Abstract
Integrin αIIbβ3 activation is critical for platelet physiology and is controlled by signal transduction through kinases and phosphatases. Compared with kinases, a role for phosphatases in platelet integrin αIIbβ3 signaling is less understood. We report that the catalytic subunit of protein phosphatase 2A (PP2Ac) associates constitutively with the integrin αIIbβ3 in resting platelets and in human embryonal kidney 293 cells expressing αIIbβ3. The membrane proximal KVGFFKR sequence within the cytoplasmic domain of integrin αIIb is sufficient to support a direct interaction with PP2Ac. Fibrinogen binding to αIIbβ3 during platelet adhesion decreased integrin-associated PP2A activity and increased the phosphorylation of a PP2A substrate, vasodilator associated phosphoprotein. Overexpression of PP2Acα in 293 cells decreased αIIbβ3-mediated adhesion to immobilized fibrinogen. Conversely, small interference RNA mediated knockdown of endogenous PP2Acα expression in 293 cells, enhanced extracellular signal-regulated kinase (ERK1/2) and p38 activation, and accelerated αIIbβ3 adhesion to fibrinogen and von Willebrand factor. Inhibition of ERK1/2, but not p38 activation, abolished the increased adhesiveness of PP2Ac α-depleted 293 cells to fibrinogen. Furthermore, knockdown of PP2Acα expression in bone marrow-derived murine megakaryocytes increased soluble fibrinogen binding induced by protease-activated receptor 4-activating peptide. These studies demonstrate that PP2Ac α can negatively regulate integrin αIIbβ3 signaling by suppressing the ERK1/2 signaling pathway.
Integrin cytoplasmic tails are devoid of any intrinsic catalytic activity. Nevertheless, integrins can transmit bidirectional signals across the plasma membrane of a cell and regulate several cellular processes, such as, adhesion, migration, and apoptosis. In the context of the major platelet integrin αIIbβ3, emerging evidence indicates that cytoplasmic tails act as a molecular scaffold for intracellular enzymes and for both cytoskeletal and adaptor proteins and can either positively or negatively regulate signaling (1). For example, during an agonist-mediated inside-out signaling process, talin interacts with the integrin β3 tail and induces integrin αIIbβ3 activation (2), whereas calcium and integrin-binding protein 1 binds to the αIIb tail and negatively regulates αIIbβ3 activation (3).
Subsequent binding of fibrinogen to the activated αIIbβ3 integrin initiates an outside-in signaling process that regulates platelet function. Outside-in signaling can be mediated by intricate interplay of a set of proteins that associate constitutively with the integrin and by others that either associate or dissociate with the integrin in response to fibrinogen binding. For instance, c-Src associates constitutively to the β3 tail (4). Fibrinogen binding to αIIbβ3 induces association of protein tyrosine phosphatase 1B, spleen tyrosine kinase, and protein kinase Cβ to the β3 tail (4–6) and calcium and integrin-binding protein 1 to the αIIb tail (7) and causes the dissociation of C terminus Src kinase from the β3 tail (4) and the catalytic subunit of protein phosphatase 1 (PP1c)6 from the αIIb tail (8). Reversible phosphorylation of multiple effector proteins that are downstream of the integrin signaling pathway is one of the mechanisms by which these αIIbβ3-associated proteins can initiate and/or transduce signals. The phosphorylation status of most signaling proteins is determined by a fine balance between the activities of kinases and phosphatases. Thus far, among the reported αIIbβ3-associated signaling molecules, kinases have outnumbered phosphatases. Consequently, in contrast to integrin-associated kinases, a role for phosphatases in platelet signaling, with rare exception (5), is not well understood.
Protein phosphatase 2A (PP2A) is a ubiquitously expressed Ser/Thr phosphatase and is implicated in β1 integrin function in cell types other than platelets (9). The PP2A holoenzyme consists of a ∼36-kDa catalytic subunit C (PP2Ac) and a ∼65-kDa structural subunit A (PP2Aa) that together form an AC core dimer (PP2Aac). The A subunit in the core dimer links multiple regulatory B subunits in a fashion that determines the substrate specificity, the subcellular location, and the catalytic activity of the phosphatase (10). Blockade by generic Ser/Thr phosphatase inhibitors like okadaic acid and calyculin A impair agonist-induced platelet aggregation, secretion (11–13), and αIIbβ3 outside-in signaling functions such as adhesion and spreading to immobilized fibrinogen. This act may be independent of β3 Thr753 phosphorylation status (14, 15); however, okadaic acid and calyculin A can inhibit multiple Ser/Thr phosphatases such as PP1, PP2A, and PP4 (16). Therefore, these agents are unable to specifically elucidate the role of PP2A in integrin αIIbβ3 signaling and function.
In this study, we show that a pool of the catalytic subunit of PP2A constitutively associates with integrin αIIbβ3. By using a genetic (gene knockdown and/or gene overexpression) approach in two distinct model systems, such as the 293 and the primary murine megakaryocytes, PP2Acα was identified to negatively regulate αIIbβ3 adhesiveness to immobilized and soluble fibrinogen. PP2Acα can negatively regulate αIIbβ3 signaling by repressing the ERK1/2 activation pathway.
MATERIALS AND METHODS
Immunoprecipitation and Western Blotting—Blood was drawn in an acid/citrate/dextrose anticoagulant from normal, healthy, fasting donors. Each donor signed an informed consent approved by the Institutional Review Board of Baylor College of Medicine, Houston, TX. Washed platelets were prepared as previously described (17). Using either 750 μg/ml 1% Triton X-100 or Igepal CA-630, lysates were obtained from either resting platelets or 293 cells expressing αIIbβ3 or the various mutants. The lysates were immunoprecipitated using anti-αIIb (Sew-8, gift from Dr. Newman, Blood Research Institute, Milwaukee, WI), anti-PP2Ac (Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit IgG (Pierce) using Protein A-Sepharose beads (Amersham Biosciences). Proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose, probed with monoclonal antibodies to αIIb (132.1), PP2Ac (Upstate Biotechnology/Millipore, Billerica, MA), or PP2Cc (Alexis Biochemicals, San Diego, CA), and developed using the ECL system (Amersham Biosciences).
