Abstract
All aspartic proteases, including retroviral proteases, share the triplet DTG critical for the active site geometry and catalytic function. These residues interact closely in the active, dimeric structure of HIV-1 protease (PR). We have systematically assessed the effect of the D25N mutation on the structure and stability of the mature PR monomer and dimer. The D25N mutation (PRD25N) increases the equilibrium dimer dissociation constant by a factor >100-fold (1.3 ± 0.09 μm) relative to PR. In the absence of inhibitor, NMR studies reveal clear structural differences between PR and PRD25N in the relatively mobile P1 loop (residues 79-83) and flap regions, and differential scanning calorimetric analyses show that the mutation lowers the stabilities of both the monomer and dimer folds by 5 and 7.3 °C, respectively. Only minimal differences are observed in high resolution crystal structures of PRD25N complexed to darunavir (DRV), a potent clinical inhibitor, or a non-hydrolyzable substrate analogue, Ac-Thr-Ile-Nle-r-Nle-Gln-Arg-NH2 (RPB), as compared with PR·DRV and PR·RPB complexes. Although complexation with RPB stabilizes both dimers, the effect on their Tm is smaller for PRD25N (6.2 °C) than for PR (8.7 °C). The Tm of PRD25N·DRV increases by only 3 °C relative to free PRD25N, as compared with a 22 °C increase for PR·DRV, and the mutation increases the ligand dissociation constant of PRD25N·DRV by a factor of ∼106 relative to PR·DRV. These results suggest that interactions mediated by the catalytic Asp residues make a major contribution to the tight binding of DRV to PR.
In HIV-1,2 the protease is synthesized as part of a 165-kDa polyprotein (Gag-Pol). Gag-Pol comprises the matrix, capsid, P2, nucleocapsid, transframe, protease (PR), reverse transcriptase, and integrase domains (1). The protease mediates its own release and the processing of the viral polyproteins, Gag and Gag-Pol, into the necessary structural and functional proteins (1-3). This spatio-temporally regulated process is crucial for the maturation and propagation of HIV (4-7). Because of this vital role, the mature protease dimer has proven to be a successful target for the development of antiviral agents. Structure-based design of drugs targeted against the mature protease has aided in the development of potent inhibitors that bind specifically to the active site (8, 9). Although several of these inhibitors are in clinical use and have curtailed the progression of the disease, the effectiveness of long term treatment has been limited due to naturally selected protease variants exhibiting lower affinity to the drugs than the wild-type enzyme, and this has been a challenge for the past decade (10). In recent years, a major emphasis in protease research has been to improve inhibitor design and treatment regimens, which include the highly active retroviral therapy, to overcome the problem of drug resistance and curb progress of the disease (11, 12).
The HIV-1 protease is composed of 99 amino acids and is a member of the family of aspartic acid proteases (1, 13). Unlike the cellular aspartic proteases that are active as monomers, catalytic activity of retroviral proteases, including HIV-1 protease, requires dimer formation (14). The active site is formed along the dimer interface, and each subunit contributes one of the two catalytic aspartic acid residues (1, 14). These residues are expected to be in opposite states of protonation for activity, and the water molecule involved in the hydrolysis of the peptide bond is proposed to be hydrogen bonded to the aspartyl residues (15, 16). Hydrolysis of the peptide bond mediated by the protease involves general base/general acid catalysis. The importance of the Asp-25 residue for protease function was confirmed in studies showing that it is required for catalytic activity and viral infectivity (2). Subsequent studies showed that coexpression of the wild-type proviral DNA with increasing amounts of the mutant proviral DNA bearing the D25N mutation results in a concomitant decrease in the proteolytic activity monitored by in vivo viral polyprotein processing (17). This suggested that the protease domain bearing the D25N mutation is capable of adopting a native-like fold to sequester the wild-type protease via heterodimer formation. Because the protease bearing this mutation is completely devoid of catalytic activity, it is a useful tool for studies of the protease under conditions where the active protease tends to undergo rapid autoproteolysis (self-degradation). Additionally, the inactivating mutation D25N makes it possible to investigate the binding of substrates to the protease without the interference of the accompanying proteolytic cleavage reaction. Thus, the mature protease and its precursor bearing the D25N mutation have been effectively used in such studies with substrates corresponding to the natural cleavage sites in the Gag and Gag-Pol polyproteins by x-ray crystallography and NMR (10, 18).
The steps in the maturation of the Gag-Pol precursor and the mechanism of the autocatalytic maturation of the protease have been studied extensively (3, 10). Upon its intramolecular maturation at its N terminus, the protease forms a stable dimer concomitant with the formation of the terminal β-sheet structure and a very low equilibrium dimer dissociation constant (Kd < 10 nm) (10). It is predicted that disrupting the terminal β-sheet arrangement of the mature protease, which contributes to ∼50% of the total dimer interface contacts, or preventing dimer formation prior to its maturation may provide an alternative avenue for inhibitor design (10, 11, 19, 20). This presumably non-competitive mode of inhibition might show a synergistic effect to the conventional active site targeted compounds with the advantage of reducing the emergence of drug-resistant strains. Several groups have reported the development of dimerization inhibitors of the mature protease; however, none of these kinds of potential lead compounds have been characterized further for possible clinical use (19). Recent complementary NMR structural and biophysical studies of the mature protease and its precursor have aimed to elucidate the role of conserved regions required for a native-like fold, dimer formation, and precursor maturation. Several subtle mutations have been identified that increase the Kd by ∼2 to >5 orders of magnitude, thus also enabling the solution structure determination of the protease monomer by NMR (10). It is anticipated that insights derived from such studies will aid in the discovery and rational design of novel inhibitors of dimerization.
In our exploration of sites that are critical for dimer formation, we had observed that a D25N mutation also significantly increases the Kd (21). In the present study we report the characterization of the effects of this mutation on the dimer stability, inhibitor/substrate binding, and the molecular structure and dynamics of the protease by a multifaceted approach comprising NMR, calorimetry, and crystallography.
EXPERIMENTAL PROCEDURES
Vector Construction and Protein Preparation—The pseudo wild type (PR) (22) and the active site mutant (PRD25N) mature protease constructs were expressed using pET11a vector and Escherichia coli BL21(DE3) host, purified from inclusion bodies and folded from a denatured state as described (22, 23). The expression and purification of PRT26A, PRD25N/T26A, and SFNFPRD25N constructs has been described previously (24). A new construct, PR5-95/D25N, was generated using the PR5-95 DNA template (23), appropriate forward and reverse primers, and the QuikChange protocol (Stratagene). The newly introduced mutation was verified both by DNA sequencing and mass spectrometry. To maintain consistency in the analysis, all constructs bear five mutations, Q7K, L33I and L63I (which restrict the autoproteolysis (self-degradation) of active proteases), and C67A and C97A, to prevent cysteine thiol oxidation (22). They differ only in their specified mutations (shown in subscripts). Proteins for NMR studies were obtained by growing the cells in minimal media containing [15N]ammonium chloride with or without [13C]glucose as the sole nitrogen and carbon sources, respectively.
Inhibitors and Substrate—The non-hydrolyzable substrate analog inhibitor RPB, Ac-Thr-Ile-Nle-r-Nle-Gln-Arg-NH2, in which r (reduced peptide bond) represents a methylene group that replaces the peptide carbonyl, was obtained from Bachem Bioscience Inc. (King of Prussia, PA). Darunavir (DRV) was a generous gift of Dr. Arun Ghosh, Purdue University, and was subsequently obtained from the National Institutes of Health (NIH) AIDS research and reference reagent program, Division of AIDS, NIAID, NIH: Reagent 11447 from Tibotec Pharmaceuticals. Substrate IV, Lys-Ala-Arg-Val-Nle-(4-nitrophenylalanine)-Glu-Ala-Nle-NH2, was purchased from California Peptide Research (Napa, CA).
NMR Experiments—All NMR data were recorded on DMX500 spectrometers with or without a cryoprobe (Bruker Instruments, Billerica, MA) at 20 °C. 1H-15N correlation spectra of PRD25N were acquired using 15 N-labeled protein at 0.5 mm in 20 mm phosphate buffer at pH 5.8 and 25 μm in 50 mm acetate buffer at pH 5.0. Backbone chemical shifts were assigned based on HNCA type experiments using a 0.5 mm 15N,13C-labeled protein at pH 5.8, similar to those used to assign chemical shifts of PR previously (25). Experiments to determine transverse relaxation time, T2, and 15N-{1H} NOE values were carried out using 0.5 mm 15N-labeled protein at pH 5.8 as described previously (26).
