Abstract
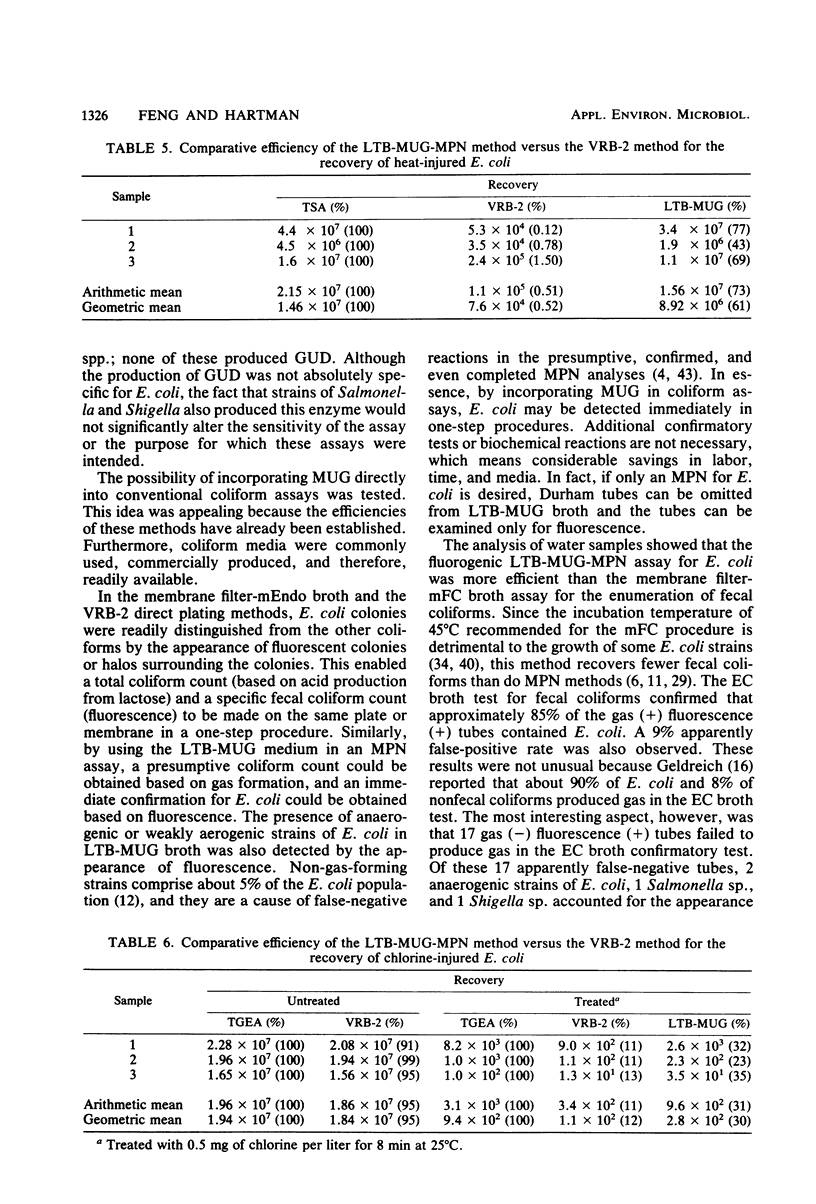
Rapid assays for Escherichia coli were developed by using the compound 4-methylumbelliferone glucuronide (MUG), which is hydrolyzed by glucuronidase to yield a fluorogenic product. The production of glucuronidase was limited to strains of E. coli and some Salmonella and Shigella strains in the family Enterobacteriaceae. For immediate confirmation of the presence of E. coli in most-probable-number tubes, MUG was incorporated into lauryl tryptose broth at a final concentration of 100 micrograms/ml. Results of both the presumptive test (gas production) and the confirmed test (fluorescence) for E. coli were obtained from a variety of food, water, and milk samples after incubation for only 24 h at 35 degrees C. Approximately 90% of the tubes showing both gas production and fluorescence contained fecal coliforms (they were positive in EC broth incubated at 45 degrees C). Few false-positive reactions were observed. The lauryl tryptose broth-MUG-most-probable-number assay was superior to violet red bile agar for the detection of heat- and chlorine-injured E. coli cells. Anaerogenic strains produced positive reactions, and small numbers of E. coli could be detected in the presence of large numbers of competing bacteria. The fluorogenic assay was sensitive and rapid; the presence of one viable cell was detected within 20 h. E. coli colonies could be distinguished from other coliforms on membrane filters and plates of violet red bile agar if MUG was incorporated into the culture media. A rapid confirmatory test for E. coli that is amenable to automation was developed by using microtitration plates filled with a nonselective medium containing MUG. Pure or mixed cultures containing E. coli produced fluorescence within 4 h (most strains) to 24 h (a few weakly positive strains).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braswell J. R., Hoadley A. W. Recovery of Escherichia coli from chlorinated secondary sewage. Appl Microbiol. 1974 Aug;28(2):328–329. doi: 10.1128/am.28.2.328-329.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady P., Dufour S. W., Shaw J., Kraeger S. J. Electrical impedance measurements: rapid method for detecting and monitoring microorganisms. J Clin Microbiol. 1978 Mar;7(3):265–272. doi: 10.1128/jcm.7.3.265-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper A. K., McFeters G. A. Chlorine injury and the enumeration of waterborne coliform bacteria. Appl Environ Microbiol. 1979 Mar;37(3):633–641. doi: 10.1128/aem.37.3.633-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. W., Ordal Z. J. Thermal injury and recovery of Salmonella typhimurium and its effect on enumeration procedures. Appl Microbiol. 1969 Sep;18(3):332–336. doi: 10.1128/am.18.3.332-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén G., Linde A. Screening plate method for detection of bacterial beta-glucuronidase. Appl Microbiol. 1973 Dec;26(6):863–866. doi: 10.1128/am.26.6.863-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., LeChevallier M. W., Waarvick C. E., Seidler R. J. Coliform species recovered from untreated surface water and drinking water by the membrane filter, standard, and modified most-probable-number techniques. Appl Environ Microbiol. 1981 Mar;41(3):657–663. doi: 10.1128/aem.41.3.657-663.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., Waarvick C. E., Seidler R. J., LeChevallier M. W. Failure of the most-probable-number technique to detect coliforms in drinking water and raw water supplies. Appl Environ Microbiol. 