Interaction of PP2Ac with the Integrin αIIb Subunit—Truncation of the cytoplasmic domain of integrin αIIb at residue 989 was generated according to the manufacturer's protocol for the QuikChange™ site-directed mutagenesis kit (Stratagene, La Jolla, CA). Integrin β3 with truncation of the cytoplasmic domain at residue 716 was kindly provided by Dr. Michael H. Kroll (Baylor College of Medicine, Houston, TX). These constructs were sequenced to confirm the presence of the desired truncations. 293 cells were transiently transfected with wild-type αIIb and β3 or with truncated integrin tails using Lipofectamine (Invitrogen) for 48 h. Cells were lysed in 1% Triton X-100, and lysates were immunoprecipitated with anti-PP2Ac antibody and immunoblotted with anti-αIIb antibody. In an alternate approach, biotinylated peptides corresponding to residues 985–995 of the integrin αIIb (LAMWKVGFFKR) and a control with scrambled sequence (LWKRVAGPFKM) were synthesized at the Baylor College of Medicine Protein Sequencing Core Facility, Houston, TX. We mixed 25–50 μg/ml peptide with 1 μg/ml purified PP2A or 750 μg/ml platelet lysate, and the mixtures were precipitated using streptavidin-agarose beads. The beads were washed three times, and bound proteins were eluted using SDS sample buffer. Proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti-PP2Ac antibody, and signals were then detected using ECL.
siRNA Construct, Transfection, and Adhesion—The following SMARTpool siRNA reagents, purchased from Dharmacon (Thermo Fisher Scientific, Lafayette, CO), were used in this study: 1) catalog # M-003598 targeting human PP2A catalytic subunit, α isoforms (NM_002715); 2) catalog # M-04065700 targeting murine PP2A catalytic subunit, α isoforms (NM_019411); and 3) catalog # D-001206-13-05 a nonspecific control pool with no sequence homology to any human or mouse sequence. According to manufacturer's instructions, we used siImporter (Upstate Biotechnology) to transfect 293 αIIbβ3 cells with 100 nm siRNA. After 48 h, the cells were used for Western blotting or adhesion experiments. For the adhesion studies, 1 × 105 cells, suspended in Tyrode's buffer containing 1.8 mm CaCl2 and 0.49 mm MgCl2, were incubated with either 5% BSA (control), 12.5 μg/ml fibrinogen (Enzyme Research Laboratories Inc., South Bend, IN), or 10 μg/ml VWF (gift from Dr. Jing-fei Dong, Baylor College of Medicine)-coated wells for varying time points. During certain experiments, the cells were pretreated with either control DMSO, 10 μm U0126 (ERK1/2 inhibitor), or 10 μm SB203580 (p38 inhibitor). Unbound cells were washed, and the adherent cells were quantified by assaying for acid phosphatase activity at 405 nm. The number of bound cells was obtained using a standard curve for absorbance versus cell number. Percent adhesion was calculated as the number of bound cells divided by the total number of cells added per well multiplied by 100. Specific fibrinogen binding was calculated after subtracting values obtained for BSA-coated wells. In some experiments, 293 αIIbβ3 cells were transiently transfected using Lipofectamine with cDNA for HA-tagged PP2Acα or the control vector (gift from Dr. A. Verin, University of Chicago, Chicago, IL). After 48 h, cells were analyzed for Western blotting and adhesion as described above. In some experiments, fibrinogenor BSA-coated dishes were incubated with 2 × 108 platelets for 30 min at 37 °C. The fibrinogen-bound platelets and the non-adherent platelets from BSA-coated plates were lysed in a phosphate-free buffer, and αIIb was immunoprecipitated. The integrin αIIb-associated PP2Ac activity assay was quantified using a PP2A phosphatase assay kit (Upstate Biotechnology). Using a malachite green assay, αIIb immunoprecipitates were evaluated for PP2Ac activity by dephosphorylation of the phosphopeptide K-Rp-I-R-R.
Megakaryocyte Culture, Transfection, and Flow Cytometry—Megakaryocytes were obtained from the bone marrow cultures of BALB/c mice as described previously (18). At day 5, megakaryocytes were transfected with 100 nm control siRNA or PP2Acα siRNA by using a transfecting agent from Mirus (Mirus Bio Corp., Madison, WI) for 48 h at 37 °C. On day 7, a portion of the differentiated megakaryocytes was used to assess the expression of PP2Acα. We mixed 50 μl of the megakaryocyte suspension with 2.5 mm agonist PAR4AP (AYPGKF, Protein Sequencing Core Facility, Baylor College of Medicine). Next, a non-blocking anti-αIIb antibody, 7-amino-actinomycin D (BD Bioscience, San Jose, CA) and Alexa 488-conjugated fibrinogen (20 μg/ml final concentration, Invitrogen) were added in the presence or absence of 10 mm EDTA at room temperature for 15–20 min. Alexa-fibrinogen binding was measured using an EPICS-XL flow cytometer (Beckman Coulter, Miami, FL). The FL1 expression was evaluated from the gated population of only large megakaryocytes (size) that expressed αIIbβ3 (FL2) and were viable (defined as negative for 7-amino-actinomycin D in the FL3 parameter).
RESULTS
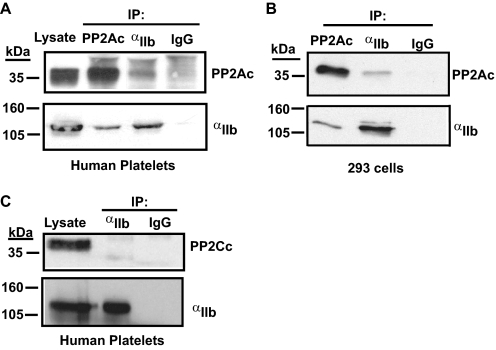
In resting platelets, a pool of the catalytic subunit of protein phosphatase 1 (PP1c) constitutively associates with αIIbβ3 complex (8). Studies from other cell types have revealed that PP2Ac can associate with integrin β1 (19). Because the primary amino acid sequence within the catalytic subunits of PP1 and PP2A are closely related, we considered whether the catalytic subunit of PP2A (PP2Ac) could associate with αIIbβ3 complex in platelets. During co-immunoprecipitation assays with lysates from resting human platelets, we detected the presence of PP2Ac in the αIIb immunoprecipitate. Conversely, in a reciprocal co-immunoprecipitation assay, αIIb was observed in the PP2Ac immunoprecipitate (Fig. 1A). Furthermore, the association of PP2Ac with integrin αIIbβ3 could be recapitulated in human embryonal kidney cell line 293 expressing αIIbβ3. Thus, suggesting the phosphatase-integrin association is intrinsic to integrin αIIbβ3 (Fig. 1B).
