Abstract
We previously found that glial cell line-derived neurotrophic factor (GDNF) in the midbrain ventral tegmental area (VTA) negatively regulates alcohol drinking (He, D. Y., McGough, N. N., Ravindranathan, A., Jeanblanc, J., Logrip, M. L., Phamluong, K., Janak, P. H., and Ron, D. (2005) J. Neurosci. 25, 619–628). Several studies suggest a role for GDNF in the regulation of tyrosine hydroxylase (TH) levels in the midbrain (Georgievska, B., Kirik, D., and Bjorklund, A. (2004) J. Neurosci. 24, 6437–6445). Up-regulation of TH levels has been reported as a hallmark of biochemical adaptations to in vivo chronic exposure to drugs of abuse, including ethanol (Ortiz, J., Fitzgerald, L. W., Charlton, M., Lane, S., Trevisan, L., Guitart, X., Shoemaker, W., Duman, R. S., and Nestler, E. J. (1995) Synapse 21, 289–298). We hypothesized that GDNF plays an important role in regulating prolonged ethanol-mediated increases in TH protein levels. Using the SH-SY5Y dopaminergic-like cell line, we found that the increase in TH levels in the presence of ethanol required the activation of the cAMP/PKA pathway and was reversed by GDNF. Ethanol treatment did not alter the mRNA level or protein translation of TH, but enhanced the stability of the protein that was decreased by GDNF. Interestingly, we observed that ethanol treatment resulted in an increase in TH association with the chaperone heat shock protein (HSP90) that was mediated by the cAMP/PKA pathway and inhibited by GDNF. Taken together, these data suggest that prolonged ethanol exposure leads to increased association of TH and HSP90 via the cAMP/PKA pathway, resulting in the stabilization and subsequent accumulation of TH. GDNF reverses this ethanol-mediated adaptation by inhibiting the interaction of TH with HSP90.
Tyrosine hydroxylase (TH)2 catalyzes the hydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine, which is the rate-limiting step in the biosynthesis of catecholamine neurotransmitters, including dopamine (6, 7). The mesolimbic dopamine system, which consists of the dopaminergic neurons in the ventral tegmental area (VTA) and projections to the nucleus accumbens and the prefrontal cortex, is the major neural structure that mediates the rewarding effects of drugs of abuse and ethanol. Biochemical adaptations in dopaminergic midbrain neurons induced by chronic exposure to drugs of abuse have been observed and implicated in relation to drug addiction (8–11). One of the most consistent adaptations to long-term exposure to drugs of abuse and ethanol is the up-regulation of TH protein levels in the VTA (2, 5, 12–15). We therefore set out to identify the molecular mechanism by which prolonged exposure to ethanol leads to an increase in TH immunoreactivity.
Several studies suggest a role for the glial cell line-derived neurotrophic factor (GDNF) in the regulation of TH levels in the midbrain. For example, GDNF overexpression by lentiviral delivery in the striatum reduced mRNA and/or protein levels of TH in the substantia nigra (SN) (3, 4) and the VTA (4). This increase in GDNF leads to a decrease in TH enzyme activity and dopamine levels in the striatum (16). Furthermore, infusion of GDNF into the VTA reverses chronic cocaine- or morphine-increased TH protein levels in this brain region (2). GDNF is a distant member of the transforming growth factor β superfamily, originally isolated from the rat B49 glial cell line (17). GDNF has been shown to promote the survival of adult midbrain dopaminergic neurons after injury (17–19). For example, repeated injections of GDNF adjacent to the SN prevented axotomy-induced loss of TH-expressing neurons in that brain region (18) and adenoviral delivery of GDNF into the SN protected against degeneration of dopamine neurons following injection of 6-hydroxydopamine in the striatum (20). Injection (19) or lentiviral delivery (21) of GDNF into the SN and striatum protected against nigrostriatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is toxic to dopaminergic neurons. GDNF selectively protects dopaminergic neurons, as compared with serotonergic neurons, against the neurotoxic effects of methamphetamine (22). More recently, GDNF has been shown to regulate behavioral responses to drugs of abuse such as cocaine, morphine, and ethanol (1, 2, 23). For example, GDNF heterozygous (+/–) mice display increased responsiveness to the rewarding effects of cocaine and increased locomotor activity after repeated exposure to cocaine or morphine as compared with their wild-type littermates (2). Messer et al. further reported that infusion of GDNF into the VTA, where GDNF-mediated signaling was decreased after chronic exposure to cocaine or morphine, reduced the rewarding effects of cocaine in rats (2).
We recently found that the expression of GDNF is highly up-regulated in vitro and in vivo in the midbrain region containing the VTA following administration of ibogaine, a psychoactive indole alkaloid extracted from the root bark of the African shrub Tabernanthe Iboga (1). Ibogaine is reported to reverse phenotypes associated with addiction to multiple drugs of abuse, including alcohol, in humans (24, 25), and various studies including ours also suggest that ibogaine attenuates drug- and ethanol-mediated behaviors in rodents (1, 26–28). We identified GDNF in the VTA as the mediator of the ability of ibogaine to decrease ethanol consumption as inhibition of GDNF signaling in the VTA by delivery of anti-GDNF neutralizing antibodies attenuated the action of ibogaine to decrease ethanol intake (1). Additionally, we showed that increasing GDNF levels in the VTA by direct injection of the growth factor reduced rat operant self-administration of ethanol (1). Together these results suggest that GDNF negatively controls responses to exposure to drugs of abuse and ethanol via its actions in the VTA. We therefore hypothesized that down-regulation of TH levels by GDNF may underlie its ability to negatively regulate adaptations to ethanol exposure. To address these questions, we used dopaminergic-like SH-SY5Y cells (29), which express GDNF and its receptors (1, 30), as a cell culture model and investigated the molecular mechanisms mediating the increase in TH levels by ethanol to determine whether and how GDNF alters ethanol action.
