (SPH3 + 1–6). Mean values of a percentage ± S.D. of CD184-, CD49d-, and CD49b-positive cells were determined as shown in Fig. 1. A shows changes in the expression of CD184. B shows changes in the expression of CD49b and CD49d. Corresponding values for a monolayer of hMSCs (hMSCs) and isotype controls are presented on each panel. Data represent an average of at least three independent experiments. Statistically significant changes in comparison with hMSCs are denoted by asterisks (*, t-test, p value <0.05).
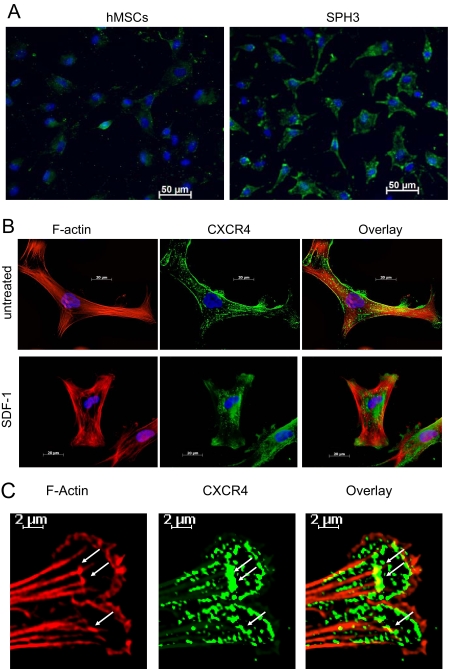
SDF-1 Induces Internalization of CXCR4—Expression of CXCR4 in a monolayer of hMSCs was too low for positive staining (Fig. 4A). Therefore, the effects of SDF-1 on intracellular localization of CXCR4 were studied with cells dissociated from the spheroids. In the absence of SDF-1, CXCR4 was localized on the cell surface (Fig. 4, A and B). Addition of SDF-1 resulted in internalization of CXCR4 (Fig. 4B). Internalized CXCR4 was targeted to two distinct locations. The majority of the receptor was detected in the perinuclear space of hMSCs. A portion of CXCR4 was found associated with filamentous structures of hMSC lamellipodias. The greater part of CXCR4 detected in lamellipodias was not co-localized but, rather, positioned along the F-actin cytoskeleton (Fig. 4C). CXCR4 showed a prominent co-localization with F-actin only at the tips of growing F-actin filaments, the place where F-actin contacts focal adhesion complexes (Fig. 4C).
FIGURE 4.
Intracellular localization of CXCR4. Cells were dissociated by trypsinization, plated on Lab-Tek II chamber CC2 glass slides, and allowed to attach for 8 h. After serum starvation, cells were treated with and without 1 μg/ml SDF-1α for 45 min. hMSCs from a monolayer (hMSCs) or 3-day-old hMSC spheroids (SPH3) were stained with anti-human CXCR4 antibody and Alexa Fluor 488-conjugated F(ab′)2 secondary antibody (green). F-actin was stained with Alexa Fluor 594-conjugated phalloidin (red). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). A shows CXCR4 staining (green) in hMSCs and SPH3. B shows CXCR4 (green) and F-actin (red) staining in SPH3 cells before (untreated) and after treatment with 1 μg/ml SDF-1α for 45 min (SDF-1). C shows staining for CXCR4 (green) and F-actin (red) in lamellipodias of cells from SPH3 treated with SDF-1. Sites of co-localization are denoted by white arrows.
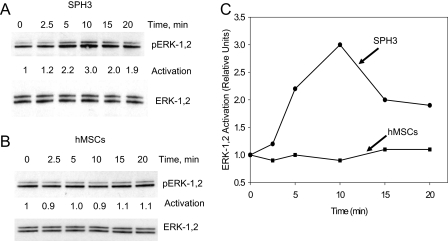
SDF-1 Activates ERK-1,2 in hMSC Spheroid Cells—To investigate activation of signaling pathways via CXCR4 in hMSCs, we tested activation of ERK, the known downstream effector of CXCR4. Treatment of cells from a monolayer with SDF-1 does not activate ERK-1,2 (Fig. 5, B and C). We hypothesize that the expression level of CXCR4 in these cells is not sufficient for activation of ERK. Treatment of cells isolated from hMSC spheroids resulted in activation of ERK-1,2, suggesting that expressed CXCR4 is functionally active (Fig. 5, A and C).