FIGURE 1.
Association of PP2Ac, but not PP2Cc, with integrin αIIbβ3. Integrin αIIb was immunoprecipitated from lysates of washed platelets (A) or 293 cells expressing αIIbβ3 (B) with control antibodies: rabbit IgG, anti-αIIb, or anti-PP2Ac. Immunoprecipitates were immunoblotted with antibodies to αIIb or PP2Ac. C, αIIb was immunoprecipitated, as described above, and the membrane was probed with anti-PP2Cc antibody. Results are representative of three to four experiments.
In contrast to PP2Ac, the catalytic subunit of a structurally distinct Ser/Thr phosphatase, protein phosphatase 2C (PP2Cc) was not detected in the αIIb immunoprecipitate, indicating the PP2Ac-αIIbβ3 interaction is specific (Fig. 1C). Although the phosphatase-integrin association may appear modest, its consequence on cellular function is not (see Fig. 4). PP2Acα and PP2Acβ are the two ubiquitously expressed isoforms of PP2Ac and share roughly 97% similarity in the primary amino acid sequence. The PP2Ac antibody used in these studies recognizes both isoforms. Thus, these studies indicate a specific and constitutive interaction of the catalytic subunit of PP2A with the resting integrin αIIbβ3.
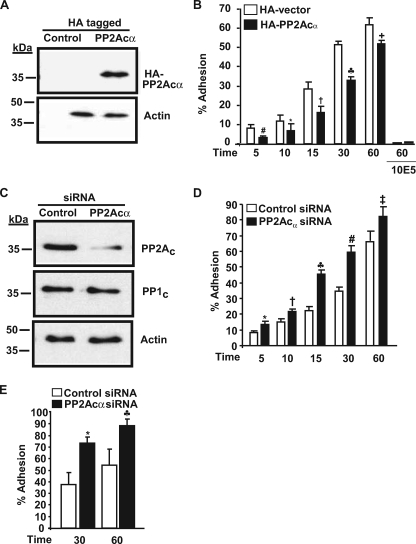
FIGURE 4.
PP2Acα negatively regulates αIIbβ3 adhesiveness in 293 cells. A, PP2Acα expression as revealed by anti-HA antibody in control and HA-tagged PP2Acα-overexpressing cells. This blot was reprobed for actin (loading control) and is a representative of four different experiments. B, effect of HA-PP2Acα overexpression on 293 cell adhesion. 293 cells transfected with control vector or vector with PP2Acα cDNA were allowed to adhere to fibrinogen in the presence and absence of 10E5 (blocking antibody to αIIbβ3), and adhesion was measured by absorbance at 405 nm. Results are mean ± S.E. of 4–7 experiments in triplicate for various time points and 2 experiments for 10E5 blocking studies. Results were significant at #, p = 0.008; *, p = 0.0031; †, p < 0.0001; ♣, p < 0.0001; +, p = 0.002 for 5-, 10-, 15-, 30-, and 60-min adhesion. Error bars were too narrow to be seen with the 10E5 inhibition. C, PP2Ac expression in control and PP2Acα-transfected siRNA. The membrane was reprobed for PP1c and actin to demonstrate siRNA specificity and equal loading respectively. Blots are representative of five different experiments. D, effect of PP2Acα knockdown on 293 cell adhesion to fibrinogen. Results are mean ± S.E. of 5–6 experiments each performed in triplicate and was significant at *, p = 0.001; †, p = 0.006; ♣, p < 0.001; #, p < 0.0001; ‡, p = 0.01 for 5-, 10-, 15-, 30-, and 60-min adhesion by t test. E, effect of PP2Acα knockdown on 293 cell adhesion to von Willebrand factor. Results are mean ± S.E. of three experiments each performed in triplicate and was significant at *, p = 0.0084; ♣, p = 0.0086, for 30 and 60 min.
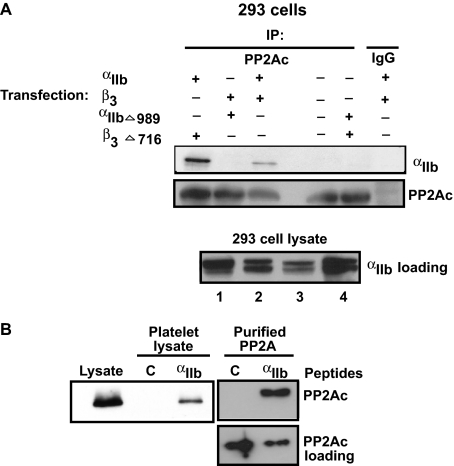
To identify whether αIIb or the β3 cytoplasmic domains support PP2Ac interaction, 293 cells were transiently transfected with wild-type αIIbβ3 or with αIIb and β3 cytoplasmic domain truncation mutants. The association of PP2Ac was then assessed by co-immunoprecipitation assays. Integrin αIIb co-immunoprecipitated with PP2Ac in 293 cells transiently expressing the wild-type (WT) αIIbβ3 (Fig. 2A). Furthermore, cells expressing the WT αIIb along with the β3 cytoplasmic truncation mutant (β3Δ716) also supported the interaction of αIIb with PP2Ac. In contrast, αIIb failed to associate with PP2Ac in 293 cells that expressed 1) no integrin αIIbβ3, 2) αIIb cytoplasmic truncation mutant (αIIbΔ989) along with the WT β3, and 3) αIIb (αIIbΔ989) and β3 (β3Δ716) cytoplasmic truncation mutants. The apparent increased αIIb association with PP2Ac in β3Δ716-expressing cells, seen in Fig. 2, was not consistently reproducible and may be due to an increased amount of immunoprecipitated PP2Ac. The inability of αIIbΔ989 mutant to support PP2Ac association was not due to a lack of αIIb expression (Fig. 2A, 293 cell lysates). These studies suggest that the cytoplasmic domain of integrin αIIb, but not β3, supports the interaction of PP2Ac.
FIGURE 2.