EXPERIMENTAL PROCEDURES
Materials—Recombinant human GDNF polypeptide and anti-GDNF monoclonal neutralizing antibodies were purchased from R&D Systems. Bisindolylmaleimide I (Bis), PP2, and H89 were purchased from EMD Calbiochem. Phosphatase Inhibitor Cocktails 1 & 2, ibogaine-HCl, phosphatidylinositol phospholipase C (PI-PLC), cycloheximide (CHX), Rp-cyclic 3′, 5′-hydrogen phosphorothioate adenosine triethylammonium salt (Rp-cAMPS) and anti-TH antibody were purchased from Sigma. Anti-heat shock protein 90αβ (HSP90) and anti-actin antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho (Ser-40) TH antibodies, anti-phospho(Ser/Thr) PKA substrate antibody and anti-Akt1/2 antibody were purchased from Cell Signaling Technology. Protein G-agarose was purchased from Invitrogen. Geldanamycin (GA) was purchased from Alexis Biochemicals. Redivue™ Pro-Mix™l-[35S] in vitro Cell Labeling Mix was purchased from GE Healthcare. The protease inhibitor mixture was purchased from Roche Applied Science. Reverse Transcription System and 2× PCR Master Mix were purchased from Promega Corp. Primers for PCR were synthesized by Sigma-Genosys.
Cell Culture—SH-SY5Y human neuroblastoma cells were cultured in the growth medium Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) plus 1× MEM non-essential amino acid solution (Invitrogen). All experiments were carried out in cells that had been incubated in a low serum medium containing 1% FBS for 2 days. GDNF stable cells overexpressing GDNF were derived from SH-SY5Y cells stably transfected with pUSE-GDNF and control cells were obtained by stable transfection with the pUSE empty vector (30). The stable cell lines were maintained in growth medium containing 500 μg/ml G418 and incubated in low serum medium for 2 days prior to experiments.
Treatments—Cells were treated with ethanol for 12–48 h in low serum medium by replacing the medium with ethanol every 12 h. GDNF or ibogaine was added to the medium for the last 12 h of 24-h treatments with ethanol, and the inhibitors GA, Bis, PP2, H89, and Rp-cAMPS were added for the last 9 h of the 24-h ethanol treatment. PI-PLC was incubated with cells at a concentration of 0.3 units/ml for 1 h as described previously (1). Anti-GDNF neutralizing antibodies were dissolved in phosphate-buffered saline and were used at a concentration of 10 μg/ml.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)—Total RNA isolation was carried out using TRIzol reagent according to the manufacturer's protocol. RNA samples were analyzed by RT-PCR with actin as an internal control as described previously (1, 30). After the RT reaction, PCR was run for 32 cycles with the TH primers as follows: upstream 5′-TTC GCG CAG TTC TCG CAG GAC ATT GGC-3′ and downstream 5′-CGT GTA CGG GTC GAA CTT CAC GGA GAA-3′.
Western Blot and Immunoprecipitation—Cells were collected and lysed in radioimmune precipitation assay buffer plus protease and phosphatase inhibitor mixtures for Western blot and immunoprecipitated as previously described (1, 30). Briefly, 15 μg of each homogenate was subjected to an SDS-PAGE gel and blotted on a nitrocellulose membrane for Western blot analysis with anti-TH antibody or other primary antibodies, followed by reprobing with anti-actin antibody. Alternatively, 500 μg of each homogenate was incubated with 5 μg of anti-TH, anti-HSP90 or anti-Akt antibodies in TBS-T buffer overnight at 4 °C, followed by a 2-h incubation with protein G-agarose beads. Immunoprecipitates were separated on an SDS-PAGE gel for Western blot analysis.
Pulse-chase Analysis—After 24 h of ethanol treatment, cells were labeled following a pulse-chase procedure as described previously (31) with modifications. Briefly, cells were incubated with methionine/cystine-free DMEM (Invitrogen) containing 1% dialyzed FBS for 1 h and then labeled in the Met/Cys-free medium containing 25 μCi of Amersham Biosciences Redivue Pro-Mix l-[35S] in vitro Cell Labeling Mix per ml for 3 h. Dialysis of FBS was performed against the Met/Cys-free medium overnight. After labeling, cells were washed twice with the normal medium and chased in the normal medium without ethanol for 2–8 h before homogenizing as described above. Homogenates were centrifuged, and protein concentrations of the supernatants were determined. An equal amount of protein from each sample was immunoprecipitated with anti-TH antibody and separated by electrophoresis on an 8% SDS gel. Autoradiography was performed by exposure of the dried gels to Amersham Biosciences Storage Phosphor Screen and visualization of radioactive signals using the Typhoon 9410 Variable Mode Imager (GE Healthcare).
Quantification and Statistical Analysis—Signals from Western blot, RT-PCR, or 35S-labeled radioactivity images were quantified using NIH Image 1.61 as described previously (1, 30). The density of TH immunoreactivity was used to estimate TH protein levels that were expressed as ratios to those of actin. TH mRNA levels were expressed as ratios to those of actin. In the pulse-chase analysis, percentage of the radioactive signals of 35S-labeled TH during the chase times was used to estimate relative stability of the protein. Results were obtained from at least three separate experiments, and the statistical significance of differences between treatments and control was analyzed using a one-sample t test (GraphPad).
RESULTS
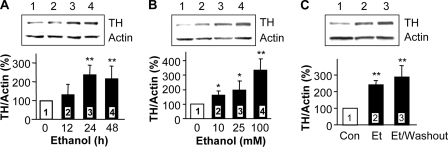
Tyrosine Hydroxylase Immunoreactivity Is Up-regulated upon Prolonged Exposure of Cells to Ethanol—We first tested whether the chronic ethanol-mediated increase in TH immunoreactivity could be mimicked in the dopaminergic-like SH-SY5Y cells. We found that prolonged ethanol treatment resulted in an increase in TH protein levels after 24 and 48 h of exposure to ethanol (Fig. 1A). The increase in TH protein levels by ethanol occurred in a dose-dependent manner (Fig. 1B). Interestingly, even 10 mm, a low, non-intoxicating dose of ethanol, produced a significant increase in the immunoreactivity of TH. Finally, the ethanol-induced increase in TH protein levels was long-lasting and could be detected 24 h after ethanol washout (Fig. 1C). These results indicate that the increase in TH levels is a long-lasting adaptation to ethanol exposure, which can be detected in SH-SY5Y cells.
FIGURE 1.