FIGURE 5.
Effects of SDF-1 on activation of ERK-1,2. Cells from a monolayer of hMSCs (hMSCs) or 3-day-old hMSC spheroids (SPH3) were dissociated, plated, and allowed to adhere for 8 h. After serum starvation, cells were treated with 1 μg/ml SDF-1α for 0–20 min and lysed. Proteins were separated on an SDS-PAG gel and analyzed by Western blot using antibodies against total ERK-1,2 and pERK-1,2. A shows pERK-1,2 and total ERK-1,2 staining for cells isolated from SPH3. B shows pERK-1,2 and total ERK-1,2 for hMSCs. C shows the results of densitometric analysis of pERK-1,2 for hMSCs and SPH3 after normalization for total ERK-1,2 in corresponding cellular lysates.
CXCR4 Regulates hMSC Adhesion to Endothelial Cells—Adhesion of cells circulating in the bloodstream to endothelial cells is the first and key event in cell homing. We investigated how expression of CXCR4 regulates adhesion of hMSCs to normoxic or pre-exposed to hypoxia endothelial cells.
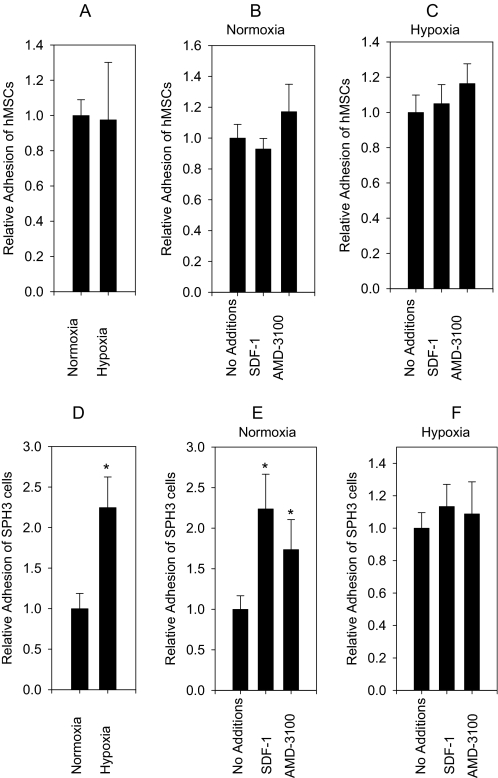
There was no statistically significant effect of HUVEC pre-exposure to hypoxia on the adhesion of hMSCs from a monolayer (Fig. 6A). Neither SDF-1 nor AMD-3100 affected the adhesion of hMSCs from a monolayer to normoxic or pre-exposed to hypoxia endothelial cells (Fig. 6, B and C). In contrast, the adhesion of cells from the spheroids was stimulated 2.2 ± 0.4-fold (t-test, p value <0.05) by pre-exposure of HUVECs to hypoxia (Fig. 6D). SDF-1 and AMD-3100 stimulated the adhesion of cells from the spheroids to normoxic endothelial cells 2.2 ± 0.4 times and 1.7 ± 0.4 times (t-test, p value <0.05), respectively (Fig. 6E). Addition of SDF-1 or AMD-3100 had no effect on the adhesion of cells from the spheroids to endothelial cells pre-exposed to hypoxia (Fig. 6F). Thus, effects of hypoxia and treatment with SDF-1 or AMD-3100 on the adhesion of cells from the spheroids to HUVECs were not additive.
FIGURE 6.
