Abstract
The interactions between brood parasitic birds and their host species provide one of the best model systems for coevolution. Despite being intensively studied, the parasite–host system provides ample opportunities to test new predictions from both coevolutionary theory as well as life-history theory in general. I identify four main areas that might be especially fruitful: cuckoo female gentes as alternative reproductive strategies, non-random and nonlinear risks of brood parasitism for host individuals, host parental quality and targeted brood parasitism, and differences and similarities between predation risk and parasitism risk. Rather than being a rare and intriguing system to study coevolutionary processes, I believe that avian brood parasites and their hosts are much more important as extreme cases in the evolution of life-history strategies. They provide unique examples of trade-offs and situations where constraints are either completely removed or particularly severe.
Keywords: brood parasitism, evolutionary equilibrium, evolutionary lag, fitness, nest-site selection, selection pressure
1. The quintessential cheat
The interactions between avian brood parasites and their hosts provide a remarkable diversity of sophisticated adaptations, but sometimes there also seems to be a surprising lack of adaptations (Davies 1999). The Common Cuckoo Cuculus canorus can be regarded as the quintessential brood parasite, laying an egg into the nest of a host species, commonly a small passerine, which subsequently acts as a foster parent and incubates and feeds the young cuckoo, even if it is six times as heavy as the host parent. Aristotle had already observed 2300 years ago that the Common Cuckoo is a brood parasite and that it reduces the breeding success of the host species to zero because the young cuckoo evicts all other eggs and chicks from the nest (Hett 1936). Egg eviction as an adaptation of the young cuckoo chick to monopolize all parental care was rediscovered by Edward Jenner in the eighteenth century (Jenner 1788), and he published his findings in this journal. At the same time, Gilbert White started to think why the cuckoo was a brood parasite, when other closely related species showed the normal reproductive strategy of nest building, egg laying and incubation, followed by chick rearing (White 1789). Darwin (1859) was the first to propose that the cuckoo's parasitic behaviour evolved from an ancestor with parental care. He knew that species closely related to the Common Cuckoo build a nest and raise their own chicks, and until today the degree of variation in parental care within the family of cuckoos is believed to be unmatched by any other bird family (Payne 2005a). Cuckoos are not the only birds that fool other species into feeding their chicks: among the 10 000 or so bird species currently recognized, around 100 are obligate brood parasites, distributed unevenly among five bird families (Winfree 1999; Davies 2000).
Because there are already excellent recent reviews on both cuckoos and cowbirds (Ortega 1998; Rothstein & Robinson 1998; Payne 2005a), I will focus more on recent discoveries and point out potential future areas of research. In doing so, I will also cover studies on brood parasitic birds beyond cuckoos and cowbirds if the findings elucidate general aspects of host–parasite coevolution.
2. The evolution of interspecific brood parasitism
On current evidence, interspecific brood parasitism has evolved independently seven times in birds (Sorenson & Payne 2002, 2005): three times among cuckoos (family Cuculidae), two times among songbirds, namely in the cowbirds (genus Molothrus, family Icteridae) and African brood parasitic finches (family Viduidae), once among the honeyguides (family Indicatoridae) and once among waterfowl (Black-headed Duck Heteronetta atricapilla). Interestingly, interspecific brood parasitism evolved only once in precocial birds, although they show a much higher occurrence of intraspecific brood parasitism, so that costs and benefits of interspecific brood parasitism are lower in precocial birds. This may also imply that the benefits of brood parasitism are much higher in altricial birds where the costs of reproduction are much higher (Lyon & Eadie 1991). In five instances, the evolution of interspecific brood parasitism is considered to be relatively old (the three instances in the cuckoos, one in the parasitic finches and one in the honeyguides: greater than 10 million years (Myr); Davies 2000; Sorenson & Payne 2002) whereas the evolution of brood parasitism in the cowbirds and the Black-headed Duck is considered to be rather recent (less than 5 Myr, Sorenson & Payne 2002). This differs from subsequent radiation of brood parasitic taxa as Sorenson et al. (2003, 2004) found that the extant parasitic finch species are less than 5 Myr old, the radiation being most probably the result of colonization of new hosts rather than cospeciation.
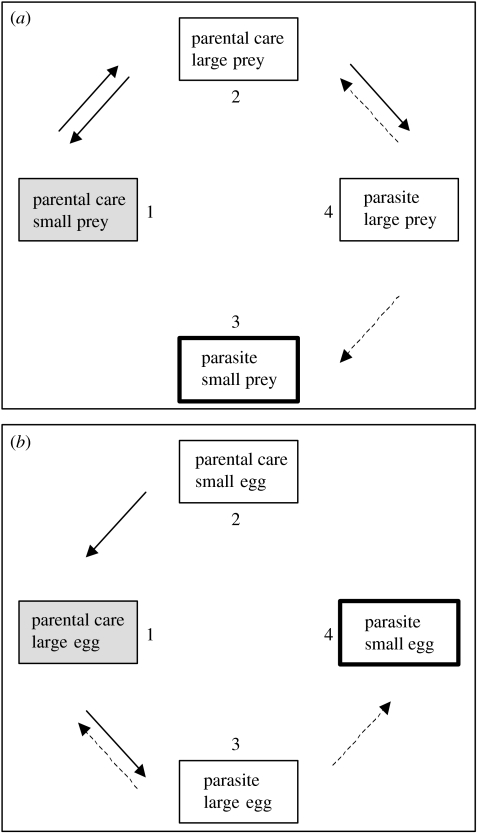
What are the changes that occurred in ecology and life history when brood parasites evolved brood parasitism from an ancestor with parental care? Did these changes precede the evolution of brood parasitism or were they consequences? These questions have been tackled in comparative analyses. Krüger & Davies (2002) explained large amounts of variation of cuckoo reproductive strategies and showed that the transition from parental care to brood parasitism was accompanied by an increase in migratory behaviour and breeding range size, but a decrease in egg size and a shift in diet towards smaller prey items. Using a maximum-likelihood approach (Pagel 1994), it was possible to construct the most likely evolutionary pathway between the presumed ancestral state and that displayed by modern brood parasitic species (figure 1). With the exception of egg size, changes in ecology were more likely to precede the evolution of brood parasitism than to result from it. Hence, the evolution of brood parasitism in cuckoos is more likely to be a later adaptation, possibly to reduce the cost of reproduction, whereas the reduction of egg size is a direct adaptation in the coevolutionary interaction with the host species. Similar to this, Mermoz & Ornelas (2004) found that parasitic cowbirds had increased egg thickness compared to non-parasitic species but did not differ in any other life-history trait they examined.
Figure 1.
Flow diagram of the most likely evolutionary pathways between cuckoo breeding strategies and prey size (a) and egg size (b). The presumed ancestral state is shaded in light grey, whereas the common current state in parasitic cuckoos is the boldly lined box. Solid arrows represent significant evolutionary pathways (p<0.05) and dashed arrows represent trends (p<0.1). Modified after Krüger & Davies (2002) and based on the phylogeny of Aragon et al. (1999).
3. Adaptations and counter-adaptations
(a) Before egg laying
There is ample experimental evidence that brood parasites and host adaptations coevolve (Rothstein 1975, 1990; Brooke & Davies 1988; Davies & Brooke 1989a,b; Moksnes et al. 1991a,b; Soler et al. 1994, 1998), but what happens before a brood parasitic egg is laid has not received that much attention.
There is now increasing evidence that individuals within and across host populations are not parasitized with equal probability (Lindholm 1999; Røskaft et al. 2002b; Hauber et al. 2004). Recent evidence from radio-tracking and habitat selection studies indicates the importance of particular habitat features for female Common Cuckoos (Nakamura & Miyazawa 1997; Honza et al. 2002; Vogl et al. 2002, 2004) and Brown-headed Cowbirds Molothrus ater (Clotfelter 1998; Jensen & Cully 2005). In other words, parasitism is non-random. Such non-randomness has been documented for nest sites of hosts: Alvarez (1993), Øien et al. (1996), Clotfelter (1998) and Moskat & Honza (2000) found that host nests closer to potential perches such as trees and those more obvious to a human observer were more likely to be parasitized, and Hauber (2001) showed that Phoebe Sayornis phoebe nests were more likely to be parasitized if located under eaves than under bridges. Hauber et al. (2004) showed that this non-randomness in parasitism can constrain the evolution of host defences because of the heterogeneities in costs associated with brood parasitism. All else being equal, parasitic females should select host parents of high quality. Indeed, Soler et al. (1995) found evidence that Great Spotted Cuckoos, Clamator glandarius, prefer to parasitize large nests of their Magpie Pica pica hosts, a large nest apparently indicating high parental quality. Brooker & Brooker (1996) reported that young and/or inexperienced Splendid Fairy-wrens Malurus splendens were most likely to be parasitized by the Horsfield's Bronze-cuckoo Chrysococcyx basalis, demonstrating non-randomness with regard to age and/or experience of the host. Smith et al. (1984) showed that old Song Sparrow Melospiza melodia females were more likely to be parasitized by Brown-headed Cowbirds, despite higher aggressive behaviour in these older females. The authors argue that old females advertise their nest location to invite parasitism and prevent subsequent predation by the cowbird, whereas young females do not show aggressive behaviour because they cannot afford to raise a cowbird chick on top of their own brood, hence they take a gamble. Such behaviour is what we would expect under Zahavi's handicap hypothesis: old females can afford to raise a cowbird chick and advertise this to both brood parasite and conspecifics in order to increase their mating success (Zahavi & Zahavi 1997). However, Payne & Payne (1998) could not detect an effect of host female age in Indigo Buntings Passerina cyanea on the probability of parasitism by Brown-headed Cowbirds.