Isothermal Titration Calorimetry—Measurements were performed using a high precision VP-ITC titration calorimetric system (MicroCal Inc.) at 28 °C. PR and PRD25N were prepared according to the quench protocol of protein folding (23) to a final concentration of 6.9 μm and 14.7 μm, respectively, by mixing 1 volume of protein in 30-50 mm formic acid with 2.3-2.5 volumes of 5 mm sodium acetate, pH 6, and then with 3.3-4 volumes of 100 mm sodium acetate, pH 5 (buffer mix). The RPB inhibitor was dissolved in the same buffer mix to a final concentration of 300 μm. For titration of PRD25N with DRV, the buffered PRD25N solution contained a final protein concentration of 11 μm. The DRV solution was prepared at a final concentration of 266 μm and contained a final concentration of 0.5% Me2SO derived from the DRV stock solution. Me2SO was added to the enzyme solution to give the same 0.5% concentration, to eliminate possible thermal effects on dilution of the organic solvent from the titrant. Analyses of the data were performed using Origin software provided with the instrument.
Differential Scanning Calorimetry—Measurements were performed using a VP-DSC microcalorimeter (MicroCal Inc.). Samples were prepared by the quench protocol (23) as above to give a final protein concentration of 26-33 μm as monomer and a buffer concentration of 50 mm sodium acetate at pH 4.8. Thermal denaturation scans were begun at a temperature of 20 or 25 °C and run at a rate of 90 °C/h, except for SFNFPRD25N (60 °C/h). The final temperature ranged from 85 to 100 °C depending on the observed Tm. Raw data from representative DSC scans of each of the protease constructs are given in supporting information (supplemental Fig. S1). Reversibility as determined by assaying PR (22) before and after scanning showed an ∼90% loss of activity after one scan. In the experiments with RPB and DRV inhibitors, the inhibitor was present at a final concentration of 30 μm (approximately twice the concentration of the dimeric proteins). For the DSC of PRD25N with substrate IV, the final substrate concentration was 360 μm. Reference (buffer) scans were subtracted from the observed traces, and the data were normalized for protein (monomer or dimer) concentration. Linear baseline segments were selected in regions of the trace before and after the transition peak, and the Origin software of the instrument was then used to generate and subtract a “progress baseline” between them that reflects the fraction of the total reaction completed at each temperature point (as described in the MicroCal data analysis manual). The apparent Tm was determined from the resultant peak maximum. Because of the lack of reversibility, rigorous thermodynamic analysis was not possible, and no attempt was made to determine ΔCp or ΔH for the transitions.
Crystallographic Analysis—Crystals were grown at 20-25 °C by vapor diffusion using the hanging drop method. Mutant PRD25N was co-crystallized with the potent clinical inhibitor, DRV, in a protein to inhibitor molar ratio of 1:2. The reservoir contained 0.2 m sodium acetate buffer, pH 5.0, and 30% sodium chloride as precipitant. PRD25N was co-crystallized with RPB in a molar ratio of 1:20 in the reservoir containing 0.1 m citrate/0.2 m phosphate buffer, pH 5.4, 10% sodium chloride as precipitant, and 5-10% Me2SO. The protein (1.8-3.5 mg/ml) was preincubated on ice. The crystallization drops had a 1:1 ratio by volume of reservoir solution and protein. The crystals grew in 1-7 days and were frozen in liquid nitrogen with a cryoprotectant of 20-30% glycerol.
X-ray diffraction data for both complexes were collected on the SER-CAT beamline of the Advanced Photon Source, Argonne National Laboratory. Data were processed using HKL2000 (27). The structures were solved by molecular replacement using AmoRe (28), refined using SHELX (29) and refitted using O (30). Alternate conformations were modeled for residues when obvious in the electron density maps. The solvent was modeled with over 100 water molecules, and ions present in the crystallization solutions, as described previously (31). Anisotropic B factors were refined for all the structures. Hydrogen atom positions were included in the last stage of refinement using all data once all other parameters, including disorder, had been modeled. The structures have been submitted to the Protein Data Bank with accession code 3BVB for PRD25N·DRV and 3BVA for PRD25N·RPB.
RESULTS AND DISCUSSION
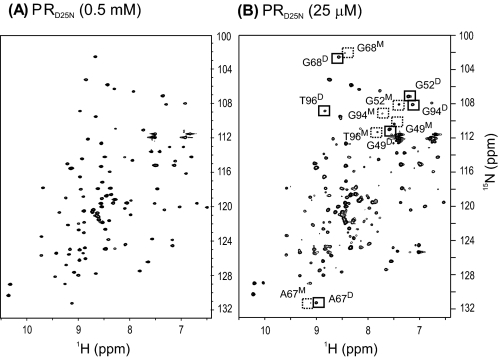
Monomer-Dimer Equilibria—Because PRD25N is not catalytically active, it was not possible to measure its Kd by analysis of the dependence of enzymatic activity on protein concentration as in the case of PR (22). We have previously observed differences in the 1H-15N HSQC spectra of protease constructs that exist predominantly as dimers or as monomers and assigned the signals characteristic of each species (32). Hence this method was used to characterize the monomer-dimer equilibrium of PRD25N. At ∼0.5 mm concentration in 20 mm sodium phosphate, pH 5.8, 20 °C, we observed a 1H-15N HSQC spectrum for PRD25N (Fig. 1A) in the absence of bound inhibitor that is similar to that observed for PR (32), indicating that it is essentially dimeric at this concentration. We have recently described a simple protocol (see quench protocol in Ref. 23) for preparing folded dimers and monomeric mutants of the protease at low concentrations of ∼20 μm, suitable for NMR and other biophysical studies. Under these conditions (an ∼20-fold lower protein concentration than in Fig. 1A), signals corresponding to a minor fraction become more evident in the PRD25N spectrum (Fig. 1B). Although the signals of the minor component were not assigned due to overlap with the dimer signals, it is apparent from comparing the previously assigned chemical shifts of PRR87K and PR1-95 monomers (24, 32) that the minor fraction observed in Fig. 1B corresponds to the PRD25N monomer. On the basis of the signal intensities of the characteristic monomer peaks and the corresponding dimer peaks, the Kd of the PRD25N dimer was determined to be 1.3 ± 0.09 μm, at pH 5.0. These values are similar to those previously determined qualitatively for PRD25N by NMR (21) and by sedimentation equilibrium analysis at pH 7.0 (33), and approximately two orders of magnitude larger than the value of ∼0.01 μm previously estimated for PR from kinetic data (22). Thus, in addition to suppression of catalytic activity, the D25N mutation significantly affects the monomer-dimer equilibrium of the mature protease.
FIGURE 1.
1H-15N HSQC spectra of PRD25N at 0. 5 mm (A) and 25 μm (B) protein concentrations. Proteins were prepared and folded as described previously (23). Peaks unique to the dimer and the monomer are shown in solid and dashed boxes, respectively.
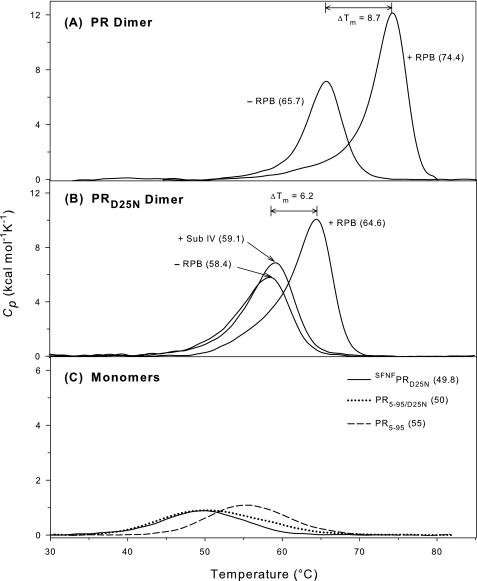
Effects on Thermal Stability—In earlier studies we had shown that the quench protocol of preparing folded protease permitted NMR and fluorescence measurements under similar conditions. Under the conditions described (23), folded protease samples can be prepared easily prior to each experiment from the same stock solutions maintained in an unfolded state, usually in 30-50 mm formic acid. This has enabled the facile preparation and comparison of the thermal stabilities of PR, PRD25N, and several related constructs (supporting information, supplemental Table S1). Consistent with the difference in dimer stability, the D25N mutation results in a significant (7.3 °C) decrease in the Tm determined from the peak maxima of the transitions for unfolding/dimer dissociation relative to PR as measured by DSC at pH 4.8 (Fig. 2, A and B). Previous observations that the midpoints of urea denaturation curves for PR (22) and PRD25N (23) both occur at ∼2 m urea had indicated no significant difference between PRD25N and PR. The present observations may result from mechanistic differences between the thermal and urea denaturation processes of the protease. Constructs that lack the terminal residues 1-4 and 96-99 critical for the dimer interface exist as folded monomers in solution (23). Thus, we examined the effect of the D25N mutation on monomer stability by comparing the thermal stabilities of PR5-95 and PR5-95/D25N. The Tm for PR5-95/D25N is 5 °C lower than for PR5-95 (Fig. 2C). These observations indicate that Asp-25 plays a significant role in stabilizing the monomer and dimer folds and is consistent with the greater sensitivity to urea denaturation of PRD25N/T26A (midpoint of the denaturation curve at ∼1.8 m urea) relative to PRT26A (midpoint at ∼2.5 m urea), both of which are folded monomers (23).