Adhesion of hMSCs to HUVECs. hMSCs from a monolayer (hMSCs) or from 3-day-old hMSC spheroids (SPH3) were dissociated by trypsinization, labeled with Calcein AM, and incubated with a monolayer of HUVECs. Prior to analysis HUVECs were exposed to normoxia or hypoxia. Adhesion of hMSCs or SPH3 cells was studied with or without 0.5 μg/ml SDF-1α or 10 μm AMD-3100. A shows the effect of HUVEC pre-exposure to hypoxia on the adhesion of hMSCs (n = 30). B shows the effects of SDF-1α and AMD-3100 on the adhesion of hMSCs to normoxic HUVECs (n = 30). C shows effects of SDF-1α and AMD-3100 on the adhesion of hMSCs to HUVECs pre-exposed to hypoxia (n = 30). There was no statistically significant difference in hMSC adhesion to HUVECs in any of the conditions described above (A–C). D shows the effect of HUVEC pre-exposure to hypoxia on the adhesion of SPH3 cells. Hypoxia increases adhesion 2.24 times (n = 36, confidence interval = 0.4, p ≤0.001, t-test). E shows the effects of SDF-1α and AMD-3100 on the adhesion of SPH3 cells to normoxic HUVECs. SDF-1α stimulates the adhesion 2.2 times (n = 36, confidence interval = 0.4). AMD-3100 stimulates the adhesion 1.7 times (n = 24, confidence interval = 0.4). The effects of SDF-1α and AMD-3100 on the adhesion of SPH3 cells are statistically significant (p value ≤0.001, analysis of variance test). There is no statistically significant difference between the adhesion in the presence of SDF-1α and AMD-3100. F shows the effects of SDF-1α (n = 36) and AMD-3100 (n = 24) on the adhesion of SPH3 cells to HUVECs pre-exposed to hypoxia. There is no statistically significant difference between adhesion of non-treated cells and cells treated with SDF-1α or AMD-3100. Statistically significant changes are denoted by asterisks, vertical intervals represent 95% CI (confidence interval).
DISCUSSION
Recently we reported that hMSCs form cellular aggregates (hMSC spheroids) when placed in hanging drops (40). In this study we investigated the difference in the expression of cell surface markers by cells from a monolayer of hMSCs and the spheroids. Antigens of cells from the spheroids were generally similar to those of hMSCs from a monolayer. Substantial changes, however, were observed in the expression of proteins responsible for cell adhesion and motility. We found that hMSCs cultured as spheroids started to express the α2 integrin subunit (CD49b) and CXCR4 (CD184) and lost the expression of the α4 integrin subunit (CD49d). These changes are consistent with the DNA microarray data reported by us previously (40). A similar increase in the expression of the α2 integrin subunit was reported for spheroid culture of ovarian cancer cells (43) and T cells incubated in three-dimensional collagen lattices (44). Both the α2 and α4 integrin subunits form a complex with the β1 integrin subunit. The α4β1 integrin interacts with fibronectin and vascular cell adhesion molecule 1 and participates in hematopoietic stem cell homing. The α2β1 integrin is a receptor for collagen and laminin and is involved in hematopoietic, endothelial, and T cell migration (44, 45). Cultured MSCs can express α2β1 integrin (3, 29) or α4β1 integrin (28). Our data demonstrate that expression of the α2 and α4 integrin subunits by hMSCs depends on culture conditions.
Several research groups have demonstrated that MSCs lose CXCR4 expression shortly after isolation and only a small fraction of culture-expanded MSCs are CXCR4-positive (30, 31, 33, 34, 36). It was hypothesized that cultured MSCs undergo transformation into more differentiated cells (33). Our results show that these changes are reversible and represent an adaptation of hMSCs to cell culture conditions. Flow cytometry and immunocytochemistry demonstrated that CXCR4 is localized on the surface of cells from the spheroids. Treatment with SDF-1 internalizes CXCR4 and activates ERK, suggesting that expressed receptor is functional. A fraction of internalized CXCR4 was detected in the lamellipodias of SDF-1-treated cells. Similar SDF-1-dependent redistribution of CXCR4 was reported for leukocytes interacting with endothelial cells (46). The majority of CXCR4 detected in lamellipodias was positioned along the F-actin cytoskeleton with some portion of the receptor being co-localized with the tips of growing F-actin filaments. The tips of F-actin filaments are the sites of attachment of focal adhesion complexes. The activation of focal adhesion kinase by SDF-1 plays an important role in adhesion of hematopoietic precursor cells (47) and results in cytoskeleton rearrangements (33, 47, 48). Association of CXCR4 with the tips of F-actin filaments correlates with the reported stimulation of focal adhesion kinase by SDF-1 and suggests that the receptor might be a part of large macromolecular complexes assembled at the points of focal adhesion.