This poses the question of whether this non-randomness results from parasite behaviour or defence behaviour by the host. A few studies have tried to determine what cues are used by brood parasites to locate host nests. The two commonly stated hypotheses are the nesting-exposure hypothesis and the nesting-cue hypothesis (Robertson & Norman 1976; Gill et al. 1997; Clotfelter 1998). Parasites either preferentially use conspicuous host nests or those where the hosts show conspicuous behaviour. As mentioned earlier, there is some evidence for the nesting-exposure hypothesis: Clotfelter (1998) found that parasitized nests were closer to trees than unparasitized nests. However, when Gill et al. (1997) and Clotfelter (1998) tested the nesting-cue hypothesis in Brown-headed Cowbird hosts, they did not find any support.
The key counter-adaptation of hosts before a parasitic egg is laid is aggression towards the parasitic female (Moksnes et al. 1991a; Røskaft et al. 2002a). Indeed, unsuitable host species have been shown to react less aggressively towards a Common Cuckoo dummy than suitable host species (Moksnes et al. 1991a) and hosts of Brown-headed Cowbirds show increasing aggression with increasing parasitism rate (Robertson & Norman 1976). This aggressive behaviour can be a specific response to parasitism: the response to predators can be similar (Grim 2005) or significantly different (Duckworth 1991; Davies et al. 2003). Aggression against parasites also ceases when the host young have fledged (Davies 2000, p. 58).
(b) The egg stage
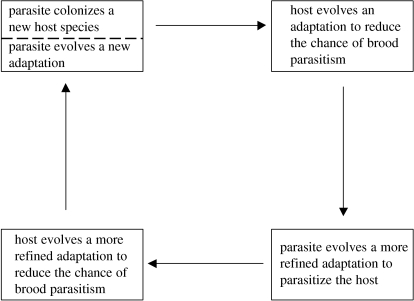
The scheme of coevolution in parasite–host systems is illustrated in figure 2, the coevolutionary stages are well illustrated by egg colouration and egg size as I shall now discuss.
Figure 2.
Scheme of the coevolutionary arms race between a brood parasite and its host.
Brood parasitism imposes costs on hosts at the egg stage: in many parasitic species the female removes a host egg when she lays (Payne 2005a), and even in those species where no host egg is removed, a reduction in host clutch size has been documented through eggs being punctured by the parasitic female (Massoni & Reboreda 1998, 2002; Hoover 2003) or cracked by the parasitic egg (Davies 2000).
Given the selection pressure on hosts to reject parasitic eggs from their nests, it comes as no surprise that a number of counter-adaptations have been documented at the egg stage. There is some evidence that hosts learn to recognize their eggs and base their rejection behaviour on this information (Victoria 1972; Lotem et al. 1992), but Amundsen et al. (2002) failed to find any evidence for learning in one host species. Some host species subsequently desert the entire nest if they are parasitized (Hill & Sealy 1994), some puncture the parasitic egg with their beak and throw it out of the nest or roll the parasitic egg out of the nest (Davies 2000; Lorenzana & Sealy 2001), and others bury the parasitic egg and their own eggs under a new layer of nest material (Sealy 1995). On a larger scale, the time of sympatry between parasite and host can explain significant amounts of variation in egg rejection behaviour, with longer times of sympatry being associated with higher rejection behaviour (Rothstein 1975; Peer & Sealy 2004).
Size also matters for parasitic eggs because host species might use differences in either colouration or size to detect a parasitic egg (Mason & Rothstein 1986; Davies & Brooke 1988). Good evidence for the importance of egg size as an adaptation to brood parasitism comes from a study on the Lesser Cuckoo Cuculus poliocephalus by Marchetti (2000). These cuckoos parasitize Tickell's Leaf Warbler Phylloscopus affinis, but not the closely related Hume's Yellow-browed Leaf Warbler Phylloscopus humei. Experiments showed that Hume's warblers rejected eggs on the basis of relative size; model eggs 75% larger in size than host eggs (but still smaller than the real cuckoo egg) were rejected. These species build domed nests and the dark environment might preclude egg colouration from being a reliable clue. The response of the host puts this cuckoo species under selection pressure to evolve a smaller egg, which was identified as a major adaptation to brood parasitism in general by Payne (1974) and Krüger & Davies (2002, 2004). Another adaptation and constraint is the composition of the parasitic egg. Kattan (1995) showed that shiny cowbird eggs have a reduced energy content to reduce incubation length. This provides the parasitic chick with a head-start but the price to pay is a lower hatchling mass. This might be a constraint to successful parasitism of larger hosts.
The evolution of a small egg could be achieved through a reduction in body size (there is a strong allometric relationship between body size and egg size in birds in general and cuckoos in particular) or through the evolution of an unusually small egg in cuckoos. Darwin (1859) commented on the small egg of the Common Cuckoo, and Payne (1974) showed that brood parasitic cuckoos lay smaller eggs than cuckoos of the same size with parental care. Krüger & Davies (2004) looked at two of the most speciose cuckoo genera, to test which mechanism explained the closer size matching of cuckoo and host eggs in the genus Chrysococcyx compared to the genus Cuculus. Chrysococcyx species are relatively small and parasitize dome-nesting host species, whereas the great majority of Cuculus species are larger and parasitize cup-nesting host species. The better size matching between parasitic and host egg in Chrysococcyx is not achieved through an unusually small egg but through reduced body size, and this might reflect a selection pressure to be small in order to enter or at least insert the lower abdomen more effectively into the domed nests of the hosts to lay. However, Payne (2005a) does reject the idea that body size in Chrysococcyx has anything to do with laying.
Apart from egg size, eggshell strength might be an another important trait. According to some studies, parasitic cuckoos lay eggs of higher eggshell strength and/or eggshell density than nesting cuckoos of the same body size (Brooker & Brooker 1991; Picman & Pribil 1997), while very comprehensive studies refute this (Schönwetter 1964; Payne 2005a). In general, brood parasites lay eggs that are more resistant to puncture (Picman 1989; Mikhailov 1997; Picman & Pribil 1997), which facilitates rapid laying by parasitic females and makes egg puncturing by hosts more difficult.
However, by far the best-studied adaptation of brood parasites to egg rejection of hosts is egg mimicry (Davies 2000; Grim 2005; Payne 2005a). In some brood parasites such as honeyguides and parasitic finches, similarities between parasitic egg and host egg are best explained by common ancestry rather than coevolution (Davies 2000), but the selection pressure that hosts impose on brood parasites to lay mimetic eggs has been recognized for almost a century (Baker 1913) and there is good evidence that hosts are more likely to accept mimetic eggs and to reject non-mimetic ones (Brooke & Davies 1988; Davies & Brooke 1988; Lotem et al. 1995; Stokke et al. 1999; Lahti & Lahti 2002). While the generalist brood parasitic cowbird species do not exhibit egg mimicry for the great majority of their hosts, many cuckoo species lay mimetic eggs, sometimes indistinguishable from the host egg for the human eye (Langmore et al. 2003) and even a good match for the host egg in the UV spectrum (Cherry & Bennett 2001; Langmore et al. 2003). The significance of the UV mimicry is currently not clear. Aviles et al. (2006) found no higher rejection rate by magpies when the UV spectrum was eliminated from Great Spotted Cuckoo eggs. But the coevolutionary process does not necessarily stop there. Brood parasites using more than one host species have been shown to evolve host-specific egg morphs (females laying a particular egg morph being often referred to as a gens), with genes coding for the egg pattern being most likely located on the female-specific W-chromosome (Gibbs et al. 2000). Hosts, meanwhile, have evolved larger inter-clutch variation but smaller intra-clutch variation (Stokke et al. 1999, 2002) which renders the evolution of egg mimicry much more difficult and might select for the evolution of egg polymorphism in the host (Takasu 2003).
As astonishing as the variety of adaptations and counter-adaptations at the egg stage is the apparent lack of adaptations in some parasite–host systems. Although it is estimated from historical records that the Common Cuckoo has parasitized Dunnocks Prunella modularis for at least 600 years (Davies & Brooke 1989a), they never reject an egg, even one that looks completely different from their own, despite the obvious fitness cost of brood parasitism. Similar situations have been documented for the Red-chested Cuckoo Cuculus solitarius, the Jacobin Cuckoo Clamator jacobinus and many cowbird hosts, despite large differences in both colouration and size (Liversidge 1970; Ortega 1998; Davies 2000). If parasite–host systems represent the coevolutionary process, these situations need an explanation. Under which circumstances could it not be the best option to reject a parasitic egg? Even deserting the brood leads to another chance to breed (Grim et al. 2003, Langmore et al. 2003), either in the current or next season, whereas raising a cuckoo chick not only reduces the reproductive success of the parents to zero, but might also reduce the chance of future reproductive success through reduced survival (but see Payne & Payne 1998). There is good evidence that rejection of parasitic eggs carries a twofold cost. First, hosts commonly damage their own eggs in the process of removing a cuckoo egg (Davies & Brooke 1988; Marchetti 1992) and secondly, they very occasionally make recognition errors and eject one or more of their own eggs from the clutch, whether they have been parasitized or not (Davies & Brooke 1988; Marchetti 1992; Lotem et al. 1995; Sealy 1995). An intriguing third cost of rejection has been suggested by Zahavi (1979): where the hosts reject a parasitic egg, and the parasite destroys the nest in retaliation. However, the studies by Soler et al. (1995, 1999) on Great Spotted Cuckoos remain the only evidence to date for such an avian Mafia and the significance of these studies has been questioned (Payne 2005a).