FIGURE 2.
DSC thermograms of dimer and monomer constructs. For conditions see “Experimental Procedures.” The numbers correspond to the apparent Tm of each protein (maximum of the transition) in °C. Data shown are baseline-corrected and normalized to the appropriate dimer or monomer concentrations. A, dimeric PR (13 μm) in the presence and absence of 30 μm of RPB inhibitor; B, dimeric PRD25N (14.5 μm) in the presence and absence of 30 μm RPB and of 360 μm substrate IV (Sub IV); C, monomeric PR5-95 (32 μm), SFNFPRD25N (25 μm) and monomeric PR5-95/D25N (33 μm). Note that the mass of protein per unit volume is approximately the same in C as in A and B, although the molarity of the monomers is double the molarity of the dimers; the ordinate scale of C is expanded for comparability with A and B.
The native transframe region (TFR) flanking the N terminus of the protease comprises two domains, the conserved transframe octapeptide followed by the 48 amino acid p6pol, both separated by a protease cleavage site (see Fig. 1 in Ref. 23). It appears that the monomer-dimer equilibrium of the protease is modulated by the N-terminally flanking TFR, such that prior to the cleavage at the p6pol/PR junction, the protease domain is mainly monomeric (Kd > 0.5 mm), exhibiting a fold that is similar to that of a single subunit of the dimer at least for the region spanning residues 10-90 (24). Residues 1-9 and 91-99 display no specific structure (24). These studies were facilitated using the precursor construct TFR-PR bearing a D25N mutation to abolish autoprocessing (24). Subsequently it was found that a four-residue extension at the N terminus of the protease (Ser-Phe-Asn-Phe corresponding to the C-terminal residues of p6pol) increased the Kd of the resulting construct SFNFPRD25N by more than three orders of magnitude (24). Because the full-length TFR portion of the larger fusion protein may possess some intrinsic structure (34) that could interfere with interpretation of the DSC results, we utilized the same SFNFPRD25N construct to investigate whether the presence of the SFNF sequence affects only the magnitude of Kd or whether it also alters the stability of the monomer fold. In fact, the DSC thermograms for SFNFPRD25N and PR5-95/D25N monomers were found to be nearly superimposable, and their Tm values are the same within experimental error (Fig. 2C). The identical Tm values for the monomer fold in the presence and absence of the SFNF modification supports the conclusion that this N-terminal tetrapeptide influences only the stability of the dimer. This is consistent with the interpretation (23) that the SFNF sequence may inhibit dimerization via interactions with protease residues 3-6 at the dimer interface, a region that does not contribute to the monomer structure.
The thermal stabilities of PR and PRD25N were also determined in the presence of a substrate-analogue inhibitor with a reduced peptide bond (RPB, see “Experimental Procedures” and Fig. 6B). Repetitive scanning indicated that the thermal transition was irreversible under these conditions. Little or no change in the heat capacity before and after the transition was apparent, possibly as a result of this irreversibility. Because the observed process was not reversible, detailed thermodynamic analysis was not possible. In the presence of 30 μm RPB, ∼two times the PR dimer concentration and at least 100 times the ligand dissociation constant (KL) for PR·RPB (see following section), the Tm values for both PR (Fig. 2A) and PRD25N (Fig. 2B) are significantly increased (by 8.7 and 6.2 °C, respectively). This qualitatively indicates that RPB binding to the active site stabilizes both proteins, although the relative stabilization is larger with PR. In light of this observation it was important to determine quantitatively the extent to which the structural factors that influence the monomer-dimer equilibrium also affect the interactions of the mutant protein with substrates and inhibitors.
FIGURE 6.
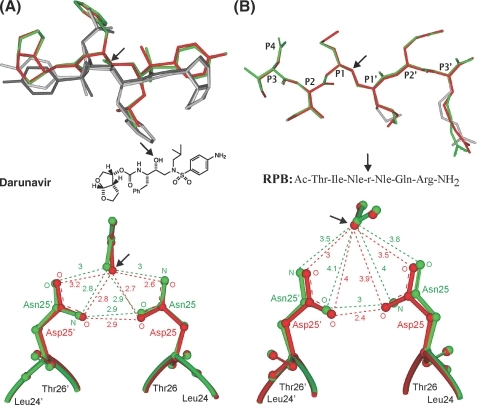
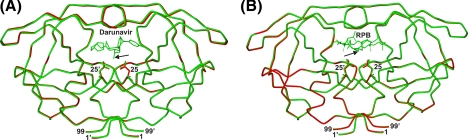
Comparison of the inhibitor structures bound to either PR (red or black) or PRD25N (green or gray). DRV binding (A) was observed in two orientations with relative occupancies of 55% (red) to 45% (black) in PR·DRV (2IEN (32)) and 76% (green) to 24% (gray) in PRD25N·DRV. RPB binding (B) was observed in a single orientation (red in PR (2AOD (37)) or green in PRD25N). Distances between the active site residues and the inhibitor (black arrows) are indicated in angstroms. Residues P4-P3′ of the RPB inhibitor are marked, and alternate conformations of P1′ and P3′ side chains are indicated in gray.
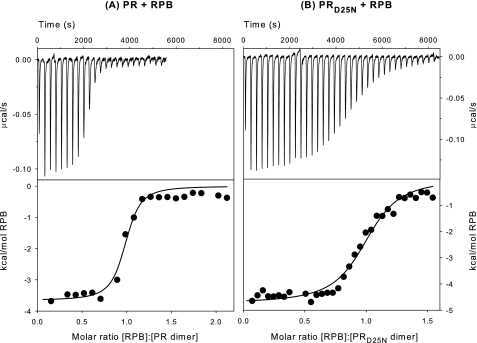
Although the affinity of PR for RPB has been measured by kinetic inhibition studies (22), this approach cannot be used to determine an analogous binding constant for the catalytically inactive protein PRD25N. Thus, ITC of PRD25N with RPB under conditions similar to those employed for the inhibition studies was used to determine KL. Fig. 3 shows the titration of PR (A) and PRD25N (B) with 300 μm RPB inhibitor at 28 °C in 50 mm sodium acetate buffer, pH 5. Curve fitting of the integrated data (Table 1) gave binding constants (Ka) of (1.86 ± 0.76) × 107 and (3.30 ± 0.56) × 106 m-1, corresponding to KL = 1/Ka values of ∼0.05 and ∼0.3 μm, for PR·RPB and PRD25N·RPB, respectively. The affinity of PRD25N for RPB falls within the ideal range for ITC (Fig. 3B) and permitted accurate determination of KL for this construct. Although the higher affinity of PR for RPB resulted in a much steeper titration curve with fewer data points in the transition region (Fig. 3A), the value of KL ∼0.05 μm for PR·RPB, while subject to greater experimental uncertainty, was reasonably consistent with the value previously determined by kinetics (22) under similar conditions. Thus the substitution of Asn for Asp at the active site of the protease decreases the affinity of the enzyme for RPB by a factor of ∼6.
FIGURE 3.
ITC of PR (A) and PRD25N (B) with RPB inhibitor in 50 mm sodium acetate buffer, pH 5, at 28 °C. A, thermal changes on addition of 3-μl aliquots of 300 μm RPB inhibitor to PR or PRD25N (6.9 and 14.7 μm, respectively) in the calorimetric cell (∼1.43 ml).) Curve fitting of the integrated data (lower panels) gave values of the dissociation constants (KL = 1/Ka; see Table 1) of ∼0.05 and ∼0.3 μm for PR·RPB and PRD25N·RPB, respectively.
TABLE 1.