Direct interaction of PP2Ac with αIIb. A, 293 cells were transiently transfected with either wild-type αIIbβ3 or truncated αIIb or β3 mutants as indicated. After 48 h, transfected cells were lysed and immunoprecipitated with control IgG or anti-PP2Ac antibody and immunoblotted with anti-αIIb and anti-PP2Ac antibodies. To demonstrate the presence of αIIb in each of the different transfections performed above, lysates obtained from 293 cells transfected with αIIb- and β3-truncated mutants (1), WT αIIb- and β3-truncated mutants (2), αIIb-truncated mutant and WT β3 (3), and WT αIIbβ3 (4) were immunoblotted with anti-αIIb antibody. B, biotinylated αIIb cytoplasmic peptide (designated “αIIb”) or control (designated C) peptide was incubated with either purified PP2A enzyme or platelet lysates. Proteins were precipitated using streptavidin-agarose beads, separated by SDS-PAGE, and immunoblotted with an anti-PP2Ac antibody. An equal aliquot of peptide/PP2Ac mixture was run simultaneously to demonstrate the presence of PP2Ac among reactions (PP2Ac loading). Blots are representative of three different experiments.
To ascertain whether PP2A could directly associate with the integrin, we examined the interaction of purified PP2A enzyme and purified αIIb cytoplasmic peptide. Because PP1c (a PP2Ac-related phosphatase) interacts with the αIIb cytoplasmic tail, containing a PP1c binding motif, 989KVXF992, we considered whether the membrane proximal, as opposed to membrane distal, residues of the αIIb subunit could also support PP2Ac interaction. Purified PP2A enzyme and PP2Ac from the resting platelet lysates bound specifically to a biotinylated αIIb peptide containing the residues 985–995 of the integrin αIIb, but not to a control peptide with scrambled sequence (Fig. 2B). These studies suggest that the αIIb membrane proximal region containing the KVGFFKR sequence can support the direct interaction of PP2Ac.
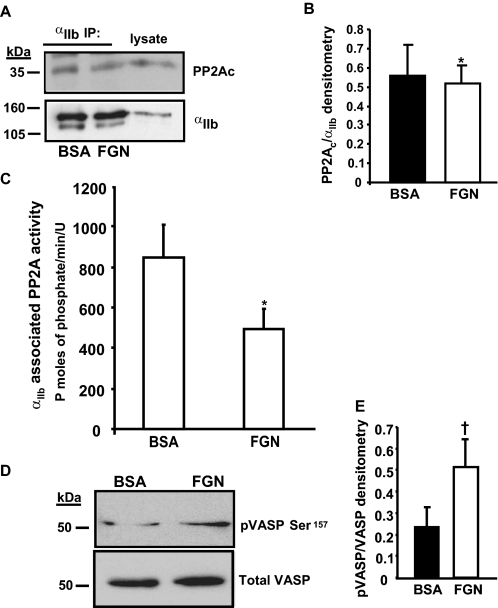
Next, we examined if αIIbβ3 activation and ligand engagement regulates PP2Ac-αIIbβ3 association or αIIbβ3-associated PP2A activity. The association of PP2Ac with the integrin was evaluated following platelet adhesion to immobilized fibrinogen, an αIIbβ3-mediated event. The association of PP2Ac with αIIbβ3 was maintained regardless of whether the platelets were held in suspension over the BSA substrate or adhered to immobilized fibrinogen (Fig. 3A). Densitometric quantification revealed that a comparable (p = 0.642) amount of PP2Ac, associated with the integrin immunoprecipitates, was obtained from platelets that either adhered to fibrinogen or suspended over BSA (Fig. 3B). Similarly, a stable association of PP2Ac with the integrin was also observed during soluble fibrinogen binding induced by Mn+2 (data not shown). Next, the activity of PP2Ac associated with αIIbβ3 was quantified in the αIIb immunoprecipitates. Platelets that adhered to fibrinogen exhibited ∼45% decreased (p = 0.02) αIIbβ3-associated PP2Ac activity compared with platelets that were maintained in suspension over the BSA substrate (Fig. 3C). Consistent with the decreased αIIbβ3-associated PP2Ac activity in fibrinogen-adhered platelets, we observed an increased Ser157 phosphorylation of vasodilator-associated phosphoprotein (VASP), a PP2Ac substrate in fibrinogen adhered platelets (Fig. 3D). By densitometry, when compared with platelets suspended over BSA, fibrinogenadhered platelets exhibited a ∼2-fold increase of VASP phosphorylation (Fig. 3E). Thus, decreased integrin-associated PP2Ac activity in fibrinogen-adhered platelets correlated with the increased phosphorylation of PP2Ac substrate VASP in platelets. Collectively, these results indicate that the integrinfibrinogen engagement may not significantly disrupt the association of a pool of phosphatase with the integrin, but rather decreases the phosphatase activity of PP2Ac associated with the αIIbβ3.
FIGURE 3.
Fibrinogen binding decreases integrin-associated PP2A activity. A, platelets were either allowed to adhere to fibrinogen (FGN) or maintained in suspension over BSA substrate. Lysates were prepared and immunoprecipitated with an αIIb antibody and immunoblotted with anti-αIIb or anti-PP2Ac antibodies. B, densitometric quantification of PP2Ac associated with the integrin αIIb (ratio of co-immunoprecipitated PP2Ac to αIIb in arbitrary units) in lysates obtained from platelets that were suspended on BSA or adhered to fibrinogen. Results are mean ± S.E., n = 3, *, p = 0.642. C, αIIb immunoprecipitates were washed in phosphatase assay buffer and evaluated for PP2Ac activity by quantifying the dephosphorylation of the phosphopeptide RKpTIRR using a malachite green PP2A phosphatase assay kit. Results are expressed as mean ± S.E. of five experiments; *, p = 0.02 by paired t test. D, lysates from platelets suspended on BSA or adhered to fibrinogen were separated by 10% SDS-PAGE and immunoblotted with anti-phospho-VASP (Ser157). The same blot was stripped and reprobed for total VASP (to assess for loading). E, densitometric quantification of VASP phosphorylation (ratio of pVASP to total VASP in arbitrary units) from suspended platelets or fibrinogen adherent platelets. Results are mean ± S.E., n = 3, †, p = 0.05.