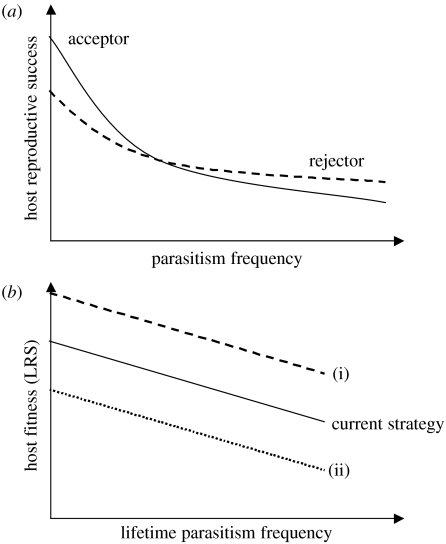
Given that both egg rejection and acceptance have costs and benefits, an optimality approach can be applied to work out a threshold frequency of brood parasitism above which egg rejection results in higher reproductive success and below which egg acceptance is favoured. Such an approach was used by Davies et al. (1996) and they showed a threshold frequency of 19% when Common Cuckoos parasitized Reed Warblers Acrocephalus scirpaceus at one site. The general approach is illustrated in figure 3 and can also be used to look at the population dynamics of a parasite–host system (Takasu et al. 1993).
Figure 3.
(a) Relationship between parasitism frequency and host reproductive success for acceptor and rejector strategies. The model assumes that there are costs of acceptance and also costs of rejecting a parasitic egg. In the absence of brood parasitism, acceptors have the highest reproductive success but at high parasitism frequencies the costs of acceptance are higher than the costs of rejecting and rejectors have a higher reproductive success. (b) Schematic relationship between lifetime parasitism frequency and host fitness. The current strategy defines a given degree of egg rejection, which can vary from 0 to 1. If a new strategy would have higher fitness (i), this would point towards evolutionary lag in the current strategy but if a new strategy would have lower fitness (ii), this would point towards evolutionary equilibrium in the current strategy. Top panel (a) modified after Takasu (1998) and Winfree (1999).
(c) The chick stage
Even before the parasitic chick hatches, adaptations increase its competitive ability. Rapid embryonic development has been observed in both cuckoos and cowbird species (Friedmann 1929; Liversidge 1961), and McMaster & Sealy (1998) showed that eggs of the Brown-headed Cowbird achieve a shorter incubation period than hosts eggs due to more efficient incubation by the host and worse incubation of the host eggs. Incubation periods shorter than those of hosts are also known for other parasites, such as parasitic finches (Payne 2005a).
One constraint that parasitic chicks often face is how to hatch from an egg that has a thick or particularly hard shell (Payne 2005a). Honza et al. (2001) showed that Common Cuckoo chicks need more time and effort to crack their egg shell compared to host chicks.
Soon after hatching, parasitic chicks of most cuckoo species and the honeyguides eliminate competition with host chicks through eviction or killing (Davies 2000; Payne 2005a). In contrast, chicks of a few cuckoo species, cowbirds and parasitic finches are commonly raised together with the host chicks. In these species, adaptations have enabled the parasitic chick to compete successfully with the host chicks. There is good evidence that both Brown-headed Cowbird and Great Spotted Cuckoo chicks are preferentially fed by their host parents (Redondo 1993; Dearborn 1998). This preferential treatment can be achieved through the parasitic chick being larger (Liversidge 1970; Dearborn 1998), providing extra stimuli that facilitate preferential treatment (Soler et al. 1995, but see Lichtenstein & Sealy 1998 for a different result) or by begging selfishly and dishonestly (Lichtenstein 2001, but see Hauber & Ramsey 2003 for a case of honest begging). The question arises as to why, given the absence of kin selection benefits between parasitic and host chicks, parasites in these species do not obligately outcompete host chicks. Kilner et al. (2004) provided a neat explanation for this in Brown-headed Cowbirds: chicks raised together with average-sized host chicks grew faster than chicks raised without nest-mates, hence parasitic chicks might use host chicks to procure resources. This discovery, however, raises the question how parasitic chicks of evicting species receive enough parental care from their host parents. Davies et al. (1998) and Kilner et al. (1999) showed that a single Common Cuckoo chick has unusually rapid begging calls that sound like many Reed Warbler chicks, and that this supernormal vocal stimulus might compensate for a subnormal visual stimulus of one gape. This ensures that the parasitic chick gets sufficient food, and similar observations have been made in chicks of honeyguides (Fry 1974). Selfish or exaggerated begging might come at a cost, and indeed Dearborn (1999) reported that begging calls at Indigo Bunting nests parasitized by Brown-headed Cowbirds were louder than at unparasitized nests and that parasitized nests had a higher predation rate, which has also been observed in parasitic finches (Payne 2005a). Recent evidence suggests that begging calls and especially the response to parental alarm calls can be host specific and differ between gentes of Common Cuckoos (Butchart et al. 2003; Davies et al. 2006), hence fine-tuning into host communication systems might be even more elaborate than previously realized.
Interestingly, despite subtle adjustments of begging behaviour, visual chick mimicry is essentially absent in brood parasites with the exception of the Screaming Cowbird Molothrus rufoaxillaris and the parasitic finches (Davies 2000; Payne 2005b). In the parasitic finches, the elaborate and colourful mouth markings in the hosts are mimicked by the parasitic chick in great detail. While common ancestry is thought to be the starting point for the mimicry, the host specificity of the mouth marking can only be explained through subsequent parasite–host coevolution (Sorenson & Payne 2002). But chick mimicry does not completely preclude colonization of new host species (Payne et al. 2001): there are hosts with non-mimetic parasitic chicks.
A previously unknown adaptation in a cuckoo chick comes from a study of the Horsfield's Hawk-cuckoo Hierococcyx hyperythrus in Japan. Tanaka & Ueda (2005) discovered that, to manipulate its foster parents, the young cuckoo has a vivid yellow skin patch on each wing-bend that matches its own gape colour. The young cuckoo flashes these yellow skin patches at the host parent to stimulate feeding, and indeed host parents sometimes try to feed the wing patch. Experimentally hiding this stimulus by paint reduced the feeding rate by the host, demonstrating the adaptive value of the skin patch.
Hosts are not completely defenceless at the chick stage. An example of how hosts can retaliate against a parasitic chick comes from Australia, where Horsfield's Bronze-cuckoo parasitizes Superb Fairy-wrens Malurus cyaneus. The cuckoo lays a highly mimetic egg and hosts accept it. However, hosts deserted 11 out of 29 parasitic chicks, sentencing them to death through starvation or cold (Langmore et al. 2003). This is partly a response to parasitic chicks because single host chicks were abandoned at a lower rate. This behaviour shifts the arms race to the chick stage, but the mechanism behind chick recognition is unclear, though Langmore et al. (2003) found that the chicks of the Shining Bronze-cuckoo were always rejected and did not mimic the begging calls of the fairy-wren chicks. While hosts can learn the appearance of the eggs through imprinting on their first clutch (Lotem et al. 1995), imprinting on the first chick has been proposed to be highly maladaptive. If parasitized in their first breeding attempt, parents would then reject all their subsequent young (Lotem 1993). However, Langmore et al. (2003) did not find evidence for this: parents that accepted a parasitic chick did not abandon their own chicks in subsequent breeding attempts. A potential solution could still be imprinting, but sibling imprinting (McLean & Maloney 1998). This means that the young chick imprints on its sibling and hence learns what its future chicks will look like. In all cuckoo–host systems, this mechanism would enable chick recognition to evolve. For those systems where the young cuckoo ejects or kills the host young, a host chick cannot imprint on a cuckoo ‘sibling’ because it is gone before imprinting could take place. Hence, false imprinting as envisaged by Lotem (1993) could not occur. Even in cuckoo–host systems where the young cuckoo does not eject, it would be adaptive as imprinting on the first clutch of eggs is adaptive. If parasitized, the defence mechanism would be ineffective but there would not be other costs. It would be interesting to test the Superb Fairy-wrens on this: one would simply have to change the appearance of siblings in the nest and examine whether recruiting individuals reject their normal chicks. Another possible means of defence against parasitism at the chick stage has been suggested by Grim et al. (2003) who found that, in some cases, Reed Warbler parents stopped feeding a Common Cuckoo chick after the cuckoo needed more food than an entire unparasitized brood. If parents were using the amount of parental care required as a discriminating mechanism, no learning or imprinting would need to be invoked.
Finally, the choice of a host species also affects offspring quality in brood parasites, as has been demonstrated by Kleven et al. (1999). Common Cuckoos of the Reed Warbler gens also parasitize the great Reed Warbler Acrocephalus arundinaceus at a study site in the Czech Republic, and cuckoo chicks raised by great Reed Warblers grew at a faster rate and fledged significantly heavier than cuckoo chicks raised by Reed Warblers. However, Kilpatrick (2002) found no growth differences in Brown-headed Cowbird chicks raised by different hosts. In a further study, Kleven et al. (2004) showed that both the hatching success of cuckoo eggs and the fledging success differed significantly among four sympatric Acrocephalus warbler hosts, with the probability of fledging being twice as high in great Reed Warbler nests when compared to Reed Warbler nests.
Apart from the quality of parental care received, the host species in which parasites are raised has important repercussions for the adult parasite. In the parasitic finches, imprinting on host song is crucial for parasitic males' later mating success (Payne et al. 2000). While cuckoo species must have an innate basis for the development of their song (Davies 2000), habitat imprinting has been suggested to be of major importance for finding suitable habitats and hosts in Common Cuckoos (Teuschl et al. 1998).
4. Coevolutionary arms races: ongoing struggle or stalemate?
Two main hypotheses have been well established which try to explain the evolutionary state of parasite–host systems in general and the lack of host defences in some host species in particular (Davies & Brooke 1989b). The evolutionary lag hypothesis proposes that it would be advantageous for hosts to counteract brood parasitism but they do not, either because there has been insufficient time for the defence to spread through the host species population or because hosts might lack the genetic variation to evolve a defence against brood parasitism (Rothstein 1975). The problem with this hypothesis is that it does not make strong predictions and is very hard to falsify. The evolutionary equilibrium hypothesis proposes that the costs of brood parasitism do not always exceed the costs of rejection and, hence, under some scenarios it is adaptive for a host to accept brood parasitism.