Expression of CXCR4 by hMSCs inversely correlates with the expression of SDF-1. According to our Affymetrix microarray data, mRNA for CXCR4 was increased 56-fold whereas the mRNA for SDF-1 was decreased 10-fold in cells from the spheroids (40). Here we demonstrate that this correlation also occurs at protein level. Similar results were reported for hMSCs seeded on a hyaluronan-based polymer scaffold (41, 42). It was suggested that the removal of SDF-1 from the hMSC microenvironment helps to mobilize the internalized CXCR4, thus increasing its functional expression (41). The exact molecular mechanisms that result in the inverse correlation between CXCR4 and SDF-1 expression by hMSCs remain unknown and need to be investigated.
Adhesion of circulating cells to endothelial cells is the first and probably the key event in hMSC homing. Molecular mechanisms of hMSC adhesion to endothelial cells remain poorly understood. From studies of T lymphocyte adhesion to endothelial cells, it can be concluded that integrins and CXCR4 may have profound effects on the cell homing. Our data suggest that configuration of cell adhesion and regulatory molecules on the surface of hMSCs from a monolayer prevent preferential recognition of endothelial cells pre-exposed to hypoxia. Due to the lack of CXCR4 expression, adhesion of hMSCs from a monolayer to HUVECs does not depend on the presence of SDF-1. These results, however, do not exclude the possibility that in vivo hMSCs may follow stimuli other than SDF-1 to target hypoxic endothelial cells. Transfer of hMSCs into the three-dimensional environment of the spheroids resulted in the expression of CXCR4 and the α2 integrin subunit, leading to preferential recognition of endothelial cells pre-exposed to hypoxia. Binding of cells from the spheroids to normoxic endothelial cells was stimulated by SDF-1 and AMD-3100. SDF-1 is an agonist of CXCR4. AMD-3100 is an anti-human immunodeficiency virus agent that blocks CXCR4-dependent virus entry (49). It remains unclear whether AMD-3100 is an antagonist (50) or a partial agonist of CXCR4 (51). In our experiments AMD-3100 performed as an agonist of CXCR4 and stimulated binding of cells from the spheroids to normoxic endothelial cells. There are no additive effects of hypoxia and addition of SDF-1 or AMD-3100 on the adhesion of cells from the spheroids to HUVECs. It is known that hypoxia up-regulates the production of SDF-1 by endothelial cells (52). Endogenous SDF-1 when immobilized on the surface of endothelial cells is far more effective than the exogenous soluble form of SDF-1 in the stimulation of T lymphocyte firm adhesion (53). We hypothesize that a mechanism similar to one postulated for T lymphocytes (53) may explain the lack of additive effects of hypoxia and SDF-1 or AMD-3100 on the adhesion of cells from the spheroids to endothelial cells.
Overall, our data demonstrate the great flexibility of hMSCs in the expression of cell surface molecules responsible for the regulation of homing. We believe that hMSC spheroids might be useful as an in vitro model that mimics cell-to-cell interactions in tissues.
Supplementary Material
Acknowledgments
We thank Todd Rueb and the staff of the flow cytometry core facility at Stony Brook University for excellent technical assistance with flow cytometry.
This work was supported, in whole or in part, by National Institutes of Health Grants HL67101 and HL28958. This work was also supported by an American Heart Association Scientist Development grant (to S. V. D.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Footnotes
The abbreviations used are: hMSC, human mesenchymal stem cell; HSC, human mesenchymal stem cell; PBS, phosphate-buffered saline; DMEM, Dulbecco's modified Eagle's medium; HBSS, Hanks' balanced salt solution; ERK, extracellular signal-regulated kinase; MOPS, 4-morpholinepropanesulfonic acid; FITC, fluorescein isothiocyanate; PE, phosphatidylethanolamine.