To explore a functional role for PP2Ac in integrin αIIbβ3 signaling, we overexpressed a HA-tagged PP2Acα in 293 αIIbβ3 cells and evaluated adhesion to immobilized fibrinogen. PP2Acα was chosen because of the reported 10-fold abundance over PP2Acβ in most tissues (21). Immunoblotting with anti-HA antibody confirmed the overexpression of PP2Acα (Fig. 4A). Compared with the vector control-treated cells, PP2Acα overexpression significantly decreased the adhesion of αIIbβ3 cells to fibrinogen (Fig. 4B). The αIIbβ3-specific blocking antibody, 10E5, inhibited the adhesion of 293 cells. Thus, indicating that the adhesion was primarily mediated through αIIbβ3. Comparable levels of integrin αIIb expression were observed by densitometry of αIIb immunoblots in control vector and PP2Acα HA-overexpressed cells (54.59 ± 7.9 versus 55.10 ± 8.02, respectively). To further verify these findings, in complementary studies, we used short interference RNA (siRNA) to knock down the expression of endogenous PP2Acα in 293 cells expressing αIIbβ3. Knockdown was maximal (∼50–60%) and specific for PP2Ac, because PP1c and actin protein levels were comparable between the control and PP2Ac siRNA-treated cells (Fig. 4C). Compared with the control siRNA-treated cells, PP2Acα knockdown significantly increased the adhesion of αIIbβ3 cells to fibrinogen (Fig. 4D). To further determine whether the increased adhesiveness exhibited by PP2Acα-depleted cells was specific to immobilized fibrinogen, we studied adhesion to immobilized VWF. PP2Acα knockdown significantly increased the adhesion of αIIbβ3 cells to VWF (Fig. 4E). This suggests the differential adhesion due to PP2Acα depletion is not ligand-specific. The mean fluorescence intensity for αIIbβ3 expression was 266.12 ± 55 and 321.3 ± 68 for control and PP2Acα siRNA-treated 293 cells, respectively (p = 0.89). Thus, integrin expression levels may not account for the observed difference in adhesion. Taken together, these results indicate that PP2Acα negatively regulates αIIbβ3 outside-in signaling function in 293 cells.
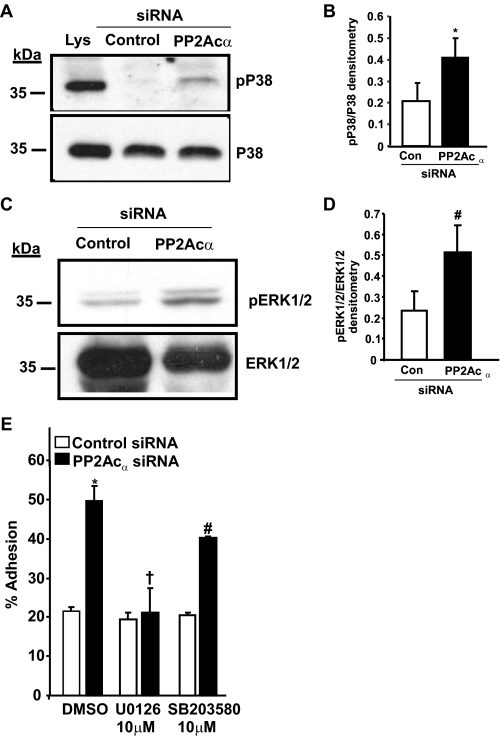
To investigate a potential mechanism by which PP2Acα can negatively regulate cell adhesion, we explored the effect of depleting endogenous PP2Acα in 293 cells on p38 and ERK (PP2Acα effectors) signaling pathways. These pathways are implicated in the modulation of cellular cytoskeletal reorganization and cell adhesiveness (22). The siRNA-mediated depletion of PP2Acα resulted in an increased activation of p38 and ERK1/2 signaling (Fig. 5, A and C). Using densitometry, the comparison of control siRNA-treated cells and PP2Acα-depleted cells exhibited a ∼2-fold (p = 0.046) increase for p38 activation and a ∼2.5-fold (p = 0.0058) increase in ERK1/2 activation (Fig. 5, B and D). Next, we ascertained whether the increased adhesiveness of PP2Acα-depleted cells was due to increased ERK1/2 or p38 signaling. Compared with control siRNA-treated cells, PP2Acα depletion significantly (p = 0.0001) increased adhesion. This increase was abolished (p = 0.373) by the ERK1/2 inhibitor (U0126) (Fig. 5E). In contrast, the p38 inhibitor, SB203580, failed to repress the increased adhesiveness of PP2Acα-depleted cells (Fig. 5E). Similar results were obtained with another p38 inhibitor (SB202190) (not shown). These studies indicate that PP2Acα may suppress αIIbβ3 adhesiveness, in part, by down-regulating ERK1/2 activation pathway.
FIGURE 5.
PP2Acα knockdown enhances p38 and ERK1/2 activation in 293 cells and blockade of ERK1/2 signaling abolishes the increased adhesiveness of PP2Acα-depleted cells. Lysates obtained from 293 αIIbβ3 cells treated with either control or PP2Acα, and siRNA was separated by 10% SDS-PAGE. Mitogen-activated protein kinase activation was assessed by immunoblotting using antibodies specific for the active (dual tyrosine and threonine-phosphorylated) forms of activated p38 (pP38) (A) and p44/42 ERK (pERK1/2) (C). Cells treated with 0.5 m sorbitol (Lys) in A serves as positive control for p38 activation. The blots were stripped and reprobed for total p38 (p38) or ERK1/2 to assess the equivalency of loading. Densitometric quantification of the enhanced activation of p38 (B) and ERK1/2 (D) in PP2Acα siRNA-treated cells compared with the control treated cells. Data are mean ± S.E. of three experiments for p38 and five experiments for ERK1/2 and was significant at *, p = 0.04 for p38 and #, p = 0.005 for ERK1/2. E, effect of ERK1/2 inhibitor (U0126) or p38 inhibitor (SB203580) on the increased adhesion of PP2Acα-depleted cells to fibrinogen. Results are mean ± S.E. of three experiments performed in triplicate with the following p values: *, p = 0.0001; †, p = 0.373; #, p = 0.012.