In theory, one could differentiate between the two hypotheses by looking at the current strategy in a population (egg acceptance or egg rejection) and at the lifetime fitness payoff of an alternative strategy (figure 3). If the fitness of the alternative strategy was higher than the fitness of the current strategy, this would support the evolutionary lag hypothesis. However, if the fitness of the alternative strategy was lower than the current strategy, this would support the evolutionary equilibrium hypothesis. This is easier said than done, given that one can hardly turn an acceptor into a rejector and hence measure the lifetime cost of egg rejection. Even documenting a lack of genetic variation in a host population with regard to egg acceptance does not provide conclusive evidence for the evolutionary lag hypothesis as suggested by Winfree (1999). Under strong selection pressure for egg acceptance, this trait would be expected to spread to fixation and no genetic variation should be detectable, even if evolutionary equilibrium operates. Rejecting hosts could, however, be experimentally turned into acceptors by adding a parasitic chick to their nest (Winfree 1999) because hosts do not reject chicks (but see Langmore et al. 2003). Another, potentially more fruitful approach is to combine detailed measurements of the benefits and costs of acceptance with modelling studies to produce probability estimates for the evolution of egg rejection and hence an indirect test of the two hypotheses.
The long-term outcomes of cuckoo–host interactions can be grouped into three cases: continued exploitation of hosts that do not show any defence; oscillatory systems, where brood parasitism frequency and host defence levels fluctuate around an evolutionary equilibrium and where fitness pay-offs of egg acceptance and egg rejection are equal; and systems where the host seems to have evolved counter-adaptations that preclude successful parasitism (Davies 2000; Rothstein 2001).
The Common Cuckoo–Dunnock system would be an example of continued exploitation with no host defences. Some hosts of Brown-headed Cowbirds also do not exhibit defences despite high parasitism rates (Ortega 1998). One can imagine host populations being stable despite heavy cowbird parasitism because cowbirds do not impose such a high fitness cost, but what about those parasites where a successful parasitism event reduces host reproductive success to zero? Barabas et al. (2004) looked at some Reed Warbler populations in Hungary, where cuckoo parasitism has been extraordinarily high at 50–66% for several decades. They showed that such host populations can only be maintained in a metapopulation framework with immigration from other, less parasitized areas. But continued exploitation of hosts can have important conservation implications in rare and localized hosts (Rothstein & Robinson 1994; Arcese et al. 1996; Trine et al. 1998). The effect of parasitism by Brown-headed Cowbirds, for example, is not restricted to reducing the reproductive success of hosts, but can skew host offspring sex-ratios (Zanette et al. 2005) and affect host population growth rate significantly (Smith et al. 2002).
There is also evidence that some parasite–host systems are in a dynamic state of oscillations. For example, the Common Cuckoo–Reed Warbler system shows dynamic behaviour at least in one location. Brooke et al. (1998) documented a decline in egg rejection behaviour in line with declining levels of brood parasitism, and the decline was so rapid that it was most likely due, not to genetic changes in the host population, but to behavioural flexibility.
Some species, which might have been cuckoo hosts in the past, evolved rather watertight defences against brood parasitism (Rothstein 2001). Hume's Leaf Warblers from India studied by Marchetti (2000) showed a very high rejection rate of the larger parasitic eggs and it is unlikely that the large parasite could ever evolve an egg small enough to mimic the tiny host's egg. In Hungary, where the Red-backed Shrike Lanius collurio was parasitized by Common Cuckoos regularly until the late 1960s, no parasitism has been recorded since. Lovaszi & Moskat (2004) found that 93% of real cuckoo eggs were rejected, so it seems that the host has won. Honza et al. (2004) regard the Blackcap Sylvia atricapilla as another winner in the arms race with the Common Cuckoo. Blackcaps react very aggressively towards a cuckoo (Røskaft et al. 2002a), and throughout Europe they reject parasitic eggs with almost 100% frequency. Honza et al. (2004) also documented very low intra-clutch variation in egg appearance, but high inter-clutch variation. The large inter-clutch variation severely constrains egg mimicry, while the low intra-clutch variation permits effective egg discrimination (Øien et al. 1995). Comparative evidence supports this as an effective mechanism against brood parasitism (Stokke et al. 2002) and Lahti (2005) showed that when the selection pressure of brood parasitism is relaxed, hosts show increased intra-clutch variation and hence decreased inter-clutch variation. Under these scenarios, the cuckoo gens that parasitized a particular host species either becomes extinct or successfully switches the host species. The best evidence that brood parasites can switch hosts comes from Japan, where Common Cuckoos started parasitizing Azure-winged Magpies Cyanopica cyana only since 1956, with current parasitism rates as high as 60% (Nakamura et al. 1998).
5. Where to go from here?
(a) Cuckoo gentes as alternative reproductive strategies
Brood parasites and their hosts provide a model system for the coevolutionary process (Rothstein 1990), but they are also very interesting models for the evolution of mating and life-history strategies. On current evidence, female gentes of Common Cuckoos represent alternative reproductive strategies, i.e. they are true genetic alternatives rather than conditional tactics (Gross 1996). Further genetic data are needed to look at mating systems and scope for sexual conflict in parasitic species (see Hauber & Dearborn 2003 for a recent review on genetic studies of cuckoo mating systems). Female reproductive success would benefit from better adaptation to the particular host species, but the cross-mating of males between female gentes compromises this. Hence, this is a scenario where the benefits of polygyny to males are higher than the benefits of a better adaptation to a particular host species. This conflict between the sexes could prove to be a fruitful target for modelling efforts, first to look at the rate of cross-mating necessary by males to prevent speciation events and, secondly, to examine the dynamic game between hosts, female and male Common Cuckoos in terms of adaptation. However, there is also good evidence that monogamy among females is very widespread (Marchetti et al. 1998; Martinez et al. 1998; Alderson et al. 1999; Woolfenden et al. 2003; Strausberger & Ashley 2003). This demands an explanation since presumably males would benefit from multiple mates and females from the potential genetic benefits of mating with several males.
(b) Nonlinearity in parasite–host relationships
Within the modelling realm, more effort could be devoted to refining current models of the costs and benefits of egg rejection or acceptance. There is good evidence that the risk of being parasitized is not equal between individuals of a host population or between host populations, and neither is it equal across an individual host's lifespan (Lotem et al. 1992; Brooker & Brooker 1996; Øien et al. 1996; Grim 2002; Røskaft et al. 2002b, Hauber et al. 2004). For the sake of simplicity, these relationships are not considered in models or analyses of costs and benefits of parasite–host coevolution (Takasu et al. 1993; Brooke et al. 1998; Servedio & Lande 2003). However, the cost of brood parasitism to a host is very likely to be nonlinear over the host's lifespan. Reproductive success in birds is commonly related to age in a nonlinear fashion (Sæther 1990; Forslund & Pärt 1995; Krüger & Lindström 2001). Hence, from a lifetime reproductive success (LRS) perspective, being parasitized in the first (but see Lotem 1993) or last breeding attempt is likely to be less costly than being parasitized during the prime years. Many host species, i.e. tropical passerines or corvid hosts, are reasonably long-lived and hence this nonlinearity should be included in quantitative models of the costs of brood parasitism. This, however, also means that brood parasites should selectively parasitize prime-aged hosts. On the other hand, some temperate host species have rather low survival rates and these differences should be explained in comparative analyses.
(c) Brood parasitism and host life-history strategies
Another trait that is commonly assumed constant is parental host quality, which affects the probability of fledging of the parasitic chick. Parasitism is non-random and it would be interesting to see whether parasitic females select hosts for their parental quality and how they assess this (see Soler et al. 1995 for one example). The effect of brood parasitism on the life-history strategy of the host species could especially merit further research. For example, Hauber (2003) used a comparative approach to document that hosts of Brown-headed Cowbirds have reduced clutch sizes, which is exactly what general life-history theory predicts under increased juvenile mortality in the host species, and Soler et al. (2001) found that magpie populations living in sympatry with the Great Spotted Cuckoo have larger clutches than those living in allopatry as an adaptation against cuckoo parasitism. This has also been shown by Cunningham & Lewis (2006), who found that Giant Cowbirds Scaphidura oryzivora select for larger clutch sizes and subsequent brood reduction in Montezuma Oropendolas Psarocolius montezuma. The larger clutches offer protection from egg removal or damage by the cowbird but necessitate brood reduction if the clutch is not parasitized. These approaches could be complemented by long-term individual-based studies to look at real fitness effects of brood parasitism by measuring LRS for host individuals. Brooker & Brooker (1996) achieved this for the Splendid Fairy-wren, host of Horsfield's Bronze-cuckoo. They reported that, despite obvious costs in any one breeding attempt, the LRS of those individuals that were never parasitized was not higher than the LRS of individuals that were parasitized once or more than once. This, however, is likely to be an effect of phenotypic correlations: high quality females were able to compensate for brood parasitism, despite it being costly. In addition, they found that brood parasitism did not lower subsequent survival probability; hence, the costs were restricted to lower reproductive success (see also Payne & Payne 1998 for a similar result in a Brown-headed Cowbird host). Given the non-randomness of parasitism that they document, this correlational study should be backed-up by experimental approaches to test whether brood parasitism at a certain age of an individual can have a fitness consequence. This experimental approach could be viewed as a perturbation analysis of a matrix analysis to test whether the reduction of a matrix element (here reproductive success) has fitness consequences (Caswell 2001). More fitness data are also needed to analyse which factors in the absence of brood parasitism explain the differences in individual fitness within a host population.