Fitted ITC data for PR and PRD25N binding to ligands in sodium acetate buffer at pH 5
| Protease construct | Ligand | Binding constant (Ka) | ΔH | ΔS | Stoichiometry |
|---|---|---|---|---|---|
| M−1 | cal/mol | cal/mol | [ligand]/[protein] | ||
| PR | RPB | (1.86 ± 0.76) × 107 | −3,661 ± 114 | 21.1 | 0.95 ± 0.02 |
| DRVa | 2.2 × 1011 | −12,100 | 10.5 | ||
| PRD25N | RPB | (3.30 ± 0.56) × 106 | −4,738 ± 87 | 14.1 | 1.00 ± 0.01 |
| DRV | (3.17 ± 0.29) × 105 | −5,812 ± 206 | 5.9 | 1.03 ± 0.03 |
For wild-type PR in 10 mm sodium acetate buffer, pH 5, at 20 °C (data from Ref. 38).
Interestingly, the enthalpies (ΔH) of RPB binding to PR and PRD25N are comparable (Table 1), whereas the entropy (ΔS) is considerably smaller for binding of RPB to PRD25N than to PR. At 28 °C, approximately two-thirds of the binding energy for PR·RPB derives from TΔS, whereas the enthalpic and entropic contributions are similar for PRD25N·RPB. Although part of the favorable entropy for formation of both complexes derives from desolvation of the inhibitor, the difference in entropy between the two systems with a common inhibitor must reflect changes in properties of the proteins upon binding to RPB. This may result in part from differences in the flap mobility of the two unliganded proteins as suggested by the NMR data below. The negative ΔS for constraining the more-mobile PRD25N flaps on binding RPB may be larger than for PR, with a net effect of decreasing the overall positive ΔS.
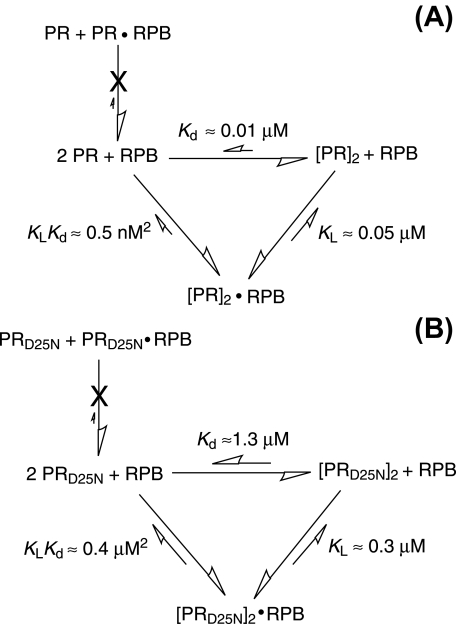
As noted above, destabilization caused by the D25N mutation results in a lowering of the Tm for unfolding/dimer dissociation by 7.3 °C (see Fig. 2, A and B). In the presence of 30 μm RPB, this lowering of the Tm is even larger (9.8 °C), because it results from both the lower affinity of PRD25N for RPB and its decreased dimer stability. Combination of the equilibrium constants for inhibitor dissociation from the dimer (KL) and for dimer dissociation to monomers (Kd), according to the thermodynamic cycle depicted in Fig. 4, gives an overall equilibrium constant for dissociation of the RPB-dimer complex that is ∼800-fold greater for PRD25N relative to PR (note micromolar versus nanomolar scale), whereas, in the absence of RPB, the equilibrium constant for dissociation of the PRD25N dimer is ∼100-fold greater relative to PR. The difference between ΔTm in the presence and absence of RPB for PR (8.7 °C) relative to the analogous ΔTm for PRD25N (6.2 °C) is clearly related to the 8- to 10-fold difference in affinity of the inhibitor for the two proteins.
FIGURE 4.
Thermodynamic cycles showing the relationship between the equilibrium constants (KLKd) for unfolding/dissociation of dimeric PR·RPB (A) and PRD25N·RPB (B) complexes, the equilibrium constants (KL) for dissociation of RPB from the dimers, and the equilibrium constants (Kd) for dissociation of the uncomplexed dimers. Binding of the inhibitor to monomeric PR or PRD25N is expected to be insignificant; thus the equilibria are shown as proceeding exclusively via inhibitor binding to the dimers. The values of KL shown for RPB inhibitor were determined by ITC (present work). Kd values were determined from kinetic data (23) and NMR spectral analysis (present work, Fig. 1) for PR and. PRD25N, respectively. PR in this scheme denotes the monomer.
Although we observed substantial effects of the D25N mutation on both the dimer stability of the protease and its ability to bind RPB, there is less of an effect on binding of a less tightly bound substrate. KL for substrate IV, a chromogenic analogue of the CA-p2 cleavage site in the Gag polyprotein, bound to PRD25N had previously been determined by NMR to be ∼270 μm (21). ITC titration of 14.5 μm PRD25N (as dimer) with 1.4 mm substrate IV under the above conditions was performed to try to confirm the magnitude of this value. Although this KL was too large (corresponding to weak binding) to permit determination by ITC, the curvature observed in a plot of the integrated thermal response (kcal/mol) versus molar ratio (0-13 mol of substrate/mol of protein) (data not shown) was consistent with the value measured by NMR. A control titration in the absence of protein showed a constant thermal response for each successive addition of substrate over the entire range of the titration. Further evidence for weak binding of substrate IV was obtained from a DSC scan of PRD25N in the presence of 360 μm substrate IV, in the concentration range used for PR enzyme assays (22). The Tm for PRD25N was virtually unaffected by the presence of this substrate (ΔTm < 1 °C; cf. Fig. 2B). The binding affinities of PRD25N (KL ∼ 270 μm from NMR, see above) and PR (Km = 177 μm) (22) for substrate IV differ by a factor of less than two, in comparison with the ∼6-fold difference in binding affinity of these two proteins for RPB. We suggest that this difference may relate to the tighter binding of the RPB inhibitor, which “freezes” both proteins into a similar conformation (see crystal structures) at some energetic cost to the mutated relative to the native protein, whereas the more loosely bound substrate gives a more flexible enzyme·substrate complex, which can better accommodate small structural differences between the proteins.
The Kd for PR has been determined from the dependence on enzyme concentration of the hydrolysis rate of substrate IV (22, 35). This method involves a potential ambiguity in that the substrate could stabilize the dimer, resulting in an observed Kd value that is smaller than the actual value for the unbound enzyme. Although it is not possible to measure the Tm for enzymatically active PR in the presence of substrate IV, which would be rapidly cleaved, our present observation that assay concentrations of this substrate contribute minimally to the stabilization of PRD25N suggests that this substrate would also not significantly stabilize the PR dimer under the same conditions and supports the conclusion that the kinetically measured Kd correctly represents the dimer dissociation constant for the unbound PR dimer.
Crystal Structures of PRD25N in Complex with Inhibitors: Comparison with PR—In light of the sizable effects of the D25N mutation on the monomer/dimer equilibrium and inhibitor binding, crystallography was used to attempt to identify a structural basis for the dimer destabilization as well as for the less effective binding of the RPB inhibitor by PRD25N relative to PR. The crystal structures of PRD25N complexed with DRV and RPB were determined and compared with the corresponding complexes (PDB accession codes 2IEN and 2AOD, respectively) of PR. The crystallographic statistics are listed in Table 2. The crystal structures of PRD25N·DRV and PRD25N·RPB were refined to an R-factor of 0.15 at the resolution of 1.3 Å and 0.14 at the resolution of 1.05 Å, respectively. Both crystal structures have one dimer in the asymmetric unit of space group P21212. The inhibitor DRV is bound in the active site cavity of PRD25N in two orientations with relative occupancies of 74%/26%, whereas the RPB inhibitor shows only one orientation in its complex with PRD25N, as had previously been observed with PR (36). Side-chain disorder was observed at P1 (Nle) and P4 (Arg) of RPB, which are flexible residues and frequently disordered in other crystal structures (36, 37). Electron density maps of DRV and RPB are provided in the supporting information (supplemental Fig. S2).
TABLE 2.