References
- 1.Deans, R. J., and Moseley, A. B. (2000) Exp. Hematol. 28 875-884 [DOI] [PubMed] [Google Scholar]
- 2.Jori, F. P., Napolitano, M. A., Melone, M. A., Cipollaro, M., Cascino, A., Altucci, L., Peluso, G., Giordano, A., and Galderisi, U. (2005) J. Cell. Biochem. 94 645-655 [DOI] [PubMed] [Google Scholar]
- 3.Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S., and Marshak, D. R. (1999) Science 284 143-147 [DOI] [PubMed] [Google Scholar]
- 4.Ball, L. M., Bernardo, M. E., Roelofs, H., Lankester, A., Cometa, A., Egeler, R. M., Locatelli, F., and Fibbe, W. E. (2007) Blood 110 2764-2767 [DOI] [PubMed] [Google Scholar]
- 5.Lazarus, H. M., Koc, O. N., Devine, S. M., Curtin, P., Maziarz, R. T., Holland, H. K., Shpall, E. J., McCarthy, P., Atkinson, K., Cooper, B. W., Gerson, S. L., Laughlin, M. J., Loberiza, F. R., Jr., Moseley, A. B., and Bacigalupo, A. (2005) Biol. Blood Marrow Transplant. 11 389-398 [DOI] [PubMed] [Google Scholar]
- 6.Le, B. K., Samuelsson, H., Gustafsson, B., Remberger, M., Sundberg, B., Arvidson, J., Ljungman, P., Lonnies, H., Nava, S., and Ringden, O. (2007) Leukemia 21 1733-1738 [DOI] [PubMed] [Google Scholar]
- 7.Horwitz, E. M., Gordon, P. L., Koo, W. K., Marx, J. C., Neel, M. D., McNall, R. Y., Muul, L., and Hofmann, T. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 8932-8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le, B. K., Gotherstrom, C., Ringden, O., Hassan, M., McMahon, R., Horwitz, E., Anneren, G., Axelsson, O., Nunn, J., Ewald, U., Norden-Lindeberg, S., Jansson, M., Dalton, A., Astrom, E., and Westgren, M. (2005) Transplantation 79 1607-1614 [DOI] [PubMed] [Google Scholar]
- 9.Wakitani, S., Imoto, K., Yamamoto, T., Saito, M., Murata, N., and Yoneda, M. (2002) Osteoarthritis Cartilage 10 199-206 [DOI] [PubMed] [Google Scholar]
- 10.Rombouts, W. J., and Ploemacher, R. E. (2003) Leukemia 17 160-170 [DOI] [PubMed] [Google Scholar]
- 11.Wang, L., Li, Y., Chen, X., Chen, J., Gautam, S. C., Xu, Y., and Chopp, M. (2002) Hematology (New York) 7 113-117 [DOI] [PubMed] [Google Scholar]
- 12.Li, Y., Chen, J., Zhang, C. L., Wang, L., Lu, D., Katakowski, M., Gao, Q., Shen, L. H., Zhang, J., Lu, M., and Chopp, M. (2005) Glia 49 407-417 [DOI] [PubMed] [Google Scholar]
- 13.Chen, S. L., Fang, W. W., Ye, F., Liu, Y. H., Qian, J., Shan, S. J., Zhang, J. J., Chunhua, R. Z., Liao, L. M., Lin, S., and Sun, J. P. (2004) Am. J. Cardiol. 94 92-95 [DOI] [PubMed] [Google Scholar]
- 14.Allers, C., Sierralta, W. D., Neubauer, S., Rivera, F., Minguell, J. J., and Conget, P. A. (2004) Transplantation 78 503-508 [DOI] [PubMed] [Google Scholar]
- 15.Barbash, I. M., Chouraqui, P., Baron, J., Feinberg, M. S., Etzion, S., Tessone, A., Miller, L., Guetta, E., Zipori, D., Kedes, L. H., Kloner, R. A., and Leor, J. (2003) Circulation 108 863-868 [DOI] [PubMed] [Google Scholar]
- 16.Gao, J., Dennis, J. E., Muzic, R. F., Lundberg, M., and Caplan, A. I. (2001) Cells Tissues Organs 169 12-20 [DOI] [PubMed] [Google Scholar]
- 17.Campagnoli, C., Roberts, I. A., Kumar, S., Bennett, P. R., Bellantuono, I., and Fisk, N. M. (2001) Blood 98 2396-2402 [DOI] [PubMed] [Google Scholar]