(d) Begging behaviour in a true life-history context
Although much research has been devoted to begging behaviour of parasitic versus host chicks (Kilner et al. 1999; Butchart et al. 2003), much more could be done. For example, Kilner et al. (2004) recently showed that Brown-headed Cowbird chicks raised with average-sized host chicks grow faster than if they are raised alone, so that they use host chicks to procure more resources. It would be interesting to know what happens between parasitic and host chicks for the non-ejecting cuckoo species and the parasitic finches. This should include further research on the interplay between hormones and begging behaviour. While two studies have shown that brood parasites do not deposit more testosterone into their eggs than their hosts (Hauber & Pilz 2003; Torök et al. 2004), Groothuis & Ros (2005) showed that testosterone reduces begging, and so there is still ample scope to test whether hormones play a part in the superior competitive ability of non-evicting brood parasites. It might also be useful to use comparative approaches to generalize how different parasitic species exploit their hosts in terms of begging behaviour. A further aspect of begging behaviour of parasites that deserves attention is host specificity. First evidence that is emerging is that some Common Cuckoo gentes also have host-specific nestling communication strategies (Davies et al. 2006) and the evolution of this might be elucidated by looking at other cuckoo species where gentes are present.
(e) Trade-offs between parasitism and predation risk in hosts
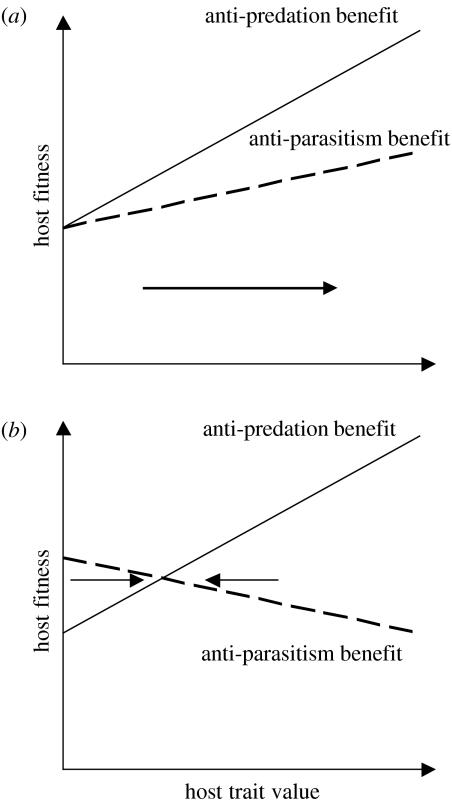
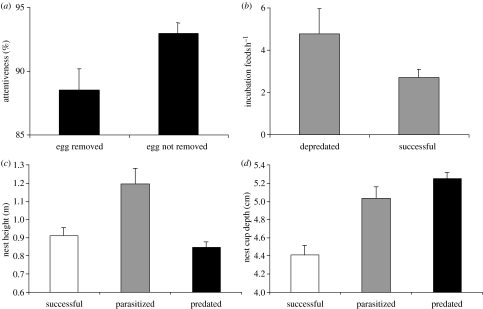
One final field that might deserve more attention could be the interactions between predation risk and parasitism risk. Brood parasitism can be viewed as a form of nest predation given that the host's reproductive success of that breeding attempt is regularly (ejecting cuckoos and the honeyguides), often (non-ejecting cuckoos, small cowbird hosts) or seldom (large cowbird hosts and parasitic finches) reduced to zero. However, brood parasites share common ground with their hosts in that they benefit if the nest is not found by a predator after they have laid an egg. Host species are under selection pressure to find nest sites and build nests in a way that minimizes the risk of both predation and parasitism simultaneously. Two scenarios are conceivable (figure 4): if a trait of a host species affects predation and parasitism risk in the same way, strong directional selection on the trait would be expected. However, if an increasing trait value decreases predation risk but increases parasitism risk, balancing selection occurs with the optimal trait value being determined by the fitness slopes for predation and parasitism and their respective frequency. Such a trade-off between the effects of parasitism and nest predation has recently been documented for a Brown-headed Cowbird host (Tewksbury et al. 2002). Increased nest attentiveness reduced the cost of brood parasitism in terms of host egg loss (figure 5a), but it necessitated more frequent nest visits, which in turn increased the predation risk (figure 5b). There is also evidence for complex trade-offs in the host–parasite system between Jacobin Cuckoo and Cape Bulbul Pycnonotus capensis (Krüger 2004). By measuring the variables describing nest-site selection and nest architecture, 70% of nests can a priori be correctly assigned into the categories successful, parasitized or predated, so that both predation and parasitism are not random events. With regard to nest height, the selection pressures from parasitism and predation are different (figure 5c), so that higher nests are much more likely to be parasitized. But with regard to a nest architecture trait such as cup depth, they are similar (figure 5d), so large and deep nests are more likely to be parasitized or predated. Hence, nest-site selection and nest architecture can influence reproductive success of the host and there are instances of stabilizing and directional selection. The overlap and difference between predation and parasitism and how they shape host reproductive success might help to gain a deeper understanding of host strategies towards brood parasitism (see Røskaft et al. 2002a,b for examples of the interactions between host behaviour, habitat structure and parasitism risk).
Figure 4.
Scheme showing the relationship between a host trait and its fitness value for both predation and parasitism risk. If a trait affects host fitness similarly under both predation and parasitism, the resulting selection pressure will be directional (a), but if predation and parasitism risk are affected in opposite directions, a balancing selection pressure is the result (b).
Figure 5.
(a) Nest attentiveness in Yellow Warbler nests parasitized by Brown-headed Cowbirds. Nests with higher attentiveness suffered a lower cost of brood parasitism because fewer host eggs were removed (p=0.018), fig. 1d from Tewksbury et al. (2002). (b) Comparison between depredated and successful Yellow Warbler nests in relation to the incubation feeding rate. A higher incubation feeding rate is associated with a higher nest predation rate (p=0.020), fig. 3d from Tewksbury et al. (2002). Differences between successful (fledging at least one host chick), parasitized and predated nests of the Cape Bulbul Pycnonotus capensis from a study in South Africa (Krüger 2004), documenting a directional selection pressure on nest architecture such as cup depth (d), but a balancing selection pressure on nest height (c). Differences in both cup depth (F2,159=23.008, p<0.001) and nest height (F2,159=9.760, p<0.001) are highly significant.
6. Conclusions
Brood parasitism and host responses continue to attract a disproportional interest from researchers because the avian parasite–host system is not only one of the best models for the coevolutionary process, but is also uniquely tractable and open to experimental manipulation.
There is overwhelming evidence for a coevolutionary arms race between brood parasites and their hosts, but it is less clear whether current outcomes of these arms races are best understood under the evolutionary lag hypothesis or under the evolutionary equilibrium hypothesis, and differentiating between the two hypotheses is extremely difficult.
Evolutionary outcomes of parasite–host interactions include continued exploitation of naive hosts, coexistence between parasite and host with dynamic behaviour of the host and fluctuating levels of brood parasitism, and finally host switch by parasites, induced by host defences that prevent continued brood parasitism. There is evidence for all the three scenarios.
Potential future directions for research might include genetic studies of the mating system and on sexual conflict between the sexes, a more comprehensive approach to understand the non-randomness of brood parasitism and the nonlinear costs of brood parasitism during the lifetime of a host individual, and further experimental work on the fitness costs of brood parasitism and the interactions between parasite chick, host chicks and host parents in non-ejecting species. In more general terms, it might be fruitful not only to treat avian parasite–host systems as a model for the coevolutionary process, but also to recognize the potential of brood parasites as models in life-history strategy evolution because many constraints are either removed completely or greatly reduced, so that their behaviour can be thought of as an extreme case which might be very elucidating.
Acknowledgements
I am grateful for comments from Mike Brooke, Nick Davies, Joah Madden, Andy Radford and Michael Sorenson which improved the paper. Graham Kerley kindly permitted research at the University of Port Elizabeth Nature Reserve. My research was funded by the Marie Curie programme of the EU, a Churchill College Junior Research Fellowship and a Royal Society Research Fellowship.