Crystallographic data statistics
| Protease construct | PRD25N | PRD25N |
| Inhibitor | DRV | RPB |
| Space group | P21212 | P21212 |
| Unit cell dimensions (Å) | ||
| a | 58.3 | 58.0 |
| b | 85.9 | 85.8 |
| c | 46.1 | 46.5 |
| Unique reflections | 51,872 | 102,685 |
| Rmerge (%) | 6.2 | 13.6 |
| All data (final shell) | (62.1) | (35.4) |
| I/σ (I) | 17.5 | 22.3 |
| All data (final shell) | (2.2) | (2.7) |
| Resolution range for refinement (Å) | 10-1.30 | 10-1.05 |
| Rwork (%) | 15.0 | 14.3 |
| Rfree (%) | 19.7 | 17.1 |
| No. of waters | 143 | 195 |
| Completeness (%) | 89.8 | 94.6 |
| All data (final shell) | (71.8) | (82.1) |
| r.m.s.d. from ideality | ||
| Bonds (Å) | 0.013 | 0.017 |
| Angle distance (Å) | 0.033 | 0.040 |
| Average B-factors (Å2) | ||
| Main chain | 18.5 | 10.3 |
| Side chain | 25.9 | 16.9 |
| Inhibitor | 22.2 | 14.1 |
| Solvent | 31.0 | 27.3 |
Alternate conformations were modeled for some side-chain atoms of the protein, e.g. residues 7, 41, 65, and 70 in one or both subunits. The B-factors of those residues were normally higher than for other residues that were smaller or had interactions with other residues. The Ile-50 at the tip of the flap had alternate conformations of the main-chain and side-chain atoms in PRD25N·DRV but not in PRD25N·RPB. Water molecules (140-195) and other solvent species, including sodium ions, chloride ions, and glycerol molecules, were modeled to fit in the electron density maps.
Overall, the conformations of PRD25N in complex with these two inhibitors, whose affinities for PR differ by >3 orders of magnitude, DRV (KL for PR ∼5 pm (38)) and RPB (KL for PR ∼30 nm, this work), are very similar to the corresponding enzyme·inhibitor complexes of PR (Fig. 5). Upon superimposition of the crystal structures, the r.m.s.d. of all Cα atoms between PR·DRV and PRD25N·DRV was 0.14 Å, and between PR·RPB and PRD25N·RPB, it was only 0.09 Å. However, the r.m.s.d. is only an indicator of macroscopic differences in the structure and folding of the protein. In general, the overall structures of HIV-1 protease constructs are very similar even when they contain mutated residues and/or different bound small molecules, although large local structural differences have been observed in some cases. For example, in PRF53L, Ile-50 at the tips of the flaps was shifted up to 2.5 Å, although the main-chain r.m.s.d. was only 0.4 Å (39). Some structural differences may also arise from crystal packing. Thus, the r.m.s.d. of main chain or Cα structures of PR is usually <0.6 Å for structures with different space groups and cell units, and <0.3 Å for structures in the same space groups and cell units (31, 37, 40). In the present structures of PRD25N, not only the overall chain conformation but also the positions of critical amino acid side chains are in most cases strikingly similar to those observed in the corresponding PR structures. In the present study we focus on two specific regions of the protease that are essential for its structural integrity and catalytic activity, namely, the dimer interface and the active site.
FIGURE 5.
Comparison of the crystal structures of PR (red)- and PRD25N (green)-inhibitor complexes. Tube representations of PR bound to DRV (A) or RPB (B) superimposed on PRD25N bound to DRV (A) or RPB (B) ranging from 1.05- to 1.4-Å resolution. Inhibitors, DRV and RPB, and the active site residue 25 are shown as stick models, and the terminal residues are indicated. The location of a central motif consisting of a hydroxyl group in DRV that can hydrogen bond to the catalytic Asp-25 is indicated by the black arrow.
The Dimer Interface—The dimer of PR is maintained by interactions between the two subunits, including the terminal residues (1-4 and 96-99), the tips of the flaps (50 and 51), Asp-29, Arg-87, and Arg-8′ (3, 10, and 20), and residues in and surrounding the active site (residues 24-27) (41). Some relatively small differences between PR and PRD25N were observed in these regions. In the terminal region, the ring of Pro-1 is bent away from the ring of Phe-99′ in both PRD25N·DRV and PRD25N·RPB complexes. The atom CG of Pro-1 is shifted by 0.8 Å away from Phe-99′ in one subunit and 0.5 Å in the other subunit in PRD25N·DRV (see supplemental Fig. S3A), whereas the shifts are 0.5 Å and 0.3 Å, respectively, in PRD25N·RPB (not shown). Thus, some CH... π interactions stabilizing the two subunits at the termini appear to be weaker in the PRD25N mutant. The distance between NH2 and OD2 of Arg-8 and Asp-29′ is 0.3 Å longer in PRD25N·DRV than in PR (see supplemental Fig. S3B). However, this contact is identical (2.8 Å) in the PRD25N and PR complexes with RPB (not shown). In general, these interactions in the dimer interface are somewhat less tight in PRD25N than in PR, which is consistent with the increased value of Kd for PRD25N. Notably, however, there are virtually no differences in the crucial “fireman's grip” region at the bottom of the active site cavity. Specifically, the inter-oxygen distances between the hydroxyl oxygen of Thr-26 and the carbonyl of Leu-24, which form a hydrogen bond that is critical for dimerization (41), are virtually identical (2.7 Å). Small differences in the orientations of the catalytic Asp-25/Asn-25 residues are discussed in the following section.
Interactions with Inhibitors at the Active Site—The inhibitor DRV is bound in the active site cavity of PRD25N in a major (74% occupancy) and a minor (26% occupancy) orientation (Fig. 6A). The same orientations were observed in the complex with PR, with a nearly equal distribution of the two populations (55%/45% (31)). For a given orientation, the conformation of DRV is similar in both the PR and PRD25N complexes (Fig. 6A), except for differences in the position of the aniline ring in the minor orientation. Detailed structures are shown in supplemental Fig. S4. Generally, the distances between atoms of DRV and residues of PR are similar or 0.2-0.4 Å longer in PRD25N than in PR. In the major orientation, the distance between aniline N1 of DRV and the peptide O atom of Asp-30 is 3.3 Å in PRD25N, whereas the equivalent distance is 2.9 Å in PR. In addition, the distance between aniline C4 and CH3 of Ile-84 in PRD25N·DRV is 0.3 Å longer than in PR·DRV. Some larger structural differences are observed in the minor orientation of DRV. The terminal aniline rings in the minor orientation do not align well on superimposition of the PR and PRD25N structures, such that their N1 atoms are displaced by 1.2 Å relative to each other (Fig. 6A and supplemental Fig. S4B). By comparison, this displacement is only 0.2 Å for the major orientation (supplemental Fig. S4A). The shift of the aniline ring causes changes in the interactions between this ring in the minor orientation and its neighboring residues of PR. A H-bond (3.2 Å) between aniline N1 and the peptide O atom of Asp-30′ in the complex PR·DRV is absent in PRD25N·DRV. However, the separation of N1 and the delta O of Asp-30′ is 2.5 Å compared with 2.9 Å in the PR complex. This is one of the few shorter distances in PRD25N than in PR. Also the distance between aniline C4 and CH3 of Ile-84′ in PRD25N is 0.8 Å longer than in PR.
In complexes with both DRV and RPB, the geometry of the catalytic Asp pair is perturbed very little on substitution by Asn. For the protein·RPB complexes, examination of the Asn-25 and Asn-25′ pair of PRD25N superimposed on the corresponding Asp pair of PR indicates a difference in position of only 0.3-0.5 Å between corresponding O and N atoms (Fig. 6B); in the DRV complexes (Fig. 6A), this difference is even smaller: 0.2-0.3 Å. The distances between RPB and the two catalytic aspartic acids in PRD25N are 0.1-0.3 Å longer than in PR. The distance between two adjacent O atoms of Asp-25 and Asp-25′ is 2.4 ÅinPR·RPB, and the corresponding distance between atom OD1 of Asn-25 and ND2 of Asn-25′ is 3.0ÅinPRD25N·RPB (Fig. 6B). This 0.6-Å increase in the distance between the closest atoms of Asp/Asn-25 and 25′ in PRD25N·RPB relative to PR·RPB is consistent with the lowered thermal stability of the mutant dimer·RPB complex. However, the equivalent distances for PR·DRV and PRD25N·DRV are identical (2.9 Å, Fig. 6A). Although the differences are small, the structural comparison of PR and PRD25N indicates that DRV has weaker overall interactions with PRD25N than with PR (see calorimetric results below).