Ethanol induces an increase in TH immunoreactivity in a time- and dose-dependent manner. A, SH-SY5Y cells were treated without (lane 1, control) or with 100 mm ethanol (lanes 2–4) for the indicated times. TH protein levels were analyzed by Western blot with anti-TH antibody, and actin protein levels were detected with anti-actin antibody as an internal control. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from seven experiments. B, cells were treated for 24 h without (lane 1, control) or with different concentrations of ethanol (lanes 2–4). The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. C, cells were treated without (lane 1, control) or with 100 mm ethanol for 24 h (lane 2), or with 100 mm ethanol for 24 h followed by washing with medium and incubation without ethanol for an additional 24 h (lane 3). The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from four experiments. *, p < 0.05; **, p < 0.01, compared with lane 1 (control).
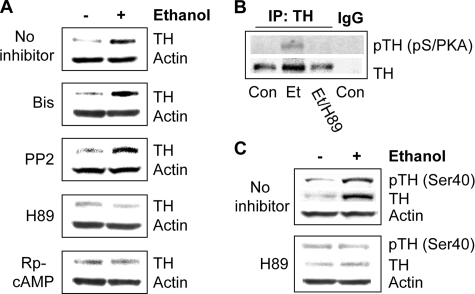
Cyclic AMP-dependent Protein Kinase A (PKA) Is Required for Ethanol Induction of TH Protein Levels—Next, we set out to identify the mechanism underlying the action of ethanol on TH protein levels. Because it is well-established that several signaling pathways are altered in response to ethanol, including those mediated by protein kinase A (PKA), Fyn, and protein kinase C (PKC) (32, 33), we investigated the possibility that kinase-mediated signaling pathways may be required for ethanol to increase TH protein levels by using inhibitors of these kinases in conjunction with chronic ethanol treatment. Application of Bis, an inhibitor of PKC, or PP2, an inhibitor of Fyn kinase, did not, while both Rp-cAMPS, an inhibitor of cAMP, and H89, an inhibitor of PKA, did block the ability of ethanol to increase TH protein levels (Fig. 2A). These results indicate that the cAMP/PKA pathway is required for the increase of TH in response to ethanol.
FIGURE 2.
Protein kinase A is required for ethanol-mediated increases in TH protein levels. A, cells were treated without or with 100 mm ethanol for 24 h, to which kinase inhibitors were added for the last 9 h of the ethanol treatment as indicated: 1 μm Bis, 1 μm PP2, 5 μm H89, and 40 μm Rp-cAMPS. TH protein levels were detected by Western blot analysis. The image is representative of six experiments. B, cells were treated without (con) or with 100 mm ethanol for 24 h alone (Et) or in combination with 1 μm H89 for the last 9 h (Et/H89). TH was immunoprecipitated using anti-TH antibody and immunoblotted with anti-phospho(Ser/Thr) PKA substrate antibody. The image is representative of four experiments. C, cells were treated without or with 100 mm ethanol for 24 h, to which 1 μm H89 was added for the last 9 h. Phosphorylation of TH was detected by Western blot analysis with anti-phospho(Ser-40)TH antibodies. The image is representative of three experiments.
PKA has been reported to phosphorylate TH (34). We therefore tested whether PKA-mediated TH phosphorylation can be detected after ethanol treatment using anti-phospho(Ser/Thr) PKA substrate antibody. This phosphospecific antibody has been used to demonstrate the phosphorylation of PKA substrates (for examples see Refs. 35, 36). As shown in Fig. 2B, we found that exposure of cells to ethanol resulted in an increase in PKA-mediated phosphorylation of TH, which was inhibited in the presence of H89. This phosphorylation was further confirmed using anti-phospho(Ser-40)TH antibodies as shown in Fig. 2C. These results suggest that ethanol treatment increases PKA-mediated phosphorylation of TH at Ser-40, which is required for up-regulation of TH protein levels by ethanol.
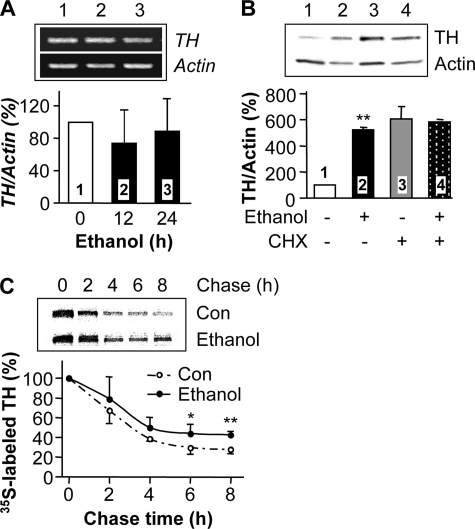
Prolonged Exposure of Cells to Ethanol Enhances the Stability of TH Protein—Next, we tested whether prolonged ethanol exposure induces an increase in TH mRNA levels or synthesis of the protein. We found that prolonged incubation with ethanol did not increase TH mRNA (Fig. 3A). In addition, CHX, a protein synthesis inhibitor, did not inhibit ethanol-mediated increases in TH protein levels (Fig. 3B). Curiously, an increase in TH protein levels was detected after long-term CHX treatment, which could be due to the inhibition of synthesis of protein components of the proteosome machinery. Nonetheless, our results suggest that ethanol exposure enhances the stability of the TH protein, resulting in its accumulation, rather than increasing transcription of the TH gene or translation of the protein. To test the possibility that prolonged exposure of cells to ethanol leads to the stabilization of the TH protein, we performed a pulse-chase analysis. After 24 h of ethanol treatment, cells were labeled with [35S]methionine/cysteine and chased in normal medium without ethanol. Results from autoradiography of 35S-labeled TH protein revealed an increase in the quantity of labeled TH protein after ethanol treatment at all points in the chase time course, compared with control (Fig. 3C). The increase in labeled TH protein amounts detected in the pulsechase analysis is relatively low compared with the increase in protein levels detected by Western blot analysis. This might be due to limitations of the pulse-chase procedure. Nevertheless, our results indicate that the ethanol treatment enhances the stability of the protein.
FIGURE 3.
Chronic ethanol does not increase the transcription and translation of TH, but enhances TH protein stability. A, cells were treated without (lane 1, control) or with 100 mm ethanol for 12 or 24 h (lanes 2 and 3). TH mRNA expression levels were analyzed by RT-PCR with actin mRNA levels as an internal control. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. B, cells were treated without (lane 1, control, and lane 3) or with 100 mm ethanol (lanes 2 and 4) for 24 h, and 30 μg/ml CHX was added as indicated for the last 12 h of the 24-h treatment (lanes 3 and 4). TH protein levels were analyzed as described above. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. **, p < 0.01, compared with control. C, cells were treated without (control) or with 100 mm ethanol for 24 h before a pulse-chase procedure as described under “Experimental Procedures.” Cells were labeled with 25 μCi of [35S]Met/Cys pro-mix cell labeling mix for 3 h and then incubated in the normal medium for the indicated chase times, followed by immunoprecipitation with anti-TH antibody, separation on an SDS-PAGE gel, and autoradiography. The histogram depicts the mean percentage change in radioactive signals of 35S-labeled TH protein at each chase time to those at initiation of chase (0 time) from six experiments. *, p < 0.05; **, p < 0.01, compared with control.