References
- Alderson G.W, Gibbs H.L, Sealy S.G. Determining the reproductive behaviour of individual brown-headed cowbirds using microsatellite DNA markers. Anim. Behav. 1999;58:895–905. doi: 10.1006/anbe.1999.1220. doi:10.1006/anbe.1999.1220 [DOI] [PubMed] [Google Scholar]
- Alvarez F. Proximity of trees facilitates parasitism by cuckoos Cuculus canorus on rufous warblers Cercotrichas galactotes. Ibis. 1993;135:331. [Google Scholar]
- Amundsen T, Brobakken P.T, Moksnes A, Røskaft E. Rejection of common cuckoo Cuculus canorus eggs in relation to female age in the bluethroat Luscinia svecica. J. Avian Biol. 2002;33:366–370. doi:10.1034/j.1600-048X.2002.02894.x [Google Scholar]
- Aragon S, Møller A.P, Soler J.J, Soler M. Molecular phylogeny of cuckoos supports a polyphyletic orgin of brood parasitism. J. Evol. Biol. 1999;12:495–506. doi:10.1046/j.1420-9101.1999.00052.x [Google Scholar]
- Arcese P, Smith J.N.M, Hatch M.I. Nest predation by cowbirds and its consequences for passerine demography. Proc. Natl Acad. Sci. USA. 1996;93:4608–4611. doi: 10.1073/pnas.93.10.4608. doi:10.1073/pnas.93.10.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles J.M, Soler J.J, Perez-Contreras T, Soler M, Mller A.P. Ultraviolet reflectance of great spotted cuckoo eggs and egg discrimination by magpies. Behav. Ecol. 2006;17:310–314. doi:10.1093/beheco/arj031 [Google Scholar]
- Baker E.C.S. The evolution of adaptation in parasitic cuckoos' eggs. Ibis. 1913;1913:384–398. [Google Scholar]
- Barabas L, Gilicze B, Takasu F, Moskat C. Survival and anti-parasite defense in a host metapopulation under heavy brood parasitism: a source–sink dynamic model. J. Ethol. 2004;22:143–151. [Google Scholar]
- Brooke M. de L, Davies N.B. Egg mimicry by cuckoos, Cuculus canorus, in relation to discrimination by hosts. Nature. 1988;335:630–632. doi:10.1038/335630a0 [Google Scholar]
- Brooke M. de L, Davies N.B, Noble D.G. Rapid decline of host defences in response to reduced cuckoo parasitism: behavioural flexibility of reed warblers in a changing world. Proc. R. Soc. B. 1998;265:1277–1282. doi:10.1098/rspb.1998.0430 [Google Scholar]
- Brooker M.G, Brooker L.C. Eggshell strength in cuckoos and cowbirds. Ibis. 1991;133:406–413. [Google Scholar]
- Brooker M.G, Brooker L.C. Acceptance by the splendid fairy-wren of parasitism by Horsfield's bronze-cuckoo: further evidence for evolutionary equilibrium in brood parasitism. Behav. Ecol. 1996;7:395–407. [Google Scholar]
- Butchart S.H.M, Kilner R.M, Fuisz T, Davies N.B. Differences in the nestling begging calls of hosts and host-races of the common cuckoo, Cuculus canorus. Anim. Behav. 2003;65:345–354. doi:10.1006/anbe.2003.2066 [Google Scholar]
- Caswell H. 2nd edn. Sinauer Associates; Sunderland, MA: 2001. Matrix population models. [Google Scholar]
- Cherry M.I, Bennett A.T.D. Egg colour matching in an African cuckoo, as revealed by ultraviolet–visible reflectance spectrophotometry. Proc. R. Soc. B. 2001;268:565–571. doi: 10.1098/rspb.2000.1414. doi:10.1098/rspb.2000.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotfelter E.D. What cues do brown-headed cowbirds use to locate red-winged blackbird host nests? Anim. Behav. 1998;55:1181–1189. doi: 10.1006/anbe.1997.0638. doi:10.1006/anbe.1997.0638 [DOI] [PubMed] [Google Scholar]
- Cunningham E.J.A, Lewis S. Parasitism of maternal investment selects for increased clutch size and brood reduction in a host. Behav. Ecol. 2006;17:126–131. doi:10.1093/beheco/arj006 [Google Scholar]
- Darwin C. John Murray; London, UK: 1859. The origin of species by means of natural selection. [Google Scholar]
- Davies N.B. Cuckoos and cowbirds versus hosts: co-evolutionary lag and equilibrium. Ostrich. 1999;70:71–79. [Google Scholar]
- Davies N.B. T. & A. D. Poyser; London, UK: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M. de L. Cuckoos versus reed warblers: adaptations and counter-adaptations. Anim. Behav. 1988;36:262–284. [Google Scholar]
- Davies N.B, Brooke M. de L. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 1989a;58:207–224. [Google Scholar]
- Davies N.B, Brooke M. de L. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 1989b;58:225–236. [Google Scholar]
- Davies N.B, Brooke M. de L, Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic eggs. Proc. R. Soc. B. 1996;263:925–931. [Google Scholar]
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;263:925–931. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Davies N.B, Butchart S.H.M, Burke T.A, Chaline N, Stewart I.R.K. Reed warblers guard against cuckoos and cuckoldry. Anim. Behav. 2003;65:285–295. doi:10.1006/anbe.2003.2049 [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M, Rutila J. A host race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. Proc. R. Soc. B. 2006;273:693–699. doi: 10.1098/rspb.2005.3324. doi:10.1098/rspb.2005.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn D.C. Begging behaviour and food acquisition by brown-headed cowbird nestlings. Behav. Ecol. Sociobiol. 1998;43:259–270. doi:10.1007/s002650050490 [Google Scholar]
- Dearborn D.C. Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk. 1999;116:448–457. [Google Scholar]
- Duckworth J.W. Responses of breeding reed warblers Acrocephalus scirpaceus to mounts of sparrowhawk Accipiter nisus, cuckoo Cuculus canorus and jay Garrulus glandarius. Ibis. 1991;133:68–74. [Google Scholar]
- Forslund P, Pärt T. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. doi:10.1016/S0169-5347(00)89141-7 [DOI] [PubMed] [Google Scholar]
- Friedmann H. C.C. Thomas; Springfield, IL: 1929. The cowbirds: a study in the biology of social parasitism. [Google Scholar]
- Fry C.H. Vocal mimesis in greater honey-guides. Bull. Br. Ornithol. Club. 1974;94:58–59. [Google Scholar]
- Gibbs H.L, Sorenson M.D, Marchetti M, Brooke M.L, Davies N.B, Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. doi:10.1038/35025058 [DOI] [PubMed] [Google Scholar]
- Gill S.A, Grieef P.M, Staib L.M, Sealy S.G. Does nest defence deter or facilitate cowbird parasitism? A test of the nesting-cue hypothesis. Ethology. 1997;103:56–71. [Google Scholar]
- Grim T. Why is mimicry in cuckoo eggs sometimes so poor? J. Avian Biol. 2002;33:202–205. doi:10.1034/j.1600-048X.2002.330312.x [Google Scholar]
- Grim T. Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 2005;84:69–78. doi:10.1111/j.1095-8312.2005.00414.x [Google Scholar]
- Grim T, Kleven O, Mikulica O. Nestling discrimination without recognition: a possible defence mechanism for hosts towards cuckoo parasitism? Proc. R. Soc. B. 2003;270(Suppl. 1):S73–S75. doi: 10.1098/rsbl.2003.0017. doi:10.1098/rsbl.2003.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G, Ros A.F.H. The hormonal control of begging and early aggressive behaviour: experiments in black-headed gull chicks. Horm. Behav. 2005;48:207–215. doi: 10.1016/j.yhbeh.2005.02.009. doi:10.1016/j.yhbeh.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Gross M.R. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Hauber M.E. Site selection and repeatability in brown-headed cowbird (Molothrus ater) parasitism of eastern phoebe (Sayornis phoebe) nests. Can. J. Zool. 2001;79:1518–1523. doi:10.1139/cjz-79-8-1518 [Google Scholar]
- Hauber M.E. Interspecific brood parasitism and the evolution of host clutch sizes. Evol. Ecol. Res. 2003;5:559–570. [Google Scholar]
- Hauber M.E, Dearborn D.C. Parentage without parental care: what to look for in genetic studies of obligate brood-parasitic mating systems. Auk. 2003;120:1–13. [Google Scholar]
- Hauber M.E, Pilz K.M. Yolk testosterone levels are not consistently higher in the eggs of obligate brood parasites than their hosts. Am. Midland Nat. 2003;149:354–362. [Google Scholar]
- Hauber M.E, Ramsey C.K. Honesty in host–parasite communication signals: the case for begging by fledgling brown-headed cowbirds Molothrus ater. J. Avian Biol. 2003;34:339–344. doi:10.1111/j.0908-8857.2003.03158.x [Google Scholar]
- Hauber M.E, Yeh P.J, Roberts J.O.L. Patterns and coevolutionary consequences of repeated brood parasitism. Proc. R. Soc. B. 2004;271(Suppl.):S317–S320. doi: 10.1098/rsbl.2004.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hett V.S. Heinemann; London, UK: 1936. Aristotle: minor works. On marvellous songs heard. [Google Scholar]
- Hill D.P, Sealy S.G. Desertion of nests parasitised by cowbirds: have clay-colored sparrows evolved an anti-parasite strategy? Anim. Behav. 1994;48:1063–1070. doi:10.1006/anbe.1994.1340 [Google Scholar]
- Honza M, Picman J, Grim T, Novak V, Capek M, Jr, Mrlik V. How to hatch from an egg of great structural strength. A case study of the common cuckoo. J. Avian Biol. 2001;32:249–255. doi:10.1111/j.0908-8857.2001.320307.x [Google Scholar]
- Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E. Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim. Behav. 2002;64:861–868. doi:10.1006/anbe.2002.1969 [Google Scholar]
- Honza M, Prochazka P, Stokke B.G, Moksnes A, Røskaft E, Capek M, Jr, Mrlik V. Are blackcaps current winners in the evolutionary struggle against the common cuckoo? J. Ethol. 2004;22:175–180. doi:10.1007/s10164-004-0119-1 [Google Scholar]
- Hoover J.P. Multiple effects of brood parasitism reduce the reproductive success of prothonotary warblers, Protonotaria citrea. Anim. Behav. 2003;65:923–934. doi:10.1006/anbe.2003.2155 [Google Scholar]
- Jenner E. Observations on the natural history of the cuckoo. Phil. Trans. R. Soc. B. 1788;78:219–237. [Google Scholar]
- Jensen W.E, Cully J.F. Density-dependent habitat selection by brown-headed cowbirds (Molothrus ater) in tallgrass prairie. Oecologia. 2005;142:136–149. doi: 10.1007/s00442-004-1709-x. doi:10.1007/s00442-004-1709-x [DOI] [PubMed] [Google Scholar]
- Kattan G.H. Mechanisms of short incubation period in brood-parasitic cowbirds. Auk. 1995;112:335–342. [Google Scholar]
- Kilner R.M, Noble D.G, Davies N.B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature. 1999;397:667–672. doi:10.1038/17746 [Google Scholar]
- Kilner R.M, Madden J.R, Hauber M.E. Brood parasitic cowbird nestlings use host young to procure resources. Science. 2004;305:877–879. doi: 10.1126/science.1098487. doi:10.1126/science.1098487 [DOI] [PubMed] [Google Scholar]
- Kilpatrick A.M. Variation in growth of brown-headed cowbird (Molothrus ater) nestlings and energetic impacts on their host parents. Can. J. Zool. 2002;80:145–153. doi:10.1139/z01-217 [Google Scholar]