Unlike DRV, the RPB inhibitor exhibits only one orientation when bound to PRand PRD25N (Fig. 6B). The conformations of RPB complexed with PR and PRD25N are very similar on superimposition of the structures, as are the Asp and Asn-25 residues. This indicates that the 6-fold reduced affinity of RPB for PRD25N relative to PR cannot be explained by steric effects alone, and is likely related to the destabilization of the fold induced by the mutation. The PRD25N·RPB complex (KL ∼ 0.3 mm) appears to exhibit a slightly better “fit” and fewer structural perturbations than the PRD25N·DRV complex (KL = 3.2 mm) on superimposition with the corresponding structures containing PR, consistent with the lower binding affinity of DRV with PRD25N (see below).
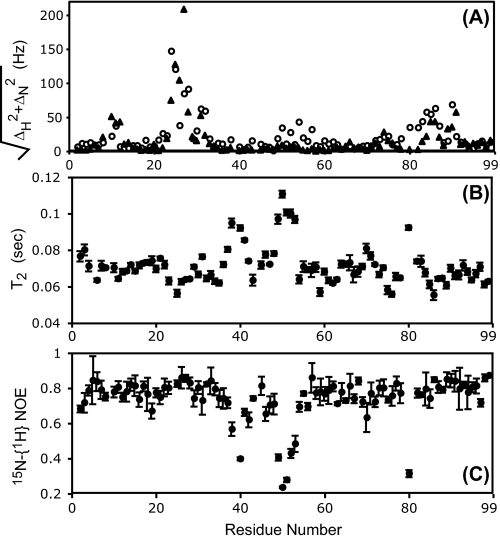
Structure and Dynamics of Free PRD25N in Solution—Earlier results have indicated that the active, mature protease crystallizes rather readily only in complex with an inhibitor or non-hydrolyzable substrate analogue, and only two crystal structures of the uninhibited wild-type mature protease have been reported so far. Similarly, we were unable to obtain crystals of free PRD25N. Thus, NMR was used to examine the conformation of the PRD25N dimer in the absence of a bound inhibitor. Furthermore, information derived from NMR techniques about protein dynamics in solution is complementary to the static information provided by the crystal structures. Fig. 7A shows a comparison of the backbone chemical shifts of PRD25N with those of PR (open circles). Most significant differences were observed in the active site region close to the site of the mutation, and these presumably result from the change in the chemical environment caused by the D25N mutation, and not from conformational changes. The flap region (residues 47-53) and the region spanning residues 80-90 that includes the P1-loop (residues 79-83) also exhibit differences in chemical shifts in locations far from the site of mutation.
FIGURE 7.
A, differences in the backbone amide chemical shifts of the dimer (PR versus PRD25N, open circles) and the monomer (PRT26A versus PRD25N/T26A, solid triangles) with or without the D25N mutation. ΔH and ΔN are the differences in chemical shifts (in hertz) for the individual 1H and 15N atoms, respectively, in the presence and absence of the D25N mutation (plotted as the square root of the sums of their squares). The transverse relaxation times, T2, and 15N-{1H} NOE values for PRD25N are shown in B and C, respectively.
These observed differences in chemical shifts are not directly correlated with the difference in the Kd for dimer dissociation of PR and PRD25N, because the chemical shift comparison does not show significant differences in the terminal β-sheet interface region. Instead, the chemical shift differences map to the upper half of the protease, i.e. above the active site (cf. Fig. 5). Protease constructs containing the T26A mutation exhibit high dimer dissociation constants (Kd > 0.5 mm (10)), and give NMR spectra characteristic of a stable monomer fold. Thus, chemical shift differences between PR and PRD25N dimers (open circles, Fig. 7A) were compared with the corresponding differences between PRT26A and PRD25N/T26A monomers (solid triangles) to determine whether the observed differences were dependent on the dimeric state of the protein. For the PRT26A and PRD25N/T26A monomers, no chemical-shift differences were found in the flap region, and the chemical-shift differences in the P1 loop were smaller than those between the PR and PRD25N dimers. Thus, the D25N mutation induces significant differences in the chemical-shift environments in the flap region and P1-loop region in the dimer, but not in the monomer. This observation is reasonable, because the side chain of residue 25 forms part of the dimer interface but is completely exposed to the solvent in the monomer. However, despite its location at the monomer surface, the D25N mutation significantly decreases the stability of the monomer fold, as shown by the 5° decrease in the Tm for PR5-95/D25N as compared with PR5-95 (Fig. 2).
15N relaxation measurements of PRD25N were utilized to determine whether there were significant differences in the dynamics of PRD25N relative to PR. For PRD25N, the transverse relaxation times, T2 (Fig. 7B) and 15N-{1H} NOE values (Fig. 7C) in the flap regions increase and decrease, respectively, showing almost opposing symmetric profiles. These observations indicate that the flap regions in PRD25N undergo a significant internal motion on the sub-nanosecond time scale. 15N relaxation studies (26) reported for an enzymatically active protease indicated that most T2 values in the flap region did not increase significantly for this construct, whereas PRD25N exhibits increased T2 values for residues 49-53. Although T2 experiments at various effective field strengths are essential to identify the contribution of chemical exchange, the difference in the T2 profiles of PRD25N and active protease indicates that the dynamics of the flap region in these two proteins differ. On the basis of both chemical shifts and relaxation measurements, it is likely that the structure of PRD25N differs from that of PR such that the flap-to-flap interaction in the free protein is somewhat diminished. Therefore, these NMR studies invoke a model in which lack of the carboxylic acid/carboxylate anion of residues 25 in PRD25N induces slight changes in the hydrophobic core of the protease through relative orientation of the P1 loop and flaps that cause the two flaps to interact less with each other in PRD25N than in PR. In the present crystal structures with bound inhibitors, a number of distances between dimer-interface residues are somewhat longer in PRD25N than in PR, suggestive of weaker interactions in the terminal β-sheet region, between the Asp/Asn-25 and -25′ in the presence of RPB and in the region comprising residues Asp-29, Arg-87, and Arg-8′. However, no significant differences in the crystal structures between the two proteins were observed in the flap region, possibly because of similar closing and tightening of the flaps in both structures upon inhibitor binding.
Effect of the D25N Mutation on PR Stabilization by DRV—The clinical inhibitor DRV is one of the most tightly binding inhibitors known for HIV-1 protease (38). This inhibitor has been reported to be effective against several drug resistant protease mutants that have emerged as a result of selective pressure in the presence of other, widely-used drugs. Interactions of DRV with enzymatically active, drug-resistant mutants of PR have thus been a subject of intensive study (10).3 Several essential residues, the most obvious of which are the catalytic Asp-25 pair, are conserved in all enzymatically active variants of the protease. Thus, comparison of inhibitor binding to active PR and inactive constructs bearing mutations at conserved residues allows assessment of the contribution made by these conserved residues to inhibitor binding. This approach is thus complementary to mapping the interactions of these inhibitors with drug-resistant PR variants in which non-conserved residues are mutated.
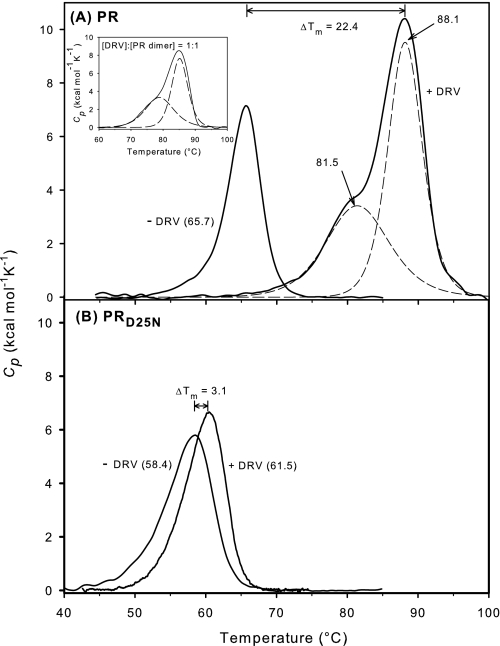
On the basis of our observations with RPB, we had anticipated that DRV would likely have a much greater effect on the stability of the folded PR dimer than that produced by the less tightly bound RPB. The effect of a ∼2-fold molar excess of DRV on the Tm of PR confirmed this expectation. At a PR:DRV ratio of 1:2 (Fig. 8A) the melting curve for PR·DRV exhibits a shoulder corresponding to Tm ∼82 °C and a major transition at 88.1 °C, with an area ratio of ∼40/60. Successive scans of the same solution showed that the unfolding of PR·DRV was not reversible under our experimental conditions. The biphasic appearance of the thermogram was independent of the scan rate at 60 and 90 °C/h, with both Tm values slightly lower (∼1 °C) at the slower scan rate. Because the concentration of DRV (28 μm) far exceeds that required for saturation of the enzyme (KL = 4.5 pm) (38), the biphasic transition cannot be ascribed to an increase in the saturation level of the remaining enzyme and consequent increase in its Tm as the inhibitor-bound enzyme unfolds and releases free inhibitor into solution (43, 44). The two transitions could result either from the two orientations of DRV in the PR active site that have been observed in the crystal structure (31), or from occupancy of the reported second binding site to give a 2:1 complex of DRV:PR (45). If we assume that binding at the second site is of lower affinity than binding in the active site, we anticipated that in the presence of a DRV:PR ratio of 1:1, all of the inhibitor should be bound at the active site, and no inhibitor would remain to form the 2:1 complex; thus the shoulder should be absent or markedly diminished if binding at a second site were responsible for the two transitions observed at the 2:1 ratio of DRV to PR. A DSC scan in the presence of ∼1 mol DRV per mol of PR (inset, Fig. 8) exhibited a slight decrease in the Tm for both transitions, as expected (44), because of the lower concentration of DRV; however, both transitions (Tm ∼ 79 and 85.2 °C) were still present in essentially the same proportion (43/56) as observed with the 2:1 inhibitor ratio. This result is most consistent with the conclusion that these two transitions are likely to be related to the two orientations of DRV in the active site.