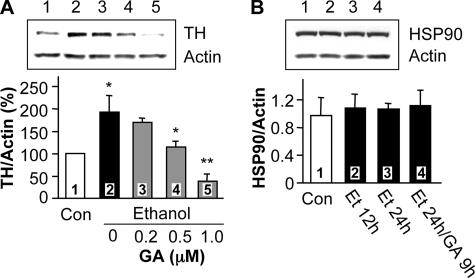
Prolonged Ethanol Exposure Enhances TH Protein Stability via Heat Shock Protein 90 (HSP90)—To elucidate the mechanism by which ethanol increases TH protein stability, we tested whether HSP90 contributes to the ethanol-mediated increase in TH immunoreactivity. HSP90 is a molecular chaperone that has extensively been shown to promote the stability and function of many signaling proteins (37). Specifically, HSP90 enhances the stability of proteins by forming an ATP-dependent complex with proteins such as steroid receptors, epidermal growth factor receptor (EGF-R), Her-2, Akt, Raf-1 kinase, p53, and cdk4, protecting them from proteasome-dependent degradation (37, 38). Disruption of the complex of HSP90 with these proteins by HSP90 inhibitors, such as GA, leads to protein degradation (37, 39–41). GA specifically inhibits the HSP90 molecular chaperone (42, 43) by binding to the ATP-binding site in the chaperone (44, 45). We hypothesized that if HSP90 activity contributes to ethanol-induced increases in TH protein levels, GA should block the action of ethanol. As shown in Fig. 4A, application of GA dose-dependently inhibited the ethanol-mediated increase in TH immunoreactivity. We further found that treatment with ethanol alone, or together with GA, did not alter the protein level of HSP90 (Fig. 4B). This suggests that the increase in TH protein levels by ethanol treatment is not due to an alteration of HSP90 protein levels, and that GA treatment specifically inhibits ethanol induction of TH protein levels.
FIGURE 4.
Geldanamycin, an inhibitor of heat shock protein 90, inhibits chronic ethanol-induced TH accumulation. A, cells were treated without (lane 1) or with 100 mm ethanol for 24 h (lane 2), or with 100 mm ethanol for 24 h to which different doses of GA were added for the last 9 h of ethanol treatment (lanes 3–5). TH protein levels were analyzed as described above. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. *, p < 0.05, lanes 2 versus 1, or 4 versus 2; **, p < 0.01, lanes 5 versus 2. B, cells were treated without (Con), or with 100 mm ethanol for 12 h (Et 12h) or 24 h (Et 24h), or 100 mm ethanol for 24 h in which 1 μm GA was added for the last 9 h (Et 24h/GA 9h). Protein levels of HSP90 and actin were measured by Western blot analysis. The histogram depicts the mean ratio of HSP90 to actin ± S.D. from three experiments.
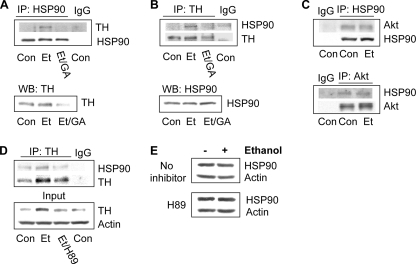
To further test whether HSP90 participates in maintaining TH stability upon ethanol treatment, we measured the possible association of TH and HSP90 in untreated and ethanol-treated cells by co-immunoprecipitation with anti-TH or anti-HSP90 antibodies. As shown in Fig. 5, A and B, ethanol treatment for 24 h induced an association of TH with HSP90, and this association was blocked in the presence of GA. This effect of ethanol is specific for the association between TH and HSP90 because the ethanol treatment did not alter the association between Akt kinase and HSP90 (Fig. 5C). Taken together, these results indicate that prolonged exposure of cells to ethanol specifically induces the formation of a complex of HSP90 and TH and thus stabilizes the TH protein. Next, we tested whether the inhibition of the cAMP/PKA pathway alters the ethanol-mediated association of HSP90 with TH. We found that H89 decreased the ethanol-mediated association of TH with HSP90 (Fig. 5D), but did not alter HSP90 protein levels (Fig. 5E). These results suggest an important role for the cAMP/PKA pathway in the regulation of the ethanol-induced increase in TH protein and its association with HSP90.
FIGURE 5.
Chronic ethanol induces an association of TH with heat shock protein 90. A and B, cells were treated without (Con) or with 100 mm ethanol for 24 h (Et), or with 100 mm ethanol for 24 h to which 1 μm GA was added for the last 9 h (Et/GA). TH was co-immunoprecipitated with HSP90 using anti-HSP90 antibody (A), and HSP90 was co-immunoprecipitated with TH using anti-TH antibody (B). HSP90 and TH levels in input samples were analyzed by Western blot. Images are representative of four experiments. C, cells were treated without (Con) or with 100 mm ethanol for 24 h (Et). Akt was co-immunoprecipitated with HSP90 using anti-HSP90 antibody (upper panel), and HSP90 was co-immunoprecipitated with Akt using anti-Akt antibody (lower panel). Images are representative of three experiments. D, cells were treated without (Con) or with 100 mm ethanol (Et) for 24 h alone or together with 5 μm H89 for the last 9 h (Et/H89). Cells were homogenized for co-immunoprecipitation of HSP90 with anti-TH antibody. The image is representative of three experiments. E, cells were treated without or with 100 mm ethanol for 24 h, to which 1 μm H89 were added for the last 9 h of the ethanol treatment. HSP90 protein levels were detected by Western blot analysis. The image is representative of six experiments.