- 18.Chute, J. P. (2006) Curr. Opin. Hematol. 13 399-406 [DOI] [PubMed] [Google Scholar]
- 19.Feng, Y., Broder, C. C., Kennedy, P. E., and Berger, E. A. (1996) Science 272 872-877 [DOI] [PubMed] [Google Scholar]
- 20.Peled, A., Petit, I., Kollet, O., Magid, M., Ponomaryov, T., Byk, T., Nagler, A., Ben-Hur, H., Many, A., Shultz, L., Lider, O., Alon, R., Zipori, D., and Lapidot, T. (1999) Science 283 845-848 [DOI] [PubMed] [Google Scholar]
- 21.Geminder, H., Sagi-Assif, O., Goldberg, L., Meshel, T., Rechavi, G., Witz, I. P., and Ben-Baruch, A. (2001) J. Immunol. 167 4747-4757 [DOI] [PubMed] [Google Scholar]
- 22.Feil, C., and Augustin, H. G. (1998) Biochem. Biophys. Res. Commun. 247 38-45 [DOI] [PubMed] [Google Scholar]
- 23.Hesselgesser, J., Halks-Miller, M., DelVecchio, V., Peiper, S. C., Hoxie, J., Kolson, D. L., Taub, D., and Horuk, R. (1997) Curr. Biol. 7 112-121 [DOI] [PubMed] [Google Scholar]
- 24.Ji, J. F., He, B. P., Dheen, S. T., and Tay, S. S. (2004) Stem Cells (Dayton) 22 415-427 [DOI] [PubMed] [Google Scholar]
- 25.Ponomaryov, T., Peled, A., Petit, I., Taichman, R. S., Habler, L., Sandbank, J., Arenzana-Seisdedos, F., Magerus, A., Caruz, A., Fujii, N., Nagler, A., Lahav, M., Szyper-Kravitz, M., Zipori, D., and Lapidot, T. (2000) J. Clin. Investig. 106 1331-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasawa, T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., Yoshida, N., Kikutani, H., and Kishimoto, T. (1996) Nature 382 635-638 [DOI] [PubMed] [Google Scholar]
- 27.Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I., and Littman, D. R. (1998) Nature 393 595-599 [DOI] [PubMed] [Google Scholar]
- 28.Conget, P. A., and Minguell, J. J. (1999) J. Cell. Physiol. 181 67-73 [DOI] [PubMed] [Google Scholar]
- 29.Majumdar, M. K., Keane-Moore, M., Buyaner, D., Hardy, W. B., Moorman, M. A., McIntosh, K. R., and Mosca, J. D. (2003) J Biomed. Sci. 10 228-241 [DOI] [PubMed] [Google Scholar]
- 30.Sordi, V., Malosio, M. L., Marchesi, F., Mercalli, A., Melzi, R., Giordano, T., Belmonte, N., Ferrari, G., Leone, B. E., Bertuzzi, F., Zerbini, G., Allavena, P., Bonifacio, E., and Piemonti, L. (2005) Blood 106 419-427 [DOI] [PubMed] [Google Scholar]
- 31.Wynn, R. F., Hart, C. A., Corradi-Perini, C., O'Neill, L., Evans, C. A., Wraith, J. E., Fairbairn, L. J., and Bellantuono, I. (2004) Blood 104 2643-2645 [DOI] [PubMed] [Google Scholar]
- 32.Kemp, K. C., Hows, J., and Donaldson, C. (2005) Leuk. Lymphoma 46 1531-1544 [DOI] [PubMed] [Google Scholar]
- 33.Honczarenko, M., Le, Y., Swierkowski, M., Ghiran, I., Glodek, A. M., and Silberstein, L. E. (2006) Stem Cells (Dayton) 24 1030-1041 [DOI] [PubMed] [Google Scholar]
- 34.Ringe, J., Strassburg, S., Neumann, K., Endres, M., Notter, M., Burmester, G. R., Kaps, C., and Sittinger, M. (2007) J. Cell Biochem. 101 135-146 [DOI] [PubMed] [Google Scholar]
- 35.Son, B. R., Marquez-Curtis, L. A., Kucia, M., Wysoczynski, M., Turner, A. R., Ratajczak, J., Ratajczak, M. Z., and Janowska-Wieczorek, A. (2006) Stem Cells (Dayton) 24 1254-1264 [DOI] [PubMed] [Google Scholar]
- 36.Von, L. I., Notohamiprodjo, M., Wechselberger, A., Peters, C., Henger, A., Seliger, C., Djafarzadeh, R., Huss, R., and Nelson, P. J. (2005) Stem Cells Dev. 14 329-336 [DOI] [PubMed] [Google Scholar]