FIGURE 8.
Effect of DRV on the thermal transitions of PR (A) and PRD25N (B). Data for comparison in the absence of inhibitor are from Fig. 2; all data are normalized to protease dimer concentration. Protease dimer concentrations in the presence of DRV (28 μm total) were 14 μm. The inset shows a thermogram for PR·DRV (1:1) measured under the same conditions at a total DRV concentration of 13 μm, with Tm values of 78.9 and 85.2 °C. The broken lines show the deconvolution (using MicroCal's version of Origin software) of the two apparent transitions observed for PR in the presence of DRV.
In remarkable contrast to PR, however, the Tm for PRD25N in the presence of the same ∼2-fold molar excess of DRV was minimally affected (Fig. 8B) and suggests that DRV binds only very weakly to PRD25N. The ΔTm of 3.1 °C induced by DRV is even smaller than that observed with RPB (6.2 °C, cf. Fig. 2B). Weak binding of DRV to PRD25N was confirmed by ITC (Fig. 9), which gave a KL of 3.2 μm at pH 5 and 28 °C, a value roughly 10-fold greater than the corresponding KL of ∼0.3 μm for PRD25N·RPB and almost 6 orders of magnitude greater than KL for PR·DRV (38). Unlike RPB, the dramatic difference in binding affinity of DRV to PR relative to PRD25N results from large favorable differences in both entropy and enthalpy (Table 1). These large differences in binding affinity and energetics are accompanied by only minor effects on the protein conformation surrounding the active site. We conclude that a major contribution to the tight binding of DRV is made by specific interactions mediated by the catalytic Asp residues. Other tight binding inhibitors contain a similar central motif consisting of a hydroxyl group that can hydrogen bond to the catalytic Asp-25, flanked by two hydrophobic moieties at the P1 and P1′ positions (indicated by the black arrow in Figs. 5A and 6A (42, 46)). These central hydroxyl-containing motifs generally align well with each other upon superimposition of crystal structures of their PR complexes. Thus we predict that the strength of the interaction with the catalytic Asp pair of PR and its contribution to the overall binding affinity should be relatively invariant for these inhibitors.
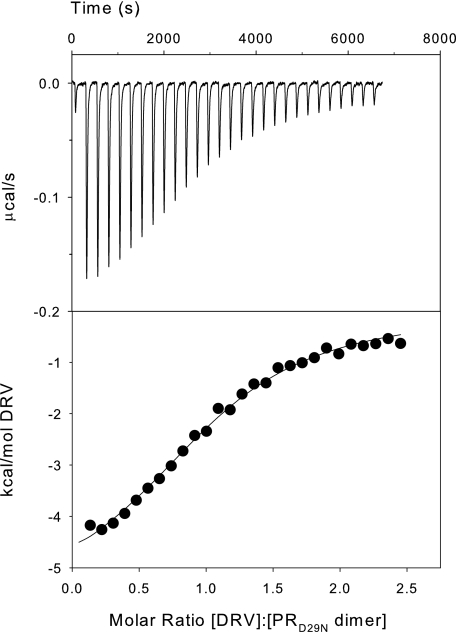
FIGURE 9.
ITC of PRD25N with DRV in 50 mm sodium acetate buffer, pH 5, at 28 °C. Upper panel, thermal changes on addition of 3-(first injection) and 5-μl (subsequent injections) aliquots of 266 μm DRV solution to 11 μm PRD25N dimer in the calorimetric cell (∼1.43 ml). Curve fitting of the integrated data (lower panel) gave a dissociation constant, KL, for DRV of ∼3.2 μm (reciprocal of Ka = 3.17 × 105 m-1; see Table 1).
Concluding Remarks—The D25N mutation lowers the Kd and Tm of PR by >100-fold and by 7.3 °C, respectively, whereas completely abolishing the dimer interface in the mutant PR5-95 lowers the Tm by 10 °C. NMR studies of the inhibitor-free PR and PRD25N suggest that the principal difference between these two proteins resides in the P1 loop and flap regions, which are known to be relatively mobile. These results indicate that the active site Asp residues make a large and specific contribution to the stabilization of the monomer fold and dimerization.
The D25N mutation produces a striking change in the interaction between the protease and the clinical inhibitor DRV, as demonstrated by the a minimal increase (3 °C) in the Tm of PRD25N·DRV as compared with a 22° increase in the Tm of PR·DRV under the same conditions, as well as the ∼106-fold less favorable binding constant for DRV to PRD25N as compared with PR. However, the high resolution structural data described here indicate only subtle differences between the active site and dimer-interface geometry of PR and PRD25N when bound to inhibitors. Thus, the critical role of Asp-25 in maintaining the dimer stability when bound to DRV appears not to be primarily steric in origin and most likely is related to an interaction of the OH of DRV with a carboxyl group that is lost on substitution with an amide.
The use of DSC and ITC to screen potential inhibitors with PRD25N or other constructs mutated at residues required for enzymatic activity is complementary to current approaches that utilize catalytically active, drug-resistant mutants. We suggest that this approach will provide a useful and hitherto unexploited tool to identify leads for inhibitors that specifically target highly conserved, catalytically and/or structurally critical residues, and are thus most likely to retain pharmacological activity against drug-resistant strains. Furthermore, the finding that D25N mutation destabilizes the dimer with a >100-fold increase in Kd may prove to be useful in screening lead compounds that block dimerization under conditions that permit observation by physical methods of the dimer-monomer equilibrium at a more convenient protein concentrations, i.e. well above the low nanomolar range exhibited by PR.
Supplementary Material
Acknowledgments
We thank D. A. Torchia, A. Y. Kovalevsky, and R. W. Harrison for helpful discussions, F. Delaglio and D. Garrett for data processing software, A. Aniana for expert technical assistance, Y.-F. Wang for help with supplemental Fig. S2, and A. Ghosh for generously providing DRV prior to its availability from the NIH AIDS research and reference reagent program, Division of AIDS, NIAID, NIH (Reagent 11447 from Tibotec Pharmaceuticals).
The atomic coordinates and structure factors (codes 3BVA and 3BVB) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was authored, in whole or in part, by National Institutes of Health staff. This work was supported, in whole or in part, by the Intramural Research Program of the NIDDK, National Institutes of Health (NIH), and NIH Grant GM62920. This work was also supported by the Molecular Basis of Disease Program, the Georgia Research Alliance, and the Georgia Cancer Coalition. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S4 and Table S1.
Footnotes
The abbreviations used are: HIV-1, human immunodeficiency virus type 1; PR, mature HIV-1 protease; r.m.s.d., root mean square deviation; Nle, norleucine; DRV, darunavir; RPB, Ac-Thr-Ile-Nle-r-Nle-Gln-Arg-NH2 non-hydrolyzable substrate analogue inhibitor with a reduced peptide bond indicated by r (carbonyl replaced by CH2); substrate IV, Lys-Ala-Arg-Val-Nle-(4-nitrophenylalanine)-Glu-Ala-Nle-NH2; ITC, isothermal titration calorimetry; DSC, differential scanning calorimetry; TFR, transframe region consisting of the N-terminal transframe octapeptide followed by the 48-amino acid p6pol. The notation used for equilibrium constants is as follows: Kd, dimer dissociation constants of dimeric protease constructs; Ka, binding (association) constants for ligands with dimeric protease constructs; KL, ligand dissociation constants defined as 1/Ka.