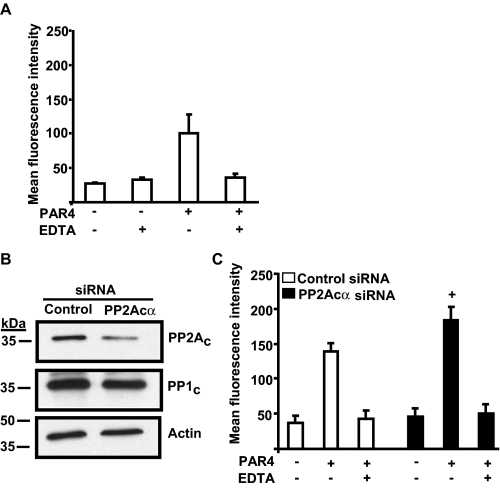
Next, we evaluated if genetic manipulation of PP2Acα negatively regulated αIIbβ3 signaling in an additional model system that has more direct relevance to platelet biology. It is not feasible to manipulate gene expression in platelets, because they are anucleate and mice lacking PP2Acα die around embryonic day 6.5, thus, precluding the study of platelets from PP2Acα null mice (23). In recent years megakaryocytes, from which platelets are derived, have emerged as an attractive system for studying integrin inside-out signaling. They express αIIbβ3, are activated by agonists, and are amenable to genetic manipulation (3, 18). Therefore, we chose to study the role of PP2Acα in murine megakaryocytes, which is a physiologically relevant model comparable to platelets. In concurrence with the previously published reports using this model system, we noticed increased fibrinogen binding in protease-activated receptor 4 activating peptide (PAR4AP)-stimulated murine megakaryocytes compared with the unstimulated megakaryocytes. Addition of EDTA, a divalent cation chelator, decreased fibrinogen binding in PAR4AP-treated megakaryocytes to the level of the unstimulated megakaryocytes. This indicates that the increased fibrinogen binding in response to PAR4AP is specific to integrin activation (Fig. 6A).
FIGURE 6.
PP2Acα negatively regulates αIIbβ3 activation in murine megakaryocytes. A, Alexa 488 fibrinogen binding in untransfected megakaryocytes. Large and alive megakaryocytes that expressed αIIb were analyzed for fibrinogen binding in response to 2.5 mm PAR4AP in the presence and absence of 10 mm EDTA by flow cytometry. B, expression of PP2Ac in control and PP2Acα-transfected siRNA. The blot was stripped and analyzed for PP1c and actin. These blots are representative of three experiments. C, increased fibrinogen binding in megakaryocytes transfected with murine PP2Acα siRNA. Fibrinogen binding was studied as described in panel A. Results are mean ± S.E. of six experiments, and the increased fibrinogen binding in megakaryocytes transfected with PP2Acα siRNA over the control siRNA was significant at +, p = 0.02 by analysis of variance.
To elucidate the role of PP2Acα in agonist-induced αIIbβ3 fibrinogen binding, we used murine siRNAs to knock down PP2Acα expression in megakaryocytes. Knockdown was maximal (∼40–50%) and specific for PP2Ac, because PP1c and actin protein levels were comparable between the control and PP2Acα siRNA-treated megakaryocytes (Fig. 6B). The residual PP2Ac signal could represent incomplete PP2Acα knockdown or expression of PP2Acβ. Knockdown of PP2Acα significantly increased binding of soluble fibrinogen in response to PAR4AP (Fig. 6C) compared with the megakaryocytes treated with control siRNA (Fig. 6C). An increased trend that did not reach statistical significance was also noted for MnCl2-induced fibrinogen binding in PP2Acα-depleted megakaryocytes. This suggests that PP2Acα may also negatively regulate αIIbβ3 outside-in signaling in megakaryocytes (data not shown). Surface expression of αIIbβ3 was not different between the control (mean fluorescence intensity, 94.67 ± 16) and PP2Acα (mean fluorescence intensity, 89.33 ± 13) siRNA-treated megakaryocytes and could not account for the observed difference in fibrinogen binding. These results suggest that PP2Acα negatively regulates integrin αIIbβ3 inside-out signaling in a murine megakaryocyte model system.
DISCUSSION
Signal transduction by kinases and phosphatases control integrin αIIbβ3 adhesiveness and activation events. However, a specific role for PP2A in integrin αIIbβ3 signaling is unclear. In this work, we show that a pool of PP2Ac associates constitutively with the integrin αIIbβ3 in resting platelets. Studies in 293 model systems revealed that PP2Acα can negatively regulate integrin αIIbβ3 adhesiveness, in part, via the suppression of ERK1/2, but not the p38 signaling pathway. Furthermore, PP2Acα negatively regulated PAR4AP-induced integrin αIIbβ3 inside-out signaling in a murine megakaryocyte model system.
Co-immunoprecipitation assays revealed a close proximal association of a pool of PP2Ac with integrin αIIbβ3. Additional studies, using integrin tail truncation mutants and integrin αIIb cytoplasmic peptides, have indicated that the αIIb membrane proximal region containing KVGFFKR is sufficient to mediate a direct interaction with PP2Ac (Fig. 2). This observation is consistent with a previous study that showed an association of the inhibitor for PP2A (I1PP2A) with the membrane proximal GFFKR motif of integrin α3Aβ1 (24). The membrane proximal region of αIIb can host the binding of calcium and integrin-binding protein 1, PP1c, and ICIn (8, 25, 26); therefore, it is conceivable that only a subpopulation of αIIbβ3 may harbor PP2Ac. Because GFFKR sequence is conserved in other α subunits, it is likely that PP2Ac association may not be limited to αIIb subunits.
Despite the similar binding motifs for PP1c and PP2Ac on integrin αIIb, integrin engagement resulted in a different effect for the two phosphatases. PP1c dissociated from the integrin complex (8), whereas a great extent of PP2Ac remained associated with the integrin (Fig. 3A). This suggests that integrin engagement may differentially regulate the two phosphatases in platelets. Interestingly, fibrinogen binding during platelet adhesion to αIIbβ3 repressed the αIIbβ3-associated PP2Ac activity (Fig. 3B). It is conceivable that the decreased PP2Ac activity, following fibrinogen binding, may be due to an increased Tyr307 phosphorylation of PP2Ac that is mediated in part by αIIbβ3-associated Src. In fact, fibrinogen binding to αIIbβ3 during platelet adhesion resulted in an increased αIIbβ3-associated Src activity (4). Also, phosphorylation of Tyr307 residue, within the catalytic subunit of PP2A, by the tyrosine kinases PP60Src or PP56Lck resulted in a reduction of the PP2A activity (27). Decreased αIIbβ3-associated PP2A activity also correlates with the increased αIIbβ3-associated Ser/Thr kinase protein kinase C activity following fibrinogen binding (6), implying αIIbβ3-associated Ser/Thr kinase and phosphatase activity are tightly controlled.