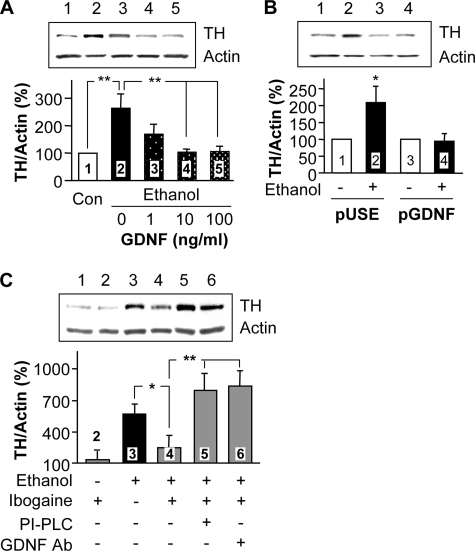
GDNF Inhibits Ethanol-mediated Increases in TH Protein Levels—To determine whether GDNF alters ethanol-mediated increases in TH levels, GDNF at concentrations of 1–100 ng/ml was added for the last 12 h of a 24-h ethanol treatment. As shown in Fig. 6A, GDNF dose-dependently decreased TH protein levels that were increased by 24-h ethanol treatment. To confirm the effect of GDNF, we examined the ability of ethanol to increase TH protein levels in cells that stably overexpress GDNF, secrete a high level of the growth factor and maintain a high basal level of GDNF signaling activity (30). As predicted, prolonged ethanol exposure did not produce an increase in TH immunoreactivity in the cells that overexpress GDNF, as compared with control cells that express the empty pUSE vector (Fig. 6B), suggesting that the high level of GDNF occludes the effect of ethanol on TH protein levels.
FIGURE 6.
GDNF or ibogaine via GDNF reverses ethanol-mediated increases in TH protein levels. A, SH-SY5Y cells were treated without (lane 1) or with 100 mm ethanol for 24 h (lane 2), or 100 mm ethanol for 24 h to which different doses of GDNF were added for the last 12 h of ethanol treatment (lanes 3–5). TH protein levels were analyzed as described above. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. **, p < 0.01. B, SH-SY5Y cells stably expressing the pUSE empty vector (pUSE, lanes 1 and 2) and cells stably expressing GDNF (pGDNF, lanes 3 and 4) were treated without (lanes 1 and 3) or with 100 mm ethanol (lanes 2 and 4) for 24 h. TH protein levels were analyzed as described above. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. *, p < 0.05, lanes 2 versus 1. C, cells were treated without (lane 1) or with 10 μm ibogaine for 12 h (lane 2), 100 mm ethanol for 24 h (lane 3) or 100 mm ethanol for 24 h to which 10 μm ibogaine was added for the last 12 h of ethanol incubation (lanes 4–6): lane 4, ethanol plus ibogaine, lane 5; ethanol plus ibogaine after 1-h preincubation with PI-PLC (see “Experimental Procedures”), lane 6; ethanol plus ibogaine together with 10 μg/ml of anti-GDNF-neutralizing antibodies. The histogram depicts the mean percentage change in the ratio of TH to actin ± S.D. from three experiments. *, p < 0.05; **, p < 0.01.
Ibogaine Reverses Ethanol-mediated Increases in TH Protein Levels via GDNF—We have previously reported that treatment with ibogaine increases GDNF gene expression and up-regulates GDNF-mediated signaling both in vitro in SH-SY5Y cells and in the midbrain region containing the VTA in vivo (1, 30). We therefore hypothesized that ibogaine, like GDNF, may reverse the ethanol-mediated induction of TH protein levels. To test this possibility, SH-SY5Y cells were treated with ethanol for 24 h in the absence and presence of ibogaine, which was added for the last 12 h of the 24-h ethanol treatment. We found that the addition of ibogaine inhibited ethanol-induced increases in TH levels (Fig. 6C). To confirm that this action of ibogaine is indeed mediated via GDNF, we applied two approaches to block the GDNF signaling pathway and measured the effects of ibogaine on TH protein levels. We found that incubation of cells with ibogaine after treatment with PI-PLC (which hydrolyzes the glycosylphosphatidylinositol link of the GDNF co-receptor GFRα1 to the membrane, Ref. 46), or in the presence of anti-GDNF inhibitory antibodies (which act as GDNF scavengers, Ref. 2, 47), inhibited ibogaine action on the ethanol-mediated induction of TH immunoreactivity (Fig. 6C). These results indicate that the GDNF signaling pathway mediates the ability of ibogaine to negatively regulate this biochemical adaptation to ethanol exposure.
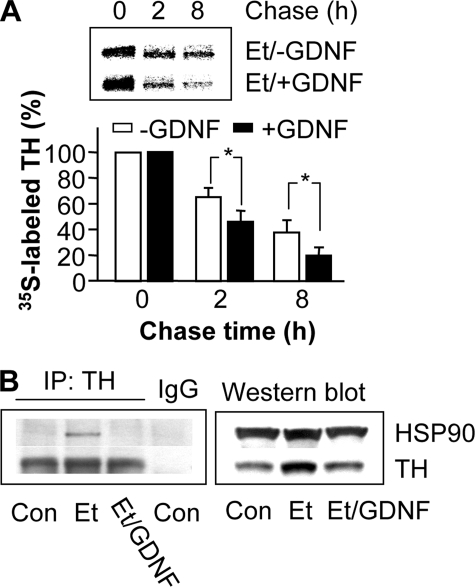
GDNF Decreases Ethanol-mediated Stabilization of TH Protein by Inhibition of the Association of TH and HSP90—Finally, we tested the hypothesis that GDNF reverses the stabilization and association of TH with HSP90 induced by ethanol treatment. In the presence of ethanol, addition of GDNF down-regulates the quantity of 35S-labeled TH at all points on the chase time course (Fig. 7A), indicating that GDNF decreases the stabilization of the TH protein. Furthermore, as shown in Fig. 7B, ethanol exposure for 24 h increased TH immunoreactivity (right panel) and its association with HSP90 (left panel). Addition of GDNF reversed both the ethanol-mediated increase in TH levels (Fig. 7B, right panel) and the association of HSP90 with TH (Fig. 7B, left panel), which was not due to alteration in HSP90 immunoreactivity (Fig. 7B, right panel). These results suggest that GDNF reduces the ethanol-mediated increases in TH protein levels by preventing the association of HSP90 with TH.
FIGURE 7.