- 37.Bhakta, S., Hong, P., and Koc, O. (2006) Cardiovasc. Revasc. Med. 7 19-24 [DOI] [PubMed] [Google Scholar]
- 38.Croitoru-Lamoury, J., Lamoury, F. M., Zaunders, J. J., Veas, L. A., and Brew, B. J. (2007) J. Interferon Cytokine Res. 27 53-64 [DOI] [PubMed] [Google Scholar]
- 39.Li, Y., Yu, X., Lin, S., Li, X., Zhang, S., and Song, Y. H. (2007) Biochem. Biophys. Res. Commun. 356 780-784 [DOI] [PubMed] [Google Scholar]
- 40.Potapova, I. A., Gaudette, G. R., Brink, P. R., Robinson, R. B., Rosen, M. R., Cohen, I. S., and Doronin, S. V. (2007) Stem Cells (Dayton) 25 1761-1768 [DOI] [PubMed] [Google Scholar]
- 41.Lisignoli, G., Toneguzzi, S., Zini, N., Piacentini, A., Cristino, S., Tschon, M., Grassi, F., Fini, M., Giardino, R., Maraldi, N. M., and Facchini, A. (2003) Chir. Organi Mov. 88 363-367 [PubMed] [Google Scholar]
- 42.Lisignoli, G., Cristino, S., Piacentini, A., Cavallo, C., Caplan, A. I., and Facchini, A. (2006) J. Cell Physiol. 207 364-373 [DOI] [PubMed] [Google Scholar]
- 43.Shield, K., Riley, C., Quinn, M. A., Rice, G. E., Ackland, M. L., and Ahmed, N. (2007) J. Carcinog. 6 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedl, P., Noble, P. B., and Zanker, K. S. (1995) J. Immunol. 154 4973-4985 [PubMed] [Google Scholar]
- 45.Sixt, M., Bauer, M., Lammermann, T., and Fassler, R. (2006) Curr. Opin. Cell Biol. 18 482-490 [DOI] [PubMed] [Google Scholar]
- 46.van Buul, J. D., Voermans, C., van Gelderen, J., Anthony, E. C., van der Schoot, C. E., and Hordijk, P. L. (2003) J. Biol. Chem. 278 30302-30310 [DOI] [PubMed] [Google Scholar]
- 47.Glodek, A. M., Le, Y., Dykxhoorn, D. M., Park, S. Y., Mostoslavsky, G., Mulligan, R., Lieberman, J., Beggs, H. E., Honczarenko, M., and Silberstein, L. E. (2007) Leukemia 21 1723-1732 [DOI] [PubMed] [Google Scholar]
- 48.Le, Y., Honczarenko, M., Glodek, A. M., Ho, D. K., and Silberstein, L. E. (2005) J. Immunol. 174 2582-2590 [DOI] [PubMed] [Google Scholar]
- 49.Donzella, G. A., Schols, D., Lin, S. W., Este, J. A., Nagashima, K. A., Maddon, P. J., Allaway, G. P., Sakmar, T. P., Henson, G., De Clercq, E., and Moore, J. P. (1998) Nat. Med. 4 72-77 [DOI] [PubMed] [Google Scholar]
- 50.Fricker, S. P., Anastassov, V., Cox, J., Darkes, M. C., Grujic, O., Idzan, S. R., Labrecque, J., Lau, G., Mosi, R. M., Nelson, K. L., Qin, L., Santucci, Z., and Wong, R. S. (2006) Biochem. Pharmacol. 72 588-596 [DOI] [PubMed] [Google Scholar]
- 51.Zhang, W. B., Navenot, J. M., Haribabu, B., Tamamura, H., Hiramatu, K., Omagari, A., Pei, G., Manfredi, J. P., Fujii, N., Broach, J. R., and Peiper, S. C. (2002) J. Biol. Chem. 277 24515-24521 [DOI] [PubMed] [Google Scholar]
- 52.Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., Capla, J. M., Galiano, R. D., Levine, J. P., and Gurtner, G. C. (2004) Nat. Med. 10 858-864 [DOI] [PubMed] [Google Scholar]
- 53.Shamri, R., Grabovsky, V., Gauguet, J. M., Feigelson, S., Manevich, E., Kolanus, W., Robinson, M. K., Staunton, D. E., von Andrian, U. H., and Alon, R. (2005) Nat. Immunol. 6 497-506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.