It is difficult to ascertain a specific role for integrin-associated PP2Ac in functional assays obtained from cells expressing either point mutations or deletions of the KVGFFKR region in αIIb, because multiple proteins dock in this region. Moreover, deletion of GFFKR sequence in αIIb can lead to integrin activation via disruption of a salt bridge between the αIIb and β3 subunits (28). Therefore, in this study, we analyzed αIIbβ3 adhesive function in cells that are depleted of PP2Acα by an siRNA approach. Although this approach can decipher a specific functional role for PP2Acα independent of other KVGFFKR-binding proteins, we cannot stringently rule out the functional contribution of PP2Acα that is also present in other subcellular locations. Nevertheless, our studies indicated that PP2Acα knockdown in 293 αIIbβ3 model cells resulted in an increased αIIbβ3 adhesion to immobilized fibrinogen and VWF. These results are in contrast to an earlier study, wherein platelets treated with PP1/PP2A inhibitor calyculin A produced a decreased adhesive phenotype to immobilized fibrinogen (14). This discrepancy could be due to inhibition of multiple phosphatases other than PP2A in calyculin A-treated platelets or could merely reflect the differences between platelets and cell lines. The siRNA approach we have undertaken provides us an opportunity to evaluate, more specifically, a role for PP2Ac independent of PP1c, because PP1c expression was not decreased in PP2Ac knockdown cells (Fig. 4C). Because platelets are anucleate and PP2Acα null mice are embryonically lethal, we employed murine megakaryocytes as a comparable model to study platelet αIIbβ3 inside-out signaling process. We observed increased fibrinogen binding in PAR4AP-treated megakaryocytes that were treated with PP2Acα siRNA (Fig. 6). Taken together with the results obtained from the 293 cell model system, these observations strengthen the conclusion that PP2Acα negatively regulates integrin signaling.
How could integrin-associated PP2Ac exert a negative regulation of integrin signaling? The association of PP2Ac with the αIIbβ3 complex does not directly regulate the integrin affinity, because the basal fibrinogen binding in PP2Acα-depleted megakaryocytes was not statistically increased (Fig. 6C). Furthermore, the association of PP2Ac with the αIIbβ3 complex does not regulate Thr753 phosphorylation of integrin β3, because we failed to observe β3 Thr753 phosphorylation in PP2Acα-depleted 293 cells (data not shown). Perhaps, multiple substrates for PP2Ac may exist in the focal adhesion protein complexes organized by αIIb andβ3 cytoplasmic tails. Suppression of the phosphorylation or activation of these proteins within the complex by the αIIbβ3-associated PP2Acα is likely to participate in limiting integrin activation and function. Indeed, we noticed that PP2Acα repressed the activation of its effectors p38 and ERK1/2 (Fig. 5). Although activation of ERK1/2 is more intensely studied as a regulator of gene expression and cell proliferation, this pathway can also regulate cellular functions. For example, cell adhesion and spreading are inhibited by dominant-negative ERK (22) and promoted by ERK activation (29). ERK and p38 are required for platelet spreading on fibrinogen (30). Cell migration is inhibited by blocking p38 activation (31). We observed that PP2Acα-depleted cells exhibited increased ERK1/2 and p38 activation and increased adhesion to fibrinogen and VWF. Inhibition of ERK1/2, but not p38 signaling, abolished the increased adhesion of PP2Acα-depleted cells (Fig. 5E). Other investigators have reported that pharmacological inhibition of PP2Ac leads to increased migration of endothelial and carcinoma cells (32, 33). Thus, the enhanced ERK1/2 and p38 signaling in PP2Acα-depleted cells would be predicted to exhibit greater adhesive and migratory properties.
Moreover, at the molecular level, evidence exists that ERK1/2 signaling can regulate focal adhesion complexes and integrin activation. Formation of peripheral actin microspikes are blocked by inhibiting ERK activation (34). After integrin engagement, active ERK is targeted to the newly forming focal adhesion via receptor for activated protein kinase C 1 (35). Interestingly, receptor for activated protein kinase C 1 also interacts with PP2A (36); therefore, it may provide the scaffold for cross-talk between PP2A and ERK signaling. Finally, the p38 and ERK signaling pathways are reported to play a critical role in the activation of integrin αIIbβ3 when induced by agonists like VWF and thrombin (20). Thus, suppression of ERK1/2 signaling by PP2A could constitute a potential mechanism for limiting integrin function.
In summary, our understanding of the role for PP2A in integrin signaling is fairly limited based on the use of pharmacological inhibitors. Using a genetic approach, our data indicate that PP2Acα negatively regulates αIIbβ3 signaling. Given that platelets are derived from megakaryocytes, this finding could be directly relevant to platelets. Moreover, such functional interactions might also extend to other α integrin subunits, thereby providing additional regulatory mechanisms to control integrin activation.
Acknowledgments
We thank Drs. Leslie Parise and Jun Liu (University of North Carolina, Chapel Hill, NC) for providing the necessary training to isolate, culture, transfect, and analyze murine megakaryocytes and Dr. Paul Bray (Jefferson Medical College, Philadelphia, PA) for scientific advice during the initial phase of this study.
This work was supported, in whole or in part, by National Institutes of Health Grants HL081613 and 0435017N. This work was also supported by the American Heart Association (to K. V. V.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PP1c, catalytic subunit of protein phosphatase 1; PAR4AP, protease-activated receptor 4-activating peptide; VASP, vasodilator-associated phosphoprotein; ERK, extracellular signal-regulated kinase; PP2Ac, catalytic subunit of protein phosphatase 2A; siRNA, short interference RNA; BSA, bovine serum albumin; VWF, von Willebrand factor; WT, wild type.