GDNF decreases ethanol-mediated stabilization and association of TH with HSP90. A, cells were treated with 100 mm ethanol for 24 h alone (Et/-GDNF) or together with 25 ng/ml GDNF added for the last 12 h (Et/+GDNF) before a pulse-chase procedure as described above with the chase times as indicated. The histogram depicts the mean percentage change in radioactive signals of 35S-labeled TH protein from three experiments. *, p < 0.05. B, cells were treated without (Con) or with 100 mm ethanol for 24 h alone (Et) or in combination with 25 ng/ml GDNF added for the last 12 h (Et/GDNF). Co-immunoprecipitation of HSP90 with TH was analyzed as described above. The image is representative of three experiments.
DISCUSSION
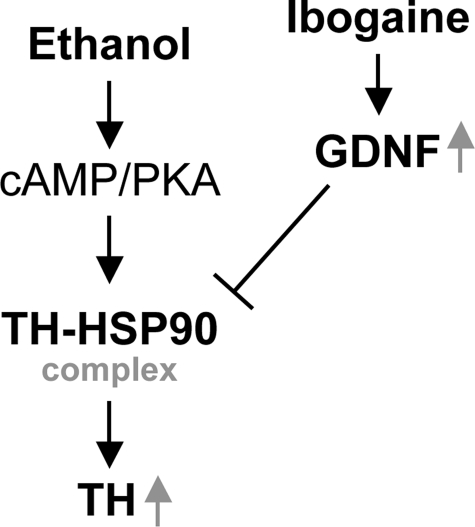
In this study we present data to suggest a novel mechanism by which prolonged exposure of the dopaminergic-like SH-SY5Y cells to ethanol induces a long-lasting increase in TH protein levels by increasing the association of TH with the chaperone protein HSP90 via the cAMP/PKA pathway, leading to the enhancement of protein stability. We further show that GDNF reverses this biochemical adaptation to ethanol exposure by inhibiting the interaction between TH and HSP90, leading to the reduction of TH protein stability (Fig. 8).
FIGURE 8.
Suggested schematic diagram of possible components that mediate the up-regulation of TH protein levels following prolonged exposure to ethanol in SH-SY5Y cells. Exposure to ethanol leads to formation of the TH-HSP90 complex via cAMP/PKA, resulting in stabilization and accumulation of TH protein. GDNF, or ibogaine via GDNF, reverses this ethanol-mediated adaptation by inhibition of the association of TH with HSP90, thus down-regulating the protein stability of TH.
Although expression of the TH gene can be regulated via different mechanisms and upon multiple simulations (48–57), the mechanism by which exposure to drugs of abuse and ethanol causes an up-regulation of TH protein levels is unclear. Here we show that chronic ethanol treatment does not induce an increase in TH mRNA levels or translation of the TH protein. Instead, our data suggest that an increase in the maintenance of TH protein stability leads to the accumulation of the protein in response to ethanol exposure. Importantly, we present a novel mechanism via HSP90 by which TH protein is stabilized following the exposure of the dopaminergic-like cells to ethanol. First, we found that the HSP90 inhibitor GA dose-dependently inhibits ethanol-mediated increases in TH protein levels. Second, ethanol treatment induces an association of TH with HSP90. Finally, we found that GA inhibits the ethanol induction of the association between TH and HSP90. HSP90 has been shown to regulate the protein stability and function of various proteins (37). However, its involvement in the maintenance of TH protein stability has not been previously reported. It would be of great interest to test whether this increase in TH protein stability is unique for ethanol or shared with other drugs of abuse, as well.
We previously found that intra-VTA application of GDNF, and ibogaine via GDNF, reduced rat ethanol drinking behaviors (1). Here we show that GDNF reverses the ethanol-mediated induction of TH immunoreactivity. Although we cannot directly tie the effects of GDNF in the behavioral paradigm to this biochemical adaptation, as the duration of the ethanol self-administration paradigm (1) is not long enough to produce changes in TH levels in vivo, our present study suggests that GDNF may act as a negative regulator of various adaptations to prolonged in vivo exposure to ethanol.
Interestingly, we found that, like the HSP90 inhibitor GA, GDNF inhibits the ethanol-mediated induction of the TH-HSP90 association. Thus, GDNF reverses the ethanol induction of TH protein levels through inhibition of the TH-HSP90 association or disruption of the TH-HSP90 complex. Further investigations revealed that cAMP/PKA signaling mediates the ethanol-induced association of TH and HSP90 and the increase in TH protein levels. However, the link between GDNF and cAMP/PKA signaling following prolonged ethanol treatment remains to be investigated in future studies.
TH can be phosphorylated at several serine residues (34), and the phosphorylation may contribute to the activity of the enzyme (34, 48). Early studies have shown that the phosphorylated enzyme is less stable than its nonphosphorylated form (58, 59). However, recent studies have demonstrated that phosphorylation of the enzyme contributes to its enhanced stability (60–62). For example, Moy and Tsai reported that phosphorylation of TH at Ser-31 by the proline-directed serine/threonine kinase cyclin-dependent kinase 5 increases TH protein stability (61). Toska et al. (60) observed that double-site phosphorylation of the enzyme at Ser-19 and Ser-40 increases the thermal stability of the enzyme and suggested that such phosphorylation at multiple sites may be more stable than the single and nonphosphorylated forms. Interestingly, sustained phosphorylation of TH at Ser-40 by PKA has been investigated in bovine adrenal chromaffin cells after treatment with the pituitary adenylate cyclase-activating polypeptide (PACAP) (63). The sustained phosphorylation of TH resulted in sustained activation of the enzyme, independent of new TH synthesis (63). In summary, in this study we found that prolonged exposure of the dopaminergic like SH-SY5Y cells to ethanol produces a similar adaptation in TH protein levels as previously reported in vivo (5). As shown in the suggested schematic diagram in Fig. 8, the up-regulation of TH protein levels results from the stabilization of the protein via the induction of a PKA-mediated association of TH with HSP90. Importantly, we show that GDNF reverses the increase in TH immunoreactivity induced by ethanol by inhibiting the interaction between TH and the chaperone. These findings provide a new insight into the molecular mechanism of both the adaptation of dopaminergic cells to ethanol exposure and its reversal by GDNF.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AA014366-02 (to D. R.). This work was also supported by the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (to D. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TH, tyrosine hydroxylase; CHX, cycloheximide; GA, geldanamycin; GDNF, glial cell line-derived neurotrophic factor; HSP90, heat shock protein 90; PI-PLC, phosphatidylinositol phospholipase C; PKA, cyclic AMP-dependent protein kinase A; Rp-cAMPS, Rp-cyclic 3′, 5′-hydrogen phosphorothioate adenosine triethylammonium salt; RT-PCR, reverse transcription-polymerase chain reaction; SN, substantia nigra; VTA, ventral tegmental area; FBS, fetal bovine serum.