- Kleven O, Moksnes A, Røskaft E, Honza M. Host species affects the growth rate of cuckoo (Cuculus carnorus) chicks. Behav. Ecol. Sociobiol. 1999;47:41–46. doi:10.1007/s002650050647 [Google Scholar]
- Kleven O, Moksnes A, Røskaft E, Rudolfsen G, Stokke B.G, Honza M. Breeding success of common cuckoos Cuculus canorus parasitising four sympatric species of Acrocephalus warblers. J. Avian Biol. 2004;35:394–398. doi:10.1111/j.0908-8857.2004.03359.x [Google Scholar]
- Krüger O. Breeding biology of the Cape Bulbul Pycnonotus capensis: a 40-year comparison. Ostrich. 2004;75:211–216. [Google Scholar]
- Krüger O, Davies N.B. The evolution of cuckoo parasitism: a comparative analysis. Proc. R. Soc. B. 2002;269:375–381. doi: 10.1098/rspb.2001.1887. doi:10.1098/rspb.2001.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger O, Davies N.B. The evolution of egg size in the brood parasitic cuckoos. Behav. Ecol. 2004;15:210–218. doi:10.1093/beheco/arg104 [Google Scholar]
- Krüger O, Lindström J. Lifetime reproductive success in common buzzard Buteo buteo: from individual variation to population demography. Oikos. 2001;93:260–273. doi:10.1034/j.1600-0706.2001.930209.x [Google Scholar]
- Lahti D.C. Evolution of bird eggs in the absence of cuckoo parasitism. Proc. Natl Acad. Sci. USA. 2005;102:18 057–18 062. doi: 10.1073/pnas.0508930102. doi:10.1073/pnas.0508930102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti D.C, Lahti A.R. How precise is egg discrimination in weaverbirds? Anim. Behav. 2002;63:1135–1142. doi:10.1006/anbe.2002.3009 [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Lichtenstein G. Selfish begging by screaming cowbirds, a mimetic brood parasite of the bay-winged cowbird. Anim. Behav. 2001;61:1151–1158. doi:10.1006/anbe.2000.1688 [Google Scholar]
- Lichtenstein G, Sealy S.G. Nestling competition, rather than supernormal stimulus, explains the success of parasitic brown-headed cowbird chicks in yellow warbler nests. Proc. R. Soc. B. 1998;265:249–254. doi:10.1098/rspb.1998.0289 [Google Scholar]
- Lindholm A.K. Brood parasitism by the cuckoo on patchy reed warbler populations in Britain. J. Anim. Ecol. 1999;68:293–309. doi:10.1046/j.1365-2656.1999.00286.x [Google Scholar]
- Liversidge R. Pre-incubation development of Clamator jacobinus. Ibis. 1961;103:624. [Google Scholar]
- Liversidge R. The biology of the Jacobin Cuckoo Clamator jacobinus. Ostrich. 1970;8(Suppl.):117–137. [Google Scholar]
- Lorenzana J.C, Sealy S.G. Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav. Ecol. 2001;12:325–329. doi:10.1093/beheco/12.3.325 [Google Scholar]
- Lotem A. Learning to recognize nestlings is maladaptive for cuckoo Cuculus carnorus hosts. Nature. 1993;362:743–745. doi:10.1038/362743a0 [Google Scholar]
- Lotem A, Nakamura H, Zahavi A. Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behav. Ecol. 1992;3:128–132. [Google Scholar]
- Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo–host co-evolution. Anim. Behav. 1995;49:1185–1209. doi:10.1006/anbe.1995.0152 [Google Scholar]
- Lovaszi P, Moskat C. Break-down of arms race between the red-backed shrike (Lanius collurio) and common cuckoo (Cuculus canorus) Behaviour. 2004;141:245–262. doi:10.1163/156853904322890843 [Google Scholar]
- Lyon B.E, Eadie J.M. Mode of development and interspecific avian brood parasitism. Behav. Ecol. 1991;2:309–318. [Google Scholar]
- Marchetti K. Costs to host defence and the persistence of parasitic cuckoos. Proc. R. Soc. B. 1992;248:41–45. doi: 10.1098/rspb.1992.0040. [DOI] [PubMed] [Google Scholar]
- Marchetti K. Egg rejection in a passerine bird: size does matter. Anim. Behav. 2000;59:877–883. doi: 10.1006/anbe.1999.1388. doi:10.1006/anbe.1999.1388 [DOI] [PubMed] [Google Scholar]
- Marchetti K, Nakamura H, Gibbs H.L. Host-race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. doi:10.1126/science.282.5388.471 [DOI] [PubMed] [Google Scholar]
- Martinez J.G, Burke T, Dawson D, Soler J.J, Soler M, Møller A.P. Microsatellite typing reveals mating patterns in the brood parasitic great spotted cuckoo Clamator glandarius. Mol. Ecol. 1998;7:289–297. doi:10.1046/j.1365-294X.1998.00348.x [Google Scholar]
- Mason P, Rothstein S.I. Coevolution and avian brood parasitism—cowbird eggs show evolutionary response to host discrimination. Evolution. 1986;40:1207–1214. doi: 10.1111/j.1558-5646.1986.tb05745.x. doi:10.2307/2408948 [DOI] [PubMed] [Google Scholar]
- Massoni V, Reboreda J.C. Costs of brood parasitism and the lack of defences in the yellow-winged blackbird–shiny cowbird system. Behav. Ecol. Sociobiol. 1998;42:273–280. doi:10.1007/s002650050439 [Google Scholar]
- Massoni V, Reboreda J.C. A neglected cost of brood parasitism: egg punctures by shiny cowbirds during inspection of potential host nests. Condor. 2002;104:407–412. [Google Scholar]
- McLean I.G, Maloney R.F. Brood parasitism, recognition, and response. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts: studies in coevolution. Oxford University Press; Oxford, UK: 1998. pp. 255–269. [Google Scholar]
- McMaster D.G, Sealy S.G. Short incubation periods of brown-headed cowbirds: how do cowbird eggs hatch before yellow warbler eggs? Condor. 1998;100:102–111. [Google Scholar]
- Mermoz M.E, Ornelas J.F. Phylogenetic analysis of life-history adaptations in parasitic cowbirds. Behav. Ecol. 2004;15:109–119. doi:10.1093/beheco/arg102 [Google Scholar]
- Mikhailov, K. E. 1997 Avian eggshells: an atlas of scanning electron micrographs Br. Ornithol. Club Occ. Pub. No. 3.
- Moksnes A, Røskaft E, Braa A.T, Korsnes L, Lampe H.M, Pedersen H.C. Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour. 1991a;116:64–89. [Google Scholar]
- Moksnes A, Røskaft E, Braa A.T. Rejection behaviour by common cuckoo hosts towards artificial brood parasitic eggs. Auk. 1991b;108:348–354. [Google Scholar]
- Moskat C, Honza M. Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography. 2000;23:335–341. doi:10.1034/j.1600-0587.2000.d01-1642.x [Google Scholar]
- Nakamura H, Miyazawa Y. Movements, space-use and social organisation of radio-tracked common cuckoos during the breeding season in Japan. Jpn. J. Ornithol. 1997;46:23–54. [Google Scholar]
- Nakamura H, Kubota S, Suzuki R. Coevolution between the common cuckoo and its major hosts in Japan: stable versus dynamic specialization on hosts. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts: studies in coevolution. Oxford University Press; Oxford, UK: 1998. pp. 94–112. [Google Scholar]
- Øien I.J, Moksnes A, Røskaft E. Evolution of variation in egg color and marking pattern in European passerines: adaptations in a coevolutionary arms race with the cuckoo, Cuculus canorus. Behav. Ecol. 1995;6:166–174. [Google Scholar]
- Øien I.J, Honza M, Moksnes A, Røskaft E. The risk of parasitism in relation to the distance from reed warbler nests to cuckoo perches. J. Anim. Ecol. 1996;65:147–153. [Google Scholar]
- Ortega C.P. University of Arizona Press; Tucson, AZ: 1998. Cowbirds and other brood parasites. [Google Scholar]
- Pagel M.D. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Payne R.B. The evolution of clutch size and reproductive rates in parasitic cuckoos. Evolution. 1974;28:169–181. doi: 10.1111/j.1558-5646.1974.tb00738.x. doi:10.2307/2407320 [DOI] [PubMed] [Google Scholar]
- Payne R.B. Oxford University Press; Oxford, UK: 2005a. The cuckoos. [Google Scholar]
- Payne R.B. Nestling mouth markings and colors of Old World finches Estrildidae: mimicry and coevolution of nesting finches and their Vidua brood parasites. Misc. Publ. Univ. Mich. Museum Zool. 194. 2005b [Google Scholar]
- Payne R.B, Payne L.L. Brood parasitism by brown-headed cowbirds: risks and costs on reproductive success and survival in indigo buntings. Behav. Ecol. 1998;9:64–73. [Google Scholar]
- Payne R.B, Payne L.L, Woods J.L, Sorenson M.D. Imprinting and the origin of parasite–host species associations in brood parasitic indigobirds Vidua chalybeata. Anim. Behav. 2000;59:69–81. doi: 10.1006/anbe.1999.1283. doi:10.1006/anbe.1999.1283 [DOI] [PubMed] [Google Scholar]
- Payne R.B, Woods J.L, Payne L.L. Parental care in estrildid finches: experimental tests of a colonization model of Vidua brood parasitism. Anim. Behav. 2001;62:473–483. doi:10.1006/anbe.2001.1773 [Google Scholar]
- Peer B.D, Sealy S.G. Correlates of egg rejection in hosts of the brown-headed cowbird. Condor. 2004;106:580–599. [Google Scholar]
- Picman J. Mechanism of increased puncture resistance of eggs of brown-headed cowbirds. Auk. 1989;106:577–583. [Google Scholar]
- Picman J, Pribil S. Is greater eggshell density an alternative mechanism by which parasitic cuckoos increase the strength of their eggs? J. Ornithol. 1997;138:531–541. doi:10.1007/BF01651384 [Google Scholar]
- Redondo T. Exploitation of host mechanisms for parental care by avian brood parasites. Etologia. 1993;3:235–297. [Google Scholar]
- Robertson R.J, Norman R.F. Behavioural defences to brood parasitism by potential hosts of the brown-headed cowbird. Condor. 1976;78:166–173. [Google Scholar]
- Røskaft E, Moksnes A, Stokke B.A, Bicik V, Moskat C. Aggression to dummy cuckoos by potential European cuckoo hosts. Behaviour. 2002a;139:613–628. doi:10.1163/15685390260136735 [Google Scholar]
- Røskaft E, Moksnes A, Stokke B.A, Moskat C, Honza M. The spatial habitat structure of host populations explains the pattern of rejection behaviour in hosts and parasitic adaptations in cuckoos. Behav. Ecol. 2002b;13:163–168. doi:10.1093/beheco/13.2.163 [Google Scholar]
- Rothstein S.I. Evolutionary rates and host defenses against avian brood parasitism. Am. Nat. 1975;109:161–176. doi:10.1086/282984 [Google Scholar]
- Rothstein S.I. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 1990;21:481–508. doi:10.1146/annurev.es.21.110190.002405 [Google Scholar]
- Rothstein S.I. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim. Behav. 2001;61:95–107. doi: 10.1006/anbe.2000.1570. doi:10.1006/anbe.2000.1570 [DOI] [PubMed] [Google Scholar]
- Rothstein S.I, Robinson S.K. Conservation and coevolutionary implications of brood parasitism by cowbirds. Trends Ecol. Evol. 1994;9:162–164. doi: 10.1016/0169-5347(94)90077-9. doi:10.1016/0169-5347(94)90077-9 [DOI] [PubMed] [Google Scholar]
- Rothstein S.I, Robinson S.K. Oxford University Press; Oxford, UK: 1998. Parasitic birds and their hosts: studies in coevolution. [Google Scholar]
- Sæther B.E. Age specific variation on reproductive performance of birds. Curr. Ornithol. 1990;7:251–283. [Google Scholar]
- Schönwetter, M. 1964 Handbuch der Oologie. Band 1, Nonpasseres. Cuculiformes: Nos. 9, 10. (ed. W. Meise). Berlin: Akademie.