HIV Drug Resistance Database: hivdb.stanford.edu/index.html.
References
- 1.Oroszlan, S., and Luftig, R. B. (1990) Curr. Top. Microbiol. Immunol. 157 153-185 [DOI] [PubMed] [Google Scholar]
- 2.Kohl, N. E., Emini, E. A., Schleif, W. A., Davis, L. J., Heimbach, J. C., Dixon, R. A., Scolnick, E. M., and Sigal, I. S. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 4686-4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis, J. M., Weber, I. T., Tozser, J., Clore, G. M., and Gronenborn, A. M. (2000) Adv. Pharmacol. 49 111-146 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan, A. H., Manchester, M., and Swanstrom, R. (1994) J. Virol. 68 6782-6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan, A. H., Zack, J. A., Knigge, M., Paul, D. A., Kempf, D. J., Norbeck, D. W., and Swanstrom, R. (1993) J. Virol. 67 4050-4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karacostas, V., Wolffe, E. J., Nagashima, K., Gonda, M. A., and Moss, B. (1993) Virology 193 661-671 [DOI] [PubMed] [Google Scholar]
- 7.Krausslich, H. G. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 3213-3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson, J. W., and Burt, S. K. (1996) Annu. Rev. Pharmacol. Toxicol. 36 545-571 [DOI] [PubMed] [Google Scholar]
- 9.Weber, I. T., Kovalevsky, A. Y., and Harrison, R. W. (2007) Frontiers Drug Design Discov. 3 45-62 [Google Scholar]
- 10.Louis, J. M., Ishima, R., Torchia, D. A., and Weber, I. T. (2007) Adv. Pharmacol. 55 261-298 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Barrios, F., and Gago, F. (2004) Curr. Top. Med. Chem. 4 991-1007 [DOI] [PubMed] [Google Scholar]
- 12.Temesgen, Z., Warnke, D., and Kasten, M. J. (2006) Expert. Opin. Pharmacother. 7 1541-1554 [DOI] [PubMed] [Google Scholar]
- 13.Pearl, L. H., and Taylor, W. R. (1987) Nature 329 351-354 [DOI] [PubMed] [Google Scholar]
- 14.Wlodawer, A., and Erickson, J. (1993) Annu. Rev. Biochem. 62 543-585 [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez, E. J., Angeles, T. S., and Meek, T. D. (1993) Biochemistry 32 12380-12385 [DOI] [PubMed] [Google Scholar]
- 16.Wlodawer, A., Miller, M., Jaskolski, M., Sathyanarayana, B. K., Baldwin, E., Weber, I. T., Selk, L. M., Clawson, L., Schneider, J., and Kent, S. B. (1989) Science 245 616-621 [DOI] [PubMed] [Google Scholar]
- 17.Babe, L. M., Rose, J., and Craik, C. S. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 10069-10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabu-Jeyabalan, M., Nalivaika, E., and Schiffer, C. A. (2000) J. Mol. Biol. 301 1207-1220 [DOI] [PubMed] [Google Scholar]
- 19.Sluis-Cremer, N., and Tachedjian, G. (2002) Eur. J. Biochem. 269 5103-5111 [DOI] [PubMed] [Google Scholar]
- 20.Weber, I. T. (1990) J. Biol. Chem. 265 10492-10496 [PubMed] [Google Scholar]
- 21.Katoh, E., Louis, J. M., Yamazaki, T., Gronenborn, A. M., Torchia, D. A., and Ishima, R. (2003) Protein Sci. 12 1376-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis, J. M., Clore, G. M., and Gronenborn, A. M. (1999) Nat. Struct. Biol. 6 868-875 [DOI] [PubMed] [Google Scholar]
- 23.Ishima, R., Torchia, D. A., and Louis, J. M. (2007) J. Biol. Chem. 282 17190-17199 [DOI] [PubMed] [Google Scholar]
- 24.Ishima, R., Torchia, D. A., Lynch, S. M., Gronenborn, A. M., and Louis, J. M. (2003) J. Biol. Chem. 278 43311-43319 [DOI] [PubMed] [Google Scholar]
- 25.Ishima, R., Louis, J. M., and Torchia, D. A. (2001) J. Mol. Biol. 305 515-521 [DOI] [PubMed] [Google Scholar]
- 26.Freedberg, D. I., Ishima, R., Jacob, J., Wang, Y. X., Kustanovich, I., Louis, J. M., and Torchia, D. A. (2002) Protein Sci. 11 221-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 28.Navaza, J. (1994) Acta Crystallogr. Sect. D Biol. Crystallogr. 50 157-163 [Google Scholar]
- 29.Sheldrick, G. M., and Schneider, T. R. (1997) Methods Enzymol. 277 319-343 [PubMed] [Google Scholar]
- 30.Jones, T. A., Zou, J. Y., Cowan, S. W., and Kjeldgaard, M. (1991) Acta Crystallogr. Sect. A 47 110-119 [DOI] [PubMed] [Google Scholar]
- 31.Tie, Y., Boross, P. I., Wang, Y. F., Gaddis, L., Hussain, A. K., Leshchenko, S., Ghosh, A. K., Louis, J. M., Harrison, R. W., and Weber, I. T. (2004) J. Mol. Biol. 338 341-352 [DOI] [PubMed] [Google Scholar]
- 32.Ishima, R., Ghirlando, R., Tozser, J., Gronenborn, A. M., Torchia, D. A., and Louis, J. M. (2001) J. Biol. Chem. 276 49110-49116 [DOI] [PubMed] [Google Scholar]
- 33.Xie, D., Gulnik, S., Gustchina, E., Yu, B., Shao, W., Qoronfleh, W., Nathan, A., and Erickson, J. W. (1999) Protein Sci. 8 1702-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beissinger, M., Paulus, C., Bayer, P., Wolf, H., Rosch, P., and Wagner, R. (1996) Eur. J. Biochem. 237 383-392 [DOI] [PubMed] [Google Scholar]
- 35.Wondrak, E. M., and Louis, J. M. (1996) Biochemistry 35 12957-12962 [DOI] [PubMed] [Google Scholar]
- 36.Tie, Y., Boross, P. I., Wang, Y. F., Gaddis, L., Liu, F., Chen, X., Tozser, J., Harrison, R. W., and Weber, I. T. (2005) FEBS J. 272 5265-5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, F., Boross, P. I., Wang, Y. F., Tozser, J., Louis, J. M., Harrison, R. W., and Weber, I. T. (2005) J. Mol. Biol. 354 789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King, N. M., Prabu-Jeyabalan, M., Nalivaika, E. A., Wigerinck, P., de Bethune, M. P., and Schiffer, C. A. (2004) J. Virol. 78 12012-12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, F., Kovalevsky, A. Y., Louis, J. M., Boross, P. I., Wang, Y. F., Harrison, R. W., and Weber, I. T. (2006) J. Mol. Biol. 358 1191-1199 [DOI] [PubMed] [Google Scholar]
- 40.Mahalingam, B., Wang, Y. F., Boross, P. I., Tozser, J., Louis, J. M., Harrison, R. W., and Weber, I. T. (2004) Eur. J. Biochem. 271 1516-1524 [DOI] [PubMed] [Google Scholar]
- 41.Strisovsky, K., Tessmer, U., Langner, J., Konvalinka, J., and Krausslich, H. G. (2000) Protein Sci. 9 1631-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klei, H. E., Kish, K., Lin, P. F., Guo, Q., Friborg, J., Rose, R. E., Zhang, Y., Goldfarb, V., Langley, D. R., Wittekind, M., and Sheriff, S. (2007) J. Virol. 81 9525-9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrake, A., and Ross, P. D. (1992) Biopolymers 32 925-940 [DOI] [PubMed] [Google Scholar]
- 44.Brandts, J. F., and Lin, L. N. (1990) Biochemistry 29 6927-6940 [DOI] [PubMed] [Google Scholar]
- 45.Kovalevsky, A. Y., Liu, F., Leshchenko, S., Ghosh, A. K., Louis, J. M., Harrison, R. H., and Weber, I. T. (2006) J. Mol. Biol. 363 161-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoll, V., Qin, W., Stewart, K. D., Jakob, C., Park, C., Walter, K., Simmer, R. L., Helfrich, R., Bussiere, D., Kao, J., Kempf, D., Sham, H. L., and Norbeck, D. W. (2002) Bioorg. Med. Chem. 10 2803-2806 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.