References
- 1.He, D. Y., McGough, N. N., Ravindranathan, A., Jeanblanc, J., Logrip, M. L., Phamluong, K., Janak, P. H., and Ron, D. (2005) J. Neurosci. 25 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messer, C. J., Eisch, A. J., Carlezon, W. A., Jr., Whisler, K., Shen, L., Wolf, D. H., Westphal, H., Collins, F., Russell, D. S., and Nestler, E. J. (2000) Neuron 26 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgievska, B., Kirik, D., and Bjorklund, A. (2004) J. Neurosci. 24 6437–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblad, C., Georgievska, B., and Kirik, D. (2003) Eur. J. Neurosci. 17 260–270 [DOI] [PubMed] [Google Scholar]
- 5.Ortiz, J., Fitzgerald, L. W., Charlton, M., Lane, S., Trevisan, L., Guitart, X., Shoemaker, W., Duman, R. S., and Nestler, E. J. (1995) Synapse 21 289–298 [DOI] [PubMed] [Google Scholar]
- 6.Nagatsu, T., Levitt, M., and Udenfriend, S. (1964) J. Biol. Chem. 239 2910–2917 [PubMed] [Google Scholar]
- 7.Levitt, M., Spector, S., Sjoerdsma, A., and Udenfriend, S. (1965) J. Pharmacol. Exp. Ther. 148 1–8 [PubMed] [Google Scholar]
- 8.Self, D. W., and Nestler, E. J. (1995) Annu. Rev. Neurosci. 18 463–495 [DOI] [PubMed] [Google Scholar]
- 9.Nestler, E. J. (1992) J. Neurosci. 12 2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koob, G. F. (1992) Trends Pharmacol. Sci. 13 177–184 [DOI] [PubMed] [Google Scholar]
- 11.Wise, R. A., and Rompre, P. P. (1989) Annu. Rev. Psychol. 40 191–225 [DOI] [PubMed] [Google Scholar]
- 12.Beitner-Johnson, D., and Nestler, E. J. (1991) J. Neurochem. 57 344–347 [DOI] [PubMed] [Google Scholar]
- 13.Sorg, B. A., Chen, S. Y., and Kalivas, P. W. (1993) J. Pharmacol. Exp. Ther. 266 424–430 [PubMed] [Google Scholar]
- 14.Haile, C. N., Hiroi, N., Nestler, E. J., and Kosten, T. A. (2001) Synapse 41 179–190 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt, E. F., Sutton, M. A., Schad, C. A., Karanian, D. A., Brodkin, E. S., and Self, D. W. (2001) J. Neurosci. 21 RC137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajadi, A., Bauer, M., Thony, B., and Aebischer, P. (2005) J. Neurochem. 93 1482–1486 [DOI] [PubMed] [Google Scholar]
- 17.Lin, L. F., Doherty, D. H., Lile, J. D., Bektesh, S., and Collins, F. (1993) Science 260 1130–1132 [DOI] [PubMed] [Google Scholar]
- 18.Beck, K. D., Valverde, J., Alexi, T., Poulsen, K., Moffat, B., Vandlen, R. A., Rosenthal, A., and Hefti, F. (1995) Nature 373 339–341 [DOI] [PubMed] [Google Scholar]
- 19.Tomac, A., Lindqvist, E., Lin, L. F., Ogren, S. O., Young, D., Hoffer, B. J., and Olson, L. (1995) Nature 373 335–339 [DOI] [PubMed] [Google Scholar]
- 20.Choi-Lundberg, D. L., Lin, Q., Chang, Y. N., Chiang, Y. L., Hay, C. M., Mohajeri, H., Davidson, B. L., and Bohn, M. C. (1997) Science 275 838–841 [DOI] [PubMed] [Google Scholar]
- 21.Kordower, J. H., Emborg, M. E., Bloch, J., Ma, S. Y., Chu, Y., Leventhal, L., McBride, J., Chen, E. Y., Palfi, S., Roitberg, B. Z., Brown, W. D., Holden, J. E., Pyzalski, R., Taylor, M. D., Carvey, P., Ling, Z., Trono, D., Hantraye, P., Deglon, N., and Aebischer, P. (2000) Science 290 767–773 [DOI] [PubMed] [Google Scholar]
- 22.Cass, W. A. (1996) J. Neurosci. 16 8132–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green-Sadan, T., Kuttner, Y., Lublin-Tennenbaum, T., Kinor, N., Boguslavsky, Y., Margel, S., and Yadid, G. (2005) Exp. Neurol. 194 97–105 [DOI] [PubMed] [Google Scholar]
- 24.Popik, P., Layer, R. T., and Skolnick, P. (1995) Pharmacol. Rev. 47 235–253 [PubMed] [Google Scholar]
- 25.Mash, D. C., Kovera, C. A., Buck, B. E., Norenberg, M. D., Shapshak, P., Hearn, W. L., and Sanchez-Ramos, J. (1998) Ann. N. Y. Acad. Sci. 844 274–292 [DOI] [PubMed] [Google Scholar]
- 26.Cappendijk, S. L., and Dzoljic, M. R. (1993) Eur J. Pharmacol. 241 261–265 [DOI] [PubMed] [Google Scholar]
- 27.Sershen, H., Hashim, A., and Lajtha, A. (1994) Pharmacol. Biochem. Behav. 47 13–19 [DOI] [PubMed] [Google Scholar]
- 28.Rezvani, A. H., Overstreet, D. H., and Lee, Y. W. (1995) Pharmacol. Biochem. Behav. 52 615–620 [DOI] [PubMed] [Google Scholar]
- 29.Biedler, J. L., Roffler-Tarlov, S., Schachner, M., and Freedman, L. S. (1978) Cancer Res. 38, 3751–3757 [PubMed] [Google Scholar]
- 30.He, D. Y., and Ron, D. (2006) Faseb J. 20 2420–2422 [DOI] [PubMed] [Google Scholar]
- 31.Ronca, F., Yee, K. S., and Yu, V. C. (1999) J. Biol. Chem. 274 18128–18134 [DOI] [PubMed] [Google Scholar]