References
- 1.Shattil, S. J., and Newman, P. J. (2004) Blood 104 1606–1615 [DOI] [PubMed] [Google Scholar]
- 2.Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H., and Calderwood, D. A. (2003) Science 302 103–106 [DOI] [PubMed] [Google Scholar]
- 3.Yuan, W., Leisner, T. M., McFadden, A. W., Wang, Z., Larson, M. K., Clark, S., Boudignon-Proudhon, C., Lam, S. C., and Parise, L. V. (2006) J. Cell Biol. 172 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obergfell, A., Eto, K., Mocsai, A., Buensuceso, C., Moores, S. L., Brugge, J. S., Lowell, C. A., and Shattil, S. J. (2002) J. Cell Biol. 157 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias-Salgado, E. G., Haj, F., Dubois, C., Moran, B., Kasirer-Friede, A., Furie, B. C., Furie, B., Neel, B. G., and Shattil, S. J. (2005) J. Cell Biol. 170 837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buensuceso, C. S., Obergfell, A., Soriani, A., Eto, K., Kiosses, W. B., Arias-Salgado, E. G., Kawakami, T., and Shattil, S. J. (2005) J. Biol. Chem. 280 644–653 [DOI] [PubMed] [Google Scholar]
- 7.Naik, U. P., and Naik, M. U. (2003) Blood 102 1355–1362 [DOI] [PubMed] [Google Scholar]
- 8.Vijayan, K. V., Liu, Y., Li, T. T., and Bray, P. F. (2004) J. Biol. Chem. 279 33039–33042 [DOI] [PubMed] [Google Scholar]
- 9.Ivaska, J., Nissinen, L., Immonen, N., Eriksson, J. E., Kahari, V. M., and Heino, J. (2002) Mol. Cell. Biol. 22 1352–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssens, V., and Goris, J. (2001) Biochem. J. 353 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa, M., Toyoda, H., Saito, M., Morita, K., Tawara, I., Deguchi, K., Kuno, T., Shima, H., Nagao, M., and Shirakawa, S. (1994) Cell Signal. 6 59–71 [DOI] [PubMed] [Google Scholar]
- 12.Hoyt, C. H., and Lerea, K. M. (1995) Biochemistry 34 9565–9570 [DOI] [PubMed] [Google Scholar]
- 13.Higashihara, M., Takahata, K., Kurokawa, K., and Ikebe, M. (1992) FEBS Lett. 307 206–210 [DOI] [PubMed] [Google Scholar]
- 14.Lerea, K. M., Cordero, K. P., Sakariassen, K. S., Kirk, R. I., and Fried, V. A. (1999) J. Biol. Chem. 274 1914–1919 [DOI] [PubMed] [Google Scholar]
- 15.Lerea, K. M., Venjara, A. Y., Olson, S. C., and Kelly, M. R. (2006) Biochim. Biophys. Acta 1773 185–191 [DOI] [PubMed] [Google Scholar]
- 16.McCluskey, A., Sim, A. T., and Sakoff, J. A. (2002) J. Med. Chem. 45 1151–1175 [DOI] [PubMed] [Google Scholar]
- 17.Vijayan, K. V., Liu, Y., Dong, J. F., and Bray, P. F. (2003) J. Biol. Chem. 278 3860–3867 [DOI] [PubMed] [Google Scholar]
- 18.Shiraga, M., Ritchie, A., Aidoudi, S., Baron, V., Wilcox, D., White, G., Ybarrondo, B., Murphy, G., Leavitt, A., and Shattil, S. (1999) J. Cell Biol. 147 1419–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankov, R., Cukierman, E., Clark, K., Matsumoto, K., Hahn, C., Poulin, B., and Yamada, K. M. (2003) J. Biol. Chem. 278 18671–18681 [DOI] [PubMed] [Google Scholar]
- 20.Li, Z., Zhang, G., Feil, R., Han, J., and Du, X. (2006) Blood 107 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khew-Goodall, Y., and Hemmings, B. A. (1988) FEBS Lett. 238 265–268 [DOI] [PubMed] [Google Scholar]
- 22.Lai, C. F., Chaudhary, L., Fausto, A., Halstead, L. R., Ory, D. S., Avioli, L. V., and Cheng, S. L. (2001) J. Biol. Chem. 276 14443–14450 [DOI] [PubMed] [Google Scholar]
- 23.Gotz, J., Probst, A., Ehler, E., Hemmings, B., and Kues, W. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 12370–12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutz, D., Weise, C., Mechai, N., Hofmann, W., Horstkorte, R., Bruning, G., and Danker, K. (2006) J. Neurosci. Res. 84 1759–1770 [DOI] [PubMed] [Google Scholar]
- 25.Barry, W. T., Boudignon-Proudhon, C., Shock, D. D., McFadden, A., Weiss, J. M., Sondek, J., and Parise, L. V. (2002) J. Biol. Chem. 277 28877–28883 [DOI] [PubMed] [Google Scholar]
- 26.Larkin, D., Murphy, D., Reilly, D. F., Cahill, M., Sattler, E., Harriott, P., Cahill, D. J., and Moran, N. (2004) J. Biol. Chem. 279 27286–27293 [DOI] [PubMed] [Google Scholar]
- 27.Chen, J., Martin, B. L., and Brautigan, D. L. (1992) Science 257 1261–1264 [DOI] [PubMed] [Google Scholar]
- 28.Hughes, P. E., Diaz-Gonzalez, F., Leong, L., Wu, C., McDonald, J. A., Shattil, S. J., and Ginsberg, M. H. (1996) J. Biol. Chem. 271 6571–6574 [DOI] [PubMed] [Google Scholar]
- 29.Zhu, X., and Assoian, R. K. (1995) Mol. Biol. Cell 6 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazharian, A., Roger, S., Berrou, E., Adam, F., Kauskot, A., Nurden, P., Jandrot-Perrus, M., and Bryckaert, M. (2007) J. Biol. Chem. 282 5478–5487 [DOI] [PubMed] [Google Scholar]
- 31.Hedges, J. C., Dechert, M. A., Yamboliev, I. A., Martin, J. L., Hickey, E., Weber, L. A., and Gerthoffer, W. T. (1999) J. Biol. Chem. 274 24211–24219 [DOI] [PubMed] [Google Scholar]
- 32.Young, M. R., Kolesiak, K., and Meisinger, J. (2002) Int. J. Cancer 100 276–282 [DOI] [PubMed] [Google Scholar]
- 33.Young, M. R., Liu, S. W., and Meisinger, J. (2003) Int. J. Cancer 103 38–44 [DOI] [PubMed] [Google Scholar]
- 34.Brunton, V. G., Fincham, V. J., McLean, G. W., Winder, S. J., Paraskeva, C., Marshall, J. F., and Frame, M. C. (2001) Neoplasia 3 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vomastek, T., Iwanicki, M. P., Schaeffer, H. J., Tarcsafalvi, A., Parsons, J. T., and Weber, M. J. (2007) Mol. Cell. Biol. 27 8296–8305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiely, P. A., O'Gorman, D., Luong, K., Ron, D., and O'Connor, R. (2006) Mol. Cell. Biol. 26 4041–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]