- Sealy S.G. Burial of cowbird eggs by parasitised yellow warblers: an empirical and experimental study. Anim. Behav. 1995;49:877–889. doi:10.1006/anbe.1995.0120 [Google Scholar]
- Servedio M.R, Lande R. Coevolution of an avian host and its parasitic cuckoo. Evolution. 2003;57:1164–1175. doi: 10.1111/j.0014-3820.2003.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Smith J.N.M, Arcese P, McLean I.G. Age, experience, and enemy recognition by wild song sparrows. Behav. Ecol. Sociobiol. 1984;14:101–106. doi:10.1007/BF00291901 [Google Scholar]
- Smith J.N.M, Taitt M.J, Zanette L. Removing brown-headed cowbirds increases seasonal fecundity and population growth in song sparrows. Ecology. 2002;83:3037–3047. [Google Scholar]
- Soler M, Soler J.J, Martinez J.G, Møller A.P. Micro-evolutionary change in host response to a brood parasite. Behav. Ecol. Sociobiol. 1994;35:295–301. doi:10.1007/s002650050100 [Google Scholar]
- Soler J.J, Soler M, Møller A.P, Martinez J.G. Does the great spotted cuckoo choose magpie hosts according to their parenting ability? Behav. Ecol. Sociobiol. 1995;36:201–206. doi:10.1007/s002650050141 [Google Scholar]
- Soler M, Soler J.J, Martinez J.G, Perez-Contreras T, Møller A.P. Micro-evolutionary change and population dynamics of a brood parasite and its primary host: the intermittent arms race hypothesis. Oecologia. 1998;117:381–390. doi: 10.1007/s004420050671. doi:10.1007/s004420050671 [DOI] [PubMed] [Google Scholar]
- Soler J.J, Sorci G, Soler M, Møller A.P. Change in host rejection behaviour mediated by the predatory behaviour of its brood parasite. Behav. Ecol. 1999;10:275–280. doi:10.1093/beheco/10.3.275 [Google Scholar]
- Soler J.J, Martinez J.G, Soler M, Møller A.P. Life history of magpie populations sympatric or allopatric with the brood-parasitic great spotted cuckoo. Ecology. 2001;82:1621–1631. doi:10.2307/2679805 [Google Scholar]
- Sorenson M.D, Payne R.B. Molecular genetic perspectives on avian brood parasitism. Integr. Comp. Biol. 2002;42:388–400. doi: 10.1093/icb/42.2.388. doi:10.1093/icb/42.2.388 [DOI] [PubMed] [Google Scholar]
- Sorenson M.D, Payne R.B. A molecular genetic analysis of cuckoo phylogeny. In: Payne R.B, editor. The cuckoos. Oxford University Press; Oxford, UK: 2005. pp. 68–94. [Google Scholar]
- Sorenson M.D, Sefc K.M, Payne R.B. Speciation by host switch in brood parasitic indigobirds. Nature. 2003;424:928–931. doi: 10.1038/nature01863. doi:10.1038/nature01863 [DOI] [PubMed] [Google Scholar]
- Sorenson M.D, Balakrishnan C.N, Payne R.B. Clade-limited colonization in brood parasitic finches (Vidua spp.) Syst. Biol. 2004;53:140–153. doi: 10.1080/10635150490265021. doi:10.1080/10635150490265021 [DOI] [PubMed] [Google Scholar]
- Stokke B.G, Moksnes A, Røskaft E, Rudolfsen G, Honza M. Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocephalus scirpaceus) Proc. R. Soc. B. 1999;266:1483–1488. doi:10.1098/rspb.1999.0804 [Google Scholar]
- Stokke B.G, Moksnes A, Røskaft E. Obligate brood parasites as selective agents for evolution of egg appearance in passerine birds. Evolution. 2002;56:199–205. doi: 10.1111/j.0014-3820.2002.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Strausberger B.M, Ashley M.V. Breeding biology of brood parasitic cowbirds characterized by parent–offspring and sibgroup reconstruction. Auk. 2003;120:433–445. [Google Scholar]
- Takasu F. Why do all host species not show defence against avian brood parasitism: evolutionary lag or equilibrium? Am. Nat. 1998;151:193–205. doi: 10.1086/286111. doi:10.1086/286111 [DOI] [PubMed] [Google Scholar]
- Takasu F. Co-evolutionary dynamics of egg appearance in avian brood parasitism. Evol. Ecol. Res. 2003;5:345–362. [Google Scholar]
- Takasu F, Kawasaki K, Nakamura H, Cohen J.E, Shigesada N. Modelling the population dynamics of a cuckoo–host association and the evolution of host defences. Am. Nat. 1993;142:819–839. doi: 10.1086/285574. doi:10.1086/285574 [DOI] [PubMed] [Google Scholar]
- Tanaka K.D, Ueda K. Horsfield's hawk cuckoo nestlings simulate multiple gapes for begging. Science. 2005;308:653. doi: 10.1126/science.1109957. doi:10.1126/science.1109957 [DOI] [PubMed] [Google Scholar]
- Teuschl Y, Taborsky B, Taborsky M. How do cuckoos find their hosts? The role of habitat imprinting. Anim. Behav. 1998;56:1425–1433. doi: 10.1006/anbe.1998.0931. doi:10.1006/anbe.1998.0931 [DOI] [PubMed] [Google Scholar]
- Tewksbury J.J, Martin T.E, Hejl S.J, Kuehn M.J, Jenkins J.W. Parental care of a cowbird host: caught between the costs of egg-removal and nest predation. Proc. R. Soc. B. 2002;269:423–429. doi: 10.1098/rspb.2001.1894. doi:10.1098/rspb.2001.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torök J, Moskat C, Michl G, Peczely P. Common cuckoos (Cuculus canorus) lay eggs with larger yolk but not more testosterone than their great reed warbler (Acrocephalus arundinaceus) hosts. Ethol. Ecol. Evol. 2004;16:271–277. [Google Scholar]
- Trine C, Robinson W.D, Robinson S.K. Consequences of brown-headed cowbird brood parasitism for host population dynamics. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts: studies in coevolution. Oxford University Press; Oxford, UK: 1998. pp. 273–295. [Google Scholar]
- Victoria J.K. Clutch characteristics and egg discriminate ability of the African village weaverbird Ploceus cucullatus. Ibis. 1972;114:367–376. [Google Scholar]
- Vogl W, Taborsky M, Taborsky B, Teuschl Y, Honza M. Cuckoo females preferentially use specific habitats when searching for host nests. Anim. Behav. 2002;64:843–850. doi:10.1006/anbe.2003.1967 [Google Scholar]
- Vogl W, Taborsky B, Taborsky M, Teuschl Y, Honza M. Habitat and space use of European cuckoo females during the egg laying period. Behaviour. 2004;141:881–898. doi:10.1163/1568539042265671 [Google Scholar]
- White G. In: The natural history of Selbourne. Mabey R, editor. Penguin Press; Harmondsworth, UK: 1789. [Google Scholar]
- Winfree R. Cuckoos, cowbirds and the persistence of brood parasitism. Trends Ecol. Evol. 1999;14:338–342. doi: 10.1016/s0169-5347(99)01643-2. doi:10.1016/S0169-5347(99)01643-2 [DOI] [PubMed] [Google Scholar]
- Woolfenden B.E, Gibbs H.L, Sealy S.G, McMaster D.G. Host use and fecundity of individual female brown-headed cowbirds. Anim. Behav. 2003;66:95–105. doi:10.1006/anbe.2003.2181 [Google Scholar]
- Zahavi A. Parasitism and nest predation in parasitic cuckoos. Am. Nat. 1979;113:157–159. doi:10.1086/283374 [Google Scholar]
- Zahavi A, Zahavi A. Oxford University Press; Oxford, UK: 1997. The handicap principle: a missing piece of Darwin's puzzle. [Google Scholar]
- Zanette L, MacDougall-Shakleton E, Clinchy M, Smith J.N.M. Brown-headed cowbirds skew host offspring sex ratios. Ecology. 2005;86:815–820. [Google Scholar]