- 32.Mailliard, W. S., and Diamond, I. (2004) Pharmacol. Ther. 101 39–46 [DOI] [PubMed] [Google Scholar]
- 33.Ron, D., and Jurd, R. (2005) Sci STKE 2005 re14. [DOI] [PubMed] [Google Scholar]
- 34.Dunkley, P. R., Bobrovskaya, L., Graham, M. E., von Nagy-Felsobuki, E. I., and Dickson, P. W. (2004) J. Neurochem. 91 1025–1043 [DOI] [PubMed] [Google Scholar]
- 35.Lei, H., Venkatakrishnan, A., Yu, S., and Kazlauskas, A. (2007) J. Biol. Chem. 282 9364–9371 [DOI] [PubMed] [Google Scholar]
- 36.Vanhoose, A. M., Clements, J. M., and Winder, D. G. (2006) J. Neurosci. 26 1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, H., and Burrows, F. (2004) J. Mol. Med. 82 488–499 [DOI] [PubMed] [Google Scholar]
- 38.Pearl, L. H., and Prodromou, C. (2006) Annu. Rev. Biochem. 75 271–294 [DOI] [PubMed] [Google Scholar]
- 39.Xiao, L., Rasouli, P., and Ruden, D. M. (2007) Curr. Med. Chem. 14 223–232 [DOI] [PubMed] [Google Scholar]
- 40.Hadden, M. K., Lubbers, D. J., and Blagg, B. S. (2006) Curr. Top Med. Chem. 6 1173–1182 [DOI] [PubMed] [Google Scholar]
- 41.Ochel, H. J., Eichhorn, K., and Gademann, G. (2001) Cell Stress Chaperones 6 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitesell, L., and Cook, P. (1996) Mol. Endocrinol. 10 705–712 [DOI] [PubMed] [Google Scholar]
- 43.Whitesell, L., Mimnaugh, E. G., De Costa, B., Myers, C. E., and Neckers, L. M. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prodromou, C., Roe, S. M., O'Brien, R., Ladbury, J. E., Piper, P. W., and Pearl, L. H. (1997) Cell 90 65–75 [DOI] [PubMed] [Google Scholar]
- 45.Grenert, J. P., Sullivan, W. P., Fadden, P., Haystead, T. A., Clark, J., Mimnaugh, E., Krutzsch, H., Ochel, H. J., Schulte, T. W., Sausville, E., Neckers, L. M., and Toft, D. O. (1997) J. Biol. Chem. 272 23843–23850 [DOI] [PubMed] [Google Scholar]
- 46.Jing, S., Wen, D., Yu, Y., Holst, P. L., Luo, Y., Fang, M., Tamir, R., Antonio, L., Hu, Z., Cupples, R., Louis, J. C., Hu, S., Altrock, B. W., and Fox, G. M. (1996) Cell 85 1113–1124 [DOI] [PubMed] [Google Scholar]
- 47.Xu, R. Y., Pong, K., Yu, Y., Chang, D., Liu, S., Lile, J. D., Treanor, J., Beck, K. D., and Louis, J. C. (1998) J. Neurochem. 70 1383–1393 [DOI] [PubMed] [Google Scholar]
- 48.Kumer, S. C., and Vrana, K. E. (1996) J. Neurochem. 67 443–462 [DOI] [PubMed] [Google Scholar]
- 49.Hagerty, T., Fernandez, E., Lynch, K., Wang, S. S., Morgan, W. W., and Strong, R. (2001) J. Neurochem. 78 1379–1388 [DOI] [PubMed] [Google Scholar]
- 50.Hagerty, T., Morgan, W. W., Elango, N., and Strong, R. (2001) J. Neurochem. 76 825–834 [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, T., Kurahashi, H., and Ichinose, H. (2004) Biochem. Biophys. Res. Commun. 315 389–396 [DOI] [PubMed] [Google Scholar]
- 52.Piech-Dumas, K. M., Best, J. A., Chen, Y., Nagamoto-Combs, K., Osterhout, C. A., and Tank, A. W. (2001) J. Neurochem. 76 1376–1385 [DOI] [PubMed] [Google Scholar]
- 53.Roe, D. F., Craviso, G. L., and Waymire, J. C. (2004) Brain Res. Mol. Brain Res. 120 91–102 [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura, R., Xu, L., Sun, B., and Tank, A. W. (2004) Brain Res. Mol. Brain Res. 126 188–197 [DOI] [PubMed] [Google Scholar]
- 55.Borba, J. C., Henze, I. P., Silveira, M. S., Kubrusly, R. C., Gardino, P. F., de Mello, M. C., Hokoc, J. N., and de Mello, F. G. (2005) Brain Res. Dev. Brain Res. 156 193–201 [DOI] [PubMed] [Google Scholar]
- 56.Gueorguiev, V. D., Cheng, S. Y., and Sabban, E. L. (2006) J. Biol. Chem. 281 10188–10195 [DOI] [PubMed] [Google Scholar]
- 57.Jeong, H., Kim, M. S., Kim, S. W., Kim, K. S., and Seol, W. (2006) J. Neurochem. 98 386–394 [DOI] [PubMed] [Google Scholar]
- 58.Lazar, M. A., Truscott, R. J., Raese, J. D., and Barchas, J. D. (1981) J. Neurochem. 36 677–682 [DOI] [PubMed] [Google Scholar]
- 59.Gahn, L. G., and Roskoski, R., Jr. (1995) Biochemistry 34 252–256 [DOI] [PubMed] [Google Scholar]
- 60.Toska, K., Kleppe, R., Armstrong, C. G., Morrice, N. A., Cohen, P., and Haavik, J. (2002) J. Neurochem. 83 775–783 [DOI] [PubMed] [Google Scholar]
- 61.Moy, L. Y., and Tsai, L. H. (2004) J. Biol. Chem. 279 54487–54493 [DOI] [PubMed] [Google Scholar]
- 62.Royo, M., Fitzpatrick, P. F., and Daubner, S. C. (2005) Arch. Biochem. Biophys. 434 266–274 [DOI] [PubMed] [Google Scholar]
- 63.Bobrovskaya, L., Gelain, D. P., Gilligan, C., Dickson, P. W., and Dunkley, P. R. (2007) Cell Signal. 19 1141–1149 [DOI] [PubMed] [Google Scholar]