1981 Jan;41(1):130–138. doi: 10.1128/aem.41.1.130-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. A., Hartman P. S., Lanz W. W. Violet red bile 2 agar for stressed coliforms. Appl Microbiol. 1975 Apr;29(4):537–539. doi: 10.1128/am.29.4.537-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol. 1977 Aug;23(8):935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- Hussong D., Colwell R. R., Weiner R. M. Rate of occurrence of false-positive results from total coliform most-probable-number analysis of shellfish and estuaries. Appl Environ Microbiol. 1980 Nov;40(5):981–983. doi: 10.1128/aem.40.5.981-983.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong D., Damaré J. M., Weiner R. M., Colwell R. R. Bacteria associated with false-positive most-probable-number coliform test results for shellfish and estuaries. Appl Environ Microbiol. 1981 Jan;41(1):35–45. doi: 10.1128/aem.41.1.35-45.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):245–251. doi: 10.1111/j.1699-0463.1976.tb01933.x. [DOI] [PubMed] [Google Scholar]
- LeChevallier M. W., Seidler R. J., Evans T. M. Enumeration and characterization of standard plate count bacteria in chlorinated and raw water supplies. Appl Environ Microbiol. 1980 Nov;40(5):922–930. doi: 10.1128/aem.40.5.922-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Evaluation of coliform tests for chlorinated secondary effluents. J Water Pollut Control Fed. 1973 Mar;45(1):498–506. [PubMed] [Google Scholar]
- MEAD J. A., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 67. The biosynthesis of the glucuronides of umbelliferone and 4-methylumbelliferone and their use in fluorimetric determination of beta-glucuronidase. Biochem J. 1955 Dec;61(4):569–574. doi: 10.1042/bj0610569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks J. L., Greenan M. J. A rapid method for identifying bacterial enzymes. J Clin Pathol. 1975 Aug;28(8):686–687. doi: 10.1136/jcp.28.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman I. J., Simon N. T., Sanders A. C., Fishbein M., Olson J. C., Jr, Read R. B. Microbiological methods. Methodology for enteropathogenic Escherichia coli. J Assoc Off Anal Chem. 1975 Mar;58(2):283–292. [PubMed] [Google Scholar]
- Olson B. H. Enchanced accuracy of coliform testing in seawater by a modification of the most-probable-number method. Appl Environ Microbiol. 1978 Sep;36(3):438–444. doi: 10.1128/aem.36.3.438-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Enumeration of Escherichia coli in frozen samples after recovery from injury. Appl Microbiol. 1973 Apr;25(4):499–503. doi: 10.1128/am.25.4.499-503.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., Geldreich E. E., Litsky W. Improved membrane filter method for fecal coliform analysis. Appl Microbiol. 1975 Apr;29(4):532–536. doi: 10.1128/am.29.4.532-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Levin R. E. Vibrios from fish pen slime which mimic Escherichia coli on violet red bile agar. Appl Microbiol. 1970 Jul;20(1):107–112. doi: 10.1128/am.20.1.107-112.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. P., Munoz E. F. Automated electrical impedance technique for rapid enumeration of fecal coliforms in effluents from sewage treatment plants. Appl Environ Microbiol. 1979 Mar;37(3):521–526. doi: 10.1128/aem.37.3.521-526.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham W., Danielsson M. L. Reliability of VRB agar and BGLB broth for enumeration of 44 degrees C coliforms in food. Nord Vet Med. 1980 Jul-Aug;32(7-8):325–331. [PubMed] [Google Scholar]
- Thompson R. E. IRREGULARITIES IN THE TEST FOR B. COLI IN WATER. J Bacteriol. 1927 Mar;13(3):209–221. doi: 10.1128/jb.13.3.209